The Use of Platelet-Rich Fibrin in Combination with Synthetic Bone Grafting: A Systematic Review
Abstract
1. Introduction
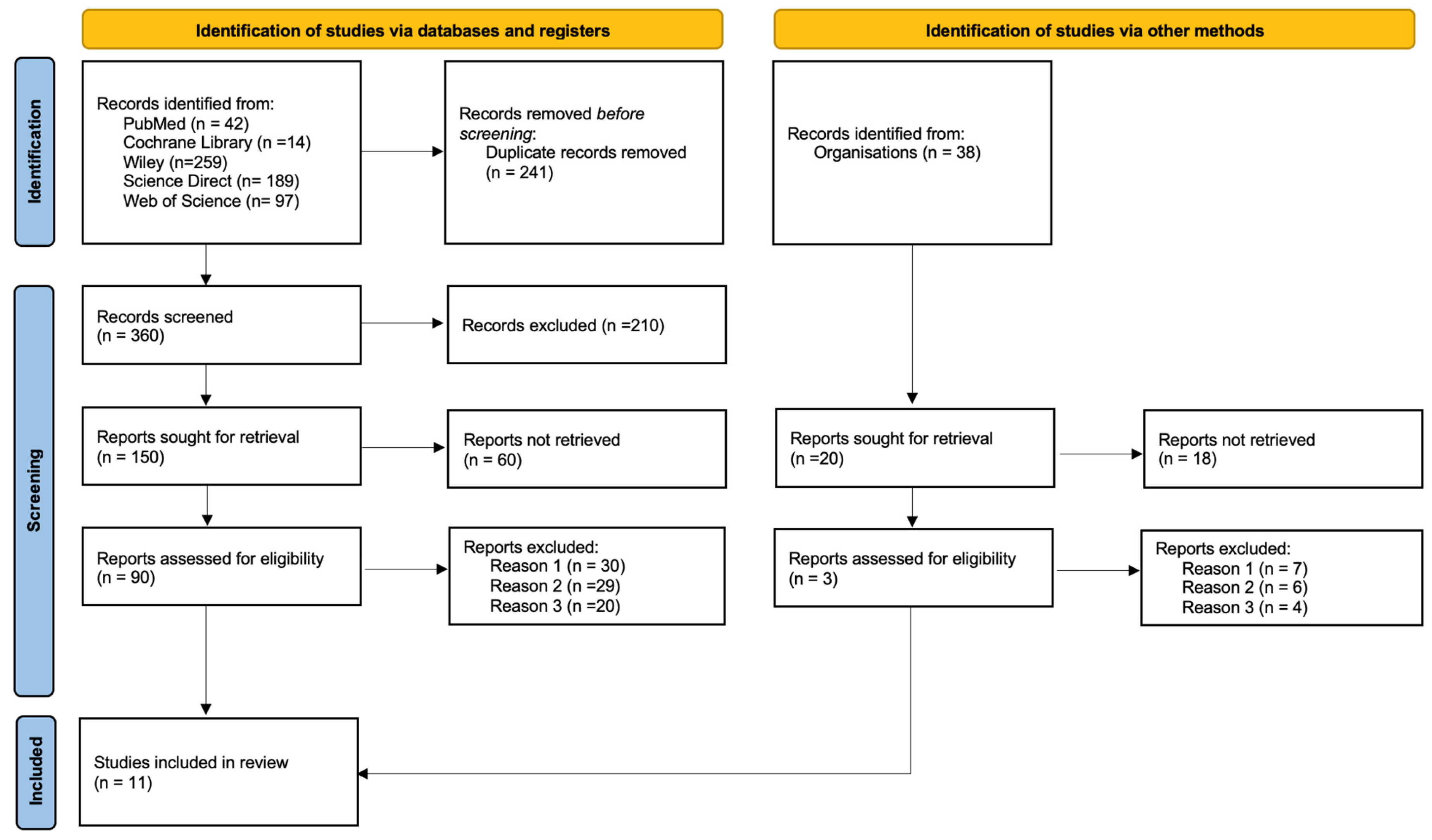
2. Materials and Methods
2.1. Sample Data Extraction
2.2. Quality Assessment and Risk of Bias
3. Results
3.1. Sample Characteristics for Study Quality
3.2. Characteristics of the Included Studies
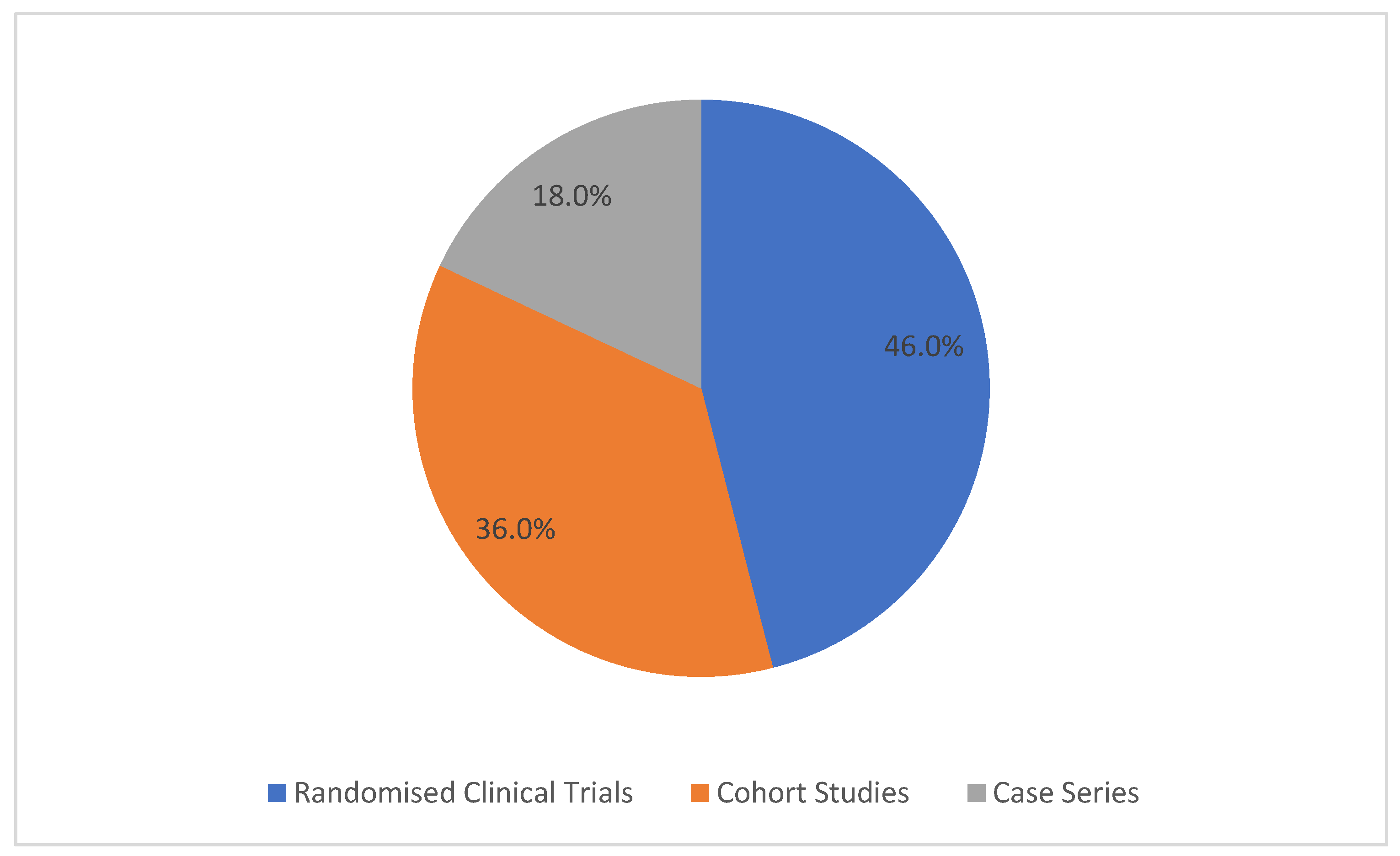
3.3. Study Designs
4. Discussion
4.1. Subantral Surgery
4.2. Synthetic Bone Substitutes in Sub-Antral Surgery
4.3. PRF and Its Application in Sub-Antral Surgeries
4.4. Synthetic Bone Versus PRF
4.5. Complications and Perforation of Schneider’s Membrane
4.6. Histology, Bone Regeneration, and New Bone Formation
Quantitative Analysis: Bone Formed, Residual Biomaterial, and Soft Tissue
4.7. Survival Rate and Stability
4.8. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-PRF | Advanced Platelet-Rich Fibrin |
| ASA | American Society of Anaesthesiologists Physical Status Classification |
| β-TCP | Beta-Tricalcium Phosphate |
| BIC | Bone-to-Implant Contact |
| BOP | Bleeding on Probing |
| CBCT | Cone Beam Computed Tomography |
| CS | Calcium Sulphate |
| HA | Hydroxyapatite |
| IT | Insertion Torque |
| JBI | Joanna Briggs Institute |
| L-PRF | Leucocyte- and Platelet-Rich Fibrin |
| NR | Not Reported |
| PB | Bacterial Plaque |
| PRF | Platelet-Rich Fibrin |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| PRGF | Plasma Rich in Growth Factors |
| PRP | Platelet-Rich Plasma |
| REC | Gingival Recession |
| RFA | Resonance Frequency Analysis |
References
- Jatana, C.; Towning, C.; Guo, X.; Ni, A.; Towning, L. The Addition of Platelet-Rich-Fibrin in Socket Preservation for Future Dental Implant Placement: A Randomized Controlled Clinical Trial. J. Oral Maxillofac. Surg. 2019, 77, e26. [Google Scholar] [CrossRef]
- Ting, M.; Rice, J.G.; Braid, S.M.; Lee, C.Y.S.; Suzuki, J.B. Maxillary Sinus Augmentation for Dental Implant Rehabilitation of the Edentulous Ridge: A Comprehensive Overview of Systematic Reviews. Implant Dent. 2017, 26, 438–464. [Google Scholar] [CrossRef]
- Barbu, H.M.; Andreescu, C.F.; Comaneanu, M.R.; Referendaru, D.; Mijiritsky, E. Maxillary Sinus Floor Augmentation to Enable One-Stage Implant Placement by Using Bovine Bone Substitute and Platelet-Rich Fibrin. BioMed Res. Int. 2018, 2018, 6562958. [Google Scholar] [CrossRef]
- Liu, R.; Yan, M.; Chen, S.; Huang, W.; Wu, D.; Chen, J. Effectiveness of Platelet-Rich Fibrin as an Adjunctive Material to Bone Graft in Maxillary Sinus Augmentation: A Meta-Analysis of Randomized Controlled Trails. BioMed Res. Int. 2019, 2019, 7267062. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.; Mendes, J.M.; Salazar, F.; Pacheco, J.J.; Rompante, P.; Câmara, M.I. Analysis of peri-implant bone defects by using cone beam computed tomography (CBCT): An integrative review. Oral Radiol. 2023, 39, 455–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tatum, H. Maxillary and sinus implant reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Kühl, S.; Kirmeier, R.; Platzer, S.; Bianco, N.; Jakse, N.; Payer, M. Transcrestal maxillary sinus augmentation: Summers’ versus a piezoelectric technique—An experimental cadaver study. Clin. Oral Implant. Res. 2016, 27, 126–129. [Google Scholar] [CrossRef]
- Aoki, N.; Maeda, M.; Kurata, M.; Hirose, M.; Ojima, Y.; Wada, K.; Shibuya, Y. Sinus floor elevation with platelet-rich fibrin alone: A Clinical retrospective study of 1–7 years. J. Clin. Exp. Dent. 2018, 10, e984–e991. [Google Scholar] [CrossRef]
- Summers, R.B. Sinus floor elevation with osteotomes. J. Esthet. Restor. Dent. 1998, 10, 164–171. [Google Scholar] [CrossRef]
- Fontes Pereira, J.; Costa, R.; Nunes Vasques, M.; Salazar, F.; Mendes, J.M.; Infante da Câmara, M. Osseodensification: An Alternative to Conventional Osteotomy in Implant Site Preparation: A Systematic Review. J. Clin. Med. 2023, 12, 7046. [Google Scholar] [CrossRef]
- Fontes Pereira, J.; Costa, R.; Nunes Vasques, M.; Relvas, M.; Braga, A.C.; Salazar, F.; Infante da Câmara, M. The Effectiveness of Osseodensification Drilling versus the Conventional Surgical Technique on Implant Stability: A Clinical Trial. J. Clin. Med. 2024, 13, 2912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa, J.A.; Mendes, J.M.; Salazar, F.; Pacheco, J.J.; Rompante, P.; Moreira, J.F.; Mesquita, J.D.; Adubeiro, N.; da Câmara, M.I. Osseodensification vs. Conventional Osteotomy: A Case Series with Cone Beam Computed Tomography. J. Clin. Med. 2024, 13, 1568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- CaramÍs, J.; Vieira, F.; Simıes, A.; Marques, H.; CaramÍs, G. A successful Schneider membrane perforation repair with leucocyte platelet rich fibrin (L-PRF)—A clinical case report. Clin. Oral Implant. Res. 2019, 30, 176–178. [Google Scholar] [CrossRef]
- Carreño, J.C.; Aguilar-Salvatierra, A.; Gómez-Moreno, G.; Carreño, E.M.G.; López-Mateos, M.L.M.; Piattelli, A.; Calvo-Guirado, J.L.; Menéndez-Núñez, M. Update of Surgical Techniques for Maxillary Sinus Augmentation: A Systematic Literature Review. Implant Dent. 2016, 25, 839–844. [Google Scholar] [CrossRef]
- Romano, M.M.; Smanio, J.A.; Ferreira, L.B.; Arana-Chavez, V.E.; Soares, M.S. Histological and Radiological Analyses of a Maxillary Sinus Lift with Extensive Drilling of the Schneider Membrane Using Xenogeneic Bone. Case Rep. Dent. 2014, 2014, 898031. [Google Scholar] [CrossRef]
- Karagah, A.; Tabrizi, R.; Mohammadhosseinzade, P.; Mirzadeh, M.; Tofangchiha, M.; Lajolo, C.; Patini, R. Effect of Sinus Floor Augmentation with Platelet-Rich Fibrin Versus Allogeneic Bone Graft on Stability of One-Stage Dental Implants: A Split-Mouth Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 9569. [Google Scholar] [CrossRef]
- Solakoglu, Ö.; Heydecke, G.; Amiri, N.; Anitua, E. The use of plasma rich in growth factors (PRGF) in guided tissue regeneration and guided bone regeneration. A review of histological, immunohistochemical, histomorphometrical, radiological and clinical results in humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef]
- Thanasut, A.; Silkosessak, O.; Subbalekha, K. Platelet-rich fibrin did not affect autologous bone graft in repairing alveolar clefts. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 402–407. [Google Scholar] [CrossRef]
- Guan, S.; Xiao, T.; Bai, J.; Ning, C.; Zhang, X.; Yang, L.; Li, X. Clinical application of platelet-rich fibrin to enhance dental implant stability: A systematic review and meta-analysis. Heliyon 2023, 9, e13196. [Google Scholar] [CrossRef]
- Strauss, F.J.; Stähli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. 18), 6–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: Sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Tsai, C.H.; Chang, Y.C. Clinical application of platelet-rich fibrin as the sole grafting material in maxillary sinus augmentation. J. Formos. Med. Assoc. 2015, 114, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Guo, T.; Ding, X.; Yu, W.; Zhao, J.; Zhou, Y. The endoscopically assisted transcrestal sinus floor elevation with platelet-rich fibrin at an immediate implantation of periapical lesion site: A case report. Medicine 2019, 98, e16251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, S.; Ahmed Bakry, S.; Abd-Elhakam, H. Platelet rich fibrin in maxillary sinus augmentation: A systematic review. J. Oral Implantol. 2015, 41, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belouka, S.-M.; Strietzel, F. Sinus Floor Elevation and Augmentation Using Synthetic Nanocrystalline and Nanoporous Hydroxyapatite Bone Substitute Materials: Preliminary Histologic Results. Int. J. Oral Maxillofac. Implant. 2016, 31, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Cömert Kılıç, S.; Güngörmüş, M.; Parlak, S.N. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-platelet-rich plasma or platelet-rich fibrin: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Amam, M.A.; Abdo, A.; Alnour, A.; Amam, A.; Jaafo, M.H. Comparison of calcium sulfate and tricalcium phosphate in bone grafting after sinus lifting for dental implantation: A randomized controlled trial. Dent. Med. Probl. 2023, 60, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.; Abdelrahman, A.R.; Attia, R.; Zahran, A. Tomographic assessment of bone changes in atrophic maxilla treated by split-crest technique and dental implants with platelet-rich fibrin and NanoBone® versus platelet-rich fibrin alone: Randomized controlled trial. BMC Oral Health 2024, 24, 691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angelo, T.; Marcel, W.; Andreas, K.; Izabela, S. Biomechanical stability of dental implants in augmented maxillary sites: Results of a randomized clinical study with four different biomaterials and PRF and a biological view on guided bone regeneration. BioMed Res. Int. 2015, 2015, 850340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolf, M.; Wurm, A.; Heinemann, F.; Gerber, T.; Reichert, C.; Jäger, A.; Götz, W. The Effect of Patient Age on Bone Formation Using a Fully Synthetic Nanocrystalline Bone Augmentation Material in Maxillary Sinus Grafting. J. Oral Maxillofac. Implant. 2014, 29, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Lorenz, J.; Obreja, K.; Choukroun, J.; Landes, C.; Sader, R.A. Nanocrystalline hydroxyapatite-based material already contributes to implant stability after 3 months: A clinical and radiologic 3-year follow-up investigation. J. Oral Implantol. 2014, 40, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Bornstein, M.M.; Carrel, J.-P.; Buser, D.; Bernard, J.-P. Maxillary Sinus Grafting with a Synthetic, Nanocrystalline Hydroxyapatite-Silica Gel in Humans: Histologic and Histomorphometric Results. Int. J. Periodontics Restor. Dent. 2014, 34, 259–267. [Google Scholar] [CrossRef] [PubMed]
- El Hage, M.; Najm, S.A.; Bischof, M.; Nedir, R.; Carrel, J.-P.; Bernard, J.-P. Graft shrinkage and survival rate of implants after sinus floor elevation using a Nanocrystalline hydroxyapatite embedded in silica gel matrix: A 1-year prospective study. Implant Dent. 2012, 21, 213–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Francisco, L.; Francisco, M.; Costa, R.; Vasques, M.N.; Relvas, M.; Rajão, A.; Monteiro, L.; Rompante, P.; Guerra, F.; da Câmara, M.I. Sinus Floor Augmentation with Synthetic Hydroxyapatite (NanoBone®) in Combination with Platelet-Rich Fibrin: A Case Series. Biomedicines 2024, 12, 1661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canullo, L.; Dellavia, C.; Heinemann, F. Maxillary sinus floor augmentation using a nano-crystalline hydroxyapatite silica gel: Case series and 3-month preliminary histological results. Ann. Anat. 2012, 194, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.; Mishra, G.; Shahi, S.; Shakarwal, P.; Singh, A.; Singh, R. Comparison between Platelet-rich Fibrin and Saline Filling after Sinus Elevation without Adjunctive Bone Graft in Dental Implants Insertion Using CBCT. J. Contemp. Dent. Pract. 2023, 24, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wiltfang, J.; Schlegel, K.A.; Schultze-Mosgau, S.; Nkenke, E.; Zimmermann, R.; Kessler, P. Sinus floor augmentation with b-tricalciumphosphate (b-TCP): Does platelet-rich plasma promote its osseous integration and degradation? Clin. Oral Implant. Res. 2003, 14, 213–218. [Google Scholar] [CrossRef]
- Ortega-Mejia, H.; Estrugo-Devesa, A.; Saka-Herrán, C.; Ayuso-Montero, R.; López-López, J.; Velasco-Ortega, E. Platelet-rich plasma in maxillary sinus augmentation: Systematic review. Materials 2020, 13, 622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olaechea, A.; Mendoza-Azpur, G.; O’vAlle, F.; Padial-Molina, M.; Martin-Morales, N.; Galindo-Moreno, P. Biphasic hydroxyapatite and β-tricalcium phosphate biomaterial behavior in a case series of maxillary sinus augmentation in humans. Clin. Oral Implant. Res. 2019, 30, 336–343. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Frenken, J.W.F.H.; Bouwman, W.F.; Bravenboer, N.; Zijderveld, S.A.; Schulten, E.A.J.M.; Bruggenkate, C.M.T. The use of Straumann® Bone Ceramic in a maxillary sinus floor elevation procedure: A clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin. Oral Implant. Res. 2010, 21, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, W.F.; Bravenboer, N.; Frenken, J.W.; Ten Bruggenkate, C.M.; Schulten, E.A. The use of a biphasic calcium phosphate in a maxillary sinus floor elevation procedure: A clinical, radiological, histological, and histomorphometric evaluation with 9- and 12-month healing times. Int. J. Implant Dent. 2017, 3, 34. [Google Scholar] [CrossRef]
- Feigin, K.; Shope, B. Use of platelet-rich plasma and platelet-rich fibrin in Dentistry and oral surgery: Introduction and review of the literature. J. Vet. Dent. 2019, 36, 109–123. [Google Scholar] [CrossRef]
- Kiliç, S.; Güngörmüş, M. Cone Beam Computed Tomography Assessment of Maxillary Sinus Floor Augmentation Using Beta-Tricalcium Phosphate Alone or in Combination with Platelet-Rich Plasma: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implant. 2016, 31, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
| P | Population | Patients requiring sub-antral bone grafting |
| I | Intervention | Use of synthetic bone |
| C | Comparison | Use of synthetic bone with PRF |
| O | Outcomes | Analysing the PRF technique in sub-antral surgery |
| Was the Randomisation Method Adequate? | Was the Allocation Method Adequate? | Were the Groups Similar at the Start of the Study? | Were the Participants Blinded? | Were the Professionals Who Administered the Interventions Blinded? | Were the Outcome Assessors Blinded? | Were the Interventions Clearly Described and Applied Equally in Both Groups? | Was the Primary Outcome Clearly Defined and Measured? | Was There an Intention-to-Treat Analysis? | Were Losses and Exclusions Described? | Were Complications or Adverse Events Reported? | Were the Study Results Accurate and Reliable? | Were the Study Results Relevant to Clinical Practice? | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angelo et al. [31], 2015 | Y | Y | Y | U | U | U | Y | Y | N | NA | N | Y | Y |
| Belouka et al. [27] 2016 | Y | Y | Y | U | U | U | Y | Y | Y | Y | Y | Y | Y |
| Cömert Kılıç et al. [28], 2017 | Y | Y | Y | N | N | Y | Y | Y | I | S | S | S | S |
| Amam et al. [29], 2023 | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y |
| Anis et al. [30], 2024 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Were the Two Groups Similar and Recruited from the Same Population? | Were Exposures Measured in a Similar Way to Assign People to Exposed and Unexposed Groups? | Was Exposure Measured in a Valid and Reliable Way? | Were Confounding Factors Identified? | Were Strategies Defined to Deal with Confounding Factors? | Were the Groups/Participants Free of the Outcome at the Start of the Study (or at the Time of Exposure)? | Were the Outcomes Measured in a Valid and Reliable Manner? | Was the Follow-Up Time Reported and Sufficient for the Outcomes to Occur? | Was the Follow-Up Complete, and If Not, Were the Reasons for Follow-Up Losses Described and Analysed? | Were Strategies Used to Deal with Incomplete Follow-Up Losses? | Was an Appropriate Statistical Analysis Used? | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dieter D. Bosshardt et al. [34], 2014 | Y | Y | Y | N | N | Y | Y | Y | U | NA | Y |
| Wolf et al. [32] 2014 | Y | Y | Y | U | N | Y | Y | Y | Y | NA | Y |
| El Hage et al. [35], 2012 | NA | NA | Y | N | N | Y | Y | Y | Y | N | N |
| Ghanaati et al. [33], 2014 | Y | Y | Y | N | N | Y | Y | Y | Y | N | Y |
| Were the Inclusion Criteria Well Defined? | Was the Condition Measured Reliably? | Were Valid Methods Used for the Condition of All Participants Included? | Did the Case Series Have Consecutive Inclusion of Participants? | Did the Case Series Include All Eligible Participants? | Was There a Clear Description of the Demographics of the Study Participants? | Was There a Clear Description of the Clinical Information of the Participants? | Were the Results or Follow-Up of the Cases Clearly Described? | Was There a Clear Description of the Demographic Information of the Site(s)/Clinic(s) Where the Study Was Conducted? | Was the Statistical Analysis Appropriate? | |
|---|---|---|---|---|---|---|---|---|---|---|
| Francisco et al. [36], 2024 | Y | Y | Y | U | Y | Y | Y | Y | N | Y |
| Canullo et al. [37], 2012 | Y | Y | Y | N | Y | N | Y | Y | N | Y |
| Author | Type of Study | Study Group | Inclusion Criteria | Exclusion Criteria | Objective | Sample | Sinuses | Complications | Implant Placement | Outcome Measurements | Results/Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| El Hage et al. [35], (2012) | Prospective cohort study | Human | Partially edentulous patients with posterior maxillary bone defects; residual bone height ≤ 3 mm | Severe systemic conditions | To evaluate the percentage of vertical resorption of NanoBone® grafts following subantral surgery, and the implant survival rate after 1 year | N = 8 Single group (five female, three male)—NanoBone® + autologous blood + collagen membrane Mean age: 53 years | 11 | Two postoperative infections; one implant loss | 12 months | Vertical graft resorption; Implant survival rate | Mean graft resorption: 8.84% ± 5.32% Implant survival rate after 1 year: 94.74% 18 implants successfully osseointegrated and rehabilitated; NanoBone® graft showed good dimensional stability |
| Canullo et al. [37], (2012) | Case series | Human | Need for rehabilitation in the posterior maxilla with residual bone height of 1–2 mm | Chronic sinusitis Acute infections Respiratory allergies Use of bisphosphonates | To histologically evaluate bone regeneration with NanoBone® and BIC after 3 months | N = 10 patients Single group—NanoBone® Mean age: 54 years | 10 | N/R | Mini-implant placed at the time of surgery to maintain space | % New Bone; Residual graft material; Bone marrow content and BIC. | New bone: 20.64% ± 2.96% Residual NanoBone®: 38.26% ± 8.07% Bone marrow: 29.23% ± 5.18% BIC: 26.02% ± 5.46% No connective tissue observed at the implant surface. |
| Ghanaati et al. [33], (2014) | Prospective cohort study | Human | Edentulous patients in the upper molar region; Severely resorbed maxilla; Age between 34 and 77 years | Chronic infections in the maxillary region Uncontrolled diabetes and other systemic conditions Use of bisphosphonates | Evaluate the impact of NanoBone® synthetic bone integration time on implant stability at 3 and 6 months after sinus lift surgery | N = 14 Group 1: 3 months: NanoBone®, sevent patients (four men, three women) Mean age: 53 years Group 2: 6 months: NanoBone®, seven patients (three men, four women) Mean age: 53 years | 14 | One implant lost in the test group | 3 or 6 months | % New Bone; Implant survival at 3 years; Periotest; Presence of osteolysis; BOP; PB; REC | Group 1: New bone: 24.89% ± 10.22% Implant survival: 94.1% Mean Periotest: 2.94 Group 2: New bone: 31.29% ± 2.29% Implant survival: 100% Mean Periotest: 2.29 No osteolysis or mobility Three months after the surgical procedure, it is already possible to achieve a stable and lasting restoration, retained by the implant, which can contribute to a reduction in healing time. |
| Dieter D. Bosshardt et al. [34], (2014) | Cohort study—Histological and histomorphometry analysis | Human | Vertical height of the edentulous maxillary ridge < 4 mm Age between 41 and 64 years Patients referred to the Department of Stomatology and Oral Surgery at the University of Geneva | Smokers; Acute or chronic sinus disease | Evaluate bone regeneration following maxillary sinus lift using nanocrystalline hydroxyapatite in a silica gel matrix | N = 8 (seven female, one male) Group 1: NanoBone® + collagen membrane (Bio-Gide®) three patients Age range: 41–64 years Group 2: NanoBone® + PRF membrane five patients Age range: 41–64 years | 16 | N/R | 7–11 months | Histomorphometry % of new bone; % of residual NanoBone®; % of soft tissue; Bone-material integration; TRAP+ cells and vascularization | Group 1: NanoBone® + Bio-Gide® New bone: 28.7% ± 5.4% Residual material: 25.5% ± 7.6% Soft tissue: 45.8% ± 3.2% Group 2: NanoBone® + PRF membrane New bone: 28.6% ± 6.9% Residual material: 25.7% ± 8.8% Soft tissue: 45.7% ± 9.3% No significant difference compared to collagen group Similar osteointegration and histological pattern New bone formation following the use of nanocrystalline hydroxyapatite for maxillary sinus floor elevation in humans is comparable to values reported in other synthetic or xenogeneic bone substitute materials. |
| Wolf et al. [32], (2014) | Cohort study | Human | Need for maxillary sinus lift prior to implant placement; Residual subantral bone height between 3 mm and 7 mm | Severe systemic diseases Uncontrolled diabetes History of radiotherapy to the head and neck region Chemotherapy Sinus disease Active periodontal disease Smoking | Evaluate whether patient age influences bone formation, biomaterial resorption, and soft tissue development after sinus lift using NanoBone® | N = 17 patients (nine male, eight female) Group 1: NanoBone®: eight patients aged 66–71 years Group 2—NanoBone®: nine patients age: 41–52 years | 20 | N/R | 7 months | % New Bone; % Residual NanoBone®; % Soft tissue; Presence of TRAP+ cells | Group 1—New bone: 20.57% ± 6.95%; Residual NanoBone®: 39.28% ± 11.58%; Soft tissue: 40.15% ± 10.93%; Group 2—New bone: 22.27% ± 4.31%; Residual NanoBone®: 39.13% ± 8.86%; Soft tissue: 38.59% ± 9.97%; TRAP+ cells were present in both groups, with no significant differences observed. |
| Angelo et al. [31], (2015) | Randomised | Human | Anterior maxillary alveolar ridge with width < 3 mm and height > 14 mm | Platelet disorders; chronic sinusitis; infectious/metabolic diseases; chemotherapy/radiotherapy; use of antibiotics or anti-inflammatory drugs. | Evaluate maxillary bone regeneration and biomechanical implant stability using self-hardening synthetic biomaterials (SHB), either combined or not with PRF. | N = 82 Control Group: native bone Group 1: HA + β-TCP (Easy-Graft® CRYSTAL) Group 2: β-TCP (Easy-Graft® CLASSIC) Group 3: β-TCP + PRF (Easy-Graft® CLASSIC + PRF) Age range: N/R | 82 | N/R | 8 months | Insertion torque (IT) and standard deviation | Control Group (native bone): Mean IT: 31.1 ± 7.3 Ncm. Grupo 1: Mean IT: 43.2 ± 7.6 Ncm; +38.6% compared to control group; Grupo 2: mean IT: 39.4 ± 8.9 Ncm; +26.7% compared to control group Grupo 3: Mean IT: 41.2 ± 5.4 Ncm; +32.5% compared to control group; The use of CS, alone or in combination with PRF, was advantageous for obtaining repaired alveolar bone with improved (bio)mechanical stability. |
| Belouka et al. [27], (2016) | Randomised | Human | Age ≥ 18 years; Need for maxillary sinus elevation for rehabilitation with implants; At least two adjacent teeth missing in the posterior maxilla. | Age < 18 years; Uncontrolled systemic diseases (ASA > 2); Drug use and alcoholism; Active periodontal disease or chronic sinusitis. Patients who refused the use of synthetic grafts. | Histomorphometric comparison of bone regeneration in maxillary sinus elevation between two types of synthetic bone: nanocrystalline hydroxyapatite (Ostim®) vs. nanoporous hydroxyapatite (NanoBone®) | N = 44 Group 1—Nanocrystalline HA (Ostim®) 22 patients (9 women, 13 men) Age: average: 63 years Group 2—Nanoporous HA (NanoBone®) 22 patients (13 women, 9 men) Average age: 63 years | 88 | Ostim®—one implant lost | Immediate | % New Bone; % of remaining biomaterial; % of soft tissue; Histology and histomorphometry at 6 months. | % New bone: NanoBone®: 34.6% ± 9.2% Ostim®: 31.8% ± 11.6% p = 0.465 % Remaining biomaterial: NanoBone®: 30.0% ± 13.0% Ostim®: 28.4% ± 18.6% p = 0.828 % Soft tissue NanoBone®: 35.4% ± 6.8% Ostim®: 39.9% ± 11.1% p = 0.159 Both synthetic bone substitute materials were found to support bone formation in sinus floor elevation by osteoconductivity. |
| Cömert Kılıç et al. [28], (2017) | Randomised | Human | Adults with atrophic maxilla; Residual bone crest height ≤ 7 mm | Infections in the maxillary sinus; Haematological, neurological, or systemic diseases; Radiotherapy/chemotherapy; Inflammatory or malignant diseases in the head/neck region | Compare histological and histomorphometric results of surgery with β-TCP alone, β-TCP + P-PRP and β-TCP + PRF | N = 26 Group 1: β-TCP + P-PRP nine patients (four women, five men) Age: 22–51 years Group 2: β-TCP + PRF eight patients (three women, five men) Age: 22–51 years Group 3-Control: β-TCP nine patients (two women, seven men) Age: 22–51 years | 26 | Five perforations: two in the control group; one in the P-PRP group; two in the PRF group | 6 months | % New Bone; % residual biomaterial; % soft tissue; Osteoblastic activity. | % of new bone: β-TCP + PRF: 35.2% ± 7.6% β-TCP + PRP: 30.4% ± 8.1% β-TCP: 27.3% ± 6.8% % of remaining biomaterial: β-TCP + PRF: 27.9% ± 7.4% β-TCP + PRP: 31.4% ± 6.3% β-TCP: 34.3% ± 5.9% % of soft tissue: β-TCP + PRF: 36.9% ± 6.3% β-TCP + PRP: 38.2% ± 5.7% β-TCP: 38.4% ± 5.4%. Intense osteoblastic activity, particularly in the PRF group. |
| Amam et al. [29], (2023) | Randomised | Human | Bilateral maxillary edentulism; Age between 45 and 70 years; Residual bone height between 0.5 and 5 mm | Metabolic diseases; Use of corticosteroids; Autoimmune, cardiovascular diseases, diabetes; Coagulation disorders. | Comparing CS and β-TCP grafts in maxillary sinus elevation | N = 9 Test group: CS + A-PRF Age: 45–70 Control group: β-TCP + A-PRF Age: 45–70 | 18 | N/R | 6 months | Vertical bone augmentation; Assessment by CBCT in the following phases: T0: preoperative; T1: immediate postoperative; T2: 6 months postoperative | Test group: CS + A-PRF Bone gain: 7.96 ± 2.78 mm (+372.8%) Control group: β-TCP + A-PRF Bone gain: 7.53 ± 1.15 mm (+353.2%) p > 0.05 in all comparisons T0: Reduced initial bone height; T1: Average increase in bone height: CS/A-PRF: +10.3 mm; β-TCP/A-PRF: +10.4 mm; T2: Reduction in bone height: CS/A-PRF: −2.35 mm; β-TCP/A-PRF: −2.79 mm. The use of CS or TCP combined with A-PRF proved to be advantageous and safe, with sufficient bone available for dental implant placement. |
| Francisco et al. [36], 2024 | Case Series | Human | ≥18 years; Posterior maxillary edentulism; Residual bone height ≤ 5 mm | Alcoholism and smoking; Diabetes; Heart disease; Use of bisphosphonates; Previous sinus pathology. | To evaluate PRF combined with NanoBone® in bone regeneration of maxillary sinus elevation. | N = 6 (three male/three female) Test Group: NanoBone®: + PRF Control Group: NanoBone®: Age: N/R | 12 | N/R | 6 months | % New Bone; % of inert particles; % Connective tissue; Histomorphometry at 6 months. | Test group—PRF + NanoBone®: New bone: 27.5% ± 4.9% Inert particles: 23.0% ± 3.7% Connective tissue: 49.4% ± 2.8% Control group—NanoBone®: -New bone: 19.5% ± 3.0% -Inert particles: 23.4% ± 5.7% Connective tissue: 57.0% ± 3.5% Mixing liquid PRF with NanoBone® appears to slightly increase the amount of new bone formation and revascularization in comparison to using NanoBone® alone. |
| Anis et al. [30], (2024) | Randomised | Human | Age > 18 years; Edentulous maxillary crest with width < 6 mm; Seibert class I defects. | Smoking; Systemic diseases; Pregnancy; Chemotherapy and radiotherapy. | Evaluating bone changes in maxillary ridges in split-crest surgeries with PRF versus PRF + NanoBone® | N = 40 Test group—20 Patients (13 women, 7 men) PRF + NanoBone®: Average age: 35 years Control group—PRF: 20 patients (11 women, 9 men) Average age: 35 years | N/R | Healing screw exposure in one case | Immediately | Crestal bone changes; Gain in horizontal bone width; Post-operative pain and oedema. | Group 1: PRF + NanoBone®: Vestibular bone resorption: 1.14 ± 0.63 mm; Lingual bone resorption: 1.47 ± 0.68 mm; Horizontal bone gain: 1.29 ± 0.73 mm; Slightly greater pain on the 2nd day; Oedema similar to the control group, total reduction on the 4th day; Group 2: PRF Vestibular bone resorption: 1.26 ± 0.58 mm; Lingual bone resorption: 1.40 ± 0.66 mm; Horizontal bone gain: 1.46 ± 0.44 mm; There was no statistically significant difference in patient morbidity or crestal and horizontal bone alterations between trial groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.; Carvalho, A.; López-Jarana, P.; Costa, V.; Relvas, M.; Salazar, F.; Infante da Câmara, T.; Nunes Vasques, M.; Infante da Câmara, M. The Use of Platelet-Rich Fibrin in Combination with Synthetic Bone Grafting: A Systematic Review. Biomedicines 2025, 13, 2266. https://doi.org/10.3390/biomedicines13092266
Costa R, Carvalho A, López-Jarana P, Costa V, Relvas M, Salazar F, Infante da Câmara T, Nunes Vasques M, Infante da Câmara M. The Use of Platelet-Rich Fibrin in Combination with Synthetic Bone Grafting: A Systematic Review. Biomedicines. 2025; 13(9):2266. https://doi.org/10.3390/biomedicines13092266
Chicago/Turabian StyleCosta, Rosana, Alicia Carvalho, Paula López-Jarana, Vitória Costa, Marta Relvas, Filomena Salazar, Tomás Infante da Câmara, Miguel Nunes Vasques, and Marco Infante da Câmara. 2025. "The Use of Platelet-Rich Fibrin in Combination with Synthetic Bone Grafting: A Systematic Review" Biomedicines 13, no. 9: 2266. https://doi.org/10.3390/biomedicines13092266
APA StyleCosta, R., Carvalho, A., López-Jarana, P., Costa, V., Relvas, M., Salazar, F., Infante da Câmara, T., Nunes Vasques, M., & Infante da Câmara, M. (2025). The Use of Platelet-Rich Fibrin in Combination with Synthetic Bone Grafting: A Systematic Review. Biomedicines, 13(9), 2266. https://doi.org/10.3390/biomedicines13092266








