Role of CD138+ Plasma Cells and Natural Killer Cells in Couple Infertility: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sources and Search Strategy
- (“Infertility”[MeSH Terms] OR “Infertility, Female”[MeSH Terms] OR infertility[Title/Abstract])
- AND (“CD138”[Title/Abstract] OR “syndecan-1”[Title/Abstract] OR “plasma cells”[MeSH Terms])
- AND (“Natural Killer Cells”[MeSH Terms] OR “NK cells”[Title/Abstract])
- AND (“Endometrium”[MeSH Terms] OR endometrium[Title/Abstract] OR uterus[Title/Abstract])
- The search was further refined to include only:
- Articles published in English.
- Full-text available studies.
- Studies conducted on human subjects.
2.3. Inclusion and Exclusion Criteria
- Observational studies (cohort, case–control, cross-sectional) and clinical trials (randomized or not) that evaluated the role of CD138+ plasma cells and/or NK cells in patients with infertility, recurrent implantation failure (RIF), or recurrent spontaneous abortion (RSA) [24].
- Studies that included either quantitative or qualitative assessment of CD138+ plasma cells (via immunohistochemistry) or NK cells (either peripheral or uterine, using flow cytometry, immunohistochemistry, or endometrial biopsy).
- Comparative studies with fertile control groups or pre-/post-treatment designs.
- Preclinical studies on animal models or in vitro cell lines.
- Abstracts without full-text availability.
- Case reports, editorials, letters to the editor, and narrative reviews (except when used as secondary references).
- Studies not directly related to infertility (e.g., oncology, systemic autoimmunity)
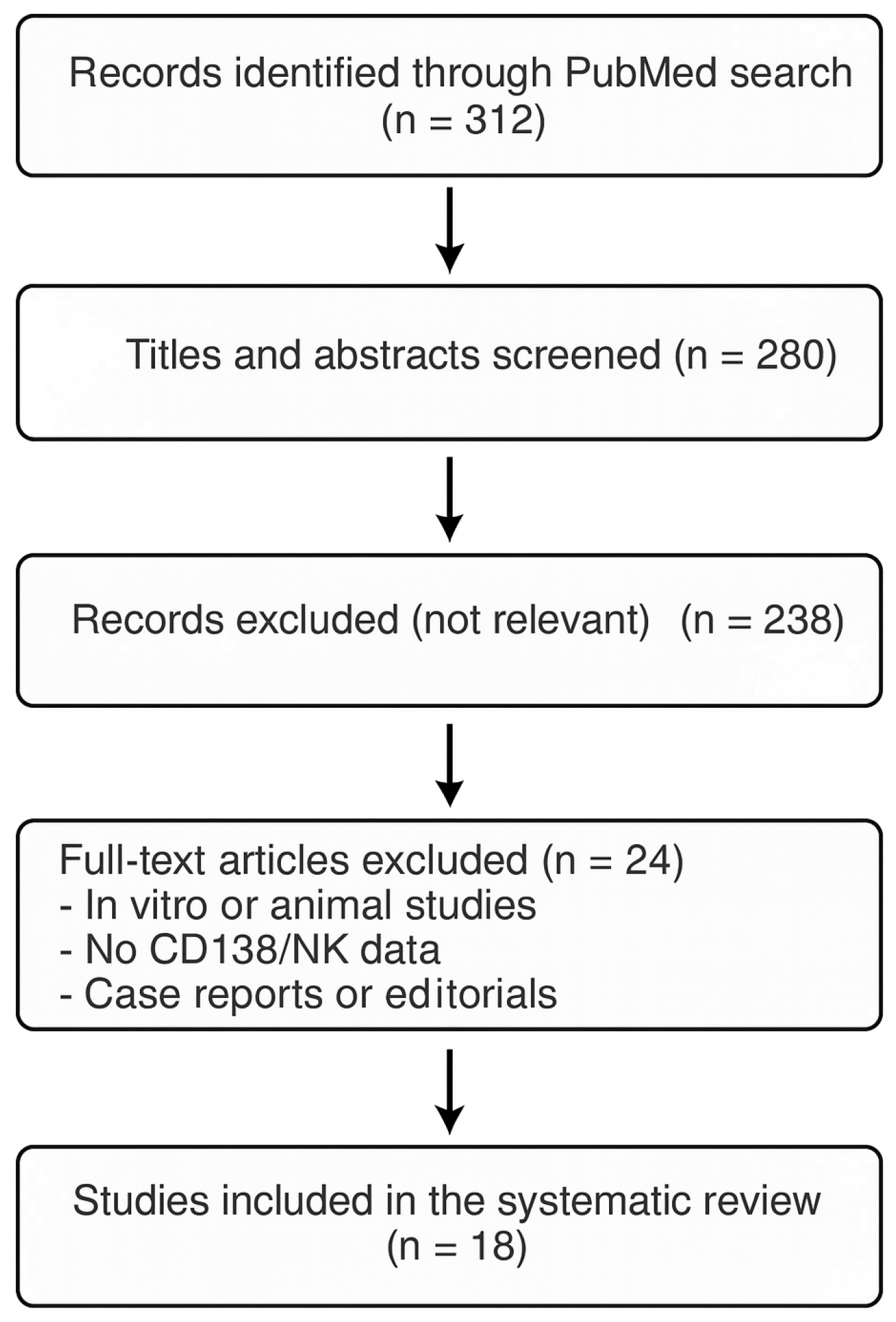
2.4. Study Selection Process
3. Results
3.1. CD138+ Plasma Cells and Chronic Endometritis: Reproductive Implications
3.2. Natural Killer Cells and Fertility: A Dual Role of Support and Cytotoxicity
3.3. Immune Interaction: CD138+ and NK Cells as Combined Biomarkers
4. Discussion
4.1. Role of CD138+ Plasma Cells in Chronic Endometritis
4.2. NK Cells: Ambiguous Regulators of Embryo Implantation
4.3. CD138+–NK Interaction: Towards an Integrated Immunological Evaluation
4.4. Strengths and Limitations of the Literature
- Lack of large-scale randomized controlled trials (RCTs) [38].
4.5. Psychological Considerations and Counseling Needs
4.6. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; van der Poel, S.; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Cousineau, T.M.; Domar, A.D. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol. 2007, 21, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.E.; McLain, A.C.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Louis, G.M.B. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331. [Google Scholar] [CrossRef]
- Cissen, M.; Bensdorp, A.J.; Cohlen, B.J.; Repping, S.; de Bruin, J.P.; van Wely, M. Assisted reproductive technologies for male subfertility. Cochrane Database Syst Rev. 2016, 2, CD000360. [Google Scholar] [CrossRef]
- Robertson, S.A. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005, 322, 43–52. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–437. [Google Scholar] [CrossRef]
- Nancy, P.; Tagliani, E.; Tay, C.-S.; Asp, P.; Levy, D.E.; Erlebacher, A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012, 336, 1317–1321. [Google Scholar] [CrossRef]
- Lédée, N.; Petitbarat, M.; Rahmati, M.; Chaouat, G.; Dubanchet, S. New pre-conception immune biomarkers for clinical practice: Interleukin-15 and interleukin-18 binding protein. J. Reprod Immunol. 2021, 88, 118–123. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Aberrant expression of cell adhesion molecules in the endometrium of infertile patients with chronic endometritis. Mod. Pathol. 2010, 23, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Resta, L.; Nicoletti, R.; Zito, A.; Piccoli, R.; Marziani, G. Detection of chronic endometritis at fluid hysteroscopy. J. Minim. Invasive Gynecol. 2005, 12, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Johnston-MacAnanny, E.B.; Hartnett, J.; Engmann, L.L.; Nulsen, J.C.; Sanders, M.M.; Benadiva, C.A. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil. Steril. 2010, 93, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Morrison, L.; Longfellow, M.; Ritson, A.; Pace, D. Granulated lymphocytes in human endometrium: Histochemical and immunohistochemical studies. Hum. Reprod. 1991, 6, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Kalumbi, C.; Bates, M.; Farquharson, R.; Vince, G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil. Steril. 2005, 84, 980–984. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediators Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, A.W.; Alfirevic, Z.; Turner, M.A.; Drury, J.A.; Li, T.C.; Quenby, S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum. Reprod. 2013, 28, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Sunkara, S.K. Natural killer cells in female infertility and recurrent miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Takahashi, T.; Maeda, E.; Ishiyama, K.; Takahashi, S.; Suganuma, R.; Matsuo, K.; Tachibana, M.; Fukuhara, R.; Shirasawa, H.; et al. Effects of localisation of uterine adenomyosis on outcome of in vitro fertilisation/intracytoplasmic sperm injection fresh and frozen-thawed embryo transfer cycles: A multicentre retrospective cohort study. Reprod. Biol. Endocrinol. 2021, 19, 84. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Huang, J.; Wang, C.-C.; Yu, M.-Y.; Laird, S.M.; Li, T.-C. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil. Steril. 2018, 109, 832–839, Erratum in: Fertil Steril. 2019, 111, 411. https://doi.org/10.1016/j.fertnstert.2018.12.004. [Google Scholar] [CrossRef]
- Changaei, M.; Javidan, M.; Ramezani Tehrani, F.; Mosaffa, N.; Noroozzadeh, M.; Hosseinzadeh, R.; Rajaei, S. Reduced expression of Il10, Stat3, Hoxa10, and Itgb3 in the embryo implantation site of rat model with prenatal androgen-induced polycystic ovary syndrome. Am. J. Reprod. Immunol. 2023, 90, e13720. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Warriner, D. How to Read a Paper: The Basics of Evidence-Based Medicine. BMJ. 2008, 336, 1381. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Hayashi, T.; Taguchi, S.; Funabiki, M.; Nakamura, Y. Comprehensive endometrial immunoglobulin subclass analysis in infertile women suffering from repeated implantation failure with or without chronic endometritis. Am. J. Reprod. Immunol. 2014, 72, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3; Cochrane: London, UK, 2022. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2012. [Google Scholar]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Sterne, J.A.C. Assessing risk of bias in a systematic review. In Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3; Cochrane: London, UK, 2022. [Google Scholar]
- Shang, J.; Wang, S.; Wang, A.; Li, F.; Zhang, J.; Wang, J.; Lv, R.; Chen, H.; Mu, X.; Zhang, K.; et al. Intra-ovarian inflammatory states and their associations with embryo quality in normal-BMI PCOS patients undergoing IVF treatment. Reprod. Biol. Endocrinol. 2024, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Kasius, J.C.; Broekmans, F.J.; Sie-Go, D.M.; Bourgain, C.; Eijkemans, M.J.; Fauser, B.C.; Devroey, P.; Fatemim, H.M. The reliability of the histological diagnosis of endometritis in asymptomatic IVF cases. Hum. Reprod. 2012, 27, 153–158. [Google Scholar] [CrossRef] [PubMed]
- McQueen, D.B.; Bernardi, L.A.; Stephenson, M.D. Chronic endometritis in women with recurrent early pregnancy loss and/or implantation failure: A study of diagnostic variability. Fertil. Steril. 2015, 104, 1521–1525. [Google Scholar] [CrossRef]
- Bouet, P.E.; El Hachem, H.; Monceau, E.; Gariépy, G.; Kadoch, I.J.; Sylvestre, C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: Prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil. Steril. 2016, 105, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Fujii, S.; Yamaguchi, E.; Kimura, H.; Sato, S.; Saito, Y. Natural killer cell subpopulations and cytotoxicity for infertile patients undergoing in vitro fertilization. Am. J. Reprod. Immunol. 1999, 41, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Coulam, C.B.; Acacio, B. Does immunotherapy for treatment of reproductive failure enhance live births? Am. J. Reprod. Immunol. 2012, 67, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Lédée, N.; Petitbarat, M.; Chevrier, L.; Vitoux, D.; Vezmar, K.; Rahmati, M.; Dubanchet, S.; Gahéry, H.; Bensussan, A.; Chaouat, G. The Uterine Immune Profile May Help Women With Repeated Unexplained Embryo Implantation Failure After In Vitro Fertilization. Am. J. Reprod. Immunol. 2016, 75, 388–401. [Google Scholar] [CrossRef]
- Lédée, N.; Petitbarat, M.; Prat-Ellenberg, L.; Dray, G.; Cassuto, G.N.; Chevrier, L.; Kazhalawi, A.; Vezmar, K.; Chaouat, G. Endometrial Immune Profiling: A Method to Design Personalized Care in Assisted Reproductive Medicine. Front. Immunol. 2020, 11, 1032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshpande, P.S.; Gupta, A.S. Causes and Prevalence of Factors Causing Infertility in a Public Health Facility. J. Hum. Reprod. Sci. 2019, 12, 287–293. [Google Scholar] [CrossRef]
- Pandian, Z.; Gibreel, A.; Bhattacharya, S. In vitro fertilisation for unexplained subfertility. Cochrane Database Syst. Rev. 2012, 4, CD003357. [Google Scholar]
- Libretti, A.; Savasta, F.; Nicosia, A.; Corsini, C.; De Pedrini, A.; Leo, L.; Laganà, A.S.; Troìa, L.; Dellino, M.; Tinelli, R.; et al. Exploring the Father’s Role in Determining Neonatal Birth Weight: A Narrative Review. Medicina 2024, 60, 1661. [Google Scholar] [CrossRef]
- Dumancic, S.; Bakotin Jakovac, M.; Mimica, M.D.; Zekic Tomas, S.; Marusic, J. CD56-Positive NK Cells and CD138-Positive Plasma Cells in Basal Decidua of Term Placentas in Singleton Pregnancies after Assisted Reproductive Technology Treatment of Endometriosis-Related Infertility. Life 2025, 15, 240. [Google Scholar] [CrossRef]
- Chiokadze, M.; Bär, C.; Pastuschek, J.; Dons’koi, B.V.; Khazhylenko, K.G.; Schleußner, E.; Markert, U.R.; Favaro, R.R. Beyond Uterine Natural Killer Cell Numbers in Unexplained Recurrent Pregnancy Loss: Combined Analysis of CD45, CD56, CD16, CD57, and CD138. Diagnostics 2020, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Dwojak, E.; Mroczek, M.; Dworacki, G.; Dobosz, P.; Ślubowska, A.; Stępień, M.; Borowczyk, M.; Filipczyńska, I.; Tomaszewska, A.; Ałtyn, R.; et al. Plasma Cells as the Key Players of IVF Failure? Unlocking the Enigma of Infertility and In Vitro Fertilization Failure in the Light of Uterine Inflammation. Int. J. Mol. Sci. 2024, 25, 13083. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, A.; Libretti, A.; Giovannini, I.; Leo, L.; Remorgida, V.; Masturzo, B.; Tinelli, R. Role of CD138+ Plasma Cells and Natural Killer Cells in Couple Infertility: A Review. Biomedicines 2025, 13, 2248. https://doi.org/10.3390/biomedicines13092248
Messina A, Libretti A, Giovannini I, Leo L, Remorgida V, Masturzo B, Tinelli R. Role of CD138+ Plasma Cells and Natural Killer Cells in Couple Infertility: A Review. Biomedicines. 2025; 13(9):2248. https://doi.org/10.3390/biomedicines13092248
Chicago/Turabian StyleMessina, Alessandro, Alessandro Libretti, Ilaria Giovannini, Livio Leo, Valentino Remorgida, Bianca Masturzo, and Raffaele Tinelli. 2025. "Role of CD138+ Plasma Cells and Natural Killer Cells in Couple Infertility: A Review" Biomedicines 13, no. 9: 2248. https://doi.org/10.3390/biomedicines13092248
APA StyleMessina, A., Libretti, A., Giovannini, I., Leo, L., Remorgida, V., Masturzo, B., & Tinelli, R. (2025). Role of CD138+ Plasma Cells and Natural Killer Cells in Couple Infertility: A Review. Biomedicines, 13(9), 2248. https://doi.org/10.3390/biomedicines13092248











