Modalities Differentiation of Pain Perception Following Ischemic Stroke: Decreased Pressure Pain Perception
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experiment 1
2.3. Experiment 2
2.4. Statistical Analysis
3. Results
3.1. Clinical and Neuroimaging Profiles of the Stroke Cohort
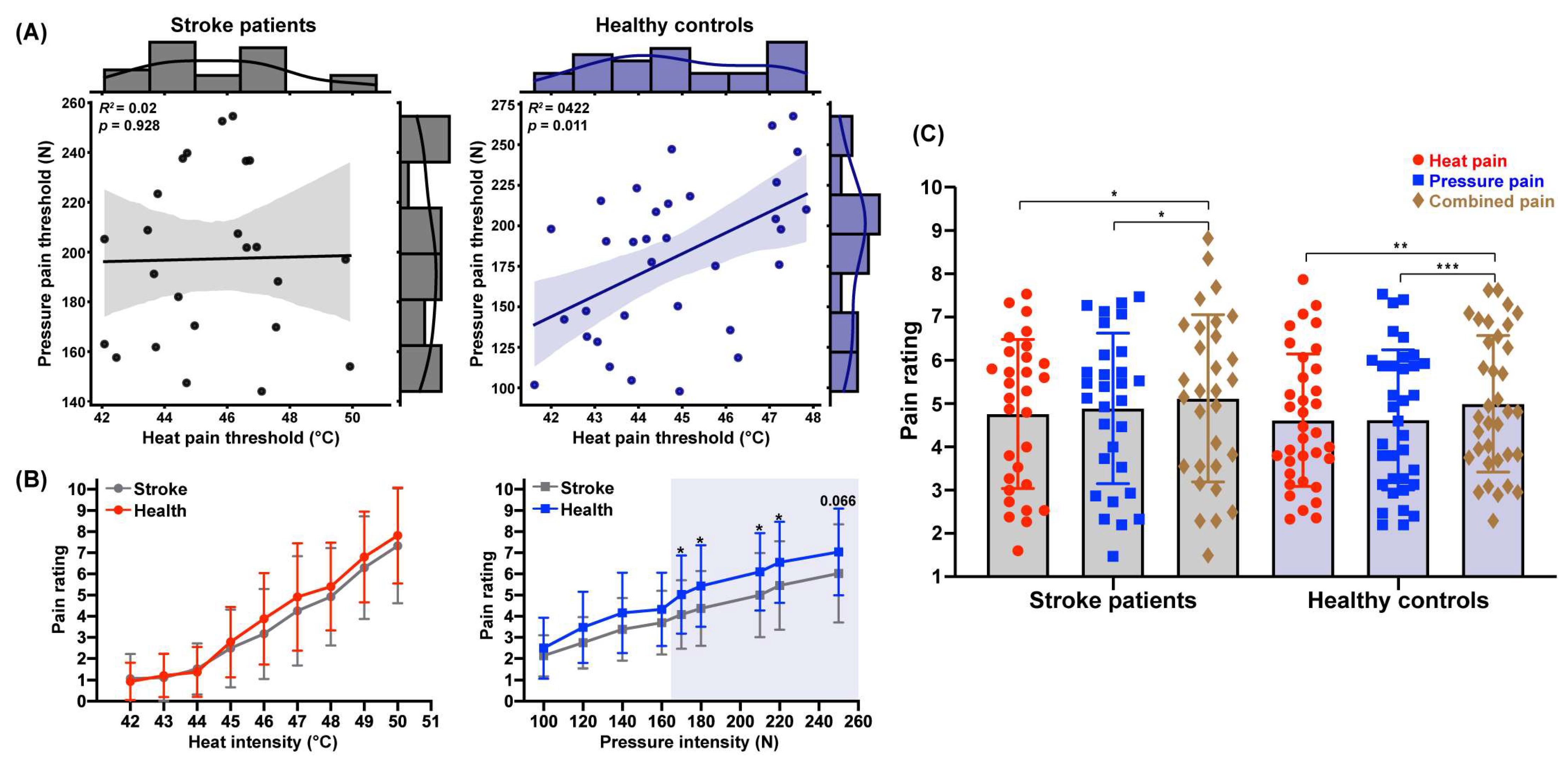
3.2. Variability in Pain Threshold Correlations Across Stimulation Modalities
3.3. Decreased Pressure Pain Perception Following Stroke
3.4. Unimpaired Pain Integration Following Stroke
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellomo, R.G.; Paolucci, T.; Saggino, A.; Pezzi, L.; Bramanti, A.; Cimino, V.; Tommasi, M.; Saggini, R. The WeReha project for an innovative home-based exercise training in chronic stroke patients: A clinical study. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520979866. [Google Scholar] [CrossRef]
- Mak-Yuen, Y.Y.; Matyas, T.A.; Carey, L.M. Characterizing touch discrimination impairment from pooled stroke samples using the Tactile Discrimination Test: Updated criteria for interpretation and brief test version for use in clinical practice settings. Brain Sci. 2023, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Astrakas, L.G.; Li, S.; Ottensmeyer, M.P.; Pusatere, C.; Moskowitz, M.A.; Tzika, A.A. Peak activation shifts in the sensorimotor cortex of chronic stroke patients following robot-assisted rehabilitation therapy. Open Neuroimaging J. 2021, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhang, J.; Pan, Y.; Liu, X.; Miao, L.; Peng, J.; Song, L.; Zou, Y.; Chen, X. Somatosensory deficits after stroke: Insights from MRI studies. Front. Neurol. 2022, 13, 891283. [Google Scholar] [CrossRef] [PubMed]
- Zahra, F.-T.; Zoghi, M.; Haslam, B.; Carey, L.M. Is there a relationship between somatosensory impairment the perception of pain in stroke survivors? An exploratory study. Int. J. Rehabil. Res. 2024, 47, 206–213. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Xu, H.-R.; Wang, Y.-C.; Hu, G.-W.; Ding, X.-Q.; Shen, X.-H.; Yang, H.; Rong, J.-F.; Wang, X.-Q. Pressure pain threshold and somatosensory abnormalities in different ages and functional conditions of post-stroke elderly. BMC Geriatr. 2022, 22, 830. [Google Scholar] [CrossRef]
- Gao, Z.; Cai, Q.; Fang, H.; He, J.; Hu, Z.; Jin, Y.; Chen, Y.; Tan, B.; Wang, Y.; Wang, J. Evaluation of relationships between corticospinal excitability and somatosensory deficits in the acute and subacute phases of stroke. J. Integr. Neurosci. 2023, 22, 61. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Wang, Y.-C.; Hu, G.-W.; Ding, X.-Q.; Shen, X.-H.; Hui, Y.; Rong, J.-F.; Wang, X.-Q. Intra-rater and inter-rater reliability of pressure pain threshold assessment in stroke patients. Eur. J. Phys. Rehabil. Med. 2022, 58, 549–557. [Google Scholar] [CrossRef]
- Kessner, S.S.; Schlemm, E.; Cheng, B.; Bingel, U.; Fiehler, J.; Gerloff, C.; Thomalla, G. Somatosensory deficits after ischemic stroke: Time course and association with infarct location. Stroke 2019, 50, 1116–1123. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, S.; Li, Y.; Zhao, J.; Li, G.; Chen, L.; Wu, Y.; Zhang, S.; Shi, X.; Chen, X. Comparison of sensory observation and somatosensory stimulation in mirror neurons and the sensorimotor network: A task-based fMRI study. Front. Neurol. 2022, 13, 916990. [Google Scholar] [CrossRef]
- Dishman, D.; Lal, T.; Silos, C.; Chen, L.; Jiang, X.; Beauchamp, J.; Aggarwal, S.; Green, C.; Savitz, S.I. A retrospective examination of pain in acute stroke at hospital discharge. J. Stroke Cerebrovasc. Dis. 2023, 32, 107370. [Google Scholar] [CrossRef]
- Pillette, L.; Lotte, F.; N’Kaoua, B.; Joseph, P.-A.; Jeunet, C.; Glize, B. Why we should systematically assess, control and report somatosensory impairments in BCI-based motor rehabilitation after stroke studies. NeuroImage Clin. 2020, 28, 102417. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, M.; Mazzone, P.; Restuccia, D.; Insola, A. Contribution of different somatosensory afferent input to subcortical somatosensory evoked potentials in humans. J. Neurol. Sci. 2021, 429, 118536. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F.A. Small fiber neuropathy. Acta Neurol. Scand. 2022, 145, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef]
- Ge, Y.; Ye, S.; Zhu, K.; Guo, T.; Su, D.; Zhang, D.; Chen, Y.; Chai, X.; Sui, X. Mediating different-diameter Aβ nerve fibers using a biomimetic 3D TENS computational model. J. Neurosci. Methods 2020, 346, 108891. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Carey, L.M.; Lamp, G.; Turville, M. The state-of-the-science on somatosensory function and its impact on daily life in adults and older adults, and following stroke: A scoping review. OTJR 2016, 36, 27S–41S. [Google Scholar] [CrossRef]
- Carey, L.M.; Matyas, T.A.; Baum, C. Effects of somatosensory impairment on participation after stroke. Am. J. Occup. Ther. 2018, 72, 7203205100p1–7203205100p10. [Google Scholar] [CrossRef]
- Gruss, S.; Geiger, M.; Werner, P.; Wilhelm, O.; Traue, H.C.; Al-Hamadi, A.; Walter, S. Multi-modal signals for analyzing pain responses to thermal and electrical stimuli. J. Vis. Exp. 2019, 146, e59057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Auer-Grumbach, M.; Matsukawa, S.; Zitzelsberger, M.; Themistocleous, A.C.; Strom, T.M.; Samara, C.; Moore, A.W.; Cho, L.T.-Y.; Young, G.T. Transcriptional regulator PRDM12 is essential for human pain perception. Nat. Genet. 2015, 47, 803–808. [Google Scholar] [CrossRef]
- Ramger, B.C.; Bader, K.A.; Davies, S.P.; Stewart, D.A.; Ledbetter, L.S.; Simon, C.B.; Feld, J.A. Effects of non-invasive brain stimulation on clinical pain intensity and experimental pain sensitivity among individuals with central post-stroke pain: A systematic review. J. Pain Res. 2019, 12, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Martín-Martín, L.; Membrilla-Mesa, M.D.; Lozano-Lozano, M.; Galiano-Castillo, N.; Fernández-Lao, C.; Arroyo-Morales, M. Association between physiological and subjective aspects of pain and disability in post-stroke patients with shoulder pain: A cross-sectional study. J. Clin. Med. 2019, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.T.; Nithya, S.; Nomier, Y.; Hassan, D.A.; Jali, A.M.; Qadri, M.; Machanchery, S. Stroke-induced central pain: Overview of the mechanisms, management, and emerging targets of central post-stroke pain. Pharmaceuticals 2023, 16, 1103. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, I.; Larssen, B.C.; Mak-Yuen, Y.Y.; Steinfort, S.; Carey, L.M.; Alahakoon, D. Profiling Somatosensory Impairment after Stroke: Characterizing Common “Fingerprints” of Impairment Using Unsupervised Machine Learning-Based Cluster Analysis of Quantitative Measures of the Upper Limb. Brain Sci. 2023, 13, 1253. [Google Scholar] [CrossRef]
- Stein, B.E.; Stanford, T.R.; Rowland, B.A. The neural basis of multisensory integration in the midbrain: Its organization and maturation. Hear. Res. 2009, 258, 4–15. [Google Scholar] [CrossRef]
- Pouget, A.; Deneve, S.; Duhamel, J.-R. A computational perspective on the neural basis of multisensory spatial representations. Nat. Rev. Neurosci. 2002, 3, 741–747. [Google Scholar] [CrossRef]
- Riera, C.E.; Dillin, A. Emerging role of sensory perception in aging and metabolism. Trends Endocrinol. Metab. 2016, 27, 294–303. [Google Scholar] [CrossRef]
- Dijkerman, H.C.; De Haan, E.H. Somatosensory processing subserving perception and action: Dissociations, interactions, and integration. Behav. Brain Sci. 2007, 30, 224–230. [Google Scholar] [CrossRef]
- Bartlett, J. Introduction to Power Analysis: A Guide to G*Power, Jamovi, and Superpower. Available online: https://osf.io/zqphw (accessed on 22 January 2023).
- Naess, H.; Lunde, L.; Brogger, J. The triad of pain, fatigue and depression in ischemic stroke patients: The Bergen Stroke Study. Cerebrovasc. Dis. 2012, 33, 461–465. [Google Scholar] [CrossRef]
- Einstad, M.S.; Saltvedt, I.; Lydersen, S.; Ursin, M.H.; Munthe-Kaas, R.; Ihle-Hansen, H.; Knapskog, A.-B.; Askim, T.; Beyer, M.K.; Næss, H. Associations between post-stroke motor and cognitive function: A cross-sectional study. BMC Geriatr. 2021, 21, 103. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhang, Y.; Zhang, Y.; Zhang, M.; Kong, Y. Age-associated changes in multimodal pain perception. Age Ageing 2024, 53, afae107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Z.; Zhong, J.; Zhang, Y.; Lin, X.; Wang, J.; Cai, H.; Kong, Y. The analgesic effect of nostalgia elicited by idiographic and nomothetic approaches on thermal stimulus. Ann. N. Y. Acad. Sci. 2022, 1517, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, K.; Zhou, J.; Chai, Y.; Long, Y.; Wang, X.; Manor, B.; Zhang, J.; Fang, J. An MRI-compatible foot-sole stimulation system enabling characterization of the brain response to walking-related tactile stimuli. Front. Neurosci. 2019, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Fong, D.Y.T.; Leung, A.Y.M.; Liao, Q.; Ruscheweyh, R.; Chau, P.H. Validation of the Mandarin Chinese version of the pain sensitivity questionnaire. Pain Pract. 2018, 18, 180–193. [Google Scholar] [CrossRef]
- Neddermeyer, T.J.; Flühr, K.; Lötsch, J. Principle components analysis of pain thresholds to thermal, electrical, and mechanical stimuli suggests a predominant common source of variance. Pain 2008, 138, 286–291. [Google Scholar] [CrossRef]
- Bhalang, K.; Sigurdsson, A.; Slade, G.D.; Maixner, W. Associations among four modalities of experimental pain in women. J. Pain 2005, 6, 604–611. [Google Scholar] [CrossRef]
- Nim, C.G.; O’Neill, S.; Geltoft, A.G.; Jensen, L.K.; Schiøttz-Christensen, B.; Kawchuk, G.N. A cross-sectional analysis of persistent low back pain, using correlations between lumbar stiffness, pressure pain threshold, and heat pain threshold. Chiropr. Man. Ther. 2021, 29, 34. [Google Scholar] [CrossRef]
- Haslam, B.S.; Butler, D.S.; Moseley, G.L.; Kim, A.S.; Carey, L.M. “My Hand Is Different”: Altered Body Perception in Stroke Survivors with Chronic Pain. Brain Sci. 2022, 12, 1331. [Google Scholar] [CrossRef]
- Beck, B.; Làdavas, E.; Haggard, P. Viewing the body modulates both pain sensations and pain responses. Exp. Brain Res. 2016, 234, 1795–1805. [Google Scholar] [CrossRef]
- Van Neerven, S.G.; Mouraux, A. Capsaicin-induced skin desensitization differentially affects a-delta and c-fiber-mediated heat sensitivity. Front. Pharmacol. 2020, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Ekholm, J.; Hansson, P. Pressure pain thresholds in different tissues in one body region. The influence of skin sensitivity in pressure algometry. Scand. J. Rehabil. Med. 1999, 31, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Defrin, R.; Givon, R.; Raz, N.; Urca, G. Spatial summation and spatial discrimination of pain sensation. Pain 2006, 126, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Turk, D.C. Chronic Pain: An Integrated Biobehavioral Approach; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015. [Google Scholar]
- Mozolic, J.L.; Hugenschmidt, C.E.; Peiffer, A.M.; Laurienti, P.J. Multisensory Integration Aging. In The Neural Bases of Multisensory Processes, 1st ed.; Murray, M.M., Wallace, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [PubMed]
- Peiffer, A.M.; Mozolic, J.L.; Hugenschmidt, C.E.; Laurienti, P.J. Age-related multisensory enhancement in a simple audiovisual detection task. Neuroreport 2007, 18, 1077–1081. [Google Scholar] [CrossRef]
- Mozolic, J.L.; Hugenschmidt, C.E.; Peiffer, A.M.; Laurienti, P.J. Modality-specific selective attention attenuates multisensory integration. Exp. Brain Res. 2008, 184, 39–52. [Google Scholar] [CrossRef]
- Guggisberg, A.G.; Koch, P.J.; Hummel, F.C.; Buetefisch, C.M. Brain networks and their relevance for stroke rehabilitation. Clin. Neurophysiol. 2019, 130, 1098–1124. [Google Scholar] [CrossRef]
- Turkeltaub, P.E. A taxonomy of brain–behavior relationships after stroke. J. Speech Lang. Hear. Res. 2019, 62, 3907–3922. [Google Scholar] [CrossRef]
- Taub, D.G.; Jiang, Q.; Pietrafesa, F.; Su, J.; Carroll, A.; Greene, C.; Blanchard, M.R.; Jain, A.; El-Rifai, M.; Callen, A.; et al. The secondary somatosensory cortex gates mechanical and heat sensitivity. Nat. Commun. 2024, 15, 1289. [Google Scholar] [CrossRef]
- Wiech, K.; Lin, C.S.; Brodersen, K.H.; Bingel, U.; Ploner, M.; Tracey, I. Anterior insula integrates information about salience into perceptual decisions about pain. J. Neurosci. 2010, 30, 16324–16331. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.G.; Peyron, R.; Mertens, P.; Gregoire, M.C.; Lavenne, F.; Bonnefoi, F.; Mauguiere, F.; Laurent, B.; Sindou, M. Positron emission tomography during motor cortex stimulation for pain control. Stereotact. Funct. Neurosurg. 1997, 68, 141–148. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Reorganization of cerebral networks after stroke: New insights from neuroimaging with connectivity approaches. Brain 2011, 134, 1264–1276. [Google Scholar] [CrossRef]
- Appelros, P.; Stegmayr, B.; Terént, A. Sex differences in stroke epidemiology: A systematic review. Stroke 2009, 40, 1082–1090. [Google Scholar] [CrossRef]
- Fang, H.; Li, M.; Yang, J.; Ma, S.; Zhang, L.; Yang, H.; Tang, Q.; Cao, J.; Yang, W. Repressing iron overload ameliorates central post-stroke pain via the Hdac2-Kv1. 2 axis in a rat model of hemorrhagic stroke. Neural Regen. Res. 2024, 19, 2708–2722. [Google Scholar] [CrossRef]

| Stroke | Health | t | p | |
|---|---|---|---|---|
| Sample size | 30 (2 female) | 35 (2 female) | ||
| Age (years) | 55.67 ± 11.31 | 54.89 ± 11.00 | 0.282 | 0.779 |
| Age range (years) | 27–77 | 27–77 | ||
| Education (years) | 17.50 ± 4.62 | 15.94 ± 3.29 | 1.581 | 0.119 |
| Age of stroke onset (years) | 55.67 ± 11.31 | – | ||
| Stroke duration (days) | 11.07 ± 8.13 | – | ||
| MRS score | 0.29 ± 0.53 | – | ||
| NIHSS score | 1.31 ± 1.47 | – | ||
| Room temperature (°C) | 24.36 ± 1.82 (Exp1) | 23.05 ± 1.52 | 3.152 | 0.002 * |
| 24.79 ± 1.75 (Exp2) | 23.86 ± 1.51 | 2.274 | 0.026 * | |
| Right foot temperature (°C) | 35.75 ± 0.61 (Exp1) | 35.91 ± 0.44 | −1.191 | 0.238 |
| 35.77 ± 0.67 (Exp2) | 35.44 ± 1.05 | 1.503 | 0.138 | |
| Left foot temperature (°C) | 35.73 ± 0.79 (Exp1) | 35.90 ± 0.38 | −1.002 | 0.312 |
| 35.94 ± 0.45 (Exp2) | 35.88 ± 0.40 | 0.609 | 0.545 | |
| PSQ score | 4.35 ± 1.92 | 5.03 ± 1.56 | −1.594 | 0.116 |
| Pain threshold | ||||
| Heat (°C) | 45.33 ± 2.77 | 44.80 ± 1.75 | 0.883 | 0.382 |
| Pressure (N) | 197.04 ± 43.59 | 178.30 ± 45.35 | 1.620 | 0.111 |
| Stimulation intensity (Exp2) | ||||
| Heat (°C) | 47.49 ± 1.83 | 46.87 ± 2.25 | 1.192 | 0.238 |
| Pressure (N) | 214.40 ± 68.30 | 184.33 ± 84.27 | 1.563 | 0.123 |
| Heat Intensity | M ± SD | t | p | Pressure Intensity | M ± SD | t | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Stroke | Health | Stroke | Health | ||||||
| 42 °C | 1.07 ± 1.15 | 0.93 ± 0.88 | 0.55 | 0.583 | 100 N | 2.14. ± 0.96 | 2.50 ± 1.44 | –1.20 | 0.236 |
| 43 °C | 1.11 ± 1.11 | 1.21 ± 1.01 | −0.37 | 0.710 | 120 N | 2.75 ± 1.21 | 3.48 ± 1.68 | −1.97 | 0.054 |
| 44 °C | 1.52 ± 1.20 | 1.37 ± 1.17 | 0.49 | 0.630 | 140 N | 3.38 ± 1.48 | 4.16 ± 1.90 | −1.82 | 0.074 |
| 45 °C | 2.49 ± 1.84 | 2.79 ± 1.65 | −0.70 | 0.488 | 160 N | 3.70 ± 1.50 | 4.32 ± 1.74 | −1.55 | 0.127 |
| 46 °C | 3.17 ± 2.12 | 3.88 ± 2.17 | −1.33 | 0.190 | 170 N | 4.08 ± 1.62 | 5.03 ± 1.85 | −2.18 | 0.033 * |
| 47 °C | 4.25 ± 2.58 | 4.91 ± 2.54 | −1.04 | 0.303 | 180 N | 4.37 ± 1.77 | 5.43 ± 1.92 | −2.31 | 0.024 * |
| 48 °C | 4.92 ± 2.30 | 5.41 ± 2.08 | −0.83 | 0.412 | 210 N | 5.00 ± 1.99 | 6.10 ± 1.83 | −2.34 | 0.023 * |
| 49 °C | 6.29 ± 2.43 | 6.80 ± 2.15 | −0.83 | 0.410 | 220 N | 5.45 ± 2.09 | 6.55 ± 1.92 | −2.20 | 0.032 * |
| 50 °C | 7.33 ± 2.71 | 7.82 ± 2.27 | −0.74 | 0.465 | 250 N | 6.03 ± 2.32 | 7.04 ± 2.05 | −1.87 | 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, Y.; Zhao, C.; Zhang, Y.; Ni, J.; Zhang, M.; Fan, D.; Kong, Y. Modalities Differentiation of Pain Perception Following Ischemic Stroke: Decreased Pressure Pain Perception. Biomedicines 2025, 13, 2241. https://doi.org/10.3390/biomedicines13092241
Zhi Y, Zhao C, Zhang Y, Ni J, Zhang M, Fan D, Kong Y. Modalities Differentiation of Pain Perception Following Ischemic Stroke: Decreased Pressure Pain Perception. Biomedicines. 2025; 13(9):2241. https://doi.org/10.3390/biomedicines13092241
Chicago/Turabian StyleZhi, Yongkang, Chen Zhao, Yu Zhang, Jianzhang Ni, Ming Zhang, Dongsheng Fan, and Yazhuo Kong. 2025. "Modalities Differentiation of Pain Perception Following Ischemic Stroke: Decreased Pressure Pain Perception" Biomedicines 13, no. 9: 2241. https://doi.org/10.3390/biomedicines13092241
APA StyleZhi, Y., Zhao, C., Zhang, Y., Ni, J., Zhang, M., Fan, D., & Kong, Y. (2025). Modalities Differentiation of Pain Perception Following Ischemic Stroke: Decreased Pressure Pain Perception. Biomedicines, 13(9), 2241. https://doi.org/10.3390/biomedicines13092241







