Abstract
Chronic inflammatory conditions often involve the dysregulation of key enzymes, including serine proteases such as transmembrane serine protease 2 (TMPRSS2) and the angiotensin converting enzyme 2 (ACE2), which are key proteins implicated in the cellular entry mechanism of SARS-CoV-2. It remains uncertain whether the gastrointestinal symptoms observed in COVID-19 patients result from direct viral infection of the gastrointestinal tract, a process that may be exacerbated by altered expression of ACE2 or TMPRSS2. In this review, we explore the interplay among ACE2 and TMPRSS2 in the context of inflammatory bowel disease (IBD), including their roles in disease pathology and response to therapy. We also examine methodological approaches for assessing whether protease alterations contribute to increased susceptibility to infection, considering that TMPRSS2 exists in inactive (zymogen) and active forms. Furthermore, while membrane-bound ACE2 facilitates viral entry, soluble ACE2 fragments may act as decoys, preventing virus–receptor interaction. Therefore, the interpretation of changes in full-length versus cleaved forms of ACE2 and related enzymes is critical for understanding vulnerability to SARS-CoV-2 infection.
Keywords:
ACE2; ADAM17; Crohn’s disease; inflammatory bowel disease; plasma; therapy; ulcerative colitis; TMPRSS2 1. Introduction
1.1. Inflammatory Bowel Disease (IBD)
At the turn of the 21st century, IBD emerged as a growing global health concern, exerting an increasing burden on healthcare systems worldwide. The prevalence of IBD now exceeds 0.3% in many regions [1], with incidence rates continuing to rise, particularly in newly industrialized countries experiencing rapid urbanization and westernization. Projections suggest that the prevalence may surpass 1% of the population in industrialized nations within the next decade [2], underscoring the urgent need for enhanced strategies in prevention, diagnosis, and long-term disease management.
Crohn’s disease (CD) and ulcerative colitis (UC) are the two principal subtypes of inflammatory bowel disease (IBD). The most prevalent clinical symptoms of IBD include chronic bloody diarrhea, abdominal pain, rectal bleeding, weight loss, and fatigue. Systemic features such as fever and malaise are also frequently reported, particularly during disease flares. Although UC and CD share overlapping clinical manifestations, these conditions differ markedly in their pathophysiology, anatomical distribution, and histopathological features. UC is characterized by a continuous mucosal inflammation that typically originates in the rectum and extends proximally through the colon, without affecting other regions of the gastrointestinal tract. Histologically, UC is associated with a reduced number of intestinal crypts and mucosal disruption, accompanied by significant infiltration of immune cells, including macrophages, lymphocytes, granulocytes, and plasma cells. In contrast, CD is defined by a transmural, segmental, and asymmetrical pattern of inflammation that can affect any part of the gastrointestinal tract, with the terminal ileum and colon being the most commonly involved sites. Histopathological examination of CD-affected tissue reveals disrupted intestinal crypts, transmural inflammatory infiltrates, and the presence of lymphoid aggregates (reviewed in [3,4]) (Figure 1A).
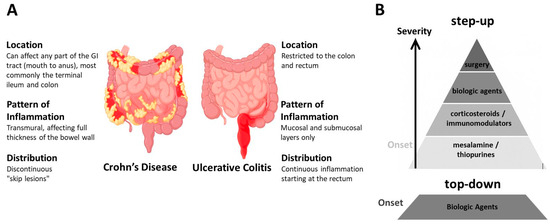
Figure 1.
Crohn’s disease and ulcerative colitis: distinguishing features and common treatment. (A) Schematic representation illustrating the key differences between the two main forms of inflammatory bowel disease (IBD): Crohn’s disease (CD) and ulcerative colitis (UC), in terms of their typical location, inflammation pattern, and distribution (highlighted in red) along the gastrointestinal tract. (B) IBD treatment is typically tailored based on disease severity, prior treatment response, and individual patient factors, following either a “step-up” or “top-down” therapeutic approach. Surgical intervention is considered in cases of complications such as strictures, perforation, failure of medical therapy, or increased cancer risk.
Currently, all therapeutic interventions for IBD are directed at controlling inflammation; however, they are ineffective in reversing established chronic bowel wall damage. Treatment decisions are primarily based on symptom severity and traditionally follow a step-up approach [5], but when treatment is personalized, it may shift to a top-down approach depending on the disease course and patient needs [6,7] (Figure 1B). The goal of treatment is to induce and maintain remission, prevent complications, and improve quality of life [8]. Accordingly, initial therapy for mild presentations of the disease often includes mesalamine/5-aminosalicylic acid [9] and immunomodulators such as thiopurines (e.g., azathioprine) [10]. Azathioprine is the immunomodulator of choice for moderately active IBD and is intended to reduce inflammation; however, its efficacy in maintaining remission is limited [11], particularly when used as monotherapy [12]. Corticosteroids are potent anti-inflammatory and immunosuppressive agents widely used to manage both acute and chronic inflammatory conditions, including moderate-to-severe IBD. Nevertheless, their use is constrained by a range of significant adverse effects [13].
When more potent therapies are required, treatment typically progresses to biologic agents, most commonly monoclonal antibodies targeting tumor necrosis factor-α (TNFα), such as infliximab and adalimumab, or targeting specific interleukins (IL-12/23), such as ustekinumab, or targeting integrins such as vedolizumab [14]. As previously noted, in the top-down therapeutic approach, biologics may be introduced early in the disease course to prevent progression and complications. The advent of biologic therapies has significantly transformed IBD management, leading to improved early treatment responses and unprecedented outcomes, including complete mucosal and histologic healing, consequently reducing hospitalization rates. Additionally, Janus kinase (JAK) inhibitors represent a novel class of therapeutic agents that modulate dysregulated immune responses by attenuating multiple cytokine signaling pathways implicated in the pathogenesis of IBD [15]. Tofacitinib, filgotinib, and upadacitinib have been approved for the treatment of moderate-to-severe UC. Among them, upadacitinib is currently the only JAK inhibitor also approved for the treatment of CD [15]. Application of opioids, probiotics or fecal microbiota is also explored in the treatment of IBD [16].
1.2. IBD and COVID-19
The spread of COVID-19 to countries with high incidence rates of IBD, along with evidence that SARS-CoV-2 can infect gastrointestinal cells (reviewed in [17]), has raised early concerns about the severity of COVID-19 in IBD patients. Although the underlying mechanisms connecting IBD and COVID-19 remain poorly understood, it has been proposed that IBD patients could be more vulnerable to COVID-19 [18]. Moreover, a large number of COVID-19 cases experience digestive symptoms [19,20], whereas gastrointestinal symptoms are part of the manifestations in long COVID-19 [21], and a case report even suggested that COVID-19 could trigger UC [22].
Anyhow, several studies have indicated that the prevalence of COVID-19 in patients with IBD was similar to the general population [23] and even to household members [24]; patients with other immune-mediated inflammatory diseases also showed lower susceptibility to COVID-19 [25]. Moreover, individuals with IBD do not have an increased risk of COVID-19 associated mortality, or with intensive care unit admission compared to the general population [26,27]. Nonetheless, most of the available evidence regarding the impact of COVID-19 in IBD derives from observational studies (reviewed in [28]), and few studies have investigated whether underlying pathobiological features in IBD patients confer increased susceptibility to local gastrointestinal SARS-CoV-2 infection.
For that reason, the potential contribution of chronic gastrointestinal inflammation, or altered expression of key proteins involved in SARS-CoV-2 cell entry, to the increase in local infection rates should not be overlooked. Indeed, chronic inflammatory conditions have been proposed as potential risk factors for increased COVID-19 severity [29], and an excessive inflammatory state increases the risk of severe disease and mortality in patients with COVID-19 [30]. Previous reviews addressed SARS-CoV-2 effects on the gastrointestinal systems, including the inflammatory response [31], however few studies investigated whether key proteins involved in SARS-CoV-2 infection are altered in association with IBD pathology. An angiotensin-converting enzyme 2 (ACE2) upregulation in active IBD tissue has been linked to compensatory anti-inflammatory responses (discussed in [32]) but may also result in increased SARS-CoV-2 vulnerability in people with gastrointestinal infections.
In this review, we examine whether the expression of key host proteins required for viral entry, namely ACE2, which serves as the cellular receptor for SARS-CoV-2, and the transmembrane serine protease 2 (TMPRSS2), which primes the viral spike protein, are altered in individuals with IBD. We discussed the implications of alterations in UC and in CD for particular molecular forms of ACE2 and TMPRSS2 in relation to gastrointestinal susceptibility to SARS-CoV-2 infection. Accordingly, we examine the accurate interpretation of changes in tissue biopsies, as well as in circulating molecular forms, where co-existing shedded fragments of ACE2 and TMPRSS2 are also present, alongside full-length species.
2. Characterization of ACE2 and TMPRSS2 Molecular Forms and Significance for COVID-19 Risk
As mentioned above, there is consensus regarding SARS-CoV entry into host cells via the gastrointestinal tract by binding to the enterocyte-expressed ACE2 receptor; and its colocalization with TMPRSS2 on cell surfaces, which facilitates viral spike fusogenic activity and also promotes viral entry [33]. ACE2 is predominantly located in human gastrointestinal-tract epithelia, and the highest levels of TMPRSS2 are also found in the gastrointestinal tract [34]. In particular, ACE2 is expressed largely in the colon and the terminal ileum [35].
2.1. ACE2 Protein Function and Proteolytic Processing
ACE2 is a monocarboxypeptidase that serves as a key counter-regulatory component of the renin-angiotensin system (RAS). The RAS is one of the most complex hormonal regulatory systems, involving several organs that interact to regulate multiple body functions [36]. The canonical role of RAS in the regulation of cardiovascular function has been extended by evidence that local RAS exists in diverse tissue types and by findings that indicate the RAS comprises two axes, the classic RAS and an alternative RAS, with antagonistic effects that are usually in equilibrium. The classic system is involved in pathologies where inflammatory, hypertrophic and fibrotic phenomena are common and are related to the development of chronic diseases that affect various body systems. In the classical RAS pathway, renin converts angiotensinogen into angiotensin I (Ang I), which is subsequently cleaved by angiotensin-converting enzyme (ACE) to form angiotensin II (Ang II). Ang II, through activation of the angiotensin II type 1 receptor (AT1R), exerts potent vasoconstrictive, pro-inflammatory, pro-thrombotic, and pro-fibrotic effects. In the alternative axis, ACE2 mitigates the effects of this axis by degrading Ang II into angiotensin-(1–7) [Ang-(1–7)], which binds to the Mas receptor and initiates signaling cascades that promote anti-fibrotic, anti-proliferative and anti-inflammatory effects [37]. By reducing Ang II bioavailability and simultaneously increasing Ang-(1–7) levels, ACE2 effectively shifts the RAS balance toward a protective balance within the system. Thus, enzymatic activity of ACE2 is essential for cardiovascular and renal physiology, as it regulates blood pressure, vascular tone, sodium and water balance, and prevents maladaptive tissue remodeling. Dysregulation of ACE2 expression or activity has been implicated in hypertension, heart failure, chronic kidney disease, acute lung injury, among others, and has potential implications in SARS-CoV vulnerability [38,39,40]. In contrast, Ang-(1–7) binds to the Mas receptor and initiates signaling cascades that promote vasodilation, anti-inflammatory responses, anti-proliferative effects, and tissue protection [41]. By reducing Ang II bioavailability and simultaneously increasing Ang-(1–7) levels, ACE2 effectively shifts the RAS balance toward a vasoprotective and homeostatic state. Moreover, ACE2 presents other physiological implications in RAS, since it can also drive cleavage of other vasoactive peptides such as the Mas receptor and AT2R [42]. ACE2 can also act as a partner for intestinal amino acid transporters [43]. For a complete review of ACE2, see [44].
The relationship between ACE2 and gastrointestinal disease will be discussed later, but components of the RAS, such as Ang-(1–7) and Mas receptor have been implicated in IBD [45], suggesting that an imbalance of the RAS may contribute to inflammation and fibrosis in IBD [46]. In this context, drugs targeting RAS have demonstrated anti-inflammatory and antifibrotic properties in the gut in preclinical studies, offering potential benefits for IBD patients (reviewed in [47]).
Some of these biological functions of ACE2 are exerted by soluble species. These soluble forms are generated by the cleavage of the membrane-resident ACE2 by the membrane-bound sheddases from the ADAM (a disintegrin and metalloprotease) family, ADAM17/TACE and ADAM10 [48], generating large ectodomain fragments that retain the carboxypeptidase [49] and the binding region to SARS-CoV-2 [50] (Figure 2A). It has been proposed that TMPRSS2 and ADAM17 will compete for ACE2 cleavage, resulting in different ACE2 cleaved products, which will exhibit differential fates [51]. ACE2 cleavage by ADAM17 and ADAM10 occurs through constitutive and regulated shedding [49], while it is unclear whether the cleavage of ACE2 by TMPRSS2 occurs in the absence of the coronavirus.
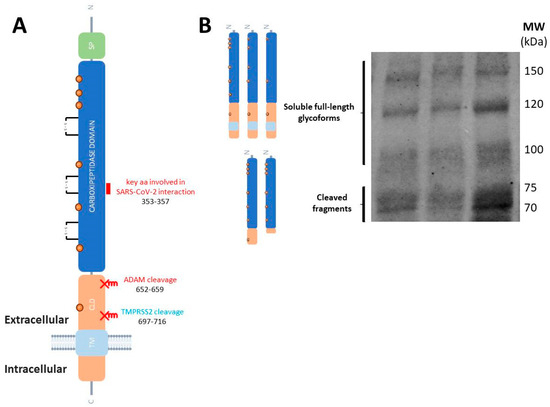
Figure 2.
Molecular species of ACE2 in human tissues and fluids. (A) Schematic representation of ACE2 as a transmembrane type I glycoprotein (not drawn to scale). The single peptide (SD), carboxypeptidase domain, collectrin-like domain (CLD) and the transmembrane (TM) domain are represented. SARS-CoV-2 S-protein binds to the carboxypeptidase ectodomain. The short domain (key amino acid residues 353-KGDFR-357) involved in the interaction with SARS-CoV-2 spike glycoprotein is represented (marked in purple). The sites of ACE2 shedding with ADAM17 are also indicated. (B) Representative blot of human plasma samples incubated with the anti-ACE2 ectodomain AF933 antibody (R&D Systems) showing the cleaved ACE2 fragments, and the soluble full-length species which diversity could be attributed to highly glycosylated forms (unpublished blot).
Interestingly, it has been demonstrated that full-length, unprocessed ACE2 retaining both its transmembrane and cytoplasmic domains is present as soluble forms in human plasma (Figure 2B), cerebrospinal fluid (CSF), and other biological fluids [52,53]. As a result, both cleaved ACE2 fragments and full-length ACE2 species may contribute in different proportions to the total soluble pool of ACE2, with variations in pathological conditions. Indeed, the presence of ACE2 fragments in circulation is expected to result from the shedding of membrane-bound ACE2, and a previous study suggested that circulating levels of full-length ACE2 may reflect its tissue expression levels [54]. Nonetheless, the potential contribution of full-length ACE2 to total plasma ACE2 levels has not been adequately considered in most studies. Notably, many of these investigations have relied on enzyme-linked immunosorbent assays (ELISA), or immunohistological analysis in intestinal biopsies, which often do not distinguish between ACE2 fragments and the full-length protein, or discriminate adequately between different fragments, thus leaving uncertainty regarding the specific species present in circulation and their contributions.
2.2. TMPRSS2 Protein Function and Proteolytic Processing
Moreover, it has been demonstrated that infection of SARS-CoV-2 in mature enterocytes expressing ACE2 is facilitated by TMPRSS2, highlighting the intestine as a potential site of SARS-CoV-2 replication [55]. TMPRSS2 is also a transmembrane glycoprotein, which is synthesized as an inactive zymogen that undergoes autoproteolytic cleavage to generate an active serine protease domain (represented in Figure 3A), which is responsible for priming the SARS-CoV-2 spike protein. Following cleavage, the active protease domain may either remain associated with the pro-domain through an interdomain disulfide bond or is released into the extracellular environment in a soluble fragment form [56,57].
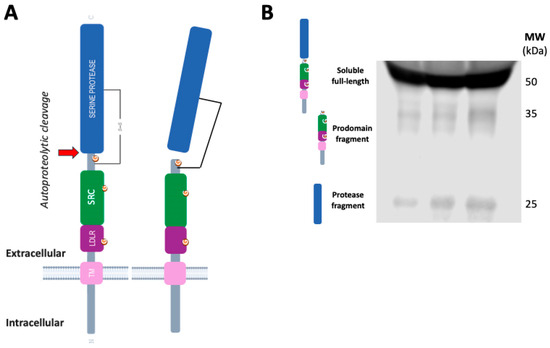
Figure 3.
Molecular species of TMPRSS2 in human tissues and fluids. (A) Schematic representation of TMPRSS2 as a transmembrane type II protein (not drawn to scale). The serine protease domain, the scavenger receptor cysteine-rich (SRC) domain and the LDL-receptor class A domain (LDLR). TMPRSS2 is expressed as a single chain zymogen that undergoes autoproteolytic cleavage at the position indicated ( , residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
, residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
 , residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
, residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
As mentioned above, TMPRSS2 displays a broad expression in epithelial cells of the respiratory and gastrointestinal tract, the prostate, and other organs, whose physiological role remains largely elusive. Several potential substrates for TMPRSS2 have been proposed, including protease-activated receptor-2 (PAR-2), which mediates protective functions in the gastrointestinal and respiratory tract; however, TMPRSS2 also cleaves pro-kallikrein-2 (KLK2) into mature KLK2 and activates the single-chain precursor of hepatocyte growth factor (HGF) (reviewed in [58]).
In human cells, the TMPRSS2 full-length zymogen is the most abundant species [55], and, surprisingly, this proteolytic unprocessed species is also the most abundant in plasma [59] and CSF [53] (Figure 3B). Therefore, it is also important to conduct an analysis by Western blotting, or techniques that discriminate between species, to estimate the levels of the protease fragment that could represent the active membrane-resident forms involved in SARS-CoV-2 cellular infection.
2.3. Significance of Particular ACE2 and TMPRSS2 Molecular Species Regarding COVID-19 Vulnerability
The importance of differentiating between tissue and circulating ACE2 regarding vulnerability to SARS-CoV-2 has been previously advised [60]. Vulnerability to SARS-CoV-2 infection should be assessed based on the relative abundance of specific circulating ACE2 species. Therefore, elevated levels of circulating ACE2 fragments could be interpreted as potentially protective, as soluble ACE2 may act as a decoy receptor by binding and neutralizing the virus [61]. On the other hand, high levels of membrane-resident ACE2 suggests increased vulnerability of the gastrointestinal tract to viral entry, and higher levels of membrane-resident ACE2 should also result in increased levels of circulating full-length ACE2 with protective effects [54]. With regard to TMPRSS2, increased vulnerability to SARS-CoV-2 should only be determined by elevated generation of active protease domains (Figure 4A includes a schematic representation).
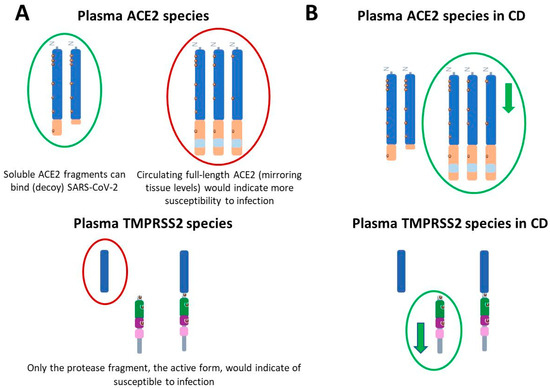
Figure 4.
Significance of changes in molecular species of ACE2 and TMPRSS2 regarding COVID-19 and its alterations in CD. (A) Changes in ACE2 and TMPRSS2 associated with chronic conditions that might increase vulnerability to SARS-CoV-2 infection. Elevated levels of cleaved ACE2 fragments could be interpreted as potentially protective (circled in green), as soluble ACE2 may act as a decoy receptor by binding and neutralizing the virus. In this sense, increased active ADAM17 (a metalloprotease that cleaves ACE2) would also indicate less susceptibility to infection. Elevated levels of full-length species of ACE2, reflecting tissue content of the full-length membrane-resident forms or elevated levels of TMPRSS2 fragments could represent increased vulnerability (circled in red). (B) Levels of ACE2 and TMPRSS2 species in plasma from individuals affected by Crohn’s disease (CD). Full-length ACE2 decreased ( ) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
 ) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
3. Levels of ACE2 and TMPRSS2 in IBD Biopsy Tissue
To date, most studies investigating potential alterations in ACE2 and TMPRSS2 expression in IBD patients have relied on transcriptional analyses, yet no clear consensus has been established. ACE2 exhibits a heterogeneous expression pattern throughout the gastrointestinal tract [35], and both ACE2 and TMPRSS2 mRNA levels vary depending on the anatomical region and the presence of inflammation [62]. For that, although intestinal inflammation is a key factor influencing the expression of ACE2 and TMPRSS2 in IBD, regional differences within the gut also contribute to the observed variability [63]. Moreover, a particular ACE2 expression pattern could be related to the physiological roles of ACE2, including an alternative function that degrades Ang II. Hence, ACE2 presents other physiological implications in RAS, since it can also drive cleavage of other vasoactive peptides such as the Mas receptor and AT2R [42]. Beyond the RAS, ACE2 displays potential catalytic activity to cleave peptides like apelin-13, dynorphin 13, des-Arg9-bradykinin peptides, β-casomorphin, neurotensin 1–8 and ghrelin [64], and some of these substrates appear to be present in the gastrointestinal tract (reviewed in [65]).
ACE2 can also act as a partner for intestinal amino acid transporters [43], potentially playing other key physiological functions related to specific cellular and subcellular localizations and shedding, which could be affected across IBD pathology. In fact, it cannot be discarded that the cleaved intracellular domain of ACE2 might act as a transcriptional regulator analogous to the intracellular domain of amyloid precursor protein or the notch receptor following ectodomain cleavage (discussed in [44]).
The intracellular cholesterol transporter Niemann Pick C1 (NPC1) protein, located in the late endosome/lysosome, also influences ACE2 abundance at the plasma membrane and its inhibition relocalizes ACE2 from the plasma membrane to the autophagosomal/lysosomal compartment [66]. Moreover, although ACE2 colocalizes with raft-resident markers [67], incubation of cells with viral spike protein revealed discrete dotted patterns at the cell surface, which consistently colocalizes with endogenous ACE2, but sparsely with a lipid raft marker [68]. In fact, it has recently been reported that SARS-CoV-2 entry and fusion are independent of ACE2 localization to lipid rafts [69]. Thus, subcellular localization of ACE2, and TMPRSS2, should play a fundamental role in their biological function and involvement in pathology, but this issue warrants more investigation.
Moreover, variances among intracellular locations of ACE2 and TMPRSS2 could also contribute to a misinterpretation of changes in these proteins in the context of IBD and susceptibility to SARS-CoV-2 infectivity. In a recent study using immunohistochemistry, a significant increase in ACE2 and TMPRSS2 was reported in colon and rectal specimens of UC [70]; although in UC, colonic ACE2 and TMPRSS2 appear as cytoplasmic in nature [71], therefore these alterations could not determine changes in vulnerability to SARS-CoV-2, at least regarding cellular penetrance of the virus. A different study that reports downregulated transcript and protein levels of ACE2 in inflamed CD ileum, and in multiple well-established mouse models, also demonstrated altered localization of intestinal epithelial cell ACE2 protein in inflamed tissues by immunostaining, with a progressive cytosolic translocation with increasing inflammation [72]. Furthermore, in primary human airway epithelium upon allergic inflammation, glycosylation of full-length ACE2 is reduced, thereby limiting expression on the apical side of ciliated cells exposed to viral infection [73].
Microarray transcriptomic analyses have reported reduced ACE2 expression in the small intestine of CD patients, while elevated levels have been observed in the colonic tissue of individuals with UC compared to controls [74]. Similarly, other studies have shown increased levels of ACE2 transcript in the inflamed colon of both UC and CD patients, but decreased expression in the inflamed ileum of CD patients [75]. In line with these findings, a database-mining study also reported elevated ACE2 expression in the colon of both CD and UC patients, and reduced levels in the ileum of CD patients [76]. Another study assessing colonic ACE2 expression using RNA sequencing and quantitative RT-PCR reported elevated levels in a subset of adult CD patients with a poor prognosis [77].
However, other studies analyzing mucosal biopsies from IBD patients have reported lower ACE2 expression compared to controls, as determined by RT-PCR [78]. These findings are consistent with previous reports showing reduced transcript levels of both ACE2 and TMPRSS2 in IBD patients [79]. In addition, no significant differences in ACE2 protein levels between IBD patients and controls have been reported in a quantitative proteomic study using intestinal tissue biopsy samples obtained during colonoscopy [80]. Similarly, another proteomic analysis found that ACE2 expression remained unchanged in the gut of patients with UC compared to control individuals [81].
In summary, analyses of intestinal biopsy samples have yielded inconsistent results, reflecting not only inter-study variability but also the inherently heterogeneous expression patterns of ACE2 and TMPRSS2 throughout the gastrointestinal tract and differences in cellular location. As such, assessing the levels of these proteins in the plasma could provide a more suitable means of quantification to determine susceptibility to SARS-CoV-2 infection.
4. Levels of ACE2 and TMPRSS2 in IBD Plasma
As mentioned, assessing the levels of ACE2 and TMPRSS2 species in the plasma of IBD patients could be potentially informative to gain a more systemic perspective on their potential vulnerability to COVID-19. Studies conducted using methodologies unable to discriminate between the different ACE2 species reported different results. As such, an early study reported higher levels of circulating ACE2 activity, estimated by an enzymatic assay, in patients with IBD compared to controls [82], whereas in a more recent study, serum sACE2 levels, estimated by ELISA, changed minimally in IBD patients [78].
In this complex scenario, we recently characterized changes in CD patients in different species of ACE2 and TMPRSS2 present in circulation [59]. In this study, only ACE2 full-length species appeared significantly decreased in the plasma of CD patients before treatment onset, as compared with age-matched controls, while levels of cleaved fragment remained unaltered. This reduction on levels of soluble full-length ACE2 may reflect decreased overall tissular expression or enhanced ACE2 shedding mediated by IBD condition. Indeed, a quotient between ACE2 fragment and ACE2 full-length species was found to discriminate between CD patients and control subjects (summarized in Figure 4B).
Moreover, there are several alternative transcripts of ACE2 encoding short isoforms, some missing parts of the cytoplasmic and collectrin domains, but also other species lacking the ectodomain SARS-CoV-2 spike protein binding sites [83]; thus, resulting in shorter forms with varying abilities to interact with the SARS-CoV-2 spike protein. Therefore, the independent estimation of these variants is relevant to determine if changes contribute to susceptibility to SARS-CoV-2 infection.
As discussed, different ACE2 species could be relevant in both a functional sense and as modulators of distinct pathologies. ACE2 can be cleaved through different means, such as the ACE2 sheddase ADAM17/TACE. This sheddase has been classically associated with inflammation, since it also processes TNFα and releases the soluble biologically active form, thus attracting interest in the IBD field, at least from a therapeutic view. A loss-of function/deletion of ADAM17 has also been associated with bowel disease [84,85]. However, alterations of ADAM17 associated with CD or UC are unclear. ADAM17 activity was demonstrated in all cell lines and in cells of controls or IBD patients, irrespective of disease activity [86], but an early study reported increased ADAM17 activity in the colonic mucosa of UC patients [87]. Further studies on ADAM17 expression in intestinal mucosa failed to demonstrate changes in IBD patients, regardless of medication [78,88]. The contribution of other members of the ADAM family to IBD has also been proposed, particularly ADAM19, the RNA transcript and protein levels of which appear upregulated in intestinal mucosa from patients with UC and, to a lesser extent, in patients with CD compared with controls [89].
As mentioned above, TMPRSS2 is also capable of cleaving ACE2, in competition with ADAM17 [51]. Therefore, elevated TMPRSS2 levels in IBD could exert a dual effect on SARS-CoV-2 susceptibility by influencing both ACE2 processing and spike protein priming. However, studies have reported decreased expression of TMPRSS2 in the intestinal mucosa of IBD patients compared to healthy controls [78,79]. In our analysis of plasma from CD patients, the active TMPRSS2 protease fragment remained unchanged, while a reduction was observed in the prodomain fragment (summarized in Figure 4B). This finding suggests a potential impairment or reduction in the zymogen-to-active protease conversion of TMPRSS2 in CD patients [59]. Therefore, if increased ACE2 shedding occurs in the context of IBD, it is likely to be independent of TMPRSS2 activity. Our data also indicates that alterations in TMPRSS2 levels are not associated with increased susceptibility to SARS-CoV-2 infection in these patients.
5. Effects of IBD Therapies on ACE2 Levels
Most of the studies commented above include patients with IBD that are under treatment, and the different therapies can differentially affect ACE2 or TMPRSS2 [90]. Immunosuppression, coupled with increased ACE2 and TMPRSS2, impairs effective immune responses against SARS-CoV-2 and increases the likelihood of severe immune response, such as cytokine storms [91]. Thus, initial concerns were raised regarding immune-suppressor therapies however observational studies conclude that treatment with biologic drugs does not represent a risk factor for the SARS-CoV-2 infection in patients with IBD [92,93], and a meta-analysis data indicates that susceptibility to COVID-19 did not increase with any drugs for IBD [27]. Thus, based on the currently available data, immunomodulatory and biological therapies can be continued in patients with IBD in remission suffering COVID-19 infection (discussed in [94]). Attention should be paid to new results on the influence on ACE2 and TMPRSS2 levels and proteolytic processing in the dynamic pandemic situation and development of new IBD therapy approaches [95].
Indeed, commonly used IBD therapies, both biologic and non-biologic, do not appear to significantly affect ACE2 or TMPRSS2 expression patterns in uninflamed intestinal tissue [62,78], although one study reported downregulation of colonic ACE2 expression in IBD patients who responded to anti-TNFα therapy [76]. None of these studies demonstrated an increased susceptibility to SARS-CoV-2 infection associated with changes in ACE2 or TMPRSS2 expression induced by IBD treatment. It is important to note that these studies did not evaluate plasma levels of these proteins or investigate alterations in their proteolytic processing, such as differences between full-length and cleaved fragments.
In our previous study [59], we found that CD patients treated with adalimumab or azathioprine exhibited a recovery of circulating full-length ACE2 species. Interestingly, patients treated with azathioprine also showed a decrease in one of the ACE2 fragments. However, patients receiving infliximab or ustekinumab continued to show reduced levels of full-length ACE2, along with decreases in its cleaved fragments. These alterations were modest in magnitude, suggesting that IBD therapies may modulate ACE2 proteolytic processing and contribute to the restoration of physiological regulation. Anyhow, none of the changes can be interpreted in the view of increased susceptibility to SARS-CoV-2 infection. Thus, no evidence points to the need for drug repositioning or the management of COVID-19 in patients with IBD.
Regarding TMPRSS2, none of the therapies studied resulted in significant changes in the levels of the active protease domain [59]. However, given that TMPRSS2 protease activity is driven by the fragment generated after autoproteolytic cleavage, not directly depending of zymogen/mRNA abundance, to determine enzyme activity assays appears revelant. Moreover, the regulation of how TMPRSS2 is able to autoactivate by autoproteolytic cleavage requires further investigation, also in the context of IBD. Interestingly, individuals with Down syndrome (DS) have TMPRSS2 triplication and show increased vulnerability to SARS-CoV-2 infection [96]. Associations between IBD and Down syndrome have been reported, although very sparsely [97,98], and poorer clinical outcomes may be related with immune dysregulation and a high prevalence of comorbidities.
Therapies for COVID-19 include use of human recombinant soluble ACE2 to act as decoy receptors for binding and neutralizing SARS-CoV-2 [99,100] and inhibition TMPRSS2 activity to prevent spike protein priming [101,102]. The potential impact of such therapies in infected IBD patients should be monitored.
Finally, further research focusing on the immune response to COVID-19 vaccination in biologically treated IBD patients, as well as on the long-term consequences of SARS-CoV-2 infection in this population, will be essential to better understand their vulnerability to COVID-19. Longitudinal studies on the expression and regulation of processing of ACE2 and TMPRSS2 over the course of the CD and UC are needed.
6. Conclusions and Final Remarks
In conclusion, individuals with IBD do not exhibit molecular changes in tissular or circulating ACE2 nor TMPRSS2 consistent with increased vulnerability to SARS-CoV-2 coronavirus cell entry in the gastrointestinal tract. Further studies addressing which species of ACE2 and TMPRSS2 could be altered in plasma from IBD patients infected by SARS-CoV2 can improve our knowledge about the role of these proteins in virus infectivity and whether this condition confers high susceptibility to SARS-CoV-2 cell entry. In this regard, it has been reported that ACE2 activity in plasma from SARS-CoV-2 positive patients is unchanged compared to SARS-CoV-2 negative patients [103]. In a cross-sectional retrospective multicenter study in COVID-19 patients with and without IBD, the levels of serum ACE2 levels were higher in IBD patients [104].
One key insight emerging from our recent studies is the critical need to evaluate not only total levels of ACE2 and TMPRSS2, but also their specific molecular forms. The distinction between ACE2 soluble species, either as proteolytic fragments or full-length forms, is essential for understanding its dual role as a viral entry receptor and a potential protective decoy in circulation. Similarly, the functional relevance of TMPRSS2 must be interpreted in light of its activation status, given that the enzymatically active protease domain appears to be selectively modulated in disease contexts. From a methodological standpoint, we advocate for future studies to employ molecular approaches that allow the discrimination of ACE2 and TMPRSS2 forms (such as quantitative Western blotting) in both tissue biopsies and peripheral fluids. Moreover, such analyses should be conducted within stratified and longitudinal patient cohorts, taking into account disease subtype, anatomical location of inflammation, treatment modality, and clinical course. Addressing this will require mechanistic and translational studies that go beyond observational data.
Finally, the potential utility of soluble ACE2 and TMPRSS2 molecular forms as surrogate biomarkers of mucosal inflammation, epithelial integrity, or viral vulnerability in IBD remains an open question. Interestingly, in our recent study [59] the estimation of a quotient between the largest ACE2 fragment and the full-length form gave the greatest discrimination between CD patients and controls. However, in plasma from COVID-19 patients [52] and in CSF of patients with COVID-19 encephalopathy [53] the shortest ACE2 fragment is the one that specifically display increases, which we also defined in an alternative quotient. Therefore, assessing the changes in ACE2 fragments could be useful for evaluating progression of IBD or of COVID-19 when IBD patients are infected.
More efforts are needed to clarify the interplay between chronic intestinal inflammation and viral pathogenesis but could also identify novel therapeutic or diagnostic avenues relevant to future pandemics and immune-mediated diseases.
Author Contributions
J.S.-V. wrote the article, J.S.-L. and C.A.-G. created the figures and the tables. M.-S.G.-A. and M.P.L. critically reviewed the content of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Fondo de Investigaciones Sanitarias (PI22/01329, co-funded by the Fondo Europeo de Desarrollo Regional, FEDER “Investing in your future”), CIBERNED (Instituto de Salud Carlos III, Spain), from the Direcció General de Ciència i Investigació, Generalitat Valenciana (AICO/2021/308) and “Severo Ochoa” Program for Centers of Excellence in R&D (CEX2021-001165-S). CAG is supported by a predoctoral contract (PRE2022-104182) funded by the Agencia Estatal de Investigación (AEI) and the Ministerio de Ciencia e Innovación (MCIN) under the Plan Estatal de I + D + I 2021–2023, co-funded by the European Social Fund (FSE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are indebted to our colleagues Ana Gutiérrez and Rubén Francés (Instituto de Investigación Sanitaria y Biomédica de Alicante, Alicante, Spain; Hepatic and Intestinal Immunobiology Group, Department of Clinical Medicine, Miguel Hernández University, San Juan de Alicante, Spain) for support and advice.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE2 | angiotensin-converting enzyme 2 |
| ADAM | a disintegrin and metalloprotease |
| Ang | angiotensin |
| CD | Crohn’s disease |
| CSF | cerebrospinal fluid |
| ELISA | enzyme-linked immunosorbent assays |
| IBD | Inflammatory Bowel Disease |
| JAK | Janus kinase |
| TNFα | tumor necrosis factor-α |
| UC | ulcerative colitis |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of inflammatory bowel disease: From 2025 to 2045. Nat. Rev. Gastroenterol. Hepatol. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Roger, J. Inflammatory Bowel Disease: Immune Function, Tissue Fibrosis and Current Therapies. Int. J. Mol. Sci. 2024, 25, 6416. [Google Scholar] [CrossRef]
- da Silva Júnior, R.T.; Apolonio, J.S.; de Souza Nascimento, J.O.; da Costa, B.T.; Malheiro, L.H.; Luz, M.S.; de Carvalho, L.S.; da Silva Santos, C.; de Melo, F.F. Crohn’s disease and clinical management today: How it does? World J. Methodol. 2023, 13, 399–413. [Google Scholar] [CrossRef]
- Hirschmann, S.; Neurath, M.F. Top-down approach to biological therapy of Crohn’s disease. Expert Opin. Biol. Ther. 2017, 17, 285–293. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Herrlinger, K.R.; Stange, E.F. Prioritization in inflammatory bowel disease therapy. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 753–767. [Google Scholar] [CrossRef]
- D’Haens, G.; van Bodegraven, A.A. Mesalazine is safe for the treatment of IBD. Gut 2004, 53, 155. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Roy, A.; Lawlor, G.; Korelitz, B.; Lichtiger, S. Thiopurines and inflammatory bowel disease: Current evidence and a historical perspective. World J. Gastroenterol. 2016, 22, 10103–10117. [Google Scholar] [CrossRef]
- Chande, N.; Patton, P.H.; Tsoulis, D.J.; Thomas, B.S.; MacDonald, J.K. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2015, 10, CD000067. [Google Scholar] [CrossRef]
- Rogler, G.; Sandborn, W.J. Is there still a role for thiopurines in Crohn’s disease? Gastroenterology 2013, 145, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef] [PubMed]
- Kapizioni, C.; Desoki, R.; Lam, D.; Balendran, K.; Al-Sulais, E.; Subramanian, S.; Rimmer, J.E.; Negro, J.D.L.R.; Pavey, H.; Pele, L.; et al. Biologic Therapy for Inflammatory Bowel Disease: Real-World Comparative Effectiveness and Impact of Drug Sequencing in 13 222 Patients within the UK IBD BioResource. J. Crohn’s Colitis 2024, 18, 790–800. [Google Scholar] [CrossRef]
- Honap, S.; Agorogianni, A.; Colwill, M.J.; Mehta, S.K.; Donovan, F.; Pollok, R.; Poullis, A.; Patel, K. JAK inhibitors for inflammatory bowel disease: Recent advances. Frontline Gastroenterol. 2023, 15, 59–69. [Google Scholar] [CrossRef]
- Long, D. Crohn’s Disease and Ulcerative Colitis: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2024, 12, 689. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Zheng, Y.; An, J.; Wen, G.; Jin, H.; Tuo, B. Digestive system infection by SARS-CoV-2: Entry mechanism, clinical symptoms and expression of major receptors (Review). Int. J. Mol. Med. 2023, 51, 19. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Matias, J.N.; Flato, U.A.P.; Pilon, J.P.G.; Bitelli, P.; Junior, M.A.P.; de Carvalho, A.C.A.; Haber, J.F.D.S.; Reis, C.H.B.; Goulart, R.A. What Do Influenza and COVID-19 Represent for Patients With Inflammatory Bowel Disease? Gastroenterol. Res. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Challa, S.R.; Singh, G.; Nanduri, S.; Ibrahim, M.; Martirosyan, Z.; Whitsell, P.; Chirumamilla, L.G.; Shayegh, N.; Watson, K.; et al. Gastrointestinal Manifestations and Their Association with Neurologic and Sleep Problems in Long COVID-19 Minority Patients: A Prospective Follow-Up Study. Dig. Dis. Sci. 2024, 69, 562–569. [Google Scholar] [CrossRef]
- Aydın, M.F.; Taşdemir, H. Ulcerative Colitis in a COVID-19 Patient: A Case Report. Turk. J. Gastroenterol. 2021, 32, 543–547. [Google Scholar] [CrossRef]
- Gubatan, J.; Levitte, S.; Balabanis, T.; Patel, A.; Sharma, A.; Habtezion, A. SARS-CoV-2 Testing, Prevalence, and Predictors of COVID-19 in Patients with Inflammatory Bowel Disease in Northern California. Gastroenterology 2020, 159, 1141–1144.e2. [Google Scholar] [CrossRef]
- Anushiravani, A.; Saberzadeh-Ardestani, B.; Vahedi, H.; Fakheri, H.; Mansour-Ghanaei, F.; Maleki, I.; Nasseri-Moghaddam, S.; Vosoghinia, H.; Ghadir, M.R.; Hormati, A.; et al. Susceptibility of Patients with Inflammatory Bowel Disease to COVID-19 Compared with Their Households. Middle East J. Dig. Dis. 2022, 14, 182–191. [Google Scholar] [CrossRef]
- Attauabi, M.; Poulsen, A.; Theede, K.; Pedersen, N.; Larsen, L.; Jess, T.; Hansen, M.R.; Verner-Andersen, M.K.; Haderslev, K.V.; Lødrup, A.B.; et al. Prevalence and Outcomes of COVID-19 Among Patients with Inflammatory Bowel Disease-A Danish Prospective Population-based Cohort Study. J. Crohn’s Colitis 2021, 15, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Taxonera, C.; Sagastagoitia, I.; Alba, C.; Mañas, N.; Olivares, D.; Rey, E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2020, 52, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Li, H.J.; Wasuwanich, P.; Kim, S.E.; Kim, J.Y.; Jeong, G.H.; Park, S.; Yang, J.W.; Kim, M.S.; Yon, D.K.; et al. COVID-19 susceptibility and clinical outcomes in inflammatory bowel disease: An updated systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2414. [Google Scholar] [CrossRef] [PubMed]
- Kogan, L.; Ungaro, R.C.; Caldera, F.; Shah, S.A. Effects of COVID-19 on Patients with Inflammatory Bowel Disease. Rhode Isl. Med. J. 2022, 105, 42–47. [Google Scholar]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Zinellu, A. Systemic inflammation index, disease severity, and mortality in patients with COVID-19: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1212998. [Google Scholar] [CrossRef]
- Saviano, A.; Brigida, M.; Petruzziello, C.; Zanza, C.; Candelli, M.; Loprete, M.R.M.; Saleem, F.; Ojetti, V. Intestinal Damage, Inflammation and Microbiota Alteration during COVID-19 Infection. Biomedicines 2023, 11, 1014. [Google Scholar] [CrossRef]
- Bourgonje, A.R. Dualistic Implications of Angiotensin-Converting Enzyme 2 (ACE2) Expression in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2025, 16, izaf131. [Google Scholar] [CrossRef]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef]
- Bach, M.L.; Jensen, B.L. Effects and regulation of ACE2 and TMPRSS2 abundance in healthy humans and in patients with SARS-CoV-2. Biochem. Soc. Trans. 2025, 53, 775–786. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Lin, W.; Liu, H.; Zhong, W.; Zhang, X.; Zhu, Y.; Wang, X.; Li, J.; Sheng, Q. SARS-CoV-2 Host Receptor ACE2 Protein Expression Atlas in Human Gastrointestinal Tract. Front. Cell Dev. Biol. 2021, 9, 659809. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.A.V.; Millán, J.M.V.; Bonilla, E.F. Renin-angiotensin system: Basic and clinical aspects-A general perspective. Endocrinol Diabetes Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1–7 Axis of the Renin-Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef]
- Sehn, F.; Büttner, H.; Godau, B.; Müller, M.; Sarcan, S.; Offermann, A.; Perner, S.; Kramer, M.W.; Merseburger, A.S.; Roesch, M.C. The alternative renin-angiotensin-system (RAS) signalling pathway in prostate cancer and its link to the current COVID-19 pandemic. Mol. Biol. Rep. 2023, 50, 1809–1816. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Martins, A.L.V.; Motta-Santos, D.; Campagnole-Santos, M.J.; Santos, R.A.S. ACE2 in the renin-angiotensin system. Clin. Sci. (Lond.) 2020, 134, 3063–3078. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Torres, E.; Oyarzún, A.; Mondaca-Ruff, D.; Azocar, A.; Castro, P.F.; Jalil, J.E.; Chiong, M.; Lavandero, S.; Ocaranza, M.P. ACE2 and vasoactive peptides: Novel players in cardiovascular/renal remodeling and hypertension. Ther. Adv. Cardiovasc. Dis. 2015, 9, 217–237. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Nalivaeva, N.N. Angiotensin-converting enzyme 2 (ACE2): Two decades of revelations and re-evaluation. Peptides 2022, 151, 170766. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.A.; Fateel, M.M.; Ananthalakshmi, K.V.; Luqmani, Y.A. Anti-Inflammatory Action of Angiotensin 1–7 in Experimental Colitis. PLoS ONE 2016, 11, e0150861. [Google Scholar] [CrossRef]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Batu, D.; Velkoska, E.; Burrell, L.M.; Patel, S.K.; Beswick, L.; Jackson, A.; et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: A novel therapeutic target? Gut 2020, 69, 841–851. [Google Scholar] [CrossRef]
- Salmenkari, H.; Korpela, R.; Vapaatalo, H. Renin-angiotensin system in intestinal inflammation-Angiotensin inhibitors to treat inflammatory bowel diseases? Basic Clin. Pharmacol. Toxicol. 2021, 129, 161–172. [Google Scholar] [CrossRef]
- Niehues, R.V.; Wozniak, J.; Wiersch, F.; Lilienthal, E.; Tacken, N.; Schumertl, T.; Garbers, C.; Ludwig, A.; Düsterhöft, S. The collectrin-like part of the SARS-CoV-1 and -2 receptor ACE2 is shed by the metalloproteinases ADAM10 and ADAM17. FASEB J. 2022, 36, e22234. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–33019. [Google Scholar] [CrossRef]
- Hayashi, T.; Abiko, K.; Mandai, M.; Yaegashi, N.; Konishi, I. Highly conserved binding region of ACE2 as a receptor for SARS-CoV-2 between humans and mammals. Vet. Q. 2020, 40, 243–249. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Moreno-Pérez, O.; García-Arriaza, J.; Ramos-Rincón, J.M.; Cortés-Gómez, M.Á.; Brinkmalm, G.; Andrés, M.; León-Ramírez, J.M.; Boix, V.; Gil, J.; et al. Plasma ACE2 species are differentially altered in COVID-19 patients. FASEB J. 2021, 35, e21745. [Google Scholar] [CrossRef]
- Lennol, M.P.; García-Ayllón, M.S.; Avilés-Granados, C.; Trasciatti, C.; Tolassi, C.; Quaresima, V.; Arici, D.; Cristillo, V.; Volonghi, I.; Caprioli, F.; et al. Increased cerebrospinal fluid ACE2 fragments as a read-out of brain infection in COVID-19 encephalopathy patients. J. Infect. Dis. 2025, 26, jiaf093. [Google Scholar]
- Lennol, M.P.; García-Ayllón, M.S.; Esteban, M.; García-Arriaza, J.; Sáez-Valero, J. Serum angiotensin-converting enzyme 2 as a potential biomarker for SARS-CoV-2 infection and vaccine efficacy. Front. Immunol. 2022, 13, 1001951. [Google Scholar] [CrossRef]
- Zang, R.; Castro, M.F.G.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef]
- Afar, D.E.; Vivanco, I.; Hubert, R.S.; Kuo, J.; Chen, E.; Saffran, D.C.; Raitano, A.B.; Jakobovits, A. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 2001, 61, 1686–1692. [Google Scholar]
- Chen, Y.W.; Lee, M.S.; Lucht, A.; Chou, F.P.; Huang, W.; Havighurst, T.C.; Kim, K.; Wang, J.K.; Antalis, T.M.; Johnson, M.D.; et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am. J. Pathol. 2010, 176, 2986–2996. [Google Scholar] [CrossRef]
- Wettstein, L.; Kirchhoff, F.; Münch, J. The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Leyva, J.; Lennol, M.P.; Avilés-Granados, C.; García-Ayllón, M.S.; Gutiérrez, A.; Francés, R.; Sáez-Valero, J. Altered plasma levels of the SARS-CoV-2-related proteins ACE2 and TMPRSS2 in patients with Crohn′s disease. Sci. Rep. 2024, 14, 30346. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.O.; Kintscher, U. ACE2 and SARS-CoV-2: Tissue or Plasma, Good or Bad? Am. J. Hypertens. 2021, 34, 274–277. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Tokuyama, M.; Wei, G.; Huang, R.; Livanos, A.; Jha, D.; Levescot, A.; Irizar, H.; Kosoy, R.; Cording, S.; et al. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps With the Pathogenesis of SARS-CoV-2-related Disease. Gastroenterology 2021, 160, 287–301.e20. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Lindstrøm, J.C.; Kalla, R.; Ricanek, P.; Halfvarson, J.; Satsangi, J. Age, Inflammation, and Disease Location Are Critical Determinants of Intestinal Expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in Inflammatory Bowel Disease. Gastroenterology 2020, 159, 1151–1154.e2. [Google Scholar] [CrossRef]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef]
- Ferreira-Duarte, M.; Estevinho, M.M.; Duarte-Araújo, M.; Magro, F.; Morato, M. Unraveling the Role of ACE2, the Binding Receptor for SARS-CoV-2, in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1787–1795. [Google Scholar] [CrossRef]
- La Rosa, P.; Tiberi, J.; Palermo, E.; Stefanelli, R.; Tiano, S.M.L.; Canterini, S.; Cortese, M.; Hiscott, J.; Fiorenza, M.T. The inactivation of the Niemann Pick C1 cholesterol transporter restricts SARS-CoV-2 entry into host cells by decreasing ACE2 abundance at the plasma membrane. Cell Biosci. 2024, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef]
- Lalioti, V.; González-Sanz, S.; Lois-Bermejo, I.; González-Jiménez, P.; Viedma-Poyatos, Á.; Merino, A.; Pajares, M.A.; Pérez-Sala, D. Cell surface detection of vimentin, ACE2 and SARS-CoV-2 Spike proteins reveals selective colocalization at primary cilia. Sci. Rep. 2022, 12, 7063. [Google Scholar] [CrossRef]
- Bolland, W.; Marechal, I.; Petiot, C.; Porrot, F.; Guivel-Benhassine, F.; Brelot, A.; Casartelli, N.; Schwartz, O.; Buchrieser, J. SARS-CoV-2 entry and fusion are independent of ACE2 localization to lipid rafts. J. Virol. 2025, 99, e0182324. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Kawamura, M.; Uchida, H.; Hiramatsu, K.; Katori, C.; Asai, H.; Egawa, S.; Yoshida, K. Increased ACE2 and TMPRSS2 expression in ulcerative colitis. Pathol. Res. Pract. 2024, 254, 155108. [Google Scholar] [CrossRef]
- McAllister, M.J.; Kirkwood, K.; Chuah, S.C.; Thompson, E.J.; Cartwright, J.A.; Russell, C.D.; Dorward, D.A.; Lucas, C.D.; Ho, G.T. Intestinal Protein Characterisation of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in Inflammatory Bowel Disease (IBD) and Fatal COVID-19 Infection. Inflammation 2022, 45, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Patankar, J.V.; Chiriac, M.T.; Lehmann, M.; Kühl, A.A.; Atreya, R.; Becker, C.; Gonzalez-Acera, M.; Schmitt, H.; Gamez-Belmonte, R.; Mahapatro, M.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Attachment Receptor Angiotensin-Converting Enzyme 2 Is Decreased in Crohn’s Disease and Regulated By Microbial and Inflammatory Signaling. Gastroenterology 2021, 160, 925–928.e4. [Google Scholar] [CrossRef]
- Stocker, N.; Radzikowska, U.; Wawrzyniak, P.; Tan, G.; Huang, M.; Ding, M.; Akdis, C.A.; Sokolowska, M. Regulation of angiotensin-converting enzyme 2 isoforms by type 2 inflammation and viral infection in human airway epithelium. Mucosal Immunol. 2023, 16, 5–16. [Google Scholar] [CrossRef]
- Potdar, A.A.; Dube, S.; Naito, T.; Li, K.; Botwin, G.; Haritunians, T.; Li, D.; Casero, D.; Yang, S.; Bilsborough, J.; et al. Altered Intestinal ACE2 Levels Are Associated With Inflammation, Severe Disease, and Response to Anti-Cytokine Therapy in Inflammatory Bowel Disease. Gastroenterology 2021, 160, 809–822.e7. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Verstockt, S.; Rahiman, S.A.; Ke, B.J.; Arnauts, K.; Cleynen, I.; Sabino, J.; Ferrante, M.; Matteoli, G.; Vermeire, S. Intestinal Receptor of SARS-CoV-2 in Inflamed IBD Tissue Seems Downregulated by HNF4A in Ileum and Upregulated by Interferon Regulating Factors in Colon. J. Crohn′s Colitis 2021, 15, 485–498. [Google Scholar] [CrossRef]
- Li, X.Z.; Qiu, Y.; Jeffery, L.; Liu, F.; Feng, R.; He, J.S.; Tan, J.Y.; Ye, Z.Y.; Lin, S.N.; Ghosh, S.; et al. Down-Regulation of Colonic ACE2 Expression in Patients With Inflammatory Bowel Disease Responding to Anti-TNF Therapy: Implications for COVID-19. Front. Med. 2021, 7, 613475. [Google Scholar] [CrossRef]
- Toyonaga, T.; Araba, K.C.; Kennedy, M.M.; Keith, B.P.; Wolber, E.A.; Beasley, C.; Steinbach, E.C.; Schaner, M.R.; Jain, A.; Long, M.D.; et al. Increased colonic expression of ACE2 associates with poor prognosis in Crohn’s disease. Sci. Rep. 2021, 11, 13533. [Google Scholar] [CrossRef]
- Pisani, L.F.; Petroni, G.A.; Crespi, G.; Mola, S.; Annunziata, M.L.; Caprioli, F.A.; Porta, C.; Pastorelli, L. Lower Expression of SARS-CoV-2 Host Cell Entry Genes in the Intestinal Mucosa of IBD Patients with Quiescent or Mildly Active Disease. Inflamm. Bowel Dis. 2025, 31, 1690–1701. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Reich, A.; Hazime, H.; Quintero, M.A.; Fernandez, I.; Fritsch, J.; Santander, A.M.; Brito, N.; Damas, O.M.; Deshpande, A.; et al. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients with IBD. Inflamm. Bowel Dis. 2020, 26, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Shan, G.; Sun, Z.; Zhang, F.; Xu, C.; Lou, X.; Li, S.; Du, H.; Chen, H.; Xu, G. Quantitative Proteomic Analysis Reveals the Deregulation of Nicotinamide Adenine Dinucleotide Metabolism and CD38 in Inflammatory Bowel Disease. Biomed Res. Int. 2019, 2019, 3950628. [Google Scholar] [CrossRef]
- Park, J.; Jeong, D.; Chung, Y.W.; Kim, D.H.; Cheon, J.H.; Ryu, J.H. Quantitative Proteomic Analysis of the Expression of SARS-CoV-2 Receptors in the Gut of Patients with Chronic Enterocolitis. Yonsei Med. J. 2020, 61, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Burrell, L.M.; Velkoska, E.; Griggs, K.; Angus, P.W.; Gibson, P.R.; Lubel, J.S. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, P.G.; Bartzoka, N.; Tsiakanikas, P.; Scorilas, A. Characterization of novel ACE2 mRNA transcripts: The potential role of alternative splicing in SARS-CoV-2 infection. Gene 2025, 936, 149092. [Google Scholar] [CrossRef]
- Blaydon, D.C.; Biancheri, P.; Di, W.L.; Plagnol, V.; Cabral, R.M.; Brooke, M.A.; van Heel, D.A.; Ruschendorf, F.; Toynbee, M.; Walne, A.; et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N. Engl. J. Med. 2011, 365, 1502–1508. [Google Scholar] [CrossRef]
- Imoto, I.; Saito, M.; Suga, K.; Kohmoto, T.; Otsu, M.; Horiuchi, K.; Nakayama, H.; Higashiyama, S.; Sugimoto, M.; Sasaki, A.; et al. Functionally confirmed compound heterozygous ADAM17 missense loss-of-function variants cause neonatal inflammatory skin and bowel disease 1. Sci. Rep. 2021, 11, 9552. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Pedersen, G.; Saermark, T.; Brynskov, J. Tumour necrosis factor-alpha converting enzyme (TACE) activity in human colonic epithelial cells. Clin. Exp. Immunol. 2004, 135, 146–153. [Google Scholar] [CrossRef]
- Brynskov, J.; Foegh, P.; Pedersen, G.; Ellervik, C.; Kirkegaard, T.; Bingham, A.; Saermark, T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 2002, 51, 37–43. [Google Scholar] [CrossRef]
- Lykowska-Szuber, L.; Walczak, M.; Skrzypczak-Zielinska, M.; Suszynska-Zajczyk, J.; Stawczyk-Eder, K.; Waszak, K.; Eder, P.; Wozniak, A.; Krela-Kazmierczak, I.; Slomski, R.; et al. Effect of Anti-TNF Therapy on Mucosal Apoptosis Genes Expression in Crohn’s Disease. Front. Immunol. 2021, 12, 615539. [Google Scholar] [CrossRef]
- Franzè, E.; Caruso, R.; Stolfi, C.; Sarra, M.; Cupi, M.L.; Ascolani, M.; Sedda, S.; Antenucci, C.; Ruffa, A.; Caprioli, F.; et al. High expression of the “A Disintegrin And Metalloprotease” 19 (ADAM19), a sheddase for TNF-α in the mucosa of patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2013, 19, 501–511. [Google Scholar] [CrossRef]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Conley, T.E.; Probert, C.; Subramanian, S. Prevalence of COVID-19 Symptoms Among Inflammatory Bowel Disease Patients Treated with Biological Agents. J. Crohn’s Colitis 2020, 14, 1794–1795. [Google Scholar] [CrossRef]
- Valenzuela, R.; Pedrosa, M.A.; Garrido-Gil, P.; Labandeira, C.M.; Navarro, G.; Franco, R.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Interactions between ibuprofen, ACE2, renin-angiotensin system, and spike protein in the lung. Implications for COVID-19. Clin. Transl. Med. 2021, 11, e371. [Google Scholar] [CrossRef]
- Bossa, F.; Carparelli, S.; Latiano, A.; Palmieri, O.; Tavano, F.; Panza, A.; Pastore, M.; Marseglia, A.; D’Altilia, M.; Latiano, T.; et al. Impact of the COVID-19 outbreak and the serum prevalence of SARS-CoV-2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig. Liver Dis. 2021, 53, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. COVID-19 and immunomodulation in IBD. Gut 2020, 69, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Fanizzi, F.; D’Amico, F.; Peyrin-Biroulet, L.; Danese, S.; Dignass, A. Treatment targets in IBD: Is it time for new strategies? Best Pract. Res. Clin. Gastroenterol. 2025, 77, 101990. [Google Scholar] [CrossRef]
- De Toma, I.; Dierssen, M. Network analysis of Down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci. Rep. 2021, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Souto-Rodríguez, R.; Barreiro-de-Acosta, M.; Domínguez-Muñoz, J.E. Down’s syndrome and inflammatory bowel disease: Is there a real link? Rev. Esp. Enferm. Dig. 2014, 106, 220–222. [Google Scholar]
- Ravel, A.; Mircher, C.; Rebillat, A.S.; Cieuta-Walti, C.; Megarbane, A. Feeding problems and gastrointestinal diseases in Down syndrome. Arch. Pediatr. 2020, 27, 53–60. [Google Scholar] [CrossRef]
- Cianfarini, C.; Hassler, L.; Wysocki, J.; Hassan, A.; Nicolaescu, V.; Elli, D.; Gula, H.; Ibrahim, A.M.; Randall, G.; Henkin, J.; et al. Soluble Angiotensin-Converting Enzyme 2 Protein Improves Survival and Lowers Viral Titers in Lethal Mouse Model of Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection with the Delta Variant. Cells 2024, 13, 203. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Popowski, K.D.; Liu, S.; Zhu, D.; Mei, X.; Li, J.; Hu, Y.; Dinh, P.C.; Wang, X.; et al. Inhalation of ACE2-expressing lung exosomes provides prophylactic protection against SARS-CoV-2. Nat. Commun. 2024, 15, 2236. [Google Scholar] [CrossRef]
- Seccia, T.M.; Shagjaa, T.; Morpurgo, M.; Caroccia, B.; Sanga, V.; Faoro, S.; Venturini, F.; Iadicicco, G.; Lococo, S.; Mazzitelli, M.; et al. Randomized Clinical Trial Of Nafamostat Mesylate; A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia. J. Clin. Med. 2023, 12, 6618. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.C.M.; Bricker, T.L.; Fritch, E.J.; Leist, S.R.; Gully, K.; Baric, R.S.; Graham, R.L.; Troan, B.V.; Mahoney, M.; Janetka, J.W. Efficacy of host cell serine protease inhibitor MM3122 against SARS-CoV-2 for treatment and prevention of COVID-19. J. Virol. 2024, 98, e0190323. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Slagman, A.; Domenig, O.; Röhle, R.; Konietschke, F.; Poglitsch, M.; Möckel, M. Plasma Angiotensin Peptide Profiling and ACE (Angiotensin-Converting Enzyme)-2 Activity in COVID-19 Patients Treated with Pharmacological Blockers of the Renin-Angiotensin System. Hypertension 2020, 76, e34–e36. [Google Scholar] [CrossRef] [PubMed]
- Shahrokh, S.; Ghavami, S.B.; Aghdaei, H.A.; Parigi, T.L.; Farmani, M.; Danese, S.; Daryani, N.E.; Vossoughinia, H.; Balaii, H.; Alborzi, F.; et al. High prevalence of SARS-Coronavirus-2 in patients with inflammatory bowel disease and the role of soluble angiotensin converting Enzyme2. Arch. Physiol. Biochem. 2024, 130, 325–332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).