Hemodynamic and Clinical Predictors of Thrombolysis in Post-COVID Venous Thromboembolism: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Inclusion and Exclusion Criteria
2.4. Data Collection
2.5. Clinical and Echocardiographic Variables
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Main Findings and Clinical Relevance
4.2. Comparison with Previous Studies
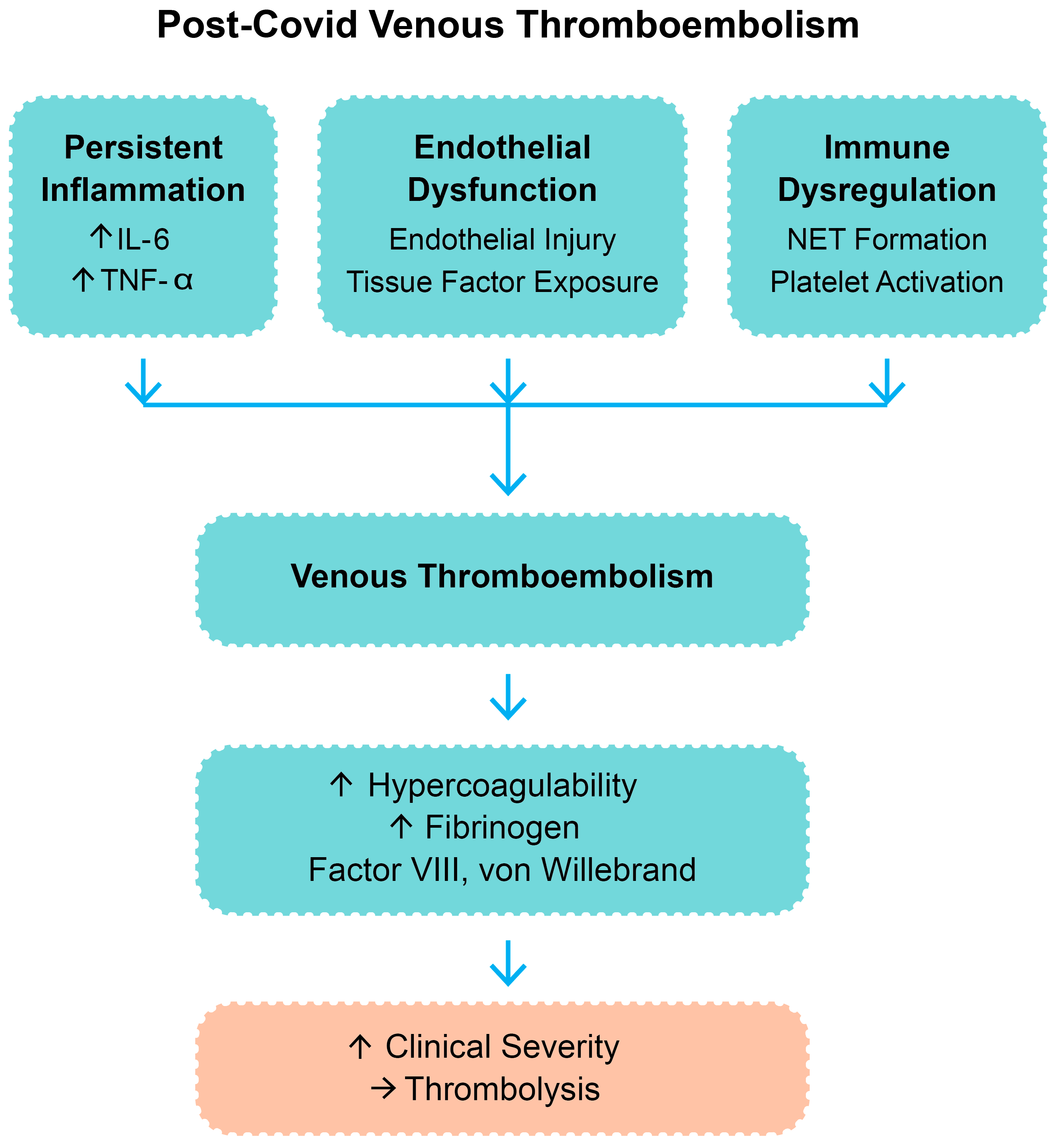
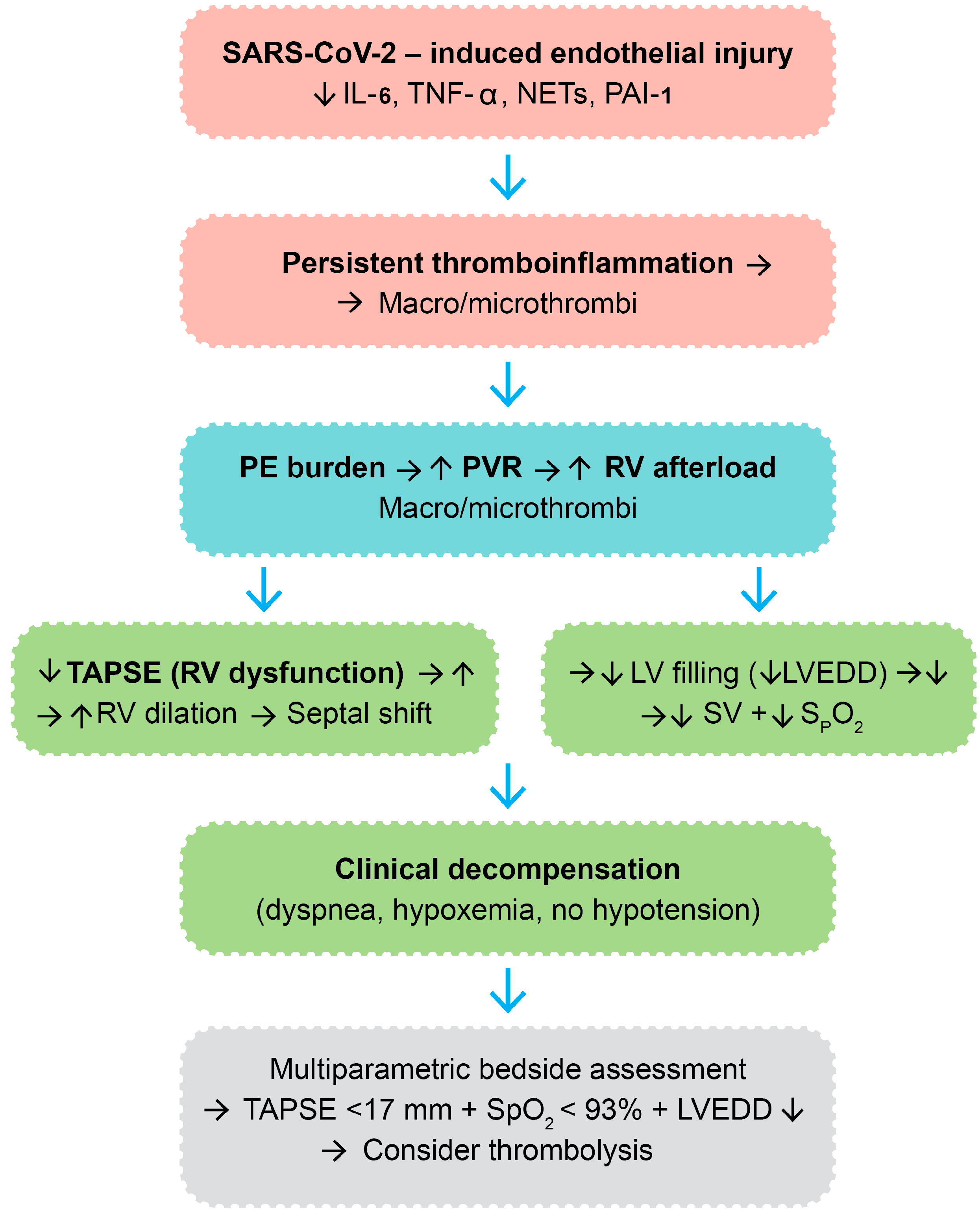
4.3. Physiological Rationale and Proposed Mechanism
4.4. Clinical Implications for Thrombolytic Decision-Making
4.5. Limitations
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef]
- Kearon, C. Epidemiology of venous thromboembolism. Semin. Vasc Med. 2001, 1, 7–26. [Google Scholar] [PubMed]
- Boyd, S.; Martin-Loeches, I. The incidence of venous thromboembolism in critically ill patients with COVID-19 compared with critically ill non-COVID patients. Ir. J. Med. Sci. 2021, 190, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; De Servi, S. The burden of thrombotic complications in critically ill patients with COVID-19: Charting the uncharted. Intern. Emerg. Med. 2020, 15, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.; Sheng Loh, K.; Lynch, J.; Alrashed, D.; Muzzammil, S.; Marsh, H.; Masoud, M.; Bin Ihsan, S.; Martin-Loeches, I. The Incidence of Venous Thromboembolism in Critically Ill Patients with SARS-CoV-2 Infection Compared with Critically Ill Influenza and Community-Acquired Pneumonia Patients: A Retrospective Chart Review. Med. Sci. 2022, 10, 30. [Google Scholar]
- Lassandro, G.; Palladino, V.; Amoruso, A.; Palmieri, V.V.; Russo, G.; Giordano, P. Children in Coronaviruses’ Wonderland: What Clinicians Need to Know. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020042. [Google Scholar] [CrossRef]
- Lassandro, G.; Palmieri, V.V.; Palladino, V.; Amoruso, A.; Faienza, M.F.; Giordano, P. Venous Thromboembolism in Children: From Diagnosis to Management. Int. J. Environ. Res. Public Health 2020, 17, 4993. [Google Scholar] [CrossRef]
- Mezoh, G.; Crowther, N.J. Endothelial Dysfunction as a Primary Consequence of SARS-CoV-2 Infection. Adv. Exp. Med. Biol. 2021, 1321, 33–43. [Google Scholar]
- Six, I.; Guillaume, N.; Jacob, V.; Mentaverri, R.; Kamel, S.; Boullier, A.; Slama, M. The Endothelium and COVID-19: An Increasingly Clear Link Brief Title: Endotheliopathy in COVID-19. Int. J. Mol. Sci. 2022, 23, 6196. [Google Scholar] [CrossRef]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J. Clin. Biochem. 2021, 36, 440–450. [Google Scholar] [CrossRef]
- Faraj, S.S.; Jalal, P.J. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: Case-control study. Ann. Med. Surg. 2023, 85, 2291–2297. [Google Scholar] [CrossRef]
- Paranga, T.G.; Mitu, I.; Pavel-Tanasa, M.; Rosu, M.F.; Miftode, I.L.; Constantinescu, D.; Obreja, M.; Plesca, C.E.; Miftode, E. Cytokine Storm in COVID-19: Exploring IL-6 Signaling and Cytokine-Microbiome Interactions as Emerging Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 11411. [Google Scholar] [CrossRef] [PubMed]
- Evangelidis, P.; Kotsiou, N.; Kalmoukos, P.; Ntova, Z.; Papadopoulou, T.; Chissan, S.; Sarvani, A.; Kokoris, S.; Grouzi, E.; Doumas, M.; et al. Prevalence and Risk Factors of Acute Ischemic Stroke in Patients with Antiphospholipid Syndrome: A Retrospective Monocenter Analysis. J. Cardiovasc. Dev. Dis. 2025, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Shao, Q. The central role of neutrophil extracellular traps (NETs) and by-products in COVID-19 related pulmonary thrombosis. Immun. Inflamm. Dis. 2023, 11, e949. [Google Scholar] [PubMed]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Ackermann, M.; Anders, H.J.; Bilyy, R.; Bowlin, G.L.; Daniel, C.; De Lorenzo, R.; Egeblad, M.; Henneck, T.; Hidalgo, A.; Hoffmann, M.; et al. Patients with COVID-19: In the dark-NETs of neutrophils. Cell Death Differ. 2021, 28, 3125–3139. [Google Scholar] [CrossRef]
- Dubey, L.; Dorosh, O.; Dubey, N.; Doan, S.; Kozishkurt, O.; Duzenko, O.; Kozlova, O.; Ievtukh, V.; Ladny, J.R.; Pruc, M.; et al. COVID-19-induced coagulopathy: Experience, achievements, prospects. Cardiol. J. 2023, 30, 453–461. [Google Scholar] [CrossRef]
- Lorini, F.L.; Di Matteo, M.; Gritti, P.; Grazioli, L.; Benigni, A.; Zacchetti, L.; Bianchi, I.; Fabretti, F.; Longhi, L. Coagulopathy and COVID-19. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2021, 23 (Suppl. E), E95–E98. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Boccatonda, A.; Campello, E.; Simion, C.; Simioni, P. Long-term hypercoagulability, endotheliopathy and inflammation following acute SARS-CoV-2 infection. Expert Rev. Hematol. 2023, 16, 1035–1048. [Google Scholar] [CrossRef]
- Martins-Gonçalves, R.; Hottz, E.D.; Bozza, P.T. Acute to post-acute COVID-19 thromboinflammation persistence: Mechanisms and potential consequences. Curr. Res. Immunol. 2023, 4, 100058. [Google Scholar] [CrossRef]
- Giaglis, S. Poised to cast wide NETs in long COVID. J. Thromb. Haemost. 2023, 21, 2362–2364. [Google Scholar] [CrossRef]
- Ro, S.K.; Sato, K.; Ijuin, S.; Sela, D.; Fior, G.; Heinsar, S.; Kim, J.Y.; Chan, J.; Nonaka, H.; Lin, A.C.W.; et al. Assessment and diagnosis of right ventricular failure-retrospection and future directions. Front. Cardiovasc. Med. 2023, 10, 1030864. [Google Scholar]
- Monitillo, F.; Di Terlizzi, V.; Gioia, M.I.; Barone, R.; Grande, D.; Parisi, G.; Brunetti, N.D.; Iacoviello, M. Right Ventricular Function in Chronic Heart Failure: From the Diagnosis to the Therapeutic Approach. J. Cardiovasc. Dev. Dis. 2020, 7, 12. [Google Scholar] [CrossRef]
- Butcovan, D.; Oboroceanu, T.; Cimpeanu, C.; Mironescu, A.; Haliga, R.E.; Pinzariu, A.C.; Lupusoru, R.V.; Popescu, E.; Mocanu, V. The Involvement of Epicardial Adiposity and Inflammation in Postoperatory Atrial Fibrilation–Immunohistochemical Qualitative and Quantitative Assessment. RevChim 2017, 68, 886–889. Available online: http://bch.ro/pdfRC/51 BUTCOVAN 4 17.pdf (accessed on 7 February 2025).
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Jiménez, D. Simplified PESI (Pulmonary Embolism Severity Index). MDCalc. Available online: https://www.mdcalc.com/calc/1247/simplified-pesi-pulmonary-embolism-severity-index (accessed on 21 November 2024).
- Pérez-Nieto, O.R.; Gómez-Oropeza, I.; Quintero-Leyra, A.; Kammar-García, A.; Zamarrón-López, É.I.; Soto-Estrada, M.; Morgado-Villaseñor, L.A.; Meza-Comparán, H.D. Hemodynamic and respiratory support in pulmonary embolism: A narrative review. Front. Med. 2023, 10, 1123793. [Google Scholar] [CrossRef] [PubMed]
- Bonnemain, J.; Ltaief, Z.; Liaudet, L. The Right Ventricle in COVID-19. J. Clin. Med. 2021, 10, 2535. [Google Scholar] [CrossRef]
- Polito, M.V.; Silverio, A.; Di Maio, M.; Bellino, M.; Scudiero, F.; Russo, V.; Rasile, B.; Alfano, C.; Citro, R.; Parodi, G.; et al. Prognostic Implications of Right Ventricular Function and Pulmonary Pressures Assessed by Echocardiography in Hospitalized Patients with COVID-19. J. Pers. Med. 2021, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Corica, B.; Marra, A.M.; Basili, S.; Cangemi, R.; Cittadini, A.; Proietti, M.; Romiti, G.F. Prevalence of right ventricular dysfunction and impact on all-cause death in hospitalized patients with COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17774. [Google Scholar] [CrossRef]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef]
- Ullah, W.; Saeed, R.; Sarwar, U.; Patel, R.; Fischman, D.L. COVID-19 Complicated by Acute Pulmonary Embolism and Right-Sided Heart Failure. JACC Case Rep. 2020, 2, 1379–1382. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Di Salvo, G.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zheng, J.B.; Jin, Y.; Tang, R.; Li, M.; Xiu, C.H.; Dai, Q.-Q.; Zuo, S.; Wang, H.-Q.; Wang, H.-L.; et al. Acute right ventricular dysfunction in severe COVID-19 pneumonia. Rev. Cardiovasc. Med. 2020, 21, 635–641. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Elliott, C.G. Acute pulmonary embolism: Part I: Epidemiology, pathophysiology, and diagnosis. Circulation 2003, 108, 2726–2729. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56, 2001634. [Google Scholar] [CrossRef]
- Kim, I.; Moon, S.O.; Kim, S.H.; Kim, H.J.; Koh, Y.S.; Koh, G.Y. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J. Biol. Chem. 2001, 276, 7614–7620. [Google Scholar] [CrossRef] [PubMed]
- Schillemans, M.; Karampini, E.; Kat, M.; Bierings, R. Exocytosis of Weibel-Palade bodies: How to unpack a vascular emergency kit. J. Thromb. Haemost. 2019, 17, 6–18. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Keller, T.T.; van Gorp, E.; ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39. [Google Scholar] [CrossRef]
- Whyte, C.S. All tangled up: Interactions of the fibrinolytic and innate immune systems. Front. Med. 2023, 10, 1212201. [Google Scholar] [CrossRef]
- de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Verma, N.; Kondoor, V.; Singh, R.; Ahuja, R. Acute RV Failure Management in Pulmonary Embolism. Tech. Vasc. Interv. Radiol. 2025, 28, 101039. [Google Scholar] [CrossRef]
- Zaidi, A.; Knight, D.S.; Augustine, D.X.; Harkness, A.; Oxborough, D.; Pearce, K.; Ring, L.; Robinson, S.; Stout, M.; Willis, J.; et al. Echocardiographic assessment of the right heart in adults: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G19–G41. [Google Scholar] [CrossRef]
- Jozwiak, M.; Teboul, J.L. Heart-Lungs interactions: The basics and clinical implications. Ann. Intensive Care 2024, 14, 122. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]

| Parameter | Thrombolysis (n = 11) | No Thrombolysis (n = 43) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 62.5 ± 13.1 | 60.9 ± 15.0 | 0.71 |
| Male gender, n (%) | 7 (63.6) | 27 (62.8) | 0.96 |
| Systolic BP, mmHg (mean ± SD) | 119.6 ± 26.0 | 129.3 ± 19.0 | 0.042 |
| Diastolic BP, mmHg (mean ± SD) | 73.3 ± 13.2 | 78.4 ± 13.4 | 0.27 |
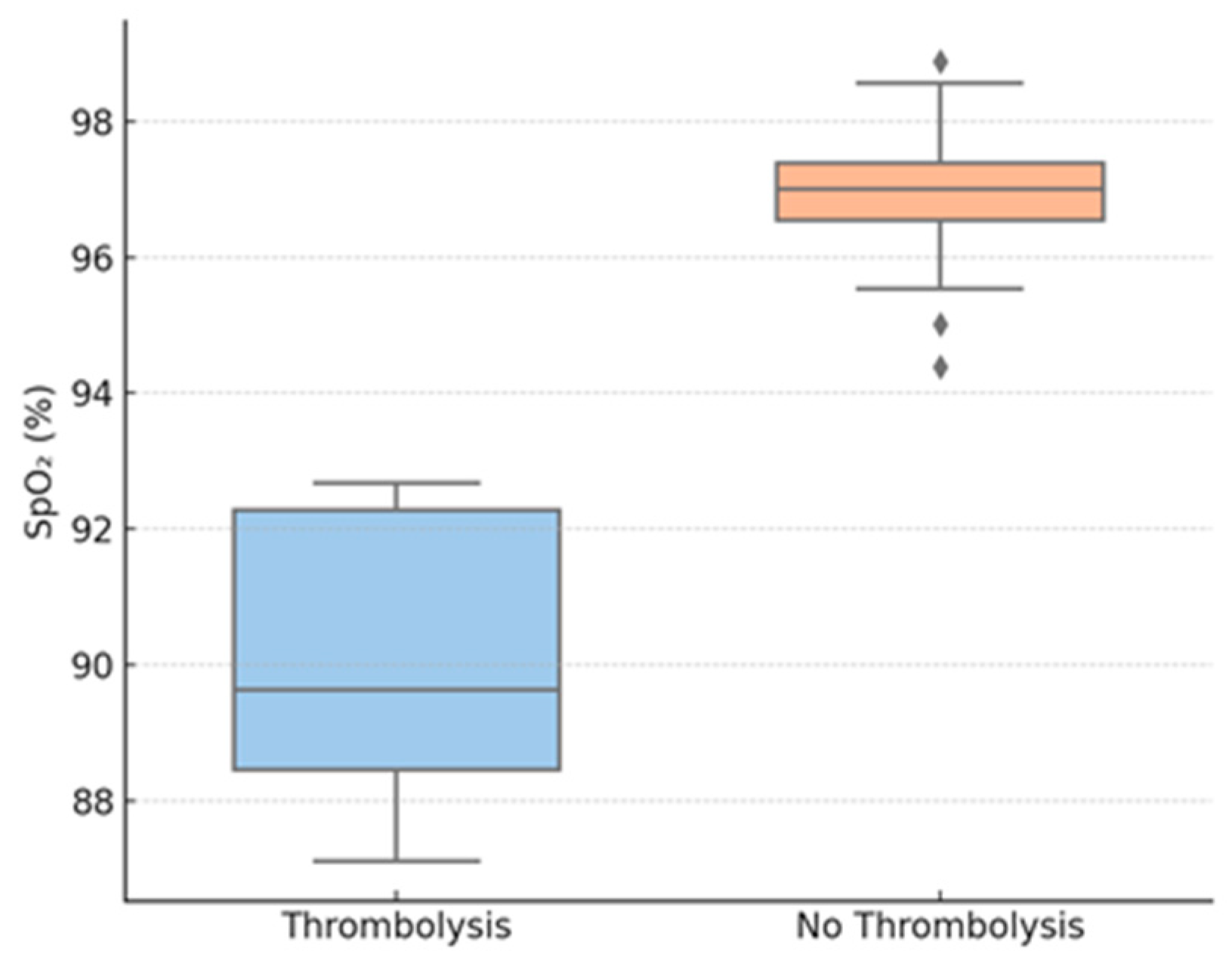
| SpO2 on admission, % (mean ± SD) | 90.1 ± 5.1 | 97.0 ± 2.8 | 0.013 * |
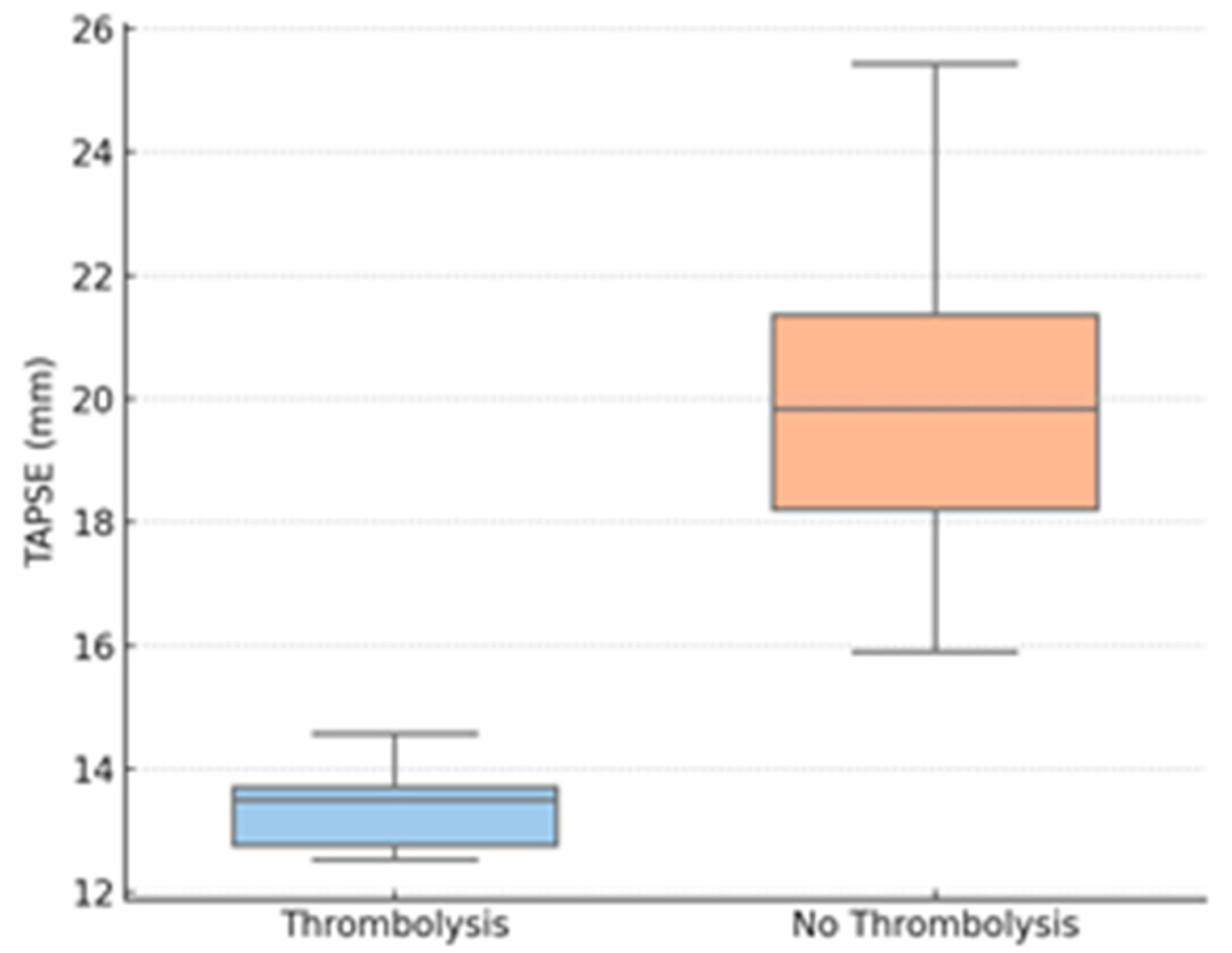
| TAPSE, mm (mean ± SD) | 13.0 ± 2.5 | 20.8 ± 5.1 | <0.001 * |
| LV diameter, mm (mean ± SD) | 24.4 ± 6.2 | 46.1 ± 8.9 | 0.009 * |
| RV diameter, mm (mean ± SD) | 34.8 ± 6.0 | 33.6 ± 7.3 | 0.87 |
| CRP, mg/L (mean ± SD) | 117.4 ± 64.3 | 105.7 ± 58.8 | 0.71 |
| Fibrinogen, mg/dL (median) | 515 | 525 | 0.86 |
| Creatinine, mg/dL (mean ± SD) | 1.13 ± 0.26 | 1.08 ± 0.31 | 0.49 |
| Group | Number of Patients (n) | Median sPESI (Mean ± SD) | Patients with ESC High-Risk Criteria | Notes |
|---|---|---|---|---|
| Thrombolysis | 11 | 3 (2.27 ± 1.4) | 8/11 (73%) | Higher frequency of systolic BP < 90 mmHg and hypoxemia |
| No thrombolysis | 43 | 1 (1.58 ± 1.3) | 19/43 (44%) | 15/43 (35%) had hypoxemia without hypotension |
| Variable | Spearman ρ (vs. Thrombolysis) | Observations |
|---|---|---|
| TAPSE (mm) | −0.71 | Strong inverse correlation; lower TAPSE increases the likelihood of thrombolysis. |
| SpO2 on admission (%) | −0.49 | Moderate inverse correlation; lower oxygen saturation increases the risk of thrombolysis. |
| LV end-diastolic diameter (mm) | −0.41 | Moderate inverse correlation; a smaller left ventricular diameter is associated with thrombolysis. |
| RV diameter (mm) | ≈0 (not significant) | No significant correlation with thrombolysis. |
| Variable | Odds Ratio per Unit (95% CI) | Interpretations |
|---|---|---|
| TAPSE (mm) | 1.58 per mm decrease (1.21–2.06) | Lower TAPSE is an independent predictor of thrombolysis. |
| SpO2 on admission (%) | 1.34 per % decrease (1.08–1.66) | Lower oxygen saturation is an independent predictor of thrombolysis. |
| LV end-diastolic diameter (mm) | 0.91 per mm increase (0.84–0.99) | Smaller LV diameter is independently associated with thrombolysis. |
| RV diameter (mm) | Not significant | Not a significant independent predictor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, G.-M.; Petriş, A.O.; Pînzariu, A.-C.; Cojocaru, T.; Coca, A.; Cojocaru, R.; Costan, C.-T.; Șorodoc, V.; Cojocaru, E. Hemodynamic and Clinical Predictors of Thrombolysis in Post-COVID Venous Thromboembolism: A Retrospective Cohort Study. Biomedicines 2025, 13, 2232. https://doi.org/10.3390/biomedicines13092232
Cojocaru G-M, Petriş AO, Pînzariu A-C, Cojocaru T, Coca A, Cojocaru R, Costan C-T, Șorodoc V, Cojocaru E. Hemodynamic and Clinical Predictors of Thrombolysis in Post-COVID Venous Thromboembolism: A Retrospective Cohort Study. Biomedicines. 2025; 13(9):2232. https://doi.org/10.3390/biomedicines13092232
Chicago/Turabian StyleCojocaru, Giulia-Mihaela, Antoniu Octavian Petriş, Alin-Constantin Pînzariu, Tudor Cojocaru, Andreea Coca, Ruxandra Cojocaru, Catherine-Teodora Costan, Victorița Șorodoc, and Elena Cojocaru. 2025. "Hemodynamic and Clinical Predictors of Thrombolysis in Post-COVID Venous Thromboembolism: A Retrospective Cohort Study" Biomedicines 13, no. 9: 2232. https://doi.org/10.3390/biomedicines13092232
APA StyleCojocaru, G.-M., Petriş, A. O., Pînzariu, A.-C., Cojocaru, T., Coca, A., Cojocaru, R., Costan, C.-T., Șorodoc, V., & Cojocaru, E. (2025). Hemodynamic and Clinical Predictors of Thrombolysis in Post-COVID Venous Thromboembolism: A Retrospective Cohort Study. Biomedicines, 13(9), 2232. https://doi.org/10.3390/biomedicines13092232











