Use of 4-Nitroquinoline 1-Oxide (4NQO) in Dysplastic and Malignant Induction: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Selection of the Cell Line
2.1.2. Induction of Malignancy and Cellular Dysplasia
2.1.3. H&E Staining
2.1.4. Immunocytochemistry
2.2. In Vivo Study
2.2.1. Ethical Considerations
2.2.2. Sample
2.2.3. Group Division
2.2.4. Induction of OL and OSCC
2.2.5. Sample Collection, Macroscopic Analysis, and Preparation
2.2.6. Histological Analysis
2.2.7. Statistical Analysis
3. Results
3.1. In Vitro Study
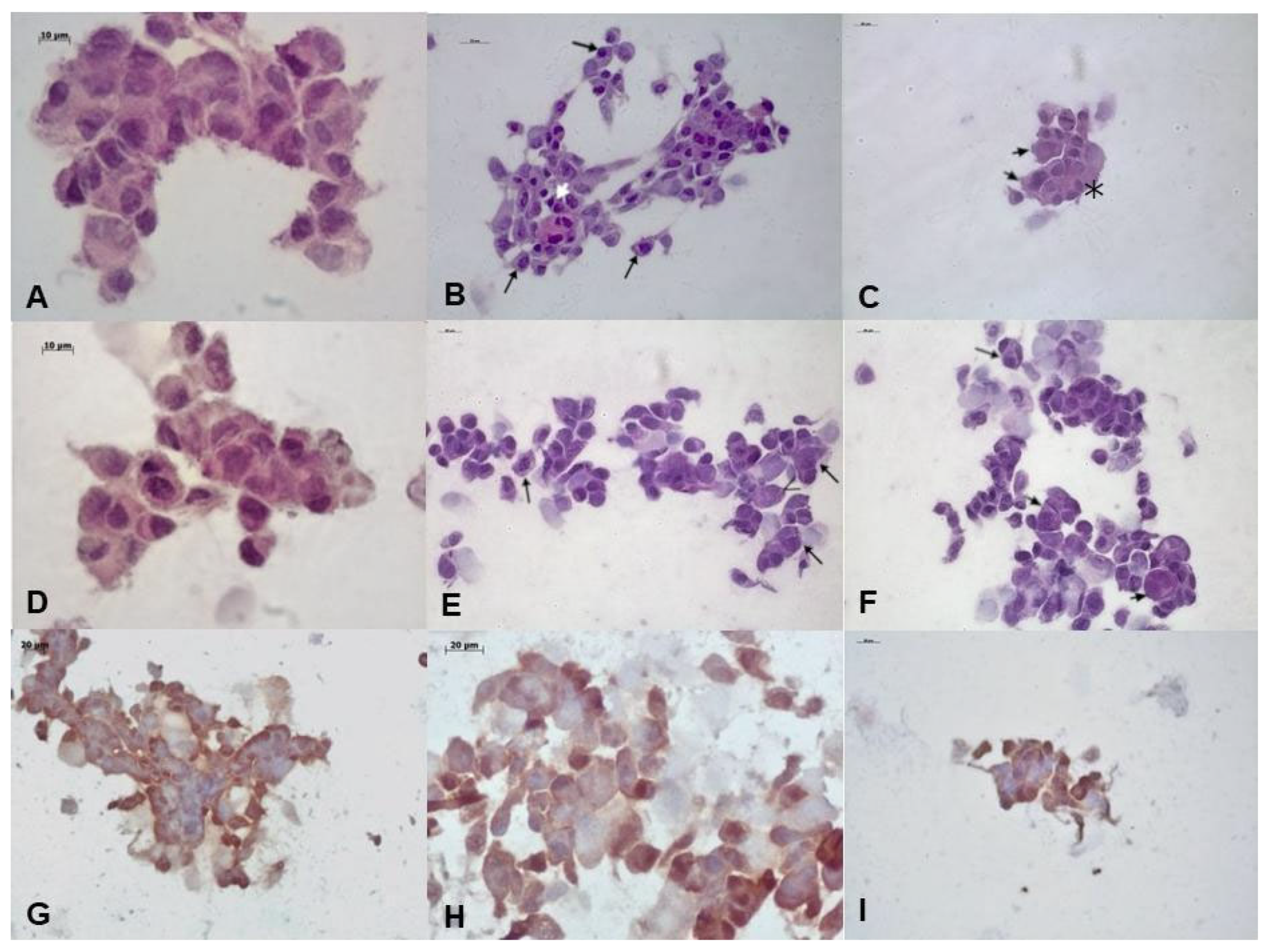
3.1.1. Microscopic Analysis of Cultured Cells
3.1.2. Hematoxylin and Eosin Analysis
3.1.3. Immunocytochemistry Analysis
3.2. In Vivo Study
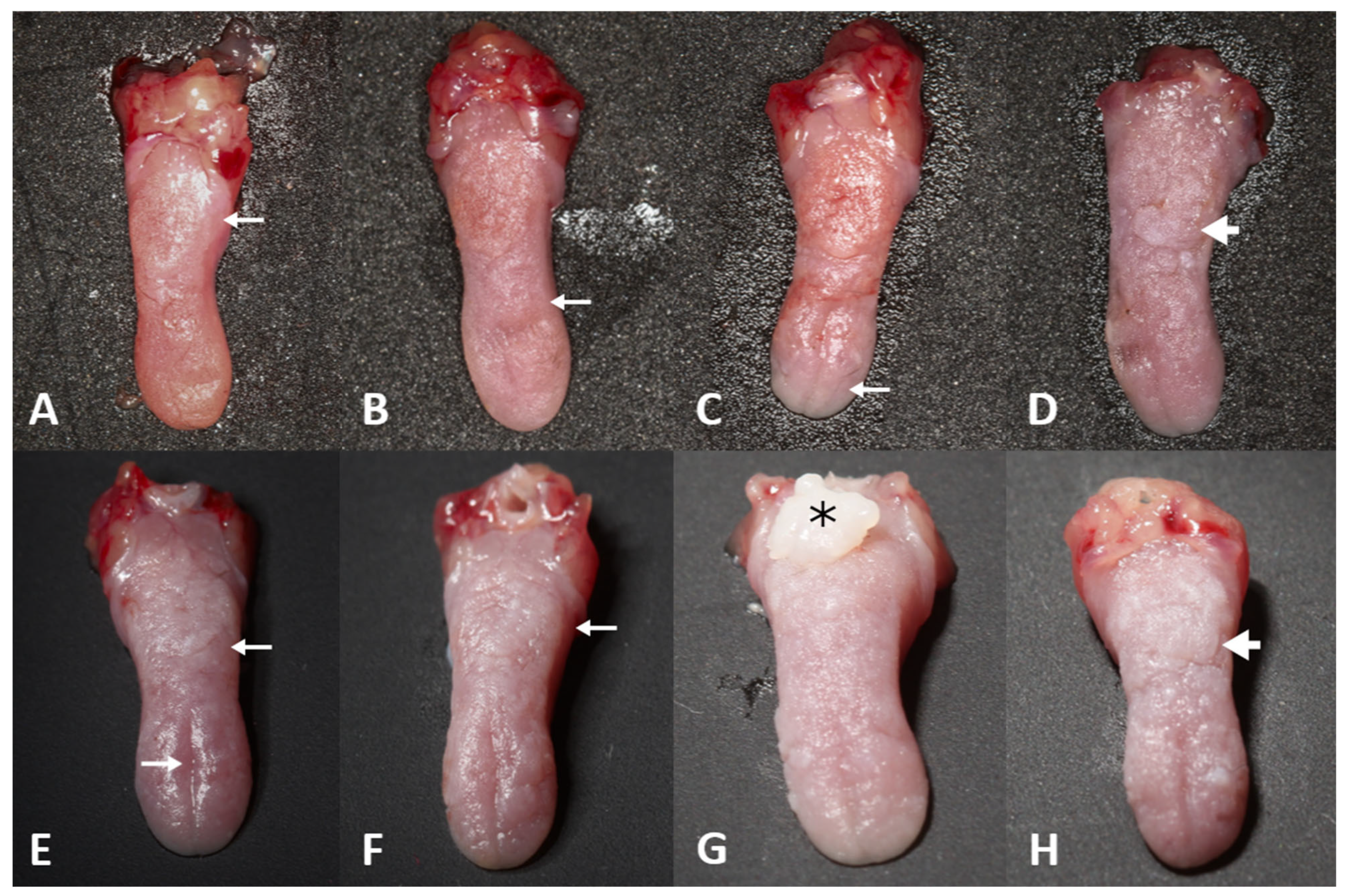
3.2.1. Macroscopic Analysis
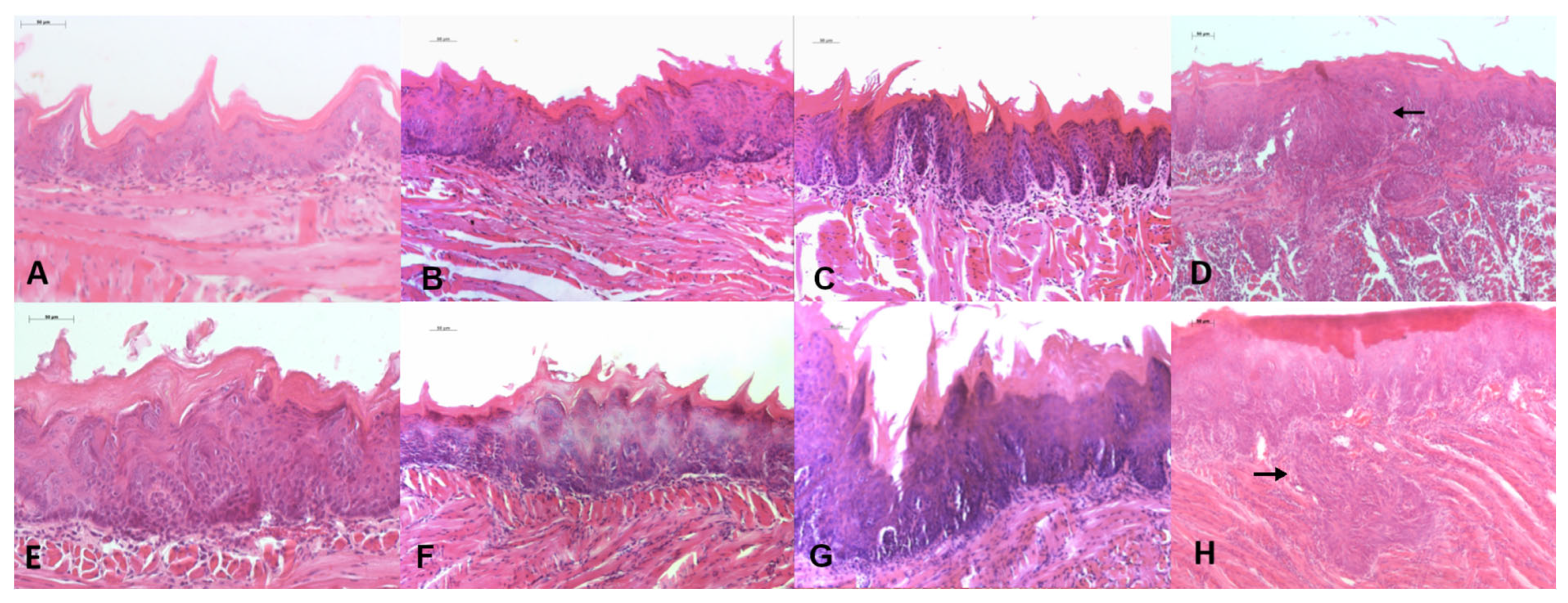
3.2.2. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and Classification of Potentially Malignant Disorders of the Oral Mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant Transformation of Oral Leukoplakia: A Systematic Review of Observational Studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef]
- Dutra, M.J.; Alves Santos, K.; Adorno-Farias, D.; Anbinder, A.L.; Kaminagakura, E. Leucoplasia verrugosa proliferativa simulando liquen plano oral: Reporte de caso y revisión de la literatura. Odontoestomatologia 2023, 25, e419. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Udaltsova, N.; Engels, E.A.; Katzel, J.A.; Yanik, E.L.; Katki, H.A.; Lingen, M.W.; Silverberg, M.J. Oral Leukoplakia and Risk of Progression to Oral Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2020, 112, 1047–1054. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially Malignant Disorders of the Oral Cavity and Oral Dysplasia: A Systematic Review and Meta-Analysis of Malignant Transformation Rate by Subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 9. [Google Scholar]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Kanojia, D.; Vaidya, M.M. 4-Nitroquinoline-1-Oxide Induced Experimental Oral Carcinogenesis. Oral Oncol. 2006, 42, 655–667. [Google Scholar] [CrossRef]
- Tan, M.T.; Wu, J.G.; Callejas-Valera, J.L.; Schwarz, R.A.; Gillenwater, A.M.; Richards-Kortum, R.R.; Vigneswaran, N. A PIK3CA Transgenic Mouse Model with Chemical Carcinogen Exposure Mimics Human Oral Tongue Tumorigenesis. Int. J. Exp. Pathol. 2020, 101, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, V.H.; Allevato, M.M.; Gilardi, M.; He, Y.; Callejas-Valera, J.L.; Vitale-Cross, L.; Martin, D.; Amornphimoltham, P.; McDermott, J.; et al. Syngeneic Animal Models of Tobacco-Associated Oral Cancer Reveal the Activity of in Situ Anti-CTLA-4. Nat. Commun. 2019, 10, 5546. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.J.; Malta, I.S.; de Almeida Lança, M.L.; de Vasconcellos, L.M.R.; Adorno-Farias, D.; Jara, J.A.; Kaminagakura, E. Effects of Artemisinin and Cisplatin on the Malignant Progression of Oral Leukoplakia—In vitro and in vivo study. J. Cancer Res. Clin. Oncol. 2024, 150, 3901–3913. [Google Scholar] [CrossRef] [PubMed]
- Crane, I.J.; Luker, J.; de Gay, L.; Rice, S.Q.; Stone, A.; Scully, C.; Prime, S.S. Transformation of Oral Keratinocytes In Vitro by 4-Nitroquinoline N-Oxide. Carcinogenesis 1988, 9, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Alves, M.G.; Carta, C.F.L.; Padín-Iruegas, M.E.; Pérez-Sayáns, M.; Suarez-Peñaranda, J.M.; Issa, J.S.; García-García, A.; Almeida, J.D. Expression of ATP6V1C1 during Oral Carcinogenesis. Biotech Histochem. 2016, 91, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kaminagakura, E.; Bonan, P.R.F.; Lopes, M.A.; Almeida, O.P. Cell Proliferation and p53 Expression in Pseudoepitheliomatous Hyperplasia of Oral Paracoccidioidomycosis. Mycoses 2006, 49, 393–396. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Ribeiro-Silva, A. Prognostic Significance of Immunohistochemical Biomarkers in Oral Squamous Cell Carcinoma. Int. J. Oral Maxillofac. Surg. 2011, 40, 298–307. [Google Scholar] [CrossRef]
- Lança, M.L.d.A.; da Conceição, N.S.C.; Malta, I.S.; Meneses, D.O.; Almeida, L.Y.; Kaminagakura, E. Chemopreventive Potential of Artemisinin and Rubus occidentalis in the Progression of Oral Leukoplakia to Oral Cancer: A Preclinical Murine Study. Int. J. Mol. Sci. 2025, 26, 8120. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Keating, A.F. Ovarian Metabolism of Xenobiotics. Exp. Biol. Med. 2011, 236, 765–771. [Google Scholar] [CrossRef]
- Plante, I. Dimethylbenz(a)anthracene-Induced Mammary Tumorigenesis in Mice. Methods Cell Biol. 2021, 163, 21–44. [Google Scholar] [CrossRef]
- Nakahara, W.; Fukuoka, F.; Sugimura, T. Carcinogenic Action of 4-Nitroquinoline-N-Oxide. Gann 1957, 48, 129–137_3. [Google Scholar]
- Brüsehafer, K.; Manshian, B.B.; Doherty, A.T.; Zaïr, Z.M.; Johnson, G.E.; Doak, S.H.; Jenkins, G.J. The Clastogenicity of 4NQO Is Cell-Type Dependent and Linked to Cytotoxicity, Length of Exposure and p53 Proficiency. Mutagenesis 2016, 31, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.R.; Thakur, S.; Peroumal, D.; Utkalaja, B.G.; Dutta, A.; Kumari, P.; Subhadarsini, I.; Acharya, N. 4-Nitroquinoline 1-Oxide Induces Immune Cells Death to Onset Early Immunosuppression During Oral Squamous Cell Carcinoma Development. Front. Immunol. 2023, 14, 1274519. [Google Scholar] [CrossRef]
- Nauta, J.M.; Roodenburg, J.L.; Nikkels, P.G.; Witjes, M.J.; Vermey, A. Comparison of Epithelial Dysplasia—The 4NQO Rat Palate Model and Human Oral Mucosa. Int. J. Oral Maxillofac. Surg. 1995, 24 Pt 1, 53–58. [Google Scholar] [CrossRef]
- MacDonald, D.G. Comparison of Epithelial Dysplasia in Hamster Cheek Pouch Carcinogenesis and Human Oral Mucosa. J. Oral Pathol. Med. 1981, 10, 186–191. [Google Scholar] [CrossRef]
- Gimenez-Conti, I.B.; Slaga, T.J. The Hamster Cheek Pouch Carcinogenesis Model. J. Cell. Biochem. 1993, 53, 83–90. [Google Scholar] [CrossRef]
- Hawkins, B.L.; Heniford, B.W.; Ackermann, D.M.; Leonberger, M.; Martinez, S.A.; Hendler, F.J. 4NQO Carcinogenesis: A Mouse Model of Oral Cavity Squamous Cell Carcinoma. Head Neck 1994, 16, 424–432. [Google Scholar] [CrossRef]
- Zigmundo, G.C.O.; Schuch, L.F.; Schmidt, T.R.; Silveira, F.M.; Martins, M.A.T.; Carrard, V.C.; Martins, M.D.; Wagner, V.P. 4-Nitroquinoline-1-Oxide (4NQO) Induced Oral Carcinogenesis: A Systematic Literature Review. Pathol. Res. Pract. 2022, 236, 153970. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Salvadori, D.M. Gingival Changes in Wistar Rats after Oral Treatment with 4-Nitroquinoline 1-Oxide. Eur. J. Dent. 2007, 1, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Hasina, R.; Martin, L.E.; Kasza, K.; Jones, C.L.; Jalil, A.; Lingen, M.W. ABT-510 Is an Effective Chemopreventive Agent in the Mouse 4-Nitroquinoline 1-Oxide Model of Oral Carcinogenesis. Cancer Prev. Res. (Phila) 2009, 2, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H.; Knudsen, B.; Bemis, D.; Tickoo, S.; Gudas, L.J. Oral Cavity and Esophageal Carcinogenesis Modeled in Carcinogen-Treated Mice. Clin. Cancer Res. 2004, 10 Pt 1, 301–313. [Google Scholar] [CrossRef]
- Kartasova, T.; Cornelissen, B.J.; Belt, P.; van de Putte, P. Effects of UV, 4-NQO and TPA on Gene Expression in Cultured Human Epidermal Keratinocytes. Nucleic Acids Res. 1987, 15, 5945–5962. [Google Scholar] [CrossRef][Green Version]
- Shishodia, G.; Toledo, R.R.G.; Rong, X.; Zimmerman, E.; Xiao, A.Y.; Harrison, L.; Nathan, C.O. 4NQO Enhances Differential Activation of DNA Repair Proteins in HPV Positive and HPV Negative HNSCC Cells. Oral Oncol. 2021, 122, 105578. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Beckett, M.A. Defective Terminal Differentiation in Culture as a Consistent and Selectable Character of Malignant Human Keratinocytes. Cell 1980, 22 Pt 2, 629–632. [Google Scholar] [CrossRef] [PubMed]


| Weeks | Classification | NQ n (%) | CM n (%) |
|---|---|---|---|
| 8 | Without dysplasia | 1 (20) | - |
| Mild/moderate dysplasia | 4 (80) | 5 (100) | |
| Severe dysplasia/carcinoma | - | - | |
| 12 | Without dysplasia | - | - |
| Mild/moderate dysplasia | 5 (100) | 4 (80) | |
| Severe dysplasia/carcinoma | - | 1 (20) | |
| 16 | Without dysplasia | - | - |
| Mild/moderate dysplasia | 5 (100) | 2 (40) | |
| Severe dysplasia/carcinoma | - | 3 (60) | |
| 20 | Without dysplasia | - | - |
| Mild/moderate dysplasia | - | - | |
| Severe dysplasia/carcinoma | 5 (100) | 5 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses, D.O.; Souza, B.d.S.N.; Dutra, M.J.; Malta, I.S.; Silva, B.O.; Cançado, I.M.; Conceição, N.S.C.; Lança, M.L.d.A.; Vasconcellos, L.M.R.d.; Kaminagakura, E. Use of 4-Nitroquinoline 1-Oxide (4NQO) in Dysplastic and Malignant Induction: In Vitro and In Vivo Studies. Biomedicines 2025, 13, 2223. https://doi.org/10.3390/biomedicines13092223
Meneses DO, Souza BdSN, Dutra MJ, Malta IS, Silva BO, Cançado IM, Conceição NSC, Lança MLdA, Vasconcellos LMRd, Kaminagakura E. Use of 4-Nitroquinoline 1-Oxide (4NQO) in Dysplastic and Malignant Induction: In Vitro and In Vivo Studies. Biomedicines. 2025; 13(9):2223. https://doi.org/10.3390/biomedicines13092223
Chicago/Turabian StyleMeneses, Daniela Oliveira, Brunna da Silva Nobrega Souza, Mateus José Dutra, Isabella Souza Malta, Bruna Oliveira Silva, Isis Moraes Cançado, Nathan Stevan Cezar Conceição, Maria Leticia de Almeida Lança, Luana Marotta Reis de Vasconcellos, and Estela Kaminagakura. 2025. "Use of 4-Nitroquinoline 1-Oxide (4NQO) in Dysplastic and Malignant Induction: In Vitro and In Vivo Studies" Biomedicines 13, no. 9: 2223. https://doi.org/10.3390/biomedicines13092223
APA StyleMeneses, D. O., Souza, B. d. S. N., Dutra, M. J., Malta, I. S., Silva, B. O., Cançado, I. M., Conceição, N. S. C., Lança, M. L. d. A., Vasconcellos, L. M. R. d., & Kaminagakura, E. (2025). Use of 4-Nitroquinoline 1-Oxide (4NQO) in Dysplastic and Malignant Induction: In Vitro and In Vivo Studies. Biomedicines, 13(9), 2223. https://doi.org/10.3390/biomedicines13092223







