Stress Hyperglycemia as a Prognostic Indicator of the Clinical Outcomes in Patients with Stroke: A Comprehensive Literature Review
Abstract
1. Introduction
2. Methods
3. SH Prevalence and Risk Factors
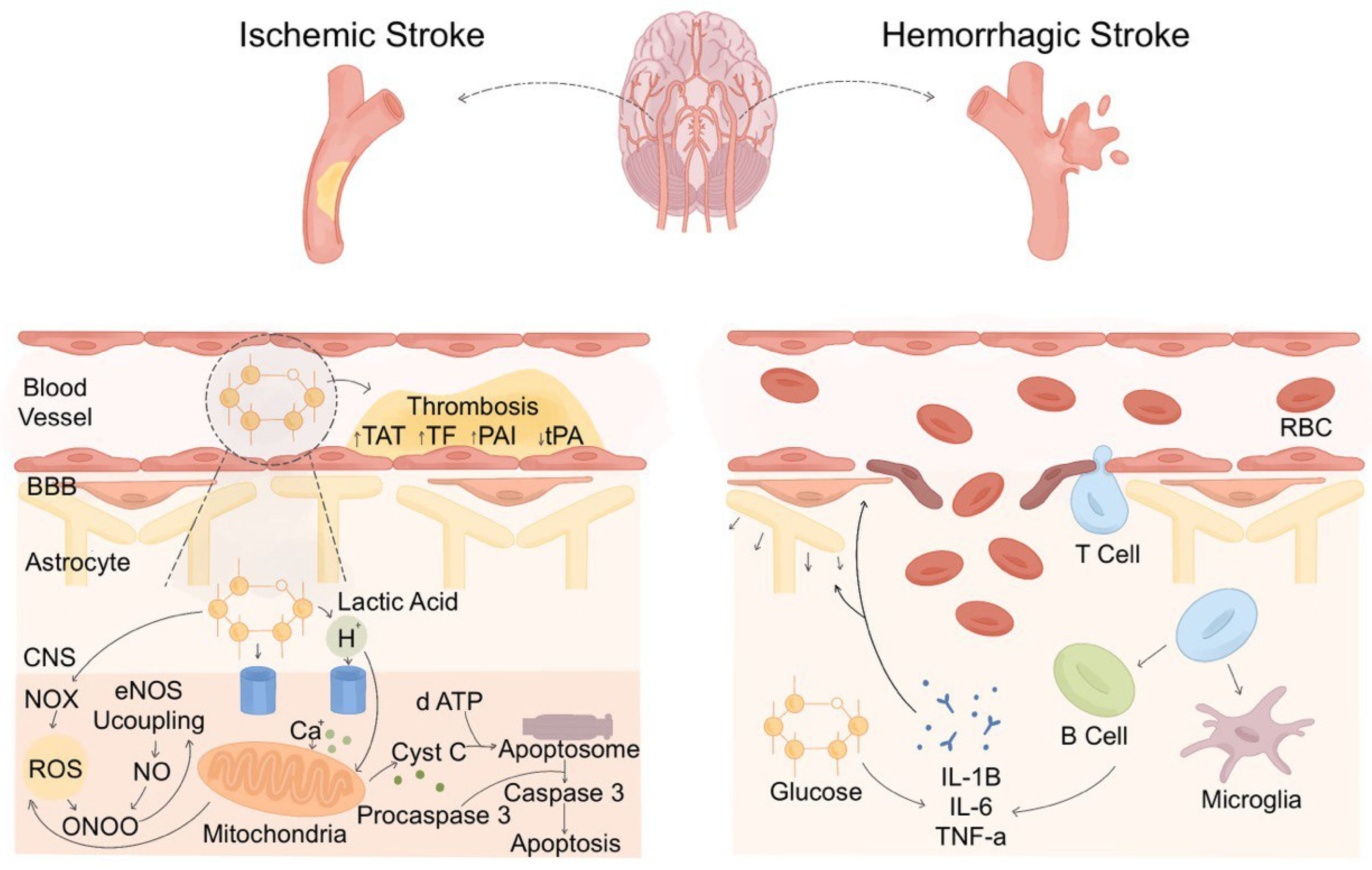
4. Overview of SH Pathophysiology in Stroke
5. Pathophysiological Variations in SH in Ischemic Versus Hemorrhagic Stroke
5.1. Inflammatory Infiltration in Different Types of Stroke
5.2. The Role of SH in Inflammatory Infiltration
6. SH as a Prognostic Indicator
6.1. Short-Term Outcome: Complications, Hospital Stay, and Death
6.2. Long-Term Outcomes: Functional and Motor Recovery and Disability
7. Impact of Chronic Versus Acute Hyperglycemia on Stroke Outcomes
8. Measurement and Assessment of SH
| Measurement Method | Ischemic Stroke | Hemorrhagic Stroke | Study | |||
|---|---|---|---|---|---|---|
| Cutoff Value | Associated Outcomes | Cutoff Value | Associated Outcomes | |||
| RBG, mg/dL | >200 | Poor functional outcomes; prolonged hospital stay; UTI; bedsores; lower respiratory tract infections | NA | NA | [45] | |
| >145 | 30-day mortality; mechanical ventilation and vasopressors requirement; hemorrhagic transformation | NA | NA | [46] | ||
| 155 | Higher admission stroke severity; 3-month poor functional outcomes; 3-month mortality; increased infarct volume | NA | NA | [57] | ||
| NA | NA | 115.2–199.8 | 3-month poor clinical outcome | [76] | ||
| FBG, mg/dL | NA | NA | 111.6–135 | Poor outcome; DCI | [77] | |
| ABG, mg/dL | NA | NA | >180 | Inpatient mortality; unfavorable discharge mRS | [78] | |
| NA | NA | NA | 30- and 90-day all-cause mortality | [79] | ||
| 110–126 | In-hospital and 30-day mortality | 120.6–126 | Not associated with short-term mortality | [49] | ||
| 130 | Higher stroke severity; functional impairment; 90-day mortality | NA | NA | [80] | ||
| 131.4 | 12-month poor functional outcomes | NA | NA | [81] | ||
| 140 | 3-month poor functional outcomes; post-stroke infection; 3-month mortality | NA | NA | [82] | ||
| 140 | 3-month poor functional outcomes; 3-month mortality; sICH | NA | NA | [83] | ||
| 140.4 | Poor functional outcomes at discharge | NA | NA | [84] | ||
| ≥140 | 3-month poor functional outcomes; sICH | NA | NA | [85] | ||
| 140.4 | 3-month poor functional outcomes | NA | NA | [86] | ||
| 140.4 | Not relevant with 3-month poor functional outcomes and sICH | NA | NA | [13] | ||
| 200 | 7-day mortality; 3-month mortality; END | NA | NA | [86] | ||
| GV | SD, mg/dL | ≥20.9 | 3-month poor functional outcomes | NA | NA | [71] |
| SD, mg/dL | NA | NA | 15.9–27.4 | Unfavorable neurological outcomes | [87] | |
| SD CoV | NA | 28- and 90-day mortality | NA | 28- and 90-day mortality | [88] | |
| CoV, % | <15 | 3-month poor functional outcomes | NA | NA | [72] | |
| J index | >21 | 3-month poor functional outcomes | NA | NA | [72] | |
| J index | ≥6.55 | 3-month cardiovascular outcomes | NA | NA | [89] | |
| MAG | NA | PSCI | NA | NA | [90] | |
| MAG | >19 | Poor neurological improvement during hospitalization | >19 | Poor neurological improvement during hospitalization | [52] | |
| MAGE | >5.6 | END | NA | NA | [73] | |
| TR | NA | 3-month poor functional outcomes; sICH | NA | NA | [91] | |
| SHR | SHR1 SHR2 SHR3 | ≥0.98 | Poor functional outcome; hemorrhagic transformation | NA | NA | [62] |
| SHR1 | ≥27.59 | Higher stroke severity; poor neurological status | NA | NA | [92] | |
| SHR2 | ≥0.97 | 3-month poor functional outcomes | NA | NA | [93] | |
| SHR2 | NA | NA | ≥0.98 | Hematoma expansion; neurological deterioration; 30-day mortality; 3-month poor mRS | [11] | |
| SHR2 | >0.89 | 3-month poor functional outcomes; hemorrhagic transformation | NA | NA | [17] | |
| SHR2 | ≥1.07 | 12-month stroke recurrence; 12-month all-cause death | NA | NA | [63] | |
| SHR2 | NA | 12-month severe neurological deficit; 12-month mortality | NA | NA | [60] | |
| SHR2 | NA | Hemorrhagic transformation | NA | NA | [94] | |
| SHR2 | >1.32 | In-hospital mortality | NA | NA | [18] | |
| SHR2 | NA | END; poor functional outcomes at discharge | NA | NA | [95] | |
| SHR2 | NA | NA | NA | In-hospital mortality; hematoma expansion | [96] | |
| SHR3 | ≥1.14 | In-hospital mortality; poor functional outcomes; stroke extension; hemorrhagic transformation | NA | NA | [61] | |
| SHR3 | ≥0.96 | 3-month poor functional outcomes | NA | NA | [54] | |
| SHR3 | NA | NA | NA | DCI; 90-days poor prognosis | [76] | |
| SHR3 | NA | NA | NA | 30-day and 1-year mortality | [97] | |
| SHR3 | >0.81 | In-hospital mortality; prolonged hospital stays | >81 | In-hospital mortality; prolonged hospital stays | [98] | |
| GA, % | NA | NA | ≥16.0 | Hematoma expansion; END; 1-month mortality; and 3-month poor functional outcomes | [75] | |
| ≥16 | Unfavorable short-term outcomes | NA | NA | [74] | ||
| ≥16 | END | [99] | ||||
| NA | 3-month and 1-year poor functional outcomes; 1-year stroke recurrence; 1-year combined vascular events | NA | NA | [100] | ||
| GG | >−0.53 | 3-month poor functional outcomes; hemorrhagic transformation | NA | NA | [17] | |
| 45 mg/dl | Higher stroke severity; poor neurological status | NA | NA | [92] | ||
| GAR | NA | Stroke recurrence, 1-year mortality | NA | NA | [63] | |
| NA | 3-month poor outcomes; 3-month mortality; sICH | NA | NA | [47] | ||
| NA | 3-month poor outcomes; 3-month mortality; sICH | NA | NA | [16] | ||
| NA | NA | ≥1.02 | Poor functional outcome | [101] | ||
| HbA1c, % | NA | NA | ≥6.0 | Unfavorable clinical outcomes | [102] | |
| 5.4–7.1 | Reduced functional independence; mortality; sICH | NA | NA | [103] | ||
| >8.2 | PSCI | NA | NA | [104] | ||
9. Management of SH in Stroke
9.1. Management Outline
9.2. Differences Between SH Management in Ischemic Versus Hemorrhagic Stroke
9.3. Effect of DM Status on SH Management in Stroke
9.4. Risk of Hypoglycemia
9.5. Treatment Options for SH in Stroke
| Management Option | Hypoglycemic Effect | Neuroprotective Effect | Study |
|---|---|---|---|
| Insulin | Reduction in gluconeogenesis; increased glucose uptake through GLUT4 transporters; glycogenesis | Suppression of neuroinflammation, ROS formation, lipolysis, and platelet aggregation; vasodilation | [122,138] |
| SGLT2 Inhibitor | Inhibiting renal SGLT2 transporter that decrease renal glucose re-absorption, increasing urinary glucose excretion | Reduction in neuroinflammation and plaque size | [133,139] |
| GLP-1 Receptor Agonists | Increased glucose-induced insulin secretion; glycogenesis | Reduction in infarct volume, apoptosis, oxidative stress, neuroinflammation, excitotoxicity, and BBB permeability; increased neurogenesis, neuroplasticity, angiogenesis, and cerebral perfusion | [131] |
| DPP-4 inhibitors | Enhanced bioavailability of GLP-1 and GIP by Inhibition of DPP-4 enzyme | Activation of the AKT/MTOR pathway; suppression of BBB disruption, oxidative stress, apoptosis, and inflammation; enhanced endothelium relaxation and cerebrovascular remodeling | [132,140] |
| Metformin | Reduction in gluconeogenesis; increased insulin receptor sensitivity; decreased glucose uptake in the intestine | Suppression of oxidative stress and apoptosis; endothelial nitric oxide synthase activation; activation of angiogenesis and neurogenesis | [134] |
| Thiazolidinediones | Enhance insulin sensitivity and reduce serum glucose by PPAR-γ activation | Reduce neuroinflammation and infarct volume | [102] |
| α-Glucosidase inhibitors | Delayed carbohydrate absorption from intestine by inhibiting α-glucosidase enzymes | Inhibitory potential on DAPK1-p53 interaction; prevention of mitochondrial and lysosomal dysfunction; favorable modulation of gene expression related to cell survival, inflammation, and regeneration | [141,142] |
10. Study Strengths and Limitations
11. Future Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Andersson, J.; Rejnö, Å.; Jakobsson, S.; Hansson, P.-O.; Nielsen, S.J.; Björck, L. Symptoms at stroke onset as described by patients: A qualitative study. BMC Neurol. 2024, 24, 150. [Google Scholar] [CrossRef]
- GBD 2021 Stroke Risk Factor Collaborators. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2021: A Systematic Analysis for the Global. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; O Martins, S.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N.; Patel, A.; Anne, K.K. Stress-Induced Hyperglycemia: Consequences and Management. Cureus 2022, 14, e26714. [Google Scholar] [CrossRef]
- Argyropoulos, T.; Korakas, E.; Gikas, A.; Kountouri, A.; Kostaridou-Nikolopoulou, S.; Raptis, A.; Lambadiari, V. Stress Hyperglycemia in Children and Adolescents as a Prognostic Indicator for the Development of Type 1 Diabetes Mellitus. Front. Pediatr. 2021, 9, 670976. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Scheen, M.; Giraud, R.; Bendjelid, K. Stress hyperglycemia, cardiac glucotoxicity, and critically ill patient outcomes current clinical and pathophysiological evidence. Physiol. Rep. 2021, 9, e14713. [Google Scholar] [CrossRef]
- Roberts, G.W.; Quinn, S.J.; Valentine, N.; Alhawassi, T.; O’DEa, H.; Stranks, S.N.; Burt, M.G.; Doogue, M.P. Relative Hyperglycemia, a Marker of Critical Illness: Introducing the Stress Hyperglycemia Ratio. J. Clin. Endocrinol. Metab. 2015, 100, 4490–4497. [Google Scholar] [CrossRef]
- Denorme, F.; Portier, I.; Kosaka, Y.; Campbell, R.A. Hyperglycemia exacerbates ischemic stroke outcome independent of platelet glucose uptake. J. Thromb. Haemost. 2021, 19, 536–546. [Google Scholar] [CrossRef]
- Chu, H.; Huang, C.; Tang, Y.; Dong, Q.; Guo, Q. The stress hyperglycemia ratio predicts early hematoma expansion and poor outcomes in patients with spontaneous intracerebral hemorrhage. Ther. Adv. Neurol. Disord. 2022, 15, 17562864211070681. [Google Scholar] [CrossRef]
- Gao, J.; Chen, X.; Huang, Q.; Gu, M.; Hong, Y.; Xu, G. Stress Hyperglycemia Is Associated with Unfavorable Outcomes After Mechanical Thrombectomy in Patients with Acute Ischemic Stroke. Brain Sci. 2025, 15, 360. [Google Scholar] [CrossRef]
- Osei, E.; Hertog, H.M.D.; Berkhemer, O.A.; Fransen, P.S.; Roos, Y.B.; Beumer, D.; van Oostenbrugge, R.J.; Schonewille, W.J.; Boiten, J.; Zandbergen, A.A.; et al. Admission Glucose and Effect of Intra-Arterial Treatment in Patients with Acute Ischemic Stroke. Stroke 2017, 48, 1299–1305. [Google Scholar] [CrossRef]
- Kes, V.B.; Solter, V.V.; Supanc, V.; Demarin, V. Impact of hyperglycemia on ischemic stroke mortality in diabetic and non-diabetic patients. Ann. Saudi Med. 2007, 27, 352–355. [Google Scholar] [CrossRef]
- Snarska, K.K.; Bachórzewska-Gajewska, H.; Kapica-Topczewska, K.; Drozdowski, W.; Chorąży, M.; Kułakowska, A.; Małyszko, J. Hyperglycemia and diabetes have different impacts on outcome of ischemic and hemorrhagic stroke. Arch. Med. Sci. 2017, 1, 100–108. [Google Scholar] [CrossRef]
- Merlino, G.; Smeralda, C.; Gigli, G.L.; Lorenzut, S.; Pez, S.; Surcinelli, A.; Marini, A.; Valente, M. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J. Thromb. Thrombolysis 2021, 51, 789–797. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Ji, Y.; Wu, K.; Wang, H.; Yuan, L.; Yang, K.; Yang, Q.; Huang, X.; Zhou, Z. New stress-induced hyperglycaemia markers predict prognosis in patients after mechanical thrombectomy. BMC Neurol. 2023, 23, 132. [Google Scholar] [CrossRef]
- Mi, D.; Li, Z.; Gu, H.; Jiang, Y.; Zhao, X.; Wang, Y.; Wang, Y. Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci. Ther. 2022, 28, 372–381. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel; Microsoft: Redmond, WA, USA, 2024. [Google Scholar]
- Savage Interactive Pty Ltd. Procreate; Savage Interactive: Hobart, TAS, Australia, 2025. [Google Scholar]
- Zhang, H.; Yue, K.; Jiang, Z.; Wu, X.; Li, X.; Luo, P.; Jiang, X. Incidence of Stress-Induced Hyperglycemia in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Brain Sci. 2023, 13, 556. [Google Scholar] [CrossRef]
- Peng, Z.; Song, J.; Li, L.; Guo, C.; Yang, J.; Kong, W.; Huang, J.; Hu, J.; Liu, S.; Tian, Y.; et al. Association between stress hyperglycemia and outcomes in patients with acute ischemic stroke due to large vessel occlusion. CNS Neurosci. Ther. 2023, 29, 2162–2170. [Google Scholar] [CrossRef]
- Luitse, M.J.; Biessels, G.J.; Rutten, G.E.; Kappelle, L.J. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012, 11, 261–271. [Google Scholar] [CrossRef]
- Katsimardou, A.; Imprialos, K.; Stavropoulos, K.; Sachinidis, A.; Doumas, M.; Athyros, V. Hypertension in Metabolic Syndrome: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 12–18. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, W.; Li, J.; Sun, W.; Zhang, J.; Xu, P.; Guo, Y.; Ning, N.; Li, J.; Zhao, B.; et al. Association of Stress Hyperglycemia Ratio and in-Hospital Mortality in Patients with Sepsis: A Two Center Retrospective Cohort Study. J. Inflamm. Res. 2024, 17, 7939–7950. [Google Scholar] [CrossRef]
- Chittawar, S.; Sharma, J.; Maniram, R.S.; Dubey, T.; Singh, A. Clinical and epidemiological study of stress hyperglycemia among medical intensive care unit patients in Central India. Indian J. Endocrinol. Metab. 2017, 21, 137–141. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, G.; Jing, J.; Wang, A.; Zhang, X.; Meng, X.; Zhao, X.; Liu, L.; Li, H.; Wang, D.; et al. Stress hyperglycemia may have higher risk of stroke recurrence than previously diagnosed diabetes mellitus. Aging 2021, 13, 9108–9118. [Google Scholar] [CrossRef]
- Yoon, N.A.; Diano, S. Hypothalamic glucose-sensing mechanisms. Diabetologia 2021, 64, 985–993. [Google Scholar] [CrossRef]
- Harrell, C.; Gillespie, C.; Neigh, G. Energetic stress: The reciprocal relationship between energy availability and the stress response. Physiol. Behav. 2016, 166, 43–55. [Google Scholar] [CrossRef]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.-C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- Stephens, J.; Pekala, P. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J. Biol. Chem. 1991, 266, 21839–21845. [Google Scholar] [CrossRef]
- Ferrari, F.; Moretti, A.; Villa, R.F. Hyperglycemia in acute ischemic stroke: Physiopathological and therapeutic complexity. Neural Regen. Res. 2022, 17, 292. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, Y.; Fu, W.; Wang, Y.; Qian, J.; Tao, C.; You, C.; Yang, M. Association between neutrophil to lymphocyte ratio and blood glucose level at admission in patients with spontaneous intracerebral hemorrhage. Sci. Rep. 2019, 9, 15623. [Google Scholar] [CrossRef]
- Yue, Y.; Li, P.; Sun, Z.; Wang, X.; Li, Z.; Zhang, Y. Unveiling the role of stress hyperglycemia in predicting mortality for critically ill hemorrhagic stroke patients: Insights from MIMIC-IV. Front. Endocrinol. 2025, 16, 1558352. [Google Scholar] [CrossRef]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation—Target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef]
- Alsbrook, D.L.; Di Napoli, M.; Bhatia, K.; Biller, J.; Andalib, S.; Hinduja, A.; Rodrigues, R.; Rodriguez, M.; Sabbagh, S.Y.; Selim, M.; et al. Neuroinflammation in Acute Ischemic and Hemorrhagic Stroke. Curr. Neurol. Neurosci. Rep. 2023, 23, 407–431. [Google Scholar] [CrossRef]
- Ohashi, S.N.; DeLong, J.H.; Kozberg, M.G.; Mazur-Hart, D.J.; van Veluw, S.J.; Alkayed, N.J.; Sansing, L.H. Role of Inflammatory Processes in Hemorrhagic Stroke. Stroke 2023, 54, 605–619. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, Q.; Song, Y.; He, M.; Zeng, Y.; Liu, Z.; Xu, J. Prognostic role of neutrophil–lymphocyte ratio in patients with acute ischemic stroke. Medicine 2017, 96, e8624. [Google Scholar] [CrossRef]
- Thålin, C.; Rudberg, A.-S.; Johansson, F.; Laska, A.C.; Nygren, A.T.; von Arbin, M.; Wallén, H.; Aspberg, S. Elevated Troponin Levels in Acute Stroke Patients Predict Long-term Mortality. J. Stroke Cerebrovasc. Dis. 2015, 24, 2390–2396. [Google Scholar] [CrossRef]
- Whiteley, W.; Chong, W.L.; Sengupta, A.; Sandercock, P. Blood Markers for the Prognosis of Ischemic Stroke. Stroke 2009, 40, e380–e389. [Google Scholar] [CrossRef]
- Van Den Berghe, G. Novel insights into the neuroendocrinology of critical illness. Eur. J. Endocrinol. 2000, 143, 1–13. [Google Scholar] [CrossRef]
- Latha, V.; Shashibhushan, J. Stress hyperglycemia as a prognostic indicator of the clinical outcome in patients with ischemic stroke. Asian J. Med. Sci. 2024, 15, 85–90. [Google Scholar] [CrossRef]
- El-Gendy, H.A.; Mohamed, M.A.; Abd-Elhamid, A.E.; Nosseir, M.A. Stress hyperglycemia as a prognostic factor in acute ischemic stroke patients: A prospective observational cohort study. Ain-Shams J. Anesthesiol. 2021, 13, 4. [Google Scholar] [CrossRef]
- Merlino, G.; Pez, S.; Gigli, G.L.; Sponza, M.; Lorenzut, S.; Surcinelli, A.; Smeralda, C.; Valente, M. Stress Hyperglycemia in Patients with Acute Ischemic Stroke Due to Large Vessel Occlusion Undergoing Mechanical Thrombectomy. Front. Neurol. 2021, 12, 725002. [Google Scholar] [CrossRef]
- Attia, S.M.; Gomaa, M.S.; Ismaeil, H.K.; Elmetwaly, A.A.S. Study of Hyperglycemia as a Prognostic Factor in Acute Ischemic Stroke. Egypt. J. Hosp. Med. 2021, 82, 641–646. [Google Scholar] [CrossRef]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Pathak, P.; Gerstein, H.C. Stress Hyperglycemia and Prognosis of Stroke in Nondiabetic and Diabetic Patients. Stroke 2001, 32, 2426–2432. [Google Scholar] [CrossRef]
- Yong, M.; Kaste, M. Dynamic of Hyperglycemia as a Predictor of Stroke Outcome in the ECASS-II Trial. Stroke 2008, 39, 2749–2755. [Google Scholar] [CrossRef]
- Kruyt, N.D.; Biessels, G.J.; DeVries, J.H.; Roos, Y.B. Hyperglycemia in acute ischemic stroke: Pathophysiology and clinical management. Nat. Rev. Neurol. 2010, 6, 145–155. [Google Scholar] [CrossRef]
- Palaiodimou, L.; Lioutas, V.-A.; Lambadiari, V.; Theodorou, A.; Themistocleous, M.; Aponte, L.; Papagiannopoulou, G.; Foska, A.; Bakola, E.; Quispe, R.; et al. Glycemic variability of acute stroke patients and clinical outcomes: A continuous glucose monitoring study. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211045876. [Google Scholar] [CrossRef]
- Rahaman, H.; Das, S.; Karmakar, A.; Biswas, L.; Pal, S.K. A study on incidence of stress hyperglycemia in acute ischemic stroke in non-diabetic patients and its prognostic significance. Asian J. Med. Sci. 2022, 13, 117–122. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Miao, J.; Zheng, W.; Yang, Q.; Ye, X.; Zhuang, X.; Peng, F. High Stress Hyperglycemia Ratio Predicts Poor Outcome after Mechanical Thrombectomy for Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1668–1673. [Google Scholar] [CrossRef]
- Yoon, J.A.; Shin, Y.-I.; Kim, D.Y.; Sohn, M.K.; Lee, J.; Lee, S.-G.; Lee, Y.-S.; Han, E.Y.; Joo, M.C.; Oh, G.-J.; et al. Post-stroke Hyperglycemia in Non-diabetic Ischemic Stroke is Related with Worse Functional Outcome: A Cohort Study. Ann. Rehabilitation Med. 2021, 45, 359–367. [Google Scholar] [CrossRef]
- Muscari, A.; Falcone, R.; Recinella, G.; Faccioli, L.; Forti, P.; Trossello, M.P.; Puddu, G.M.; Spinardi, L.; Zoli, M. Prognostic significance of diabetes and stress hyperglycemia in acute stroke patients. Diabetol. Metab. Syndr. 2022, 14, 126. [Google Scholar] [CrossRef]
- Fuentes, B.; Castillo, J.; José, B.S.; Leira, R.; Serena, J.; Vivancos, J.; DávAlos, A.; Gil NuñeZ, A.; Egido, J.; DíeZ-Tejedor, E. The Prognostic Value of Capillary Glucose Levels in Acute Stroke. Stroke 2009, 40, 562–568. [Google Scholar] [CrossRef]
- Baird, T.A.; Parsons, M.W.; Phan, T.; Butcher, K.S.; Desmond, P.M.; Tress, B.M.; Colman, P.G.; Chambers, B.R.; Davis, S.M. Persistent Poststroke Hyperglycemia Is Independently Associated with Infarct Expansion and Worse Clinical Outcome. Stroke 2003, 34, 2208–2214. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Huang, W.; Lobanova, I.; Chandrasekaran, P.N.; Hanley, D.F.; Hsu, C.Y.; Martin, R.H.; Steiner, T.; Suarez, J.I.; Yamamoto, H.; et al. Effect of Moderate and Severe Persistent Hyperglycemia on Outcomes in Patients with Intracerebral Hemorrhage. Stroke 2022, 53, 1226–1234. [Google Scholar] [CrossRef]
- Li, J.; Quan, K.; Wang, Y.; Zhao, X.; Li, Z.; Pan, Y.; Li, H.; Liu, L.; Wang, Y. Effect of Stress Hyperglycemia on Neurological Deficit and Mortality in the Acute Ischemic Stroke People With and Without Diabetes. Front. Neurol. 2020, 11, 576895. [Google Scholar] [CrossRef]
- Roberts, G.; Sires, J.; Chen, A.; Thynne, T.; Sullivan, C.; Quinn, S.; Chen, W.S.; Meyer, E. A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J. Diabetes 2021, 13, 1034–1042. [Google Scholar] [CrossRef]
- Shen, C.-L.; Xia, N.-G.; Wang, H.; Zhang, W.-L. Association of Stress Hyperglycemia Ratio with Acute Ischemic Stroke Outcomes Post-thrombolysis. Front. Neurol. 2022, 12, 785428. [Google Scholar] [CrossRef]
- Zhu, B.; Pan, Y.; Jing, J.; Meng, X.; Zhao, X.; Liu, L.; Wang, Y.; Wang, Y.; Wang, Z. Stress Hyperglycemia and Outcome of Non-diabetic Patients After Acute Ischemic Stroke. Front. Neurol. 2019, 10, 1003. [Google Scholar] [CrossRef]
- Cai, Z.-M.; Zhang, M.-M.; Feng, R.-Q.; Zhou, X.-D.; Chen, H.-M.; Liu, Z.-P.; Wu, Y.-Z.; Lin, Q.-L.; Ye, S.-L.; Liao, C.-W.; et al. Fasting blood glucose-to-glycated hemoglobin ratio and all-cause mortality among Chinese in-hospital patients with acute stroke: A 12-month follow-up study. BMC Geriatr. 2022, 22, 508. [Google Scholar] [CrossRef]
- Poppe, A.Y.; Majumdar, S.R.; Jeerakathil, T.; Ghali, W.; Buchan, A.M.; Hill, M.D. Admission Hyperglycemia Predicts a Worse Outcome in Stroke Patients Treated With Intravenous Thrombolysis. Diabetes Care 2009, 32, 617–622. [Google Scholar] [CrossRef]
- Merlino, G.; Romoli, M.; Ornello, R.; Foschi, M.; Del Regno, C.; Toraldo, F.; Marè, A.; Cordici, F.; Trosi, A.; Longoni, M.; et al. Stress hyperglycemia is associated with futile recanalization in patients with anterior large vessel occlusion undergoing mechanical thrombectomy. Eur. Stroke J. 2024, 9, 613–622. [Google Scholar] [CrossRef]
- Goyal, N. Non Diabetic and Stress Induced Hyperglycemia [SIH] in Orthopaedic Practice What do we know so Far? J. Clin. Diagn. Res. 2014, 8, LH01–LH03. [Google Scholar] [CrossRef]
- Pilackas, K.; El-Oshar, S.; Carter, C. Clinical Reliability of Point-of-Care Glucose Testing in Critically Ill Patients. J. Diabetes Sci. Technol. 2020, 14, 65–69. [Google Scholar] [CrossRef]
- Wernly, B.; Lichtenauer, M.; Hoppe, U.C.; Jung, C. Hyperglycemia in septic patients: An essential stress survival response in all, a robust marker for risk stratification in some, to be messed with in none. J. Thorac. Dis. 2016, 8, E621–E624. [Google Scholar] [CrossRef]
- Masrur, S.; Cox, M.; Bhatt, D.L.; Smith, E.E.; Ellrodt, G.; Fonarow, G.C.; Schwamm, L. Association of Acute and Chronic Hyperglycemia With Acute Ischemic Stroke Outcomes Post-Thrombolysis: Findings From Get With The Guidelines-Stroke. J. Am. Heart Assoc. 2015, 4, e002193. [Google Scholar] [CrossRef]
- Baudu, J.; Gerbaud, E.; Catargi, B.; Montaudon, M.; Beauvieux, M.-C.; Sagnier, S.; Debruxelles, S.; Renou, P.; Poli, M.; Olindo, S.; et al. High glycemic variability: An underestimated determinant of stroke functional outcome following large vessel occlusion. Rev. Neurol. 2022, 178, 732–740. [Google Scholar] [CrossRef]
- Kim, J.-T.; Lee, S.-Y.; Yoo, D.-S.; Lee, J.S.; Kim, S.-H.; Choi, K.-H.; Park, M.-S.; Cho, K.-H. Clinical Implications of Serial Glucose Measurements in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Sci. Rep. 2018, 8, 11761. [Google Scholar] [CrossRef]
- Hui, J.; Zhang, J.; Mao, X.; Li, Z.; Li, X.; Wang, F.; Wang, T.; Yuan, Q.; Wang, S.; Pu, M.; et al. The initial glycemic variability is associated with early neurological deterioration in diabetic patients with acute ischemic stroke. Neurol. Sci. 2018, 39, 1571–1577. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.-H.; Kang, M.K.; Kim, T.J.; Jeong, H.-Y.; Lee, E.-J.; Bae, J.; Jeon, K.; Nam, K.-W.; Yoon, B.-W. Glycated Albumin, a Novel Biomarker for Short-Term Functional Outcomes in Acute Ischemic Stroke. Brain Sci. 2021, 11, 337. [Google Scholar] [CrossRef]
- Lee, S.-H.; Sohn, J.-H.; Kim, C.; Kim, Y.J.; Jeon, J.P.; Yang, J.; Park, S.Y.; Choi, H.J. Pre-stroke glycemic variability estimated by glycated albumin predicts hematoma expansion and poor outcomes in patients with spontaneous intracerebral hemorrhage. Sci. Rep. 2023, 13, 12848. [Google Scholar] [CrossRef]
- Wang, M.; Wang, P.; Liu, Y.; Chen, C.; Zhi, Z.; Wang, Y.; Liu, F.; Zhao, L. Stress hyperglycemia ratio is associated with delayed cerebrovascular ischemia and poor prognosis in patients with aneurysmal subarachnoid hemorrhage undergoing neurointerventional therapy. Clin. Neurol. Neurosurg. 2025, 249, 108769. [Google Scholar] [CrossRef]
- Kruyt, N.D.; Roos, Y.W.B.M.; Mees, S.M.D.; Bergh, W.M.v.D.; Algra, A.; E Rinkel, G.J.; Biessels, G.J. High mean fasting glucose levels independently predict poor outcome and delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1382–1385. [Google Scholar] [CrossRef]
- Gilotra, K.; Basem, J.; Janssen, M.; Swarna, S.; Mani, R.; Ren, B.; Dashti, R. Stress-Induced Hyperglycemia Predicts Poor Outcomes in Primary Intracerebral Hemorrhage Patients. NeuroSci 2025, 6, 12. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Y.; Zhang, Q. Admission Hyperglycemia Predicts Long-Term Mortality in Critically Ill Patients With Subarachnoid Hemorrhage: A Retrospective Analysis of the MIMIC-III Database. Front. Neurol. 2021, 12, 678998. [Google Scholar] [CrossRef]
- Stead, L.G.; Gilmore, R.M.; Bellolio, M.F.; Mishra, S.; Bhagra, A.; Vaidyanathan, L.; Decker, W.W.; Brown, R.D. Hyperglycemia as An Independent Predictor of Worse Outcome in Non-diabetic Patients Presenting with Acute Ischemic Stroke. Neurocritical Care 2009, 10, 181–186. [Google Scholar] [CrossRef]
- Ntaios, G.; Egli, M.; Faouzi, M.; Michel, P. J-Shaped Association Between Serum Glucose and Functional Outcome in Acute Ischemic Stroke. Stroke 2010, 41, 2366–2370. [Google Scholar] [CrossRef]
- Zonneveld, T.P.; Nederkoorn, P.J.; Westendorp, W.F.; Brouwer, M.C.; van de Beek, D.; Kruyt, N.D.; For the PASS Investigators; PASS Investigators; Vermeij, J.-D.; Zock, E.; et al. Hyperglycemia predicts poststroke infections in acute ischemic stroke. Neurology 2017, 88, 1415–1421. [Google Scholar] [CrossRef]
- Goyal, N.; Tsivgoulis, G.; Pandhi, A.; Dillard, K.; Katsanos, A.H.; Magoufis, G.; Chang, J.J.; Zand, R.; Hoit, D.; Safouris, A.; et al. Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J. NeuroInterventional Surg. 2018, 10, 112–117. [Google Scholar] [CrossRef]
- Osei, E.; Hertog, H.D.; Berkhemer, O.; Fransen, P.; Roos, Y.; Beumer, D.; van Oostenbrugge, R.; Schonewille, W.; Boiten, J.; Zandbergen, A.; et al. Increased admission and fasting glucose are associated with unfavorable short-term outcome after intra-arterial treatment of ischemic stroke in the MR CLEAN pretrial cohort. J. Neurol. Sci. 2016, 371, 1–5. [Google Scholar] [CrossRef]
- Saqqur, M.; Shuaib, A.; Alexandrov, A.V.; Sebastian, J.; Khan, K.; Uchino, K. The Correlation between Admission Blood Glucose and Intravenous rt-PA-Induced Arterial Recanalization in Acute Ischemic Stroke: A Multi-Centre TCD Study. Int. J. Stroke 2015, 10, 1087–1092. [Google Scholar] [CrossRef]
- Fang, H.-J.; Pan, Y.-S.; Wang, Y.-J.; Wang, C.-X.; Wang, Y.-L.; Zhong, L.-Y.; Hao, X.-Y. Prognostic value of admission hyperglycemia on outcomes of thrombolysis in ischemic stroke patients with or without diabetes. Chin. Med. J. 2020, 133, 2244–2246. [Google Scholar] [CrossRef]
- Okazaki, T.; Hifumi, T.; Kawakita, K.; Shishido, H.; Ogawa, D.; Okauchi, M.; Shindo, A.; Kawanishi, M.; Tamiya, T.; Kuroda, Y. Blood Glucose Variability: A Strong Independent Predictor of Neurological Outcomes in Aneurysmal Subarachnoid Hemorrhage. J. Intensiv. Care Med. 2018, 33, 189–195. [Google Scholar] [CrossRef]
- Lin, J.; Cai, C.; Xie, Y.; Yi, L. Acute glycemic variability and mortality of patients with acute stroke: A meta-analysis. Diabetol. Metab. Syndr. 2022, 14, 69. [Google Scholar] [CrossRef]
- Yoon, J.-E.; Sunwoo, J.-S.; Kim, J.S.; Roh, H.; Ahn, M.-Y.; Woo, H.-Y.; Lee, K.B. Poststroke glycemic variability increased recurrent cardiovascular events in diabetic patients. J. Diabetes its Complicat. 2017, 31, 390–394. [Google Scholar] [CrossRef]
- Lim, J.-S.; Kim, C.; Oh, M.S.; Lee, J.-H.; Jung, S.; Jang, M.U.; Lee, S.-H.; Kim, Y.J.; Kim, Y.; Suh, S.W.; et al. Effects of glycemic variability and hyperglycemia in acute ischemic stroke on post-stroke cognitive impairments. J. Diabetes its Complicat. 2018, 32, 682–687. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, J.S.; Park, S.-H.; Ko, S.-B. Short-term glycemic variability and hemorrhagic transformation after successful endovascular thrombectomy. Transl. Stroke Res. 2021, 12, 968–975. [Google Scholar] [CrossRef]
- Yang, C.-J.; Liao, W.-I.; Wang, J.-C.; Tsai, C.-L.; Lee, J.-T.; Peng, G.-S.; Lee, C.-H.; Hsu, C.-W.; Tsai, S.-H. Usefulness of glycated hemoglobin A1c-based adjusted glycemic variables in diabetic patients presenting with acute ischemic stroke. Am. J. Emerg. Med. 2017, 35, 1240–1246. [Google Scholar] [CrossRef]
- Ngiam, J.N.; Cheong, C.W.S.; Leow, A.S.T.; Wei, Y.-T.; Thet, J.K.X.; Lee, I.Y.S.; Sia, C.-H.; Tan, B.Y.Q.; Khoo, C.-M.; Sharma, V.K.; et al. Stress hyperglycaemia is associated with poor functional outcomes in patients with acute ischaemic stroke after intravenous thrombolysis. Qjm Int. J. Med. 2022, 115, 7–11. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, S.; Ruan, Y.; Liu, Y.; Cheng, H.; Zeng, Y.; Chen, Y.; Cheng, Q.; Huang, G.; He, W.; et al. The Stress Hyperglycemia Ratio is Associated with Hemorrhagic Transformation in Patients with Acute Ischemic Stroke. Clin. Interv. Aging 2021, 16, 431–442. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Q.; Hu, T.; Wang, N.; Wei, X.; Wu, T.; Bi, X. Impact of Stress Hyperglycemia on Early Neurological Deterioration in Acute Ischemic Stroke Patients Treated With Intravenous Thrombolysis. Front. Neurol. 2022, 13, 870872. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Gu, H.; Zhou, Q.; Li, Z.; Zhao, X. Comparison of stress hyperglycemia ratio and glycemic gap on acute ICH in-hospital outcomes. Ann. Clin. Transl. Neurol. 2024, 11, 1492–1501. [Google Scholar] [CrossRef]
- Liang, S.; Tian, X.; Gao, F.; Man, M.; Wang, Q.; Li, J.; Li, L.; Yang, Y. Prognostic significance of the stress hyperglycemia ratio and admission blood glucose in diabetic and nondiabetic patients with spontaneous intracerebral hemorrhage. Diabetol. Metab. Syndr. 2024, 16, 58. [Google Scholar] [CrossRef]
- Shen, D.; Cai, X.; Zhu, Q.; Heizhati, M.; Hu, J.; Song, S.; Yang, W.; Hong, J.; Li, N. Increased stress hyperglycemia ratio at hospital admission in stroke patients are associated with increased in-hospital mortality and length of stay. Diabetol. Metab. Syndr. 2024, 16, 69. [Google Scholar] [CrossRef]
- Nam, K.-W.; Han, J.H.; Kim, C.K.; Kwon, H.-M.; Lee, Y.-S.; Oh, K.; Lee, K.-J.; Park, B. High glycated albumin is associated with early neurological deterioration in patients with acute ischemic stroke. BMC Neurol. 2024, 24, 278. [Google Scholar] [CrossRef]
- Mao, J.; Wang, M.; Wang, C.; Gu, H.; Meng, X.; Jiang, Y.; Yang, X.; Zhang, J.; Xiong, Y.; Zhao, X.; et al. Glycated albumin levels are associated with adverse stroke outcomes in patients with acute ischemic stroke in China. J. Diabetes 2024, 16, e13600. [Google Scholar] [CrossRef]
- Liu, J.; Gao, B.-B.; Clermont, A.C.; Blair, P.; Chilcote, T.J.; Sinha, S.; Flaumenhaft, R.; Feener, E.P. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat. Med. 2011, 17, 206–210. [Google Scholar] [CrossRef]
- Sundararajan, S.; Gamboa, J.; Victor, N.; Wanderi, E.; Lust, W.; Landreth, G. Peroxisome proliferator-activated receptor-γ ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience 2005, 130, 685–696. [Google Scholar] [CrossRef]
- Diprose, W.K.; Wang, M.T.M.; McFetridge, A.; Sutcliffe, J.; Barber, P.A. Glycated hemoglobin (HbA1c) and outcome following endovascular thrombectomy for ischemic stroke. J. NeuroInterventional Surg. 2020, 12, 30–32. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, Q.; Du, Y.; Huang, L.-W.; Yu, M. Nonlinear relationship between glycated hemoglobin and cognitive impairment after acute mild ischemic stroke. BMC Neurol. 2023, 23, 116. [Google Scholar] [CrossRef]
- Kernan, W.N.; Forman, R.; Inzucchi, S.E. Caring for Patients with Diabetes in Stroke Neurology. Stroke 2023, 54, 894–904. [Google Scholar] [CrossRef]
- Stroke and Transient Ischaemic Attack in Over 16s: Diagnosis and Initial Management. NICE Guideline. 2019. Available online: www.nice.org.uk/guidance/ng128 (accessed on 17 July 2025).
- Gladstone, D.J.; Lindsay, M.P.; Douketis, J.; Smith, E.E.; Dowlatshahi, D.; Wein, T.; Bourgoin, A.; Cox, J.; Falconer, J.B.; Graham, B.R.; et al. Canadian Stroke Best Practice Recommendations: Secondary Prevention of Stroke Update 2020. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2022, 49, 315–337. [Google Scholar] [CrossRef]
- Baker, L.; Juneja, R.; Bruno, A. Management of Hyperglycemia in Acute Ischemic Stroke. Curr. Treat. Options Neurol. 2011, 13, 616–628. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Molyneaux, B.J.; Hill, M.D.; Jovin, T.G. Care of the Post-Thrombectomy Patient. Stroke 2018, 49, 2801–2807. [Google Scholar] [CrossRef]
- Demaerschalk, B.M.; Kleindorfer, D.O.; Adeoye, O.M.; Demchuk, A.M.; Fugate, J.E.; Grotta, J.C.; Khalessi, A.A.; Levy, E.I.; Palesch, Y.Y.; Prabhakaran, S.; et al. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke. Stroke 2016, 47, 581–641. [Google Scholar] [CrossRef]
- Ahmed, N.; Dávalos, A.; Eriksson, N.; Ford, G.A.; Glahn, J.; Hennerici, M.; Mikulik, R.; Kaste, M.; Lees, K.R.; Lindsberg, P.J.; et al. Association of Admission Blood Glucose and Outcome in Patients Treated with Intravenous Thrombolysis. Arch. Neurol. 2010, 67, 1123–1130. [Google Scholar] [CrossRef]
- Southerland, A.M.; Johnston, K.C. Considering hyperglycemia and thrombolysis in the Stroke Hyperglycemia Insulin Network Effort (SHINE) trial. Ann. New York Acad. Sci. 2012, 1268, 72–78. [Google Scholar] [CrossRef]
- Gentile, N.T.; Seftchick, M.W.; Huynh, T.; Kruus, L.K.; Gaughan, J. Decreased Mortality by Normalizing Blood Glucose after Acute Ischemic Stroke. Acad. Emerg. Med. 2006, 13, 174–180. [Google Scholar] [CrossRef]
- Sacco, S.; Foschi, M.; Ornello, R.; De Santis, F.; Pofi, R.; Romoli, M. Prevention and treatment of ischaemic and haemorrhagic stroke in people with diabetes mellitus: A focus on glucose control and comorbidities. Diabetologia 2024, 67, 1192–1205. [Google Scholar] [CrossRef]
- Ma, L.; Hu, X.; Song, L.; Chen, X.; Ouyang, M.; Billot, L.; Li, Q.; Malavera, A.; Muñoz-Venturelli, P.; de Silva, A.; et al. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): An international, stepped wedge cluster randomised controlled trial. Lancet 2023, 402, 27–40. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Johnston, K.C.; Bruno, A.; Pauls, Q.; Hall, C.E.; Barrett, K.M.; Barsan, W.; Fansler, A.; Van de Bruinhorst, K.; Janis, S.; Durkalski-Mauldin, V.L.; et al. Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients with Acute Ischemic Stroke. JAMA 2019, 322, 326–335. [Google Scholar] [CrossRef]
- Johnston, K.C.; Hall, C.E.; Kissela, B.M.; Bleck, T.P.; Conaway, M.R. Glucose Regulation in Acute Stroke Patients (GRASP) Trial. Stroke 2009, 40, 3804–3809. [Google Scholar] [CrossRef]
- Rosso, C.; Corvol, J.-C.; Pires, C.; Crozier, S.; Attal, Y.; Jacqueminet, S.; Deltour, S.; Multlu, G.; Leger, A.; Meresse, I.; et al. Intensive Versus Subcutaneous Insulin in Patients with Hyperacute Stroke. Stroke 2012, 43, 2343–2349. [Google Scholar] [CrossRef]
- Bruno, A.; Kent, T.A.; Coull, B.M.; Shankar, R.R.; Saha, C.; Becker, K.J.; Kissela, B.M.; Williams, L.S. Treatment of Hyperglycemia In Ischemic Stroke (THIS). Stroke 2008, 39, 384–389. [Google Scholar] [CrossRef]
- Sulaiman, W.A.W.; Hashim, H.Z.; Abdullah, S.T.C.; Hoo, F.K.; Basri, H. Mini review: Managing post stroke hyperglycaemia: Moderate glycaemic control is better? An update. EXCLI J. 2014, 13, 825–833. [Google Scholar]
- McDonnell, M.E.; Umpierrez, G.E. Insulin Therapy for the Management of Hyperglycemia in Hospitalized Patients. Endocrinol. Metab. Clin. N. Am. 2012, 41, 175–201. [Google Scholar] [CrossRef]
- Ellahham, S. Insulin therapy in critically ill patients. Vasc. Health Risk Manag. 2010, 6, 1089. [Google Scholar] [CrossRef]
- Bruno, A.; Saha, C.; Williams, L.S.; Shankar, R. IV insulin during acute cerebral infarction in diabetic patients. Neurology 2004, 62, 1441–1442. [Google Scholar] [CrossRef]
- Walters, M.; Weir, C.; Lees, K. A Randomised, Controlled Pilot Study to Investigate the Potential Benefit of Intervention with Insulin in Hyperglycaemic Acute Ischaemic Stroke Patients. Cerebrovasc. Dis. 2006, 22, 116–122. [Google Scholar] [CrossRef]
- Nau, K.C.; Lorenzetti, R.C.; Cucuzzella, M.; Devine, T.; Kline, J. Glycemic Control in Hospitalized Patients Not in Intensive Care: Beyond Sliding-Scale Insulin. Am. Fam. Physician 2010, 81, 1130–1135. [Google Scholar] [PubMed]
- Korytkowski, M.T.; Muniyappa, R.; Antinori-Lent, K.; Donihi, A.C.; Drincic, A.T.; Hirsch, I.B.; Luger, A.; E McDonnell, M.; Murad, M.H.; Nielsen, C.; et al. Management of Hyperglycemia in Hospitalized Adult Patients in Non-Critical Care Settings: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2022, 107, 2101–2128. [Google Scholar] [CrossRef]
- Southerland, A.M.; Mayer, S.A.; Chiota-McCollum, N.A.; Bolte, A.C.; Pauls, Q.; Pettigrew, L.C.; Bleck, T.P.; Conaway, M.; Johnston, K.C. Glucose Control and Risk of Symptomatic Intracerebral Hemorrhage Following Thrombolysis for Acute Ischemic Stroke. Neurology 2024, 102, e209323. [Google Scholar] [CrossRef]
- Gray, C.S.; Hildreth, A.J.; A Sandercock, P.; E O’COnnell, J.; E Johnston, D.; Cartlidge, N.E.; Bamford, J.M.; James, O.F.; Alberti, K.G.M. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: The UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. 2007, 6, 397–406. [Google Scholar] [CrossRef]
- Long, B.; Koyfman, A.; Gottlieb, M.; Zehtabchi, S. Effect of Tight Glycemic Control on Patients with Ischemic Stroke. Acad. Emerg. Med. 2020, 28, 255–257. [Google Scholar] [CrossRef]
- Vergès, B.; Aboyans, V.; Angoulvant, D.; Boutouyrie, P.; Cariou, B.; Hyafil, F.; Mohammedi, K.; Amarenco, P. Protection against stroke with glucagon-like peptide-1 receptor agonists: A comprehensive review of potential mechanisms. Cardiovasc. Diabetol. 2022, 21, 242. [Google Scholar] [CrossRef]
- Darsalia, V.; Klein, T.; Nyström, T.; Patrone, C. Glucagon-like receptor 1 agonists and DPP-4 inhibitors: Anti-diabetic drugs with anti-stroke potential. Neuropharmacology 2018, 136, 280–286. [Google Scholar] [CrossRef]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE −/− mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef]
- Arbeláez-Quintero, I.; Palacios, M. To Use or Not to Use Metformin in Cerebral Ischemia: A Review of the Application of Metformin in Stroke Rodents. Stroke Res. Treat. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Shaheryar, Z.A.; Khan, M.A.; Hameed, H.; Zaidi, S.A.A.; Anjum, I.; Rahman, M.S.U. Lauric acid provides neuroprotection against oxidative stress in mouse model of hyperglycaemic stroke. Eur. J. Pharmacol. 2023, 956, 175990. [Google Scholar] [CrossRef]
- Wang, F.; He, Q.; Wang, J.; Yuan, Q.; Guo, H.; Chai, L.; Wang, S.; Hu, L.; Zhang, Y. Neuroprotective effect of salvianolate lyophilized injection against cerebral ischemia in type 1 diabetic rats. BMC Complement. Altern. Med. 2017, 17, 258. [Google Scholar] [CrossRef]
- Li, S.; Sun, X.; Xu, L.; Sun, R.; Ma, Z.; Deng, X.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Baicalin attenuates in vivo and in vitro hyperglycemia-exacerbated ischemia/reperfusion injury by regulating mitochondrial function in a manner dependent on AMPK. Eur. J. Pharmacol. 2017, 815, 118–126. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Zhang, G.; Kim, S.; Gu, X.; Yu, S.P.; Wei, L. DPP-4 Inhibitor Linagliptin is Neuroprotective in Hyperglycemic Mice with Stroke via the AKT/mTOR Pathway and Anti-apoptotic Effects. Neurosci. Bull. 2020, 36, 407–418. [Google Scholar] [CrossRef]
- Das, J.; Mahammad, F.S.; Krishnamurthy, R.G. An integrated chemo-informatics and in vitro experimental approach repurposes acarbose as a post-ischemic neuro-protectant. 3 Biotech 2022, 12, 71. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdali, M.M.; Alrasheed, A.S.; Alghirash, F.A.; Almaqboul, T.M.; Alhashim, A.; Aljaafari, D.T.; Alqarni, M.A. Stress Hyperglycemia as a Prognostic Indicator of the Clinical Outcomes in Patients with Stroke: A Comprehensive Literature Review. Biomedicines 2025, 13, 1834. https://doi.org/10.3390/biomedicines13081834
Alabdali MM, Alrasheed AS, Alghirash FA, Almaqboul TM, Alhashim A, Aljaafari DT, Alqarni MA. Stress Hyperglycemia as a Prognostic Indicator of the Clinical Outcomes in Patients with Stroke: A Comprehensive Literature Review. Biomedicines. 2025; 13(8):1834. https://doi.org/10.3390/biomedicines13081834
Chicago/Turabian StyleAlabdali, Majed Mohammad, Abdulrahim Saleh Alrasheed, Fatimah Ahmed Alghirash, Taif Mansour Almaqboul, Ali Alhashim, Danah Tareq Aljaafari, and Mustafa Ahmed Alqarni. 2025. "Stress Hyperglycemia as a Prognostic Indicator of the Clinical Outcomes in Patients with Stroke: A Comprehensive Literature Review" Biomedicines 13, no. 8: 1834. https://doi.org/10.3390/biomedicines13081834
APA StyleAlabdali, M. M., Alrasheed, A. S., Alghirash, F. A., Almaqboul, T. M., Alhashim, A., Aljaafari, D. T., & Alqarni, M. A. (2025). Stress Hyperglycemia as a Prognostic Indicator of the Clinical Outcomes in Patients with Stroke: A Comprehensive Literature Review. Biomedicines, 13(8), 1834. https://doi.org/10.3390/biomedicines13081834






