The Role of Immunotherapy and Radiation Therapy in the Treatment of Breast Cancer
Abstract
1. Introduction
1.1. Surgical Advances in Breast Cancer
1.2. Developments in Radiation Therapy for Breast Cancer
1.3. The Evolution of Systemic Therapy in Breast Cancer
1.4. The Evolution of Immunotherapy in Breast Cancer Treatment
| Phase; IO Agent; Enrollment | Disease Stage/ Hormone Receptor Status | Treatment Arms | Primary/ Secondary Endpoints | Treatment Sequence of IO and RT | Study Results and Conclusion or Estimated Completion Date | |
|---|---|---|---|---|---|---|
| KEYNOTE-119: Study of Single Agent Pembrolizumab (MK-3475) Versus Single-Agent Chemotherapy for Metastatic Triple Negative Breast Cancer [48] | III; pembrolizumab; 1098 patients | Metastatic TNBC | Pembrolizumab vs. investigator’s choice of single-drug CHT | OS | No radiation therapy within at least two weeks |
|
| KEYNOTE-355: A Randomized, Double-Blind, Phase III Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs. Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer [45] | III; pembrolizumab; 847 patients | Metastatic or locally recurrent inoperable TNBC | Pembrolizumab + investigator’s choice of CHT vs. placebo + CHT | OS, PFS, Percentage of patients with AE and those who discontinued study drug due to an AE | N/a |
|
| KEYNOTE-522: Study of Pembrolizumab (MK-3475) Plus Chemotherapy vs. Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs. Placebo as Adjuvant Therapy in Participants With Triple-Negative Breast Cancer [32,61,62] | III; pembrolizumab; 1174 patients | Previously untreated stage II-III TNBC | Pembrolizumab + CHT (paclitaxel and carboplatin) vs. placebo + CHT | pCR; EFS | No radiation therapy within the past 12 months |
|
| IMpassion130: A Study of Atezolizumab in Combination With Nab-Paclitaxel Compared With Placebo With Nab-Paclitaxel for Participants With Previously Untreated Metastatic Triple-Negative Breast Cancer [31,63] | III; atezolizumab; 902 patients | Previously untreated metastatic TNBC | Atezolizumab + nab-paclitaxel vs. placebo + nab-paclitaxel | PFS; OS | N/a |
|
2. Breast Cancer Immune Microenvironment
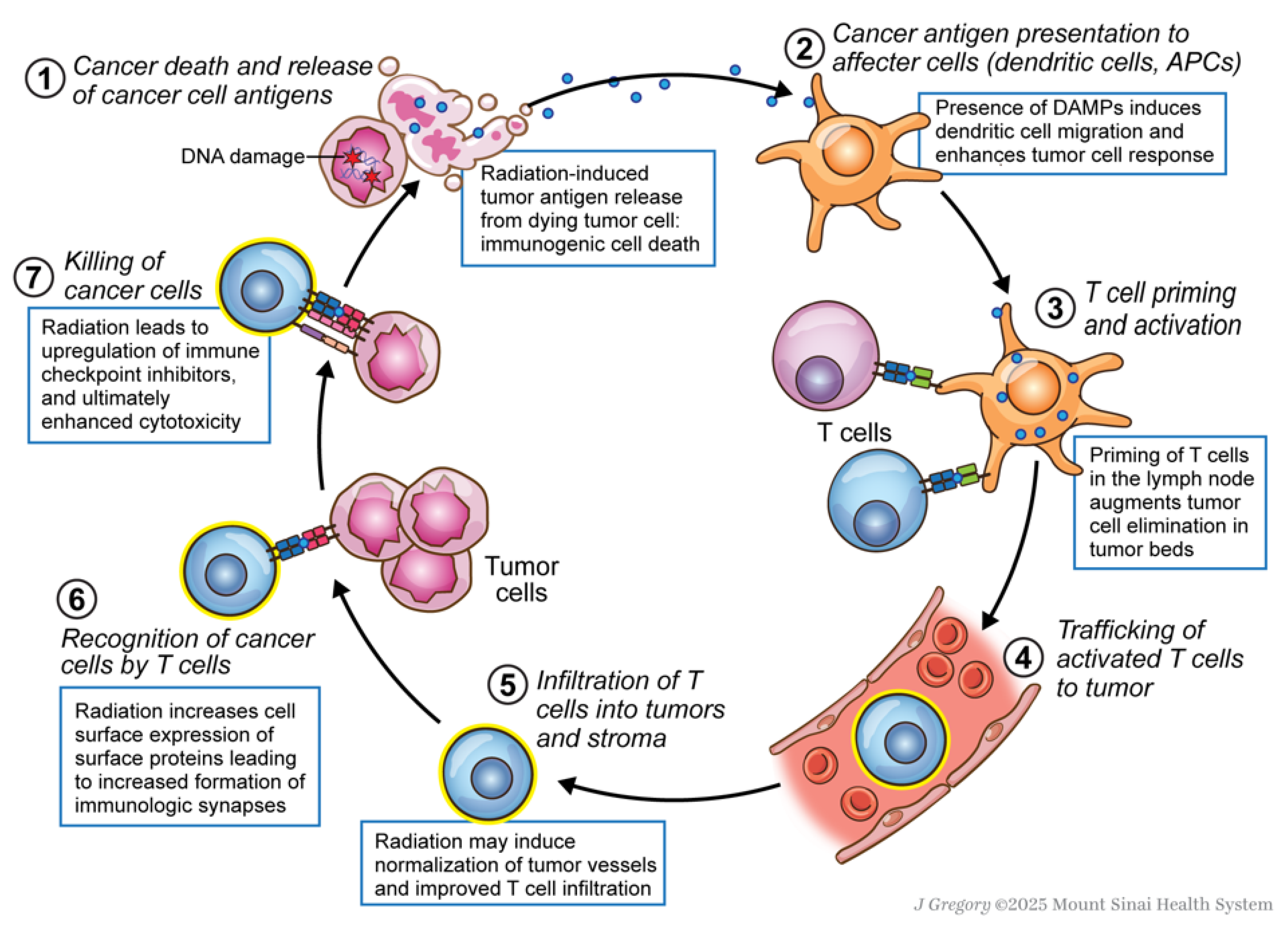
3. Mechanism and Rationale for Use of Immunotherapy and Radiation Therapy
3.1. Immunogenic Cell Death and DAMP Signaling
3.2. Activation of the STING Pathway
3.3. Upregulation of Immune Checkpoints
4. Immunotherapy and Radiation Therapy for Early-Stage and Locally Advanced Breast Cancer
5. Immunotherapy and Radiation Therapy in the Recurrent and Metastatic Setting
5.1. Metastatic TNBC
5.2. Hormone Receptor-Positive Metastatic Breast Cancer
6. Immunotherapy and Radiation Therapy and Other Systemic Agents
6.1. Poly (ADP-Ribose) Polymerase
6.2. Nanoparticle Radioenhancers
7. Tolerability and Toxicities
8. Discussion and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| APC | Antigen-Presenting Cell |
| CA | Cancer |
| CI | Confidence Interval |
| CPS | Combined Positive Score |
| DFS | Disease-Free Survival |
| EBCTCG | Early Breast Cancer Trialists’ Collaborative Group |
| EFS | Event-Free Survival |
| FAST | Fractionation of Adjuvant Radiotherapy in Breast Cancer |
| fx | Fraction |
| Gy | Gray |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HF | Hypofractionated |
| HR | Hormone Receptor/Hazard Ratio |
| ICD | Immunogenic Cell Death |
| ICI | Immune Checkpoint Inhibitor |
| IMPORT | Intensity-Modulated Partial Breast Radiotherapy |
| IO | Immunotherapy |
| IORT | Intraoperative Radiation Therapy |
| irAEs | Immune-Related Adverse Events |
| MHC | Major Histocompatibility Complex |
| NCT | National Clinical Trial |
| NSABP | National Surgical Adjuvant Breast and Bowel Project |
| OS | Overall Survival |
| PBI | Partial Breast Irradiation |
| pCR | Pathologic Complete Response |
| PD-1 | Programmed Death-1 |
| PDL-1 | Programmed Death Ligand 1 |
| PFS | Progression-Free Survival |
| RAPID | Randomized Trial of Accelerated Partial Breast Irradiation |
| RT | Radiation Therapy |
| SBRT | Stereotactic Body Radiation Therapy |
| SRS | Stereotactic Radiosurgery |
| TILs | Tumor-infiltrating lymphocytes |
| TNBC | Triple-Negative Breast Cancer |
| WBI | Whole Breast Irradiation |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Murtagh, S.; Han, Y.; Wan, F.; Toriola, A.T. Breast Cancer Incidence Among US Women Aged 20 to 49 Years by Race, Stage, and Hormone Receptor Status. JAMA Netw. Open 2024, 7, e2353331. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Nicholson, W.K.; Silverstein, M.; Wong, J.B.; Barry, M.J.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Jaén, C.R.; Krousel-Wood, M.; et al. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2024, 331, 1918–1930. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Fisher, B.; Bauer, M.; Margolese, R.; Poisson, R.; Pilch, Y.; Redmond, C.; Fisher, E.; Wolmark, N.; Deutsch, M.; Montague, E. Five-Year Results of a Randomized Clinical Trial Comparing Total Mastectomy and Segmental Mastectomy with or without Radiation in the Treatment of Breast Cancer. N. Engl. J. Med. 1985, 312, 665–673. [Google Scholar] [CrossRef]
- Fisher, B.; Dignam, J.; Wolmark, N.; Mamounas, E.; Costantino, J.; Poller, W.; Fisher, E.R.; Wickerham, D.L.; Deutsch, M.; Margolese, R.; et al. Lumpectomy and Radiation Therapy for the Treatment of Intraductal Breast Cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J. Clin. Oncol. 1998, 16, 441–452. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; et al. Effect of Radiotherapy after Mastectomy and Axillary Surgery on 10-Year Recurrence and 20-Year Breast Cancer Mortality: Meta-Analysis of Individual Patient Data for 8135 Women in 22 Randomised Trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.A.; Barrett, J.M.; Barrett-Lee, P.J.; Bliss, J.M.; Brown, J.; Dewar, J.A.; Dobbs, H.J.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: A Randomised Trial. Lancet Oncol. 2008, 9, 331–341. [Google Scholar] [CrossRef]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.A.; Barrett, J.M.; Barrett-Lee, P.J.; Bentzen, S.M.; Bliss, J.M.; Brown, J.; Dewar, J.A.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: A Randomised Trial. Lancet 2008, 371, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) Trials of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: 10-Year Follow-up Results of Two Randomised Controlled Trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated Breast Radiotherapy for 1 Week versus 3 Weeks (FAST-Forward): 5-Year Efficacy and Late Normal Tissue Effects Results from a Multicentre, Non-Inferiority, Randomised, Phase 3 Trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Whelan, T.J.; Julian, J.A.; Berrang, T.S.; Kim, D.-H.; Germain, I.; Nichol, A.M.; Akra, M.; Lavertu, S.; Germain, F.; Fyles, A.; et al. External Beam Accelerated Partial Breast Irradiation versus Whole Breast Irradiation after Breast Conserving Surgery in Women with Ductal Carcinoma in Situ and Node-Negative Breast Cancer (RAPID): A Randomised Controlled Trial. Lancet 2019, 394, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Cecchini, R.S.; White, J.R.; Arthur, D.W.; Julian, T.B.; Rabinovitch, R.A.; Kuske, R.R.; Ganz, P.A.; Parda, D.S.; Scheier, M.F.; et al. Long-Term Primary Results of Accelerated Partial Breast Irradiation after Breast-Conserving Surgery for Early-Stage Breast Cancer: A Randomised, Phase 3, Equivalence Trial. Lancet 2019, 394, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.E.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.S.; Brunt, A.M.; Ciurlionis, L.; Chan, C.; et al. Partial-Breast Radiotherapy after Breast Conservation Surgery for Patients with Early Breast Cancer (UK IMPORT LOW Trial): 5-Year Results from a Multicentre, Randomised, Controlled, Phase 3, Non-Inferiority Trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Marrazzo, L.; Saieva, C.; Desideri, I.; Scotti, V.; Simontacchi, G.; Bonomo, P.; Greto, D.; Mangoni, M.; Scoccianti, S.; et al. Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J. Clin. Oncol. 2020, 38, 4175–4183. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Wenz, F.; Bulsara, M.; Tobias, J.S.; Joseph, D.J.; Keshtgar, M.; Flyger, H.L.; Massarut, S.; Alvarado, M.; Saunders, C.; et al. Risk-Adapted Targeted Intraoperative Radiotherapy versus Whole-Breast Radiotherapy for Breast Cancer: 5-Year Results for Local Control and Overall Survival from the TARGIT-A Randomised Trial. Lancet 2014, 383, 603–613. [Google Scholar] [CrossRef]
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative Radiotherapy versus External Radiotherapy for Early Breast Cancer (ELIOT): A Randomised Controlled Equivalence Trial. Lancet Oncol. 2013, 14, 1269–1277. [Google Scholar] [CrossRef]
- Pignol, J.-P.; Olivotto, I.; Rakovitch, E.; Gardner, S.; Sixel, K.; Beckham, W.; Vu, T.T.T.; Truong, P.; Ackerman, I.; Paszat, L. A Multicenter Randomized Trial of Breast Intensity-Modulated Radiation Therapy to Reduce Acute Radiation Dermatitis. J. Clin. Oncol. 2008, 26, 2085–2092. [Google Scholar] [CrossRef]
- Zagar, T.M.; Kaidar-Person, O.; Tang, X.; Jones, E.E.; Matney, J.; Das, S.K.; Green, R.L.; Sheikh, A.; Khandani, A.H.; McCartney, W.H.; et al. Utility of Deep Inspiration Breath Hold for Left-Sided Breast Radiation Therapy in Preventing Early Cardiac Perfusion Defects: A Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 903–909. [Google Scholar] [CrossRef]
- Van Parijs, H.; Cecilia-Joseph, E.; Gorobets, O.; Storme, G.; Adriaenssens, N.; Heyndrickx, B.; Verschraegen, C.; Nguyen, N.P.; De Ridder, M.; Vinh-Hung, V. Lung-Heart Toxicity in a Randomized Clinical Trial of Hypofractionated Image Guided Radiation Therapy for Breast Cancer. Front. Oncol. 2023, 13, 1211544. [Google Scholar] [CrossRef]
- De-Colle, C.; Kirby, A.; Russell, N.; Shaitelman, S.F.; Currey, A.; Donovan, E.; Hahn, E.; Han, K.; Anandadas, C.N.; Mahmood, F.; et al. Adaptive Radiotherapy for Breast Cancer. Clin. Transl. Radiat. Oncol. 2023, 39, 100564. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Shah, C.; Mehta, M.P. Clinical Outcomes and Toxicity of Proton Radiotherapy for Breast Cancer. Clin. Breast Cancer 2016, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.A.; Dagan, R.; Ho, M.W.; Rutenberg, M.; Morris, C.G.; Li, Z.; Mendenhall, N.P. Initial Report of a Prospective Dosimetric and Clinical Feasibility Trial Demonstrates the Potential of Protons to Increase the Therapeutic Ratio in Breast Cancer Compared With Photons. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 411–421. [Google Scholar] [CrossRef]
- Flejmer, A.M.; Edvardsson, A.; Dohlmar, F.; Josefsson, D.; Nilsson, M.; Witt Nyström, P.; Dasu, A. Respiratory Gating for Proton Beam Scanning versus Photon 3D-CRT for Breast Cancer Radiotherapy. Acta Oncol. 2016, 55, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Manikhas, A.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Vazquez, F.; Byakhow, M.; Lichinitser, M.; et al. Neoadjuvant Chemotherapy with Trastuzumab Followed by Adjuvant Trastuzumab versus Neoadjuvant Chemotherapy Alone, in Patients with HER2-Positive Locally Advanced Breast Cancer (the NOAH Trial): A Randomised Controlled Superiority Trial with a Parallel HER2-Negative Cohort. Lancet 2010, 375, 377–384. [Google Scholar] [CrossRef]
- Fisher, B.; Dignam, J.; Wolmark, N.; DeCillis, A.; Emir, B.; Wickerham, D.L.; Bryant, J.; Dimitrov, N.V.; Abramson, N.; Atkins, J.N.; et al. Tamoxifen and Chemotherapy for Lymph Node-Negative, Estrogen Receptor-Positive Breast Cancer. J. Natl. Cancer Inst. 1997, 89, 1673–1682. [Google Scholar] [CrossRef]
- Henderson, I.C.; Berry, D.A.; Demetri, G.D.; Cirrincione, C.T.; Goldstein, L.J.; Martino, S.; Ingle, J.N.; Cooper, M.R.; Hayes, D.F.; Tkaczuk, K.H.; et al. Improved Outcomes from Adding Sequential Paclitaxel but Not from Escalating Doxorubicin Dose in an Adjuvant Chemotherapy Regimen for Patients with Node-Positive Primary Breast Cancer. J. Clin. Oncol. 2003, 21, 976–983. [Google Scholar] [CrossRef]
- Mackey, J.R.; Martin, M.; Pienkowski, T.; Rolski, J.; Guastalla, J.-P.; Sami, A.; Glaspy, J.; Juhos, E.; Wardley, A.; Fornander, T.; et al. Adjuvant Docetaxel, Doxorubicin, and Cyclophosphamide in Node-Positive Breast Cancer: 10-Year Follow-up of the Phase 3 Randomised BCIRG 001 Trial. Lancet Oncol. 2013, 14, 72–80. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kümmel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Goetz, M.P.; Olson, J.A.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical Implications of the Intrinsic Molecular Subtypes of Breast Cancer. Breast 2015, 24 (Suppl. S2), S26–S35. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab Monotherapy for Previously Untreated, PD-L1-Positive, Metastatic Triple-Negative Breast Cancer: Cohort B of the Phase II KEYNOTE-086 Study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab Monotherapy for Previously Treated Metastatic Triple-Negative Breast Cancer: Cohort A of the Phase II KEYNOTE-086 Study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus Investigator-Choice Chemotherapy for Metastatic Triple-Negative Breast Cancer (KEYNOTE-119): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016, 29, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Systemic Effects of Local Radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation Modulates the Peptide Repertoire, Enhances MHC Class I Expression, and Induces Successful Antitumor Immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA Exonuclease Trex1 Regulates Radiotherapy-Induced Tumour Immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune Induction Strategies in Metastatic Triple-Negative Breast Cancer to Enhance the Sensitivity to PD-1 Blockade: The TONIC Trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). A Randomized, Double-Blind, Phase III Trial of Taxane/Trastuzumab/Pertuzumab with Atezolizumab or Placebo in First-Line HER2-Positive Metastatic Breast Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT03199885 (accessed on 22 August 2025).
- Weill Medical College of Cornell University. CIMER: Combined Immunotherapies in Metastatic ER+ Breast Cancer. 2025. Available online: https://clinicaltrials.gov/study/NCT04563507 (accessed on 22 August 2025).
- Maria Sklodowska-Curie National Research. Institute of Oncology. A Phase II Study of Preoperative Stereotactic Radiation Therapy Boost Combined with Short-Course Immunotherapy (Pembrolizumab Versus Placebo, Randomized, Double-Blind) and Standard Chemiotherapy in Patients with Newly Diagnosed HER2-Negative Nonmetastatic Breast Cancer with Lack of Early Metabolic Response in 18-fluorodeoxyglucoseFDG-PET/CT After 1st Chemoterapy Cycle. 2024. Available online: https://clinicaltrials.gov/study/NCT06472583 (accessed on 22 August 2025).
- Tison, T.; Loap, P.; Arnaud, E.; Cao, K.; Bringer, S.; Kissel, M.; Maaradji, S.; Mainguene, J.; Pierga, J.-Y.; Lerebours, F.; et al. Tolerance of Concurrent Adjuvant Radiation Therapy and Pembrolizumab for Triple Negative Breast Cancer: Real Life Experience. Adv. Radiat. Oncol. 2024, 9, 101384. [Google Scholar] [CrossRef]
- Peter MacCallum Cancer Centre Australia. A Pilot Study of Stereotactic Ablation for Oligometastatic Breast Neoplasia in Combination with the Anti-PD-1 Antibody MK-3475. 2017. Available online: https://www.clinicaltrials.gov/study/NCT02303366 (accessed on 22 August 2025).
- Weill Medical College of Cornell University. Pembrolizumab and Stereotactic Radiosurgery (Srs) of Selected Brain Metastases in Breast Cancer Patients. 2024. Available online: https://clinicaltrials.gov/study/NCT03449238 (accessed on 22 August 2025).
- Ye, J.C.; Formenti, S.C. Integration of Radiation and Immunotherapy in Breast Cancer-Treatment Implications. Breast 2018, 38, 66–74. [Google Scholar] [CrossRef]
- Rodig, N.; Ryan, T.; Allen, J.A.; Pang, H.; Grabie, N.; Chernova, T.; Greenfield, E.A.; Liang, S.C.; Sharpe, A.H.; Lichtman, A.H.; et al. Endothelial Expression of PD-L1 and PD-L2 down-Regulates CD8+ T Cell Activation and Cytolysis. Eur. J. Immunol. 2003, 33, 3117–3126. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and Adaptive Immune Cells in the Tumor Microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical Relevance of Host Immunity in Breast Cancer: From TILs to the Clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef]
- Dieci, M.V.; Mathieu, M.C.; Guarneri, V.; Conte, P.; Delaloge, S.; Andre, F.; Goubar, A. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in Two Phase III Randomized Adjuvant Breast Cancer Trials. Ann. Oncol. 2015, 26, 1698–1704. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin with Doxorubicin-Based Chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Angelico, G.; Broggi, G.; Tinnirello, G.; Puzzo, L.; Vecchio, G.M.; Salvatorelli, L.; Memeo, L.; Santoro, A.; Farina, J.; Mulé, A.; et al. Tumor Infiltrating Lymphocytes (TILS) and PD-L1 Expression in Breast Cancer: A Review of Current Evidence and Prognostic Implications from Pathologist’s Perspective. Cancers 2023, 15, 4479. [Google Scholar] [CrossRef]
- Ho, A.Y.; Wright, J.L.; Blitzblau, R.C.; Mutter, R.W.; Duda, D.G.; Norton, L.; Bardia, A.; Spring, L.; Isakoff, S.J.; Chen, J.H.; et al. Optimizing Radiation Therapy to Boost Systemic Immune Responses in Breast Cancer: A Critical Review for Breast Radiation Oncologists. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 227–241. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Role of Tumor-Associated Myeloid Cells in Breast Cancer. Cells 2020, 9, 1785. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef]
- Golden, E.B.; Apetoh, L. Radiotherapy and Immunogenic Cell Death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The Cancer-Immunity Cycle: Indication, Genotype, and Immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Wang, J.-S.; Wang, H.-J.; Qian, H.-L. Biological Effects of Radiation on Cancer Cells. Mil. Med. Res. 2018, 5, 20. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.R.; Shen, Y.; Qian, D.C.; Buchwald, Z.S. The Abscopal Effect: A Review of Pre-Clinical and Clinical Advances. Int. J. Mol. Sci. 2021, 22, 11061. [Google Scholar] [CrossRef]
- Hauth, F.; Ho, A.Y.; Ferrone, S.; Duda, D.G. Radiotherapy to Enhance Chimeric Antigen Receptor T-Cell Therapeutic Efficacy in Solid Tumors. JAMA Oncol. 2021, 7, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, P.; Zhang, C.; Zhou, S.; Liang, L.; Guo, S.; Yin, Z.; Cheng, S.; Gan, Z.; Xia, Y.; et al. Nifuroxazide Suppresses PD-L1 Expression and Enhances the Efficacy of Radiotherapy in Hepatocellular Carcinoma. eLife 2024, 12, RP90911. [Google Scholar] [CrossRef] [PubMed]
- Elewaut, A.; Estivill, G.; Bayerl, F.; Castillon, L.; Novatchkova, M.; Pottendorfer, E.; Hoffmann-Haas, L.; Schönlein, M.; Nguyen, T.V.; Lauss, M.; et al. Cancer Cells Impair Monocyte-Mediated T Cell Stimulation to Evade Immunity. Nature 2025, 637, 716–725. [Google Scholar] [CrossRef]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-Induced Tumor Immune Microenvironments and Potential Targets for Combination Therapy. Signal Transduct. Target. Ther. 2023, 8, 205. [Google Scholar] [CrossRef]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-Mediated Inhibition of Metastases after Treatment with Local Radiation and CTLA-4 Blockade in a Mouse Model of Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 728–734. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and Anti-PD-L1 Treatment Synergistically Promote Antitumor Immunity in Mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Tchou, J.; Clark, A.; Taunk, N.; Freedman, G.; Xu, N.; Minn, A.; Bradbury, A.; Gross, A.D.; Domchek, S.; Knollman, H.; et al. 644: Major Pathologic Response after a Single Radiotherapy Fraction + a Single Pembrolizumab Dose given Preoperatively in Patients with cT1N0 Triple Negative Breast Cancer (TNBC)—Preliminary Results of a Phase 1b/2 Study (NCT04454528). J. Immunother. Cancer 2022, 10, A1–A1603. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S. Preoperative Combination of Pembrolizumab and Radiation Therapy in Patients with Operable Breast Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT03366844 (accessed on 22 August 2025).
- Nederlof, I.; Isaeva, O.I.; de Graaf, M.; Gielen, R.C.A.M.; Bakker, N.A.M.; Rolfes, A.L.; Garner, H.; Boeckx, B.; Traets, J.J.H.; Mandjes, I.A.M.; et al. Neoadjuvant Nivolumab or Nivolumab plus Ipilimumab in Early-Stage Triple-Negative Breast Cancer: A Phase 2 Adaptive Trial. Nat. Med. 2024, 30, 3223–3235. [Google Scholar] [CrossRef]
- Rapid Fire Session 3|FRIDAY, DECEMBER 13|12-12:50 Pm. Location: Hall 1 (Kopie)-Onlineportal Für Onkologie Und Hämatologie. Available online: https://oncoletter.ch/kongressberichte/id-2024/sabcs-rapid-fire-session-3.html (accessed on 26 February 2025).
- CDC. Metastatic Female Breast Cancer Incidence. Available online: https://www.cdc.gov/united-states-cancer-statistics/publications/metastatic-breast-cancer.html (accessed on 13 February 2025).
- Luke, J.J.; Lemons, J.M.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Al-Hallaq, H.A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [CrossRef]
- Kesireddy, M.; Elsayed, L.; Shostrom, V.K.; Agarwal, P.; Asif, S.; Yellala, A.; Krishnamurthy, J. Overall Survival and Prognostic Factors in Metastatic Triple-Negative Breast Cancer: A National Cancer Database Analysis. Cancers 2024, 16, 1791. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Stanton, S.E. Triple-Negative Breast Cancer: Immune Modulation as the New Treatment Paradigm. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2015, 35, e25–e30. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A Phase 2 Clinical Trial assessing the efficacy and Safety of Pembrolizumab and Radiotherapy in Patients with Metastatic Triple-Negative Breast Cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Krop, I.E.; Trippa, L.; Tan-Wasielewski, Z.; Li, T.; Osmani, W.; Andrews, C.; Dillon, D.; Richardson, E.T.; Pastorello, R.G.; et al. A Phase II Study of Pembrolizumab in Combination With Palliative Radiotherapy for Hormone Receptor-Positive Metastatic Breast Cancer. Clin. Breast Cancer 2020, 20, 238–245. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D. Biomarkers for Breast Cancer Immunotherapy: PD-L1, TILs, and Beyond. Expert. Opin. Investig. Drugs 2022, 31, 549–555. [Google Scholar] [CrossRef]
- Césaire, M.; Thariat, J.; Candéias, S.M.; Stefan, D.; Saintigny, Y.; Chevalier, F. Combining PARP Inhibition, Radiation, and Immunotherapy: A Possible Strategy to Improve the Treatment of Cancer? Int. J. Mol. Sci. 2018, 19, 3793. [Google Scholar] [CrossRef]
- Rosado, M.M.; Pioli, C. Radiotherapy, PARP Inhibition, and Immune-Checkpoint Blockade: A Triad to Overcome the Double-Edged Effects of Each Single Player. Cancers 2023, 15, 1093. [Google Scholar] [CrossRef]
- Isakoff, S.J. A Phase II Study of NirAparib, Dostarlimab and Radiotherapy in Metastatic, PD-L1 Negative or Immunotherapy-Refractory Triple-Negative Breast Cancer (NADiR). 2024. Available online: https://clinicaltrials.gov/study/NCT04837209 (accessed on 22 August 2025).
- Torres, M.A. A Multi-Institutional Phase II Study to Evaluate Efficacy and Safety of TAlazoparib, Radiotherapy and Atezolizumab in gBRCA 1/2 Negative Patients with PD-L1+ Metastatic Triple Negative Breast Cancer. 2023. Available online: https://clinicaltrials.gov/study/NCT04690855 (accessed on 22 August 2025).
- Memorial Sloan Kettering Cancer Center. Phase II Study of Pembrolizumab and Ablative Radiotherapy with or Without Olaparib in Metastatic Triple-Negative or Hormone-Receptor Positive/Her2 Negative Breast Cancers: Initial Test Cohorts of a Platform Trial to Sequentially Investigate Immunotherapy Combinations for the Augmentation of Immune Responses. 2024. Available online: https://clinicaltrials.gov/study/NCT04683679 (accessed on 22 August 2025).
- Nanobiotix. A Phase I Dose Escalation/Dose Expansion Study of NBTXR3 Activated by Radiotherapy for Patients with Advanced Cancers Treated with an Anti-PD-1 Therapy. 2025. Available online: https://clinicaltrials.gov/study/NCT03589339 (accessed on 22 August 2025).
- Hopwood, P.; Haviland, J.S.; Sumo, G.; Mills, J.; Bliss, J.M.; Yarnold, J.R. START Trial Management Group Comparison of Patient-Reported Breast, Arm, and Shoulder Symptoms and Body Image after Radiotherapy for Early Breast Cancer: 5-Year Follow-up in the Randomised Standardisation of Breast Radiotherapy (START) Trials. Lancet Oncol. 2010, 11, 231–240. [Google Scholar] [CrossRef]
- Taylor, C.W.; Kirby, A.M. Cardiac Side-Effects From Breast Cancer Radiotherapy. Clin. Oncol. 2015, 27, 621–629. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side Effects of Adjuvant Treatment of Breast Cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Ellithi, M.; Elnair, R.; Chang, G.V.; Abdallah, M.A. Toxicities of Immune Checkpoint Inhibitors: Itis-Ending Adverse Reactions and More. Cureus 2020, 12, e6935. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lomba, E.; Molina-López, I.; Suárez-Fernández, R.; Baniandrés-Rodríguez, O. Vitiligo-like Lesions and Immune Checkpoint Inhibition Therapy: Is It Truly an Adverse Event Exclusive to Patients with Melanoma? Clin. Exp. Dermatol. 2018, 43, 598–599. [Google Scholar] [CrossRef]
- Shaverdian, N.; Beattie, J.; Thor, M.; Offin, M.; Shepherd, A.F.; Gelblum, D.Y.; Wu, A.J.; Simone, C.B.; Hellmann, M.D.; Chaft, J.E.; et al. Safety of Thoracic Radiotherapy in Patients with Prior Immune-Related Adverse Events from Immune Checkpoint Inhibitors. Ann. Oncol. 2020, 31, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Switchenko, J.M.; Buchwald, Z.S.; Patel, P.R.; Shelton, J.W.; Kahn, S.E.; Pillai, R.N.; Steuer, C.E.; Owonikoko, T.K.; Behera, M.; et al. Lung Stereotactic Body Radiation Therapy and Concurrent Immunotherapy: A Multicenter Safety and Toxicity Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 304–313. [Google Scholar] [CrossRef]
- Fang, P.; Jiang, W.; Allen, P.; Glitza, I.; Guha, N.; Hwu, P.; Ghia, A.; Phan, J.; Mahajan, A.; Tawbi, H.; et al. Radiation Necrosis with Stereotactic Radiosurgery Combined with CTLA-4 Blockade and PD-1 Inhibition for Treatment of Intracranial Disease in Metastatic Melanoma. J. Neuro Oncol. 2017, 133, 595–602. [Google Scholar] [CrossRef]
- Ahmed, K.A.; Kim, S.; Arrington, J.; Naghavi, A.O.; Dilling, T.J.; Creelan, B.C.; Antonia, S.J.; Caudell, J.J.; Harrison, L.B.; Sahebjam, S.; et al. Outcomes Targeting the PD-1/PD-L1 Axis in Conjunction with Stereotactic Radiation for Patients with Non-Small Cell Lung Cancer Brain Metastases. J. Neuro Oncol. 2017, 133, 331–338. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Abrencillo, R.; Gandhi, S.; Altan, M.; Sheshadri, A. Immunotherapy-Related Pneumonitis and the Synergic Impact of Thoracic Radiation and Preexisting Interstitial Lung Disease. Curr. Opin. Pulm. Med. 2023, 29, 248–255. [Google Scholar] [CrossRef]
- Verma, S.; Young, S.; Boldt, G.; Blanchette, P.; Lock, M.; Helou, J.; Raphael, J. Immunotherapy and Radiation Therapy Sequencing in Breast Cancer: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 1422–1434. [Google Scholar] [CrossRef]
- McArthur, H.; Cortés, J.; Dent, R.; O’Shaughnessy, J.; Pusztai, L.; Küemmel, S.; Foukakis, T.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Abstract PD3-01: Neoadjuvant Pembrolizumab + Chemotherapy vs Placebo + Chemotherapy Followed by Adjuvant Pembrolizumab vs Placebo for Early TNBC: Post Hoc Analysis of Adjuvant Radiation Therapy in the Phase 3 KEYNOTE-522 Study. Cancer Res. 2023, 83, PD3-01. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Elemento, O.; Leslie, C.; Lundin, J.; Tourassi, G. Artificial Intelligence in Cancer Research, Diagnosis and Therapy. Nat. Rev. Cancer 2021, 21, 747–752. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]

| Trial | Key Toxicities Reported | Toxicity Notes |
|---|---|---|
| KEYNOTE-522 | Neutropenia, fatigue, diarrhea, pneumonitis | Higher irAEs vs. control; manageable with steroids |
| IMpassion130 | Peripheral neuropathy, neutropenia, rash | No RT; increased rash and irAEs in PD-L1+ subgroup |
| TONIC | Fatigue, cytokine release, hypothyroidism | Pre-treatment with SBRT increased immune response |
| KEYNOTE-355 | Fatigue, nausea, immune-mediated AEs | No RT; highlights systemic IO safety profile |
| Trial (IO Agent [if Specified]) | Phase; ClinicalTrials.gov ID | Description (Patient Population, Control Arm, Experimental Arm) | Trial Status | Expected Completion Date/Year |
|---|---|---|---|---|
| Radiotherapy for Extracranial Oligometastatic Breast Cancer [92] | III NCT04646564 | Population: patients with extracranial oligometastatic breast cancer Control arm: Standard systemic therapy (including immunotherapy) Experimental arm: above + local radiotherapy (SBRT of 30–50 Gy in 5 fx or conventional RT of 60 Gy in 25 fx) | Recruiting | 30 April 2026 |
| SBRT Combined With PD-1 Inhibitor and Chemotherapy in Early-stage TNBC [93] | III NCT06627712 | Population: patients with early-stage TNBC Control arm: PD-1 Inhibitor + chemotherapy Experimental arm: above + SBRT (24 Gy in 3 fx) | Not yet recruiting | 1 November 2031 |
| Neoadjuvant Treatment of Triple-Negative Breast Cancer with Stereotactic Radiotherapy, PD-1 Monoclonal Antibody, and Chemotherapy (pembrolizumab) [94] | II NCT06691594 | Population: patients with TNBC Experimental arm (single arm study): neoadjuvant SBRT (10 Gy in 1 fx) + pembrolizumab and chemotherapy | Not yet recruiting | November 2030 |
| Capecitabine Plus Pembrolizumab in Patients With Triple-Negative Breast Cancer After Chemo-immunotherapy and Surgery (CAPPA) (pembrolizumab) [95] | II NCT05973864 | Population: patients with TNBC with residual disease after neoadjuvant chemo-immunotherapy External cohort * receiving standard of care of pembrolizumab as adjuvant therapy Experimental arm *: pembrolizumab + capecitabine as adjuvant therapy. * Local radiotherapy as per standard practice if indicated | Not yet recruiting | August 2028 |
| Neoadjuvant Chemotherapy + PD-1 Inhibitor + Different Radiotherapy Fractionations for HR+/HER2- Breast Cancer [72] | II NCT06639672 | Population: patients with HR-positive/HER2-negative breast cancer Experimental arm 1: chemotherapy + PD-1 inhibitor + RT (24 Gy in 3 fx) Experimental arm 2: chemotherapy + PD-1 inhibitor + RT (16 Gy in 1 fx) Experimental arm 3: chemotherapy + PD-1 inhibitor + RT (41.4 Gy in 15 fx) Experimental arm 4: chemotherapy + PD-1 inhibitor + RT (6–9 Gy in 12–18 fx) | Not yet recruiting | 1 May 2031 |
| Radiotherapy Followed by Chemotherapy Combined With Toripalimab in Local Advanced HR-positive, HER2-negative BC (triplimab) [96] | II NCT06705127 | Population: patients with locally advanced HR-positive/HER2-negative breast cancer Experimental arm (single arm study): neoadjuvant SBRT (24 Gy in 3 fx) followed by chemotherapy + triplimab | Recruiting | 1 July 2028 |
| Investigating the Effectiveness of Stereotactic Body Radiotherapy (SBRT) in Addition to Standard of Care Treatment for Cancer That Has Spread Beyond the Original Site of Disease (PROMISE-005) [97] | II NCT03808337 | Population: patients with breast cancer or NSCLC with 1–5 metastases Control arm: standard of care (may include immunotherapy) Experimental arm: above + SBRT (min dose of 30 Gy in 5 fx) | Recruiting | January 2026 |
| SBRT, Chemotherapy, and AK104 Neoadjuvant Therapy for Triple-negative Breast Cancer (TNBC) (AK104, aka cadonilimab) [98] | II NCT06401005 | Population: patients with TNBC Experimental arm (single arm): SBRT (24 Gy in 3 fx or 18 Gy in 3 fx), then chemotherapy + cadonilimab, then surgery, then adjuvant immunotherapy with or without adjuvant radiotherapy | Recruiting | 1 September 2027 |
| SBRT, Chemotherapy, and AK112 Neoadjuvant Therapy for Luminal-type Breast Cancer (AK112, aka Ivonescimab) [99] | II NCT06402435 | Population: patients with luminal-type breast cancer Experimental arm (single arm): SBRT (24 Gy in 3 fx or 18 Gy in 3 fx), then chemotherapy + cadonilimab, then surgery | Recruiting | 1 September 2027 |
| A Study of Radiation Therapy With Pembrolizumab and Olaparib or Other Radiosensitizers in Women Who Have Triple-Negative or Hormone Receptor-Positive/Her2 Negative Breast Cancer (pembrolizumab) [85] | II NCT04683679 | Population: patients with metastatic TNBC or HR-positive/HER2-negative breast cancer Experimental arm A: pembrolizumab + olaparib + RT (24–27 Gy in 3 fx or 30 Gy in 5 fx for large tumors) Experimental arm B: above without olaparib | Recruiting | January 2026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panse, D.; Hsieh, K.; Arons, D.; Runnels, J.; Wassel, M.; Shah, A.; Patel, R.; Tiersten, A.; Nehlsen, A.D.; Marshall, D.; et al. The Role of Immunotherapy and Radiation Therapy in the Treatment of Breast Cancer. Biomedicines 2025, 13, 2209. https://doi.org/10.3390/biomedicines13092209
Panse D, Hsieh K, Arons D, Runnels J, Wassel M, Shah A, Patel R, Tiersten A, Nehlsen AD, Marshall D, et al. The Role of Immunotherapy and Radiation Therapy in the Treatment of Breast Cancer. Biomedicines. 2025; 13(9):2209. https://doi.org/10.3390/biomedicines13092209
Chicago/Turabian StylePanse, Drishti, Kristin Hsieh, Danielle Arons, Juliana Runnels, Monica Wassel, Anuja Shah, Rima Patel, Amy Tiersten, Anthony D. Nehlsen, Deborah Marshall, and et al. 2025. "The Role of Immunotherapy and Radiation Therapy in the Treatment of Breast Cancer" Biomedicines 13, no. 9: 2209. https://doi.org/10.3390/biomedicines13092209
APA StylePanse, D., Hsieh, K., Arons, D., Runnels, J., Wassel, M., Shah, A., Patel, R., Tiersten, A., Nehlsen, A. D., Marshall, D., Samstein, R. M., Green, S., & Bloom, J. (2025). The Role of Immunotherapy and Radiation Therapy in the Treatment of Breast Cancer. Biomedicines, 13(9), 2209. https://doi.org/10.3390/biomedicines13092209






