Clinical Significance of Tumor Infiltrating T-Helper and Regulatory Cells in Bulgarian Cervical Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Pathological Examination
2.3. Deconvoluted Transcriptomic Data
2.4. Statistical Analysis
3. Results
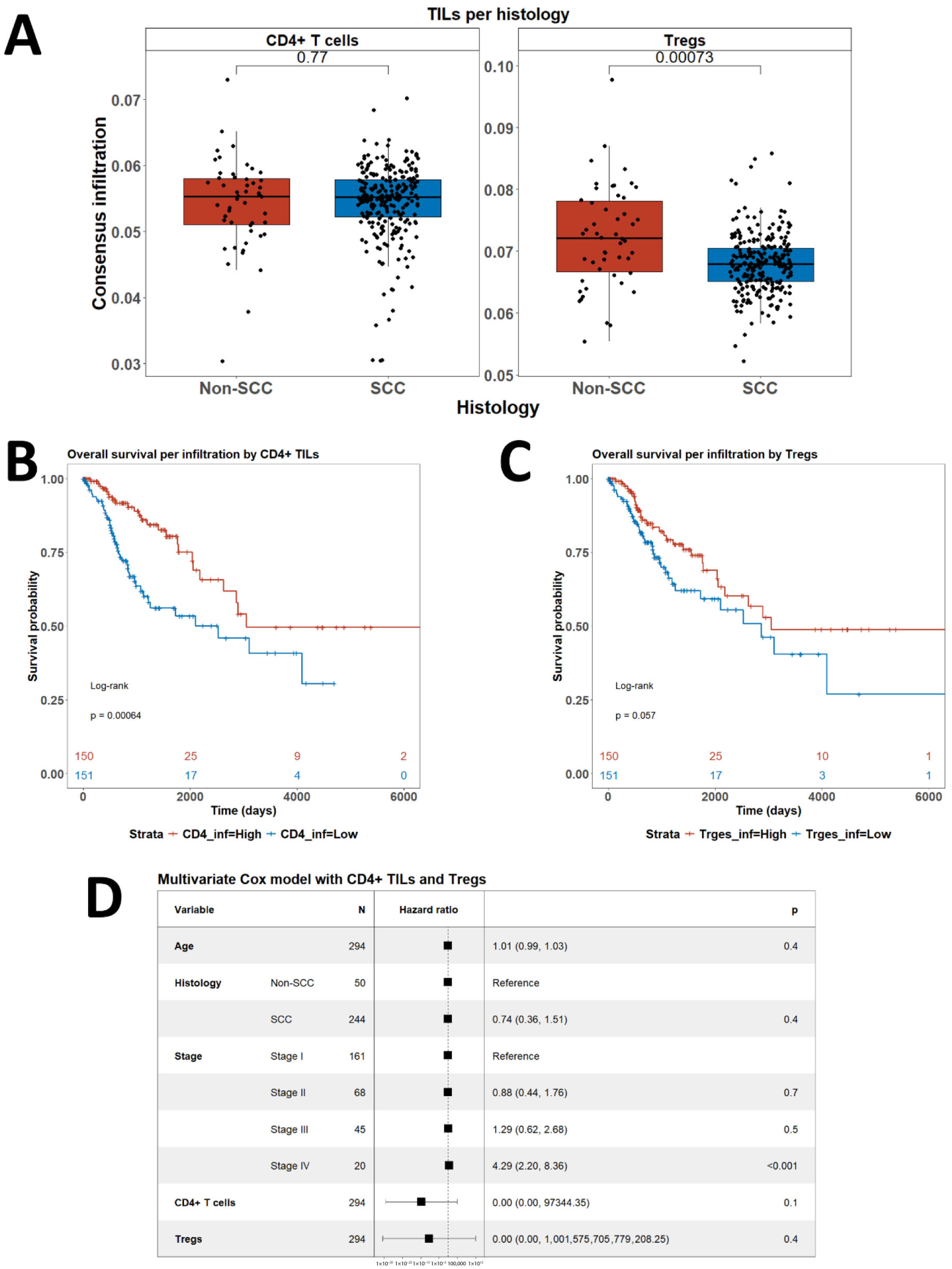
3.1. Consensus Deconvolution of Transcriptomic Data to Demonstrate the Role of CD4+ TILs and Tregs in CC
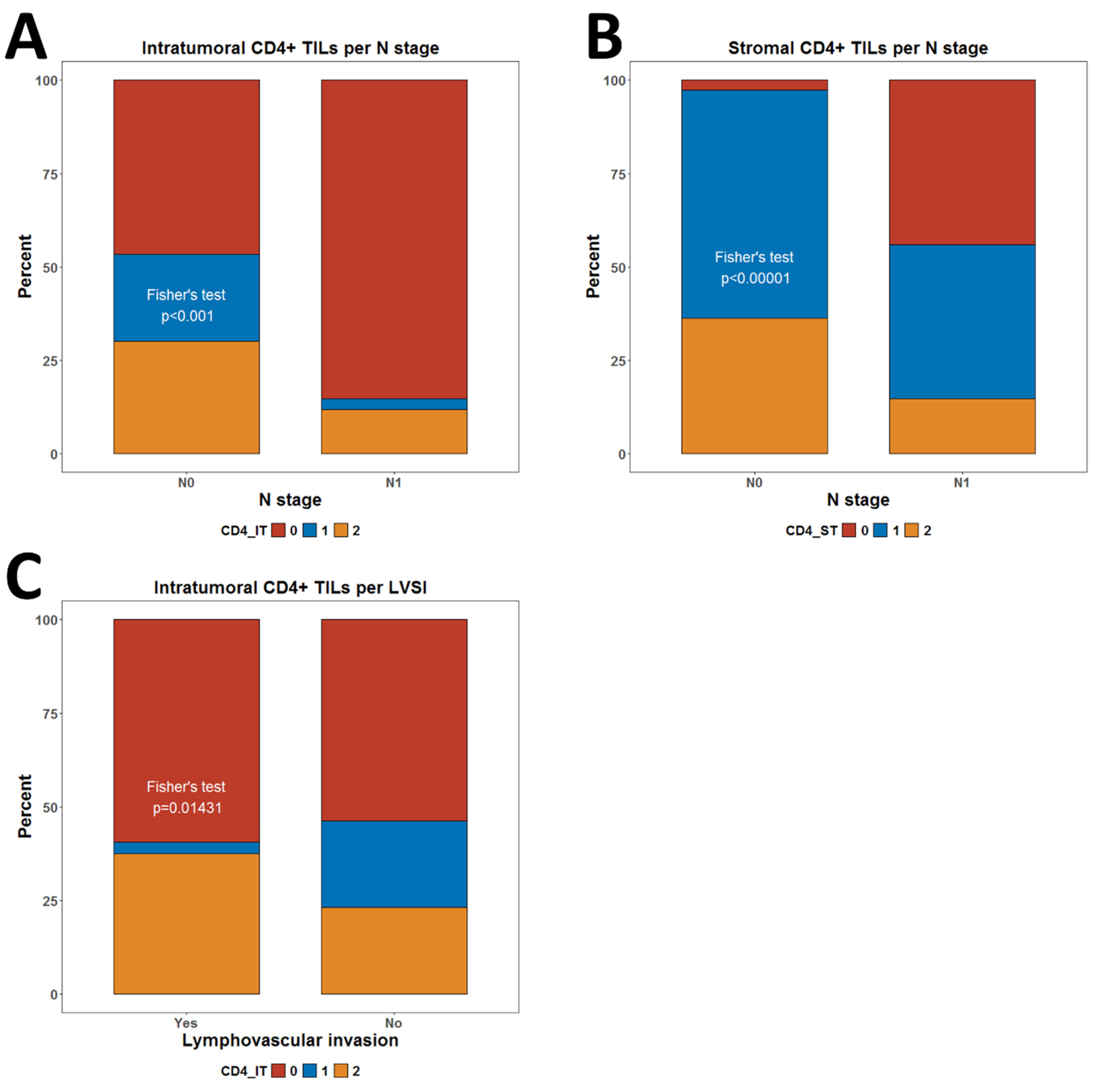
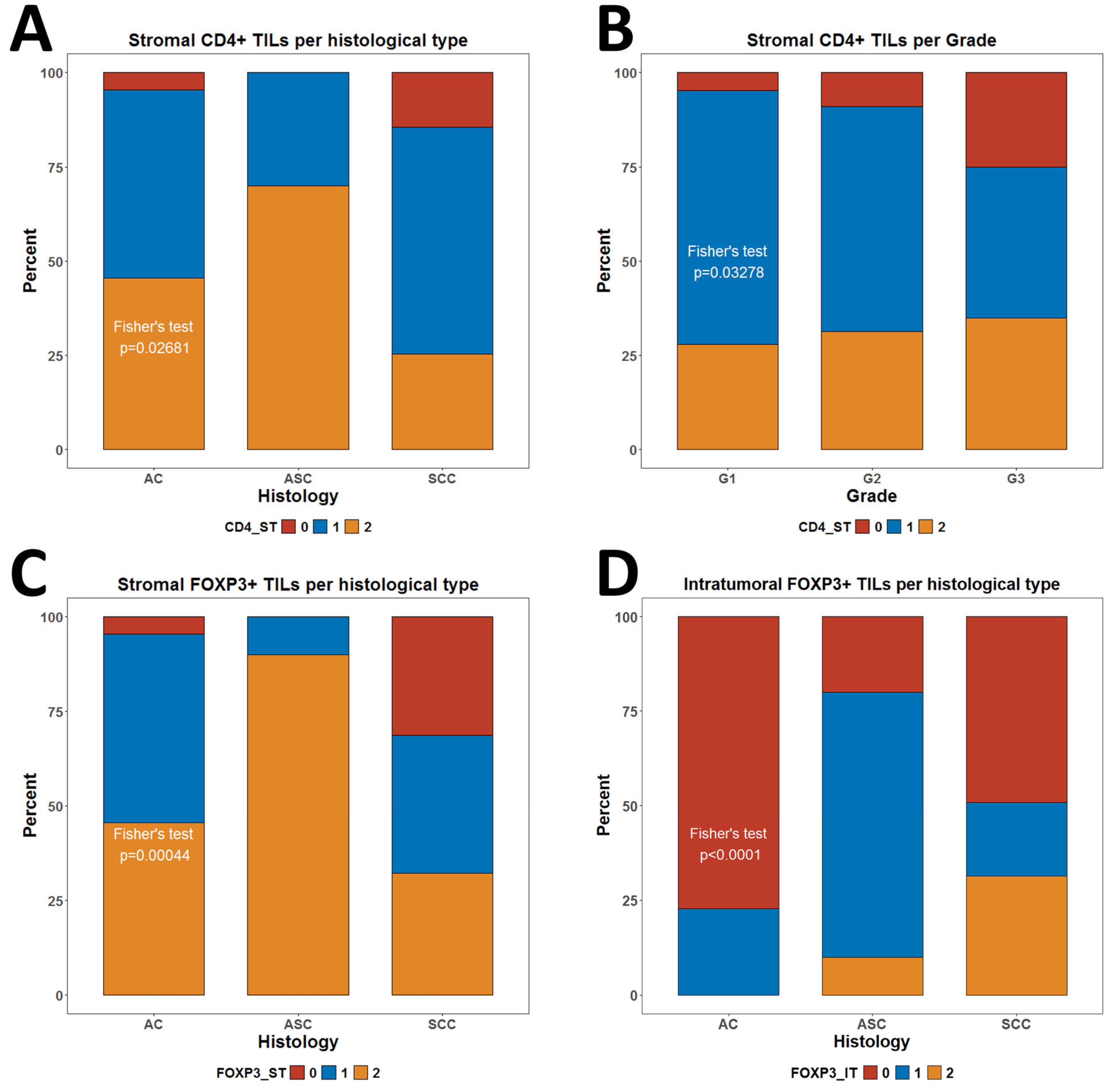
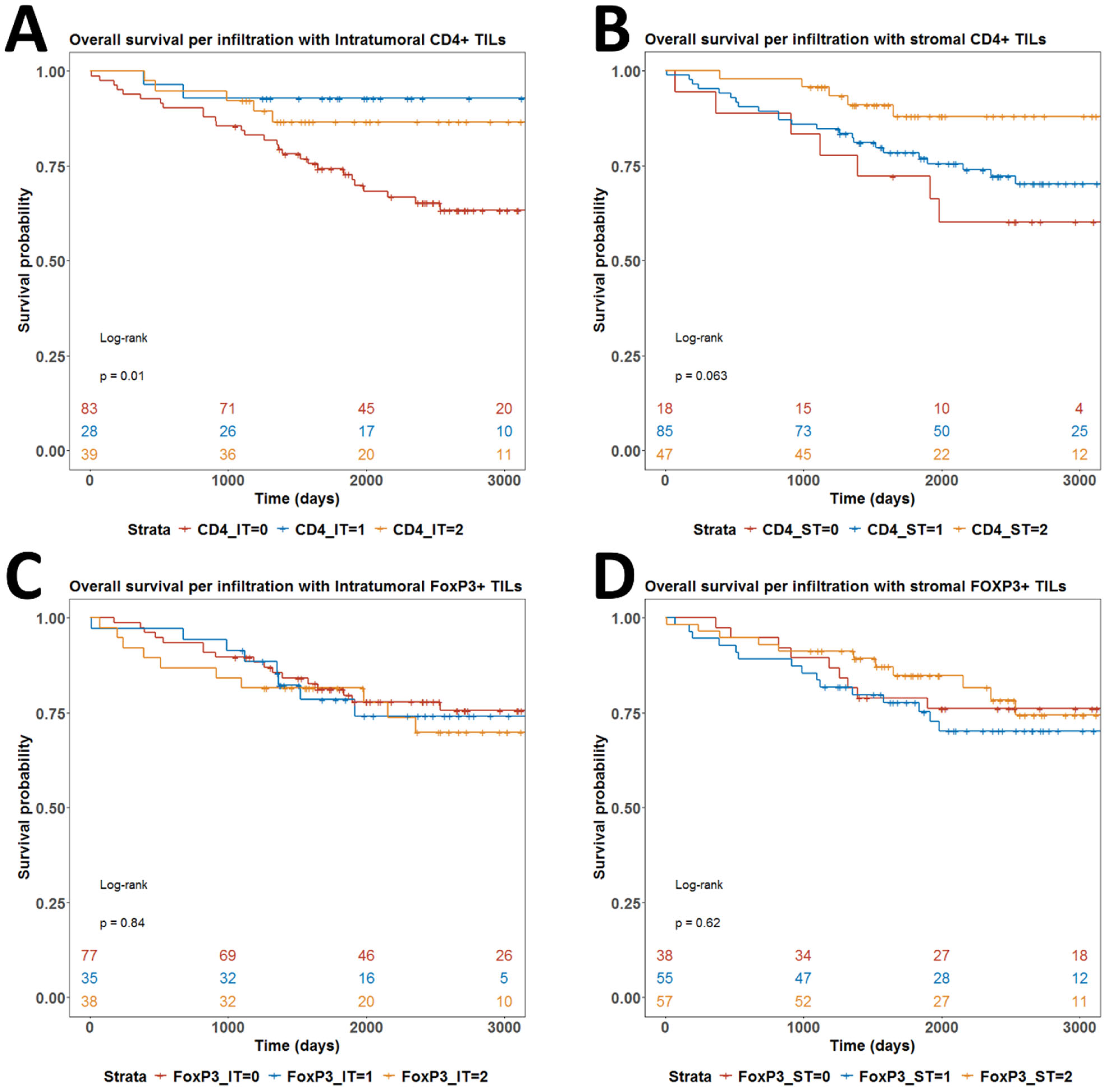
3.2. Resolving Spatial Infiltration by CD4+ and FOXP3+ TILs by IHC
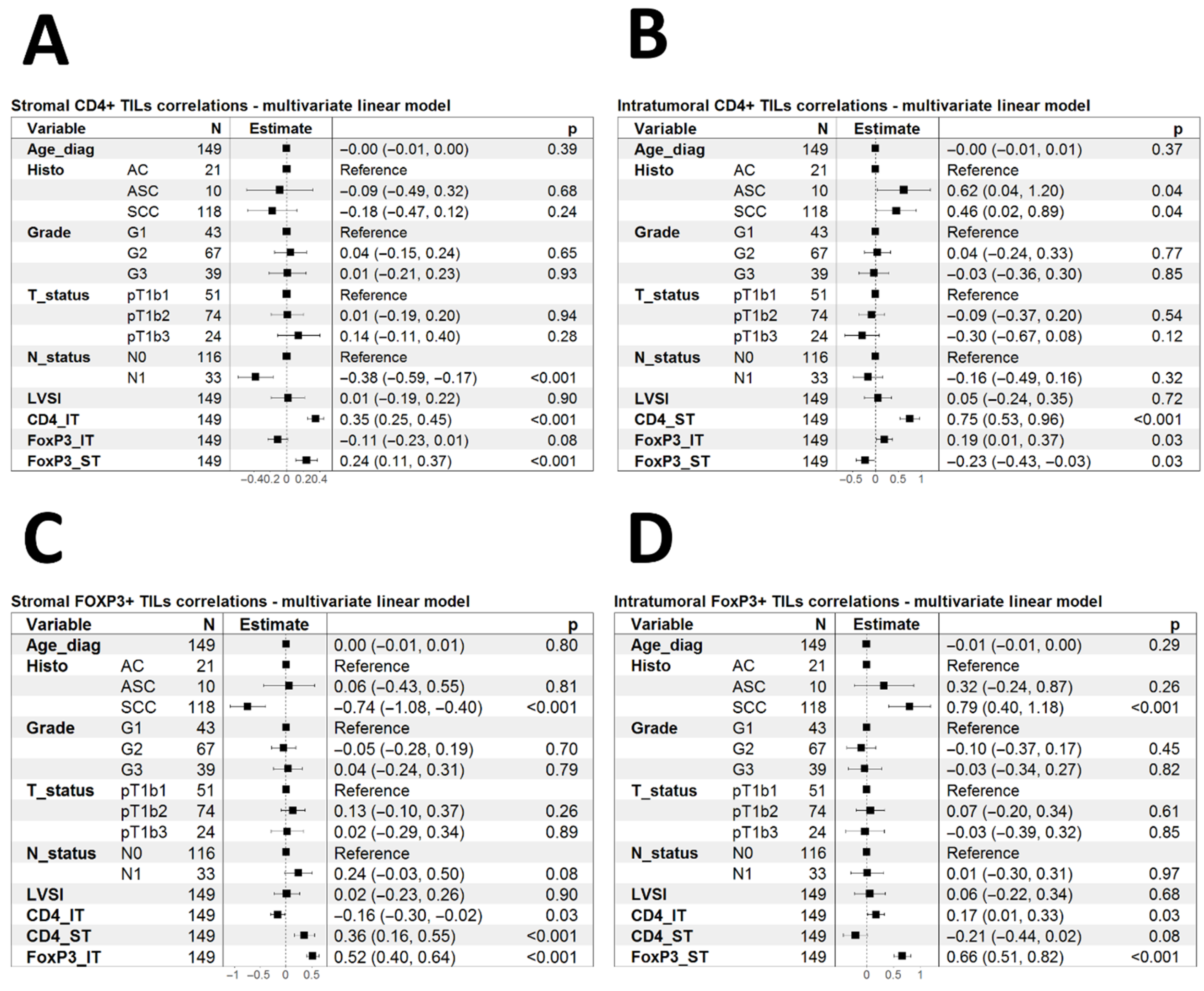
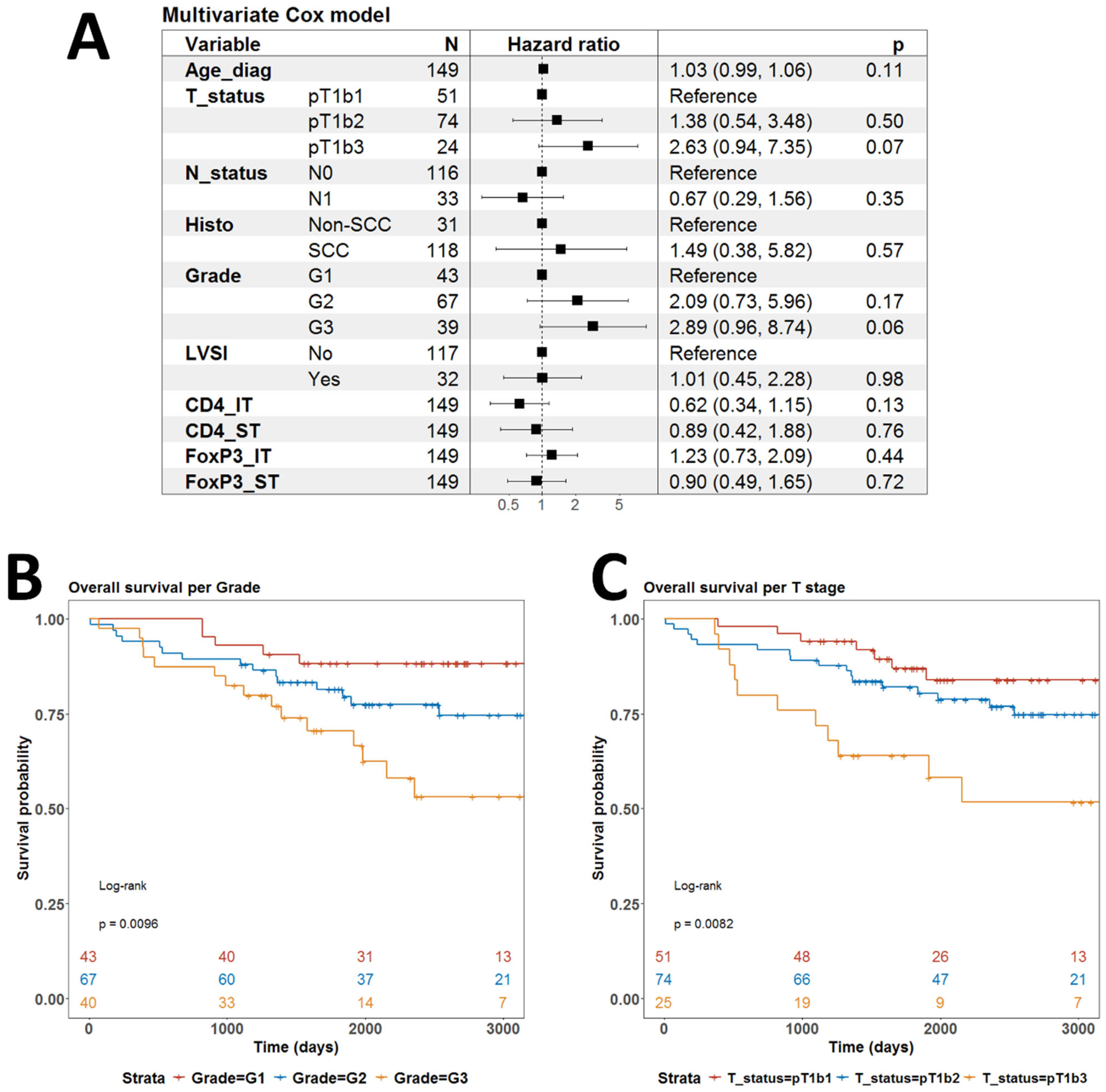
3.3. Association of CD4+ and FOXP3+ TILs with Clinical Characteristics
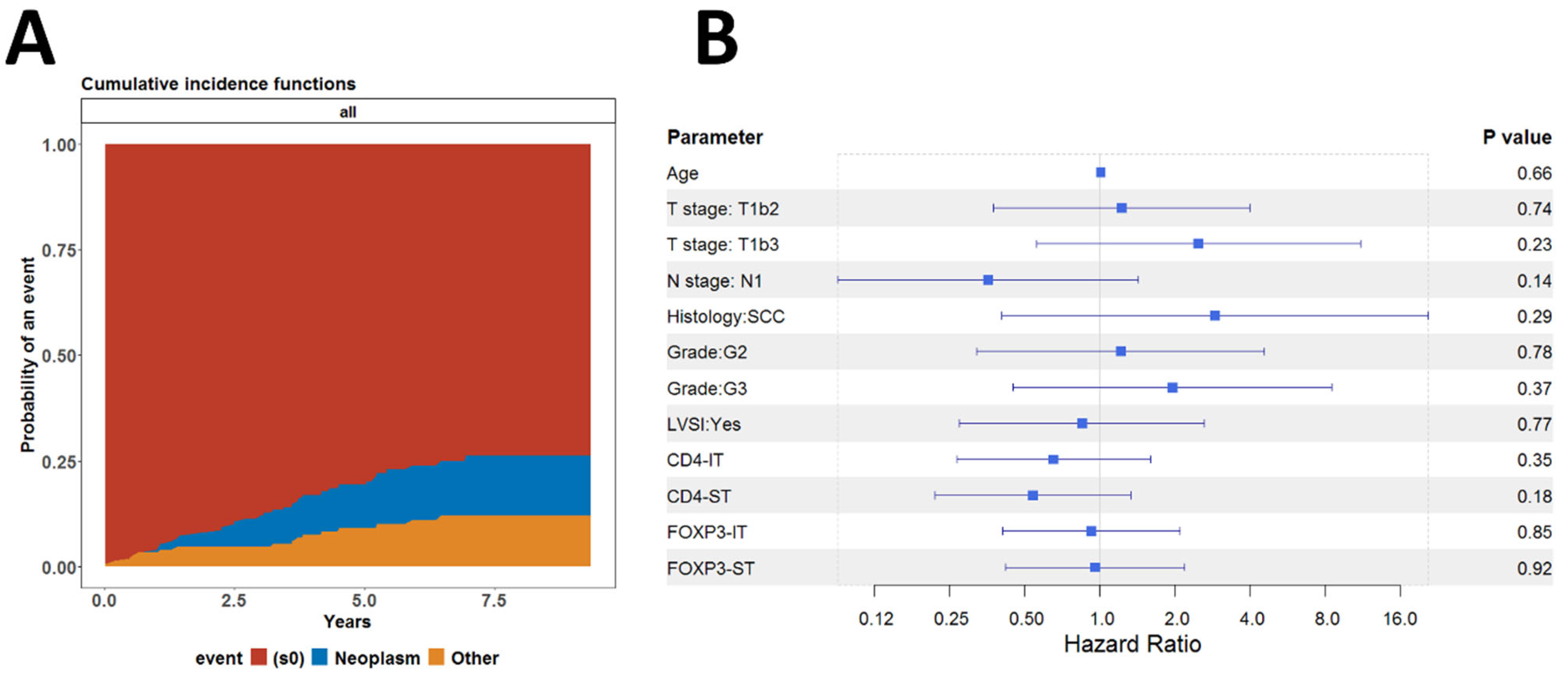
3.4. Associations Between CD4+ TILs and Tregs and Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sherer, M.V.; Kotha, N.V.; Williamson, C.; Mayadev, J. Advances in immunotherapy for cervical cancer: Recent developments and future directions. Int. J. Gynecol. Cancer 2022, 32, 281–287. [Google Scholar] [CrossRef]
- Grau-Bejar, J.F.; Garcia-Duran, C.; Garcia-Illescas, D.; Mirallas, O.; Oaknin, A. Advances in immunotherapy for cervical cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231163836. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Georges, D.; Man, I.; Baussano, I.; Clifford, G.M. Causal attribution of human papillomavirus genotypes to invasive cervical cancer worldwide: A systematic analysis of the global literature. Lancet 2024, 404, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar]
- Shamseddine, A.A.; Burman, B.; Lee, N.Y.; Zamarin, D.; Riaz, N. Tumor immunity and immunotherapy for HPV-related cancers. Cancer Discov. 2021, 11, 1896–1912. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, B.S.; Lee, S.-K. Regulatory T cells in tumor microenvironment and approach for anticancer immunotherapy. Immune Netw. 2020, 20, e4. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wang, X.; Chen, L.; Liu, W.; Zhang, Y.; Liu, D.; Zhou, C.; Shi, S.; Dong, J.; Lai, Z.; Zhao, B. TIMEDB: Tumor immune micro-environment cell composition database with automatic analysis and interactive visualization. Nucleic Acids Res. 2023, 51, D1417–D1424. [Google Scholar]
- Network, C.G.A.R. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Alkhazraji, A.; Elgamal, M.; Ang, S.H.; Shivarov, V. All cancer hallmarks lead to diversity. Int. J. Clin. Exp. Med. 2019, 12, 132–157. [Google Scholar]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Christodoulou, M.-I.; Zaravinos, A. Single-cell analysis in immuno-oncology. Int. J. Mol. Sci. 2023, 24, 8422. [Google Scholar] [CrossRef]
- Widodo, S.S.; Hutchinson, R.A.; Fang, Y.; Mangiola, S.; Neeson, P.J.; Darcy, P.K.; Barrow, A.D.; Hovens, C.M.; Dinevska, M.; Stylli, S.S. Toward precision immunotherapy using multiplex immunohistochemistry and in silico methods to define the tumor immune microenvironment. Cancer Immunol. Immunother. 2021, 70, 1811–1820. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.-H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A. The immune landscape of cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Zhang, G.; Cao, K.; Yang, M.; Liu, H. The prognostic significance of tumor-infiltrating lymphocytes in cervical cancer. J. Gynecol. Oncol. 2021, 32, e32. [Google Scholar] [CrossRef]
- Luca, B.A.; Steen, C.B.; Matusiak, M.; Azizi, A.; Varma, S.; Zhu, C.; Przybyl, J.; Espín-Pérez, A.; Diehn, M.; Alizadeh, A.A. Atlas of clinically distinct cell states and ecosystems across human solid tumors. Cell 2021, 184, 5482–5496.e28. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Yao, Y.; Jie, W.; Zhang, M.; Hu, X.; Zhao, Y.; Wang, S.; Yin, J.; Song, Y. Up-regulation of Foxp3 participates in progression of cervical cancer. Cancer Immunol. Immunother. 2013, 62, 481–487. [Google Scholar] [PubMed]
- Ssedyabane, F.; Niyonzima, N.; Ngonzi, J.; Najjuma, J.N.; Mudondo, H.; Okeny, C.; Nuwashaba, D.; Tusubira, D. FOXP3 serum concentration; a likely predictor of CIN and cervical cancer: Secondary analysis from a case control study at a clinic in South western Uganda. Gynecol. Oncol. Rep. 2024, 55, 101466. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yang, Z.; Wang, Z.; Li, Z.; Li, H.; Yin, J.; Deng, M.; Zhu, W.; Zeng, C. Foxp3 is correlated with VEGF-C expression and lymphangiogenesis in cervical cancer. World J. Surg. Oncol. 2017, 15, 173. [Google Scholar] [CrossRef]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 2009, 4, e6412. [Google Scholar]
- Frey, D.M.; Droeser, R.A.; Viehl, C.T.; Zlobec, I.; Lugli, A.; Zingg, U.; Oertli, D.; Kettelhack, C.; Terracciano, L.; Tornillo, L. High frequency of tumor-infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int. J. Cancer 2010, 126, 2635–2643. [Google Scholar]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Pénault-Llorca, F. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar]
- Yordanov, A.; Damyanova, P.; Vasileva-Slaveva, M.; Hasan, I.; Kostov, S.; Shivarov, V. Integrated Analysis of Phagocytic and Immunomodulatory Markers in Cervical Cancer Reveals Constellations of Potential Prognostic Relevance. Int. J. Mol. Sci. 2024, 25, 9117. [Google Scholar] [CrossRef]
- Yordanov, A.; Shivarov, V.; Kostov, S.; Ivanova, Y.; Dimitrova, P.; Popovska, S.; Tsoneva, E.; Vasileva-Slaveva, M. Prognostic utility of CD47 in cancer of the uterine cervix and the sensitivity of immunohistochemical scores. Diagnostics 2022, 13, 52. [Google Scholar] [CrossRef]
- Dimitrova, P.; Vasileva-Slaveva, M.; Shivarov, V.; Hasan, I.; Yordanov, A. Infiltration by Intratumor and Stromal CD8 and CD68 in Cervical Cancer. Medicina 2023, 59, 728. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.C.; Hsu, S.M.; Ho, H.N.; Lin, R.H.; Torng, P.L.; Huang, S.C. Reversed CD4/CD8 ratios of tumor-infiltrating lymphocytes are correlated with the progression of human cervical carcinoma. Cancer 1999, 86, 1537–1543. [Google Scholar] [PubMed]
- Piersma, S.J.; Jordanova, E.S.; Van Poelgeest, M.I.; Kwappenberg, K.M.; Van Der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.; Kenter, G.G.; Fleuren, G.J.; Offringa, R. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Jordanova, E.S.; Gorter, A.; Ayachi, O.; Prins, F.; Durrant, L.G.; Kenter, G.G.; Van Der Burg, S.H.; Fleuren, G.J. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: Which variable determines survival of cervical cancer patients? Clin. Cancer Res. 2008, 14, 2028–2035. [Google Scholar]
- Loddenkemper, C.; Hoffmann, C.; Stanke, J.; Nagorsen, D.; Baron, U.; Olek, S.; Huehn, J.; Ritz, J.P.; Stein, H.; Kaufmann, A.M. Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer. Cancer Sci. 2009, 100, 1112–1117. [Google Scholar]
- Vattai, A.; Kremer, N.; Meister, S.; Beyer, S.; Keilmann, L.; Hester, A.; Temelkov, M.; Heidegger, H.; Schmoeckel, E.; Kessler, M. Role of FoxP3-positive regulatory T-cells in regressive and progressive cervical dysplasia. J. Cancer Res. Clin. Oncol. 2022, 148, 377–386. [Google Scholar]
- Visser, J.; Nijman, H.; Hoogenboom, B.; Jager, P.; Van Baarle, D.; Schuuring, E.; Abdulahad, W.; Miedema, F.; Van Der Zee, A.; Daemen, T. Frequencies and role of regulatory T cells in patients with (pre) malignant cervical neoplasia. Clin. Exp. Immunol. 2007, 150, 199–209. [Google Scholar] [CrossRef]
- Mitiļdžans, A.; Zablocka, T.; Isajevs, S.; Gordjušina, V.; Rezeberga, D. Upregulation of FOXP3+ Regulatory T Lymphocytes and CD8+ Lymphocytes in Patients with High-Grade Squamous Intraepithelial Lesions Correlated with HPV Infection. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2022, 76, 218–224. [Google Scholar]
- Adurthi, S.; Krishna, S.; Mukherjee, G.; Bafna, U.D.; Devi, U.; Jayshree, R.S. Regulatory T cells in a spectrum of HPV-induced cervical lesions: Cervicitis, cervical intraepithelial neoplasia and squamous cell carcinoma. Am. J. Reprod. Immunol. 2008, 60, 55–65. [Google Scholar]
- Bedoya, A.M.; Jaramillo, R.; Baena, A.; Castaño, J.; Olaya, N.; Zea, A.H.; Herrero, R.; Sanchez, G.I. Location and density of immune cells in precursor lesions and cervical cancer. Cancer Microenviron. 2013, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shima, T.; Saeki, A.; Hidaka, T.; Nakashima, A.; Takikawa, O.; Saito, S. Expression of indoleamine 2,3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007, 98, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Li, Z.; Ma, D.; Zhang, W.; Zhang, Y.; Zhang, T.; Kong, B.; Cui, B. Distribution of Th17 cells and Foxp3-expressing T cells in tumor-infiltrating lymphocytes in patients with uterine cervical cancer. Clin. Chim. Acta 2012, 413, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Piersma, S.J. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Microenviron. 2011, 4, 361–375. [Google Scholar] [CrossRef]
- Shah, W.; Yan, X.; Jing, L.; Zhou, Y.; Chen, H.; Wang, Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4+FOXP3+ regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell. Mol. Immunol. 2011, 8, 59–66. [Google Scholar] [CrossRef]
- Punt, S.; van Vliet, M.E.; Spaans, V.M.; de Kroon, C.D.; Fleuren, G.J.; Gorter, A.; Jordanova, E.S. FoxP3+ and IL-17+ cells are correlated with improved prognosis in cervical adenocarcinoma. Cancer Immunol. Immunother. 2015, 64, 745–753. [Google Scholar]
- Ohno, A.; Iwata, T.; Katoh, Y.; Taniguchi, S.; Tanaka, K.; Nishio, H.; Nakamura, M.; Morisada, T.; Chen, G.; Saito, M. Tumor-infiltrating lymphocytes predict survival outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy. Gynecol. Oncol. 2020, 159, 329–334. [Google Scholar] [CrossRef]
- Gultekin, M.; Beduk Esen, C.S.; Ates Ozdemir, D.; Yildirim, S.; Yuce, D.; Usubutun, A.; Yildiz, F. Stromal or intraepithelial tumor-infiltrating lymphocytes: Which one has more prognostic significance in cervical cancer? Arch. Gynecol. Obstet. 2023, 307, 969–980. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Yan, X.; Ni, Y.; Gong, Y.; Wang, C.; Zhang, X.; Wan, L.; Yang, H.; Ge, C. Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci. Adv. 2023, 9, eadd8977. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Ao, J.; Li, Y.; Shen, J.; He, X.; Tang, D.; Chu, C.; Liu, C.; Weng, L. Single-cell profiling reveals the intratumor heterogeneity and immunosuppressive microenvironment in cervical adenocarcinoma. bioRxiv 2024. [Google Scholar] [CrossRef]
- Qiu, J.; Qu, X.; Wang, Y.; Guo, C.; Lv, B.; Jiang, Q.; Su, W.; Wang, L.; Hua, K. Single-cell landscape highlights heterogenous microenvironment, novel immune reaction patterns, potential biomarkers and unique therapeutic strategies of cervical squamous carcinoma, Human Papillomavirus-Associated (HPVA) and Non-HPVA Adenocarcinoma. Adv. Sci. 2023, 10, 2204951. [Google Scholar]
- Li, C.; Liu, D.; Zhao, Y.; Ding, Y.; Hua, K. Diverse intratumoral heterogeneity and immune microenvironment of two HPV-related cervical cancer types revealed by single-cell RNA sequencing. J. Med. Virol. 2023, 95, e28857. [Google Scholar] [PubMed]
- Li, X.; Zhang, M.; Lei, T.; Zou, W.; Huang, R.; Wang, F.; Huang, Q.; Wang, C.; Liu, C. Single-cell RNA-sequencing dissects cellular heterogeneity and identifies two tumor-suppressing immune cell subclusters in HPV-related cervical adenosquamous carcinoma. J. Med. Virol. 2022, 94, 6047–6059. [Google Scholar] [PubMed]
- Li, C.; Wu, H.; Guo, L.; Liu, D.; Yang, S.; Li, S.; Hua, K. Single-cell transcriptomics reveals cellular heterogeneity and molecular stratification of cervical cancer. Commun. Biol. 2022, 5, 1208. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, D.; Yang, S.; Hua, K. Integrated single-cell transcriptome analysis of the tumor ecosystems underlying cervical cancer metastasis. Front. Immunol. 2022, 13, 966291. [Google Scholar] [CrossRef]
- Li, C.; Hua, K. Dissecting the single-cell transcriptome network of immune environment underlying cervical premalignant lesion, cervical cancer and metastatic lymph nodes. Front. Immunol. 2022, 13, 897366. [Google Scholar] [CrossRef]
- Cao, G.; Yue, J.; Ruan, Y.; Han, Y.; Zhi, Y.; Lu, J.; Liu, M.; Xu, X.; Wang, J.; Gu, Q. Single-cell dissection of cervical cancer reveals key subsets of the tumor immune microenvironment. EMBO J. 2023, 42, e110757. [Google Scholar] [CrossRef]
- Lin, S.; Sun, Y.; Cao, C.; Zhu, Z.; Xu, Y.; Liu, B.; Hu, B.; Peng, T.; Zhi, W.; Xu, M. Single-nucleus RNA sequencing reveals heterogenous microenvironments and specific drug response between cervical squamous cell carcinoma and adenocarcinoma. EBioMedicine 2023, 97, 104846. [Google Scholar] [CrossRef]
- Sheng, B.; Pan, S.; Ye, M.; Liu, H.; Zhang, J.; Zhao, B.; Ji, H.; Zhu, X. Single-cell RNA sequencing of cervical exfoliated cells reveals potential biomarkers and cellular pathogenesis in cervical carcinogenesis. Cell Death Dis. 2024, 15, 130. [Google Scholar] [CrossRef]
- Ou, Z.; Lin, S.; Qiu, J.; Ding, W.; Ren, P.; Chen, D.; Wang, J.; Tong, Y.; Wu, D.; Chen, A. Single-nucleus RNA sequencing and spatial transcriptomics reveal the immunological microenvironment of cervical squamous cell carcinoma. Adv. Sci. 2022, 9, 2203040. [Google Scholar]
- Van der Burg, S.H.; Piersma, S.J.; De Jong, A.; Van Der Hulst, J.M.; Kwappenberg, K.M.; Van Den Hende, M.; Welters, M.J.; Van Rood, J.J.; Fleuren, G.J.; Melief, C.J. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc. Natl. Acad. Sci. USA 2007, 104, 12087–12092. [Google Scholar] [CrossRef]
- de Vos van Steenwijk, P.J.; Heusinkveld, M.; Ramwadhdoebe, T.H.; Löwik, M.J.; Van Der Hulst, J.M.; Goedemans, R.; Piersma, S.J.; Kenter, G.G.; Van Der Burg, S.H. An unexpectedly large polyclonal repertoire of HPV-specific T cells is poised for action in patients with cervical cancer. Cancer Res. 2010, 70, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ye, S.; Goswami, S.; Li, T.; Wu, J.; Cao, C.; Ma, J.; Lu, B.; Pei, X.; Chen, Y. Highly immunosuppressive HLADRhi regulatory T cells are associated with unfavorable outcomes in cervical squamous cell carcinoma. Int. J. Cancer 2020, 146, 1993–2006. [Google Scholar]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Oaknin, A.; Moore, K.; Meyer, T.; González, J.L.-P.; Devriese, L.A.; Amin, A.; Lao, C.D.; Boni, V.; Sharfman, W.H.; Park, J.C. Nivolumab with or without ipilimumab in patients with recurrent or metastatic cervical cancer (CheckMate 358): A phase 1–2, open-label, multicohort trial. Lancet Oncol. 2024, 25, 588–602. [Google Scholar]
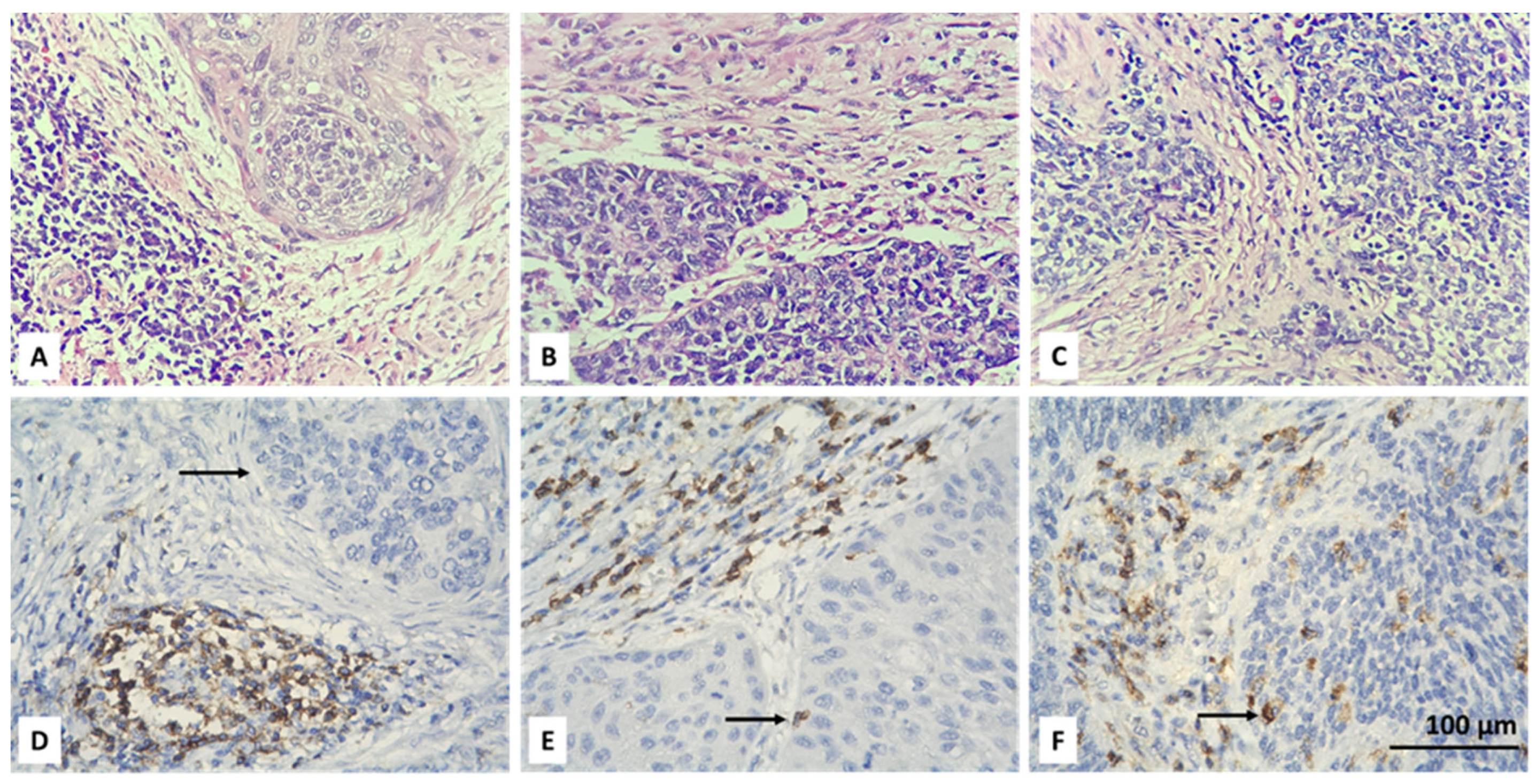
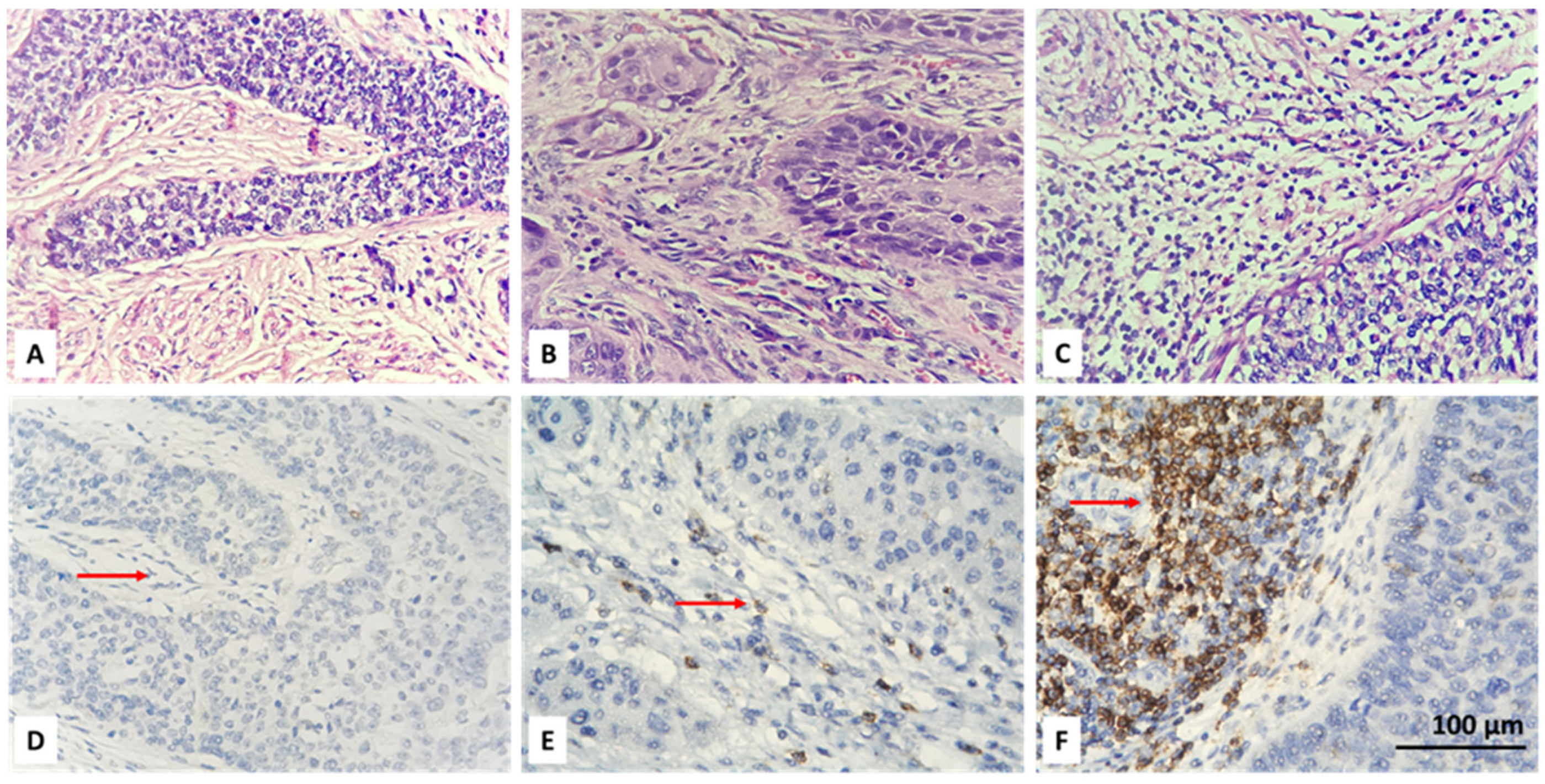
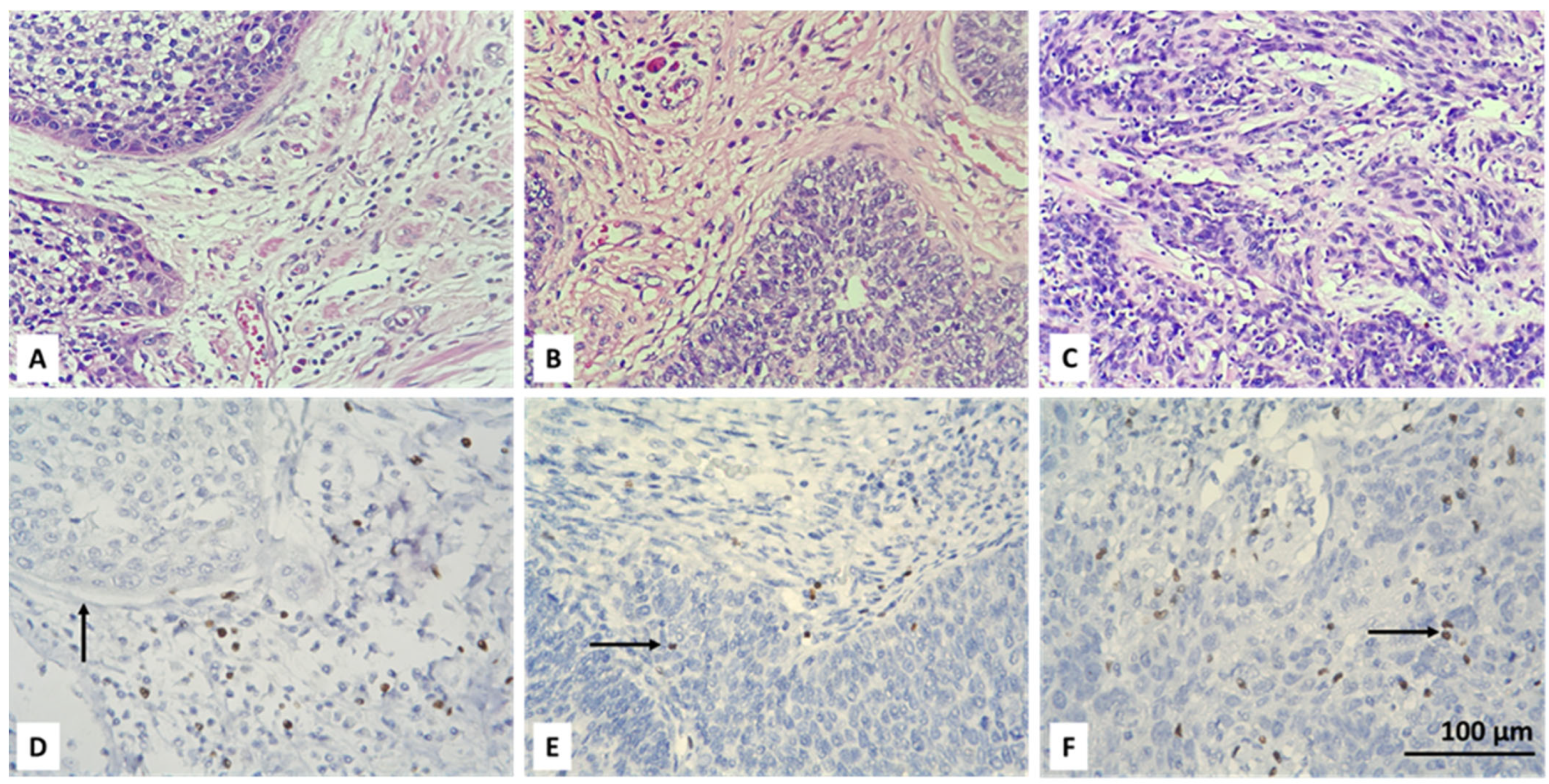
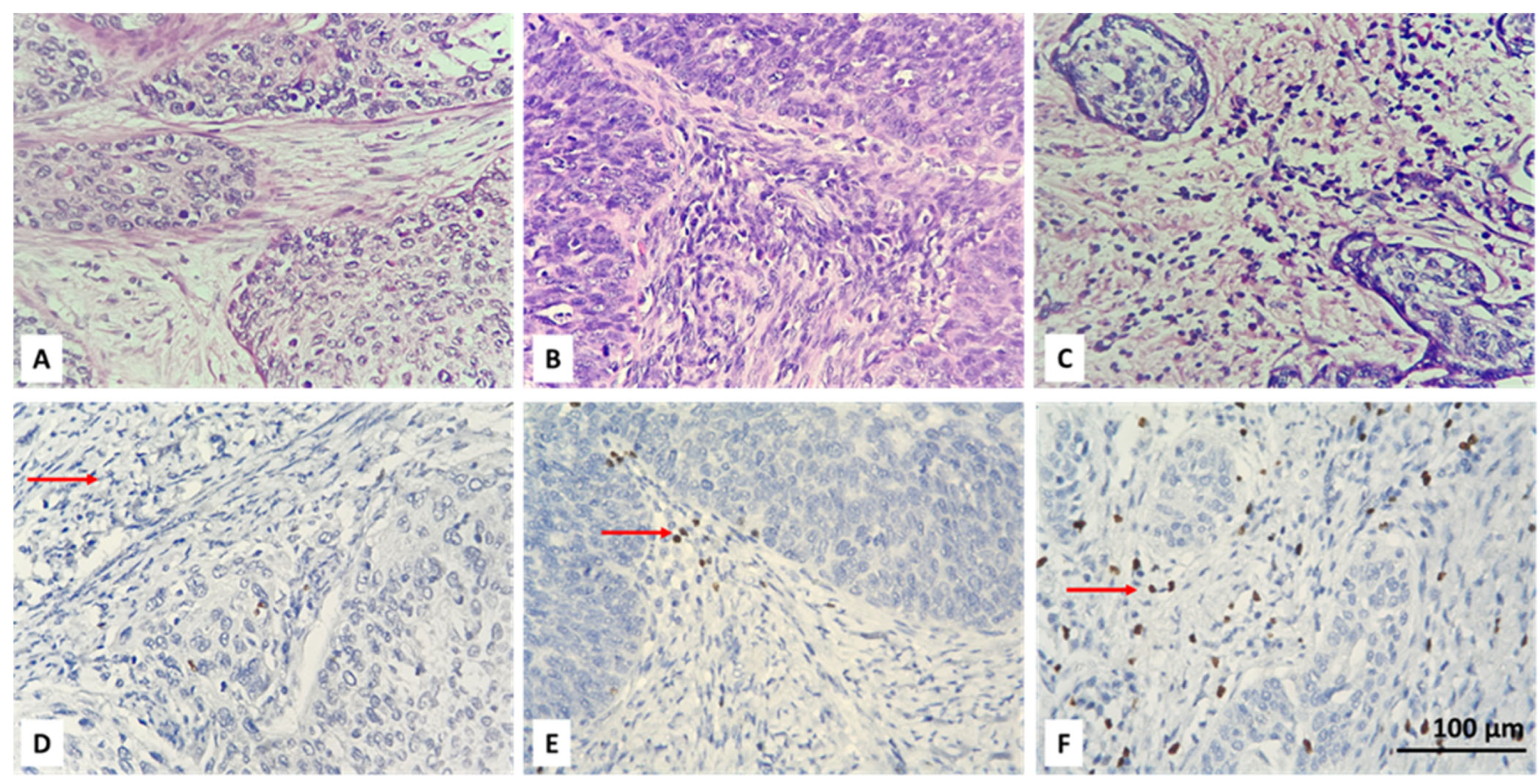
| Parameter | Count | Percent |
|---|---|---|
| Age | ||
| ≤50 years | 78 | 52 |
| >50 years | 72 | 48 |
| T stage | ||
| T1b1 | 51 | 34 |
| T1b2 | 74 | 49.33 |
| T1b3 | 25 | 16.67 |
| N stage | ||
| N0 | 116 | 77.33 |
| N1 | 34 | 22.67 |
| FIGO stage | ||
| FIGO I | 116 | 77.33 |
| FIGO III | 34 | 22.67 |
| Histology | ||
| AC | 22 | 14.67 |
| ASC | 10 | 6.66 |
| SCC | 118 | 78.67 |
| Grade | ||
| G1 | 45 | 30 |
| G2 | 65 | 43.33 |
| G3 | 40 | 26.67 |
| LVSI | ||
| Yes | 32 | 21.33 |
| No | 117 | 78 |
| Unknown | 1 | 0.67 |
| Total | 150 | 100 |
| CD4IT | CD4ST | FOXP3IT | FOXP3ST | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | Total | p-Value | 0 | 1 | 2 | Total | p-Value | 0 | 1 | 2 | Total | p-Value | 0 | 1 | 2 | Total | p-Value | |
| Age | ||||||||||||||||||||
| ≤50 years | 43 | 13 | 22 | 78 | 0.7167 | 7 | 43 | 28 | 78 | 0.324 | 33 | 24 | 21 | 78 | 0.03796 | 16 | 28 | 34 | 78 | 0.2433 |
| >50 years | 40 | 15 | 17 | 72 | 11 | 42 | 19 | 72 | 44 | 11 | 17 | 72 | 22 | 27 | 23 | 72 | ||||
| Total | 83 | 28 | 39 | 150 | 18 | 85 | 47 | 150 | 77 | 35 | 38 | 150 | 38 | 55 | 57 | 150 | ||||
| T stage | ||||||||||||||||||||
| T1b1 | 25 | 13 | 13 | 51 | 0.4952 | 4 | 28 | 19 | 51 | 0.6499 | 31 | 13 | 7 | 51 | 0.08425 | 13 | 20 | 18 | 51 | 0.6768 |
| T1b2 | 41 | 12 | 21 | 74 | 11 | 41 | 22 | 74 | 31 | 18 | 25 | 74 | 16 | 27 | 31 | 74 | ||||
| T1b3 | 17 | 3 | 5 | 25 | 3 | 16 | 6 | 25 | 15 | 4 | 6 | 25 | 9 | 8 | 8 | 25 | ||||
| Total | 83 | 28 | 39 | 150 | 18 | 85 | 47 | 150 | 77 | 35 | 38 | 150 | 38 | 55 | 57 | 150 | ||||
| N stage | ||||||||||||||||||||
| N0 | 54 | 27 | 35 | 116 | 0.00014 | 3 | 71 | 42 | 116 | 8.25 × 10−9 | 63 | 26 | 27 | 116 | 0.3464 | 32 | 38 | 46 | 116 | 0.1892 |
| N1 | 29 | 1 | 4 | 34 | 15 | 14 | 5 | 34 | 14 | 9 | 11 | 34 | 6 | 17 | 11 | 34 | ||||
| Total | 83 | 28 | 39 | 150 | 18 | 85 | 47 | 150 | 77 | 35 | 38 | 150 | 38 | 55 | 57 | 150 | ||||
| FIGO stage | ||||||||||||||||||||
| FIGO I | 54 | 27 | 35 | 116 | 0.00014 | 3 | 71 | 42 | 116 | 8.25 × 10−9 | 63 | 26 | 27 | 116 | 0.3464 | 32 | 38 | 46 | 116 | 0.1892 |
| FIGO III | 29 | 1 | 4 | 34 | 15 | 14 | 5 | 34 | 14 | 9 | 11 | 34 | 6 | 17 | 11 | 34 | ||||
| Total | 83 | 28 | 39 | 150 | 18 | 85 | 47 | 150 | 77 | 35 | 38 | 150 | 38 | 55 | 57 | 150 | ||||
| Histology | ||||||||||||||||||||
| AC | 16 | 2 | 4 | 22 | 0.1775 | 1 | 11 | 10 | 22 | 0.02681 | 17 | 5 | 0 | 22 | 6.12 × 10−5 | 1 | 11 | 10 | 22 | 0.000441 |
| ASC | 3 | 2 | 5 | 10 | 0 | 3 | 7 | 10 | 2 | 7 | 1 | 10 | 0 | 1 | 9 | 10 | ||||
| SCC | 64 | 24 | 30 | 118 | 17 | 71 | 30 | 118 | 58 | 23 | 37 | 118 | 37 | 43 | 38 | 118 | ||||
| Total | 83 | 28 | 39 | 150 | 18 | 85 | 47 | 150 | 77 | 35 | 38 | 150 | 38 | 55 | 57 | 150 | ||||
| Grade | ||||||||||||||||||||
| G1 | 22 | 10 | 11 | 43 | 0.6464 | 2 | 29 | 12 | 43 | 0.03278 | 21 | 10 | 12 | 43 | 0.9259 | 9 | 19 | 15 | 43 | 0.7016 |
| G2 | 35 | 13 | 19 | 67 | 6 | 40 | 21 | 67 | 37 | 15 | 15 | 67 | 20 | 22 | 25 | 67 | ||||
| G3 | 26 | 5 | 9 | 40 | 10 | 16 | 14 | 40 | 19 | 10 | 11 | 40 | 9 | 14 | 17 | 40 | ||||
| Total | 83 | 28 | 39 | 150 | 18 | 85 | 47 | 150 | 77 | 35 | 38 | 150 | 38 | 55 | 57 | 150 | ||||
| LVSI | ||||||||||||||||||||
| Yes | 19 | 1 | 12 | 32 | 0.01431 | 4 | 18 | 10 | 32 | 0.9589 | 13 | 9 | 10 | 32 | 0.3949 | 8 | 10 | 14 | 32 | 0.7415 |
| No | 63 | 27 | 27 | 117 | 13 | 67 | 37 | 117 | 63 | 26 | 28 | 117 | 29 | 45 | 43 | 117 | ||||
| Total | 82 | 28 | 39 | 149 | 17 | 85 | 47 | 149 | 76 | 35 | 38 | 149 | 37 | 55 | 57 | 149 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanov, A.; Damyanova, P.; Vasileva-Slaveva, M.; Karakadieva, K.; Kostov, S.; Shivarov, V. Clinical Significance of Tumor Infiltrating T-Helper and Regulatory Cells in Bulgarian Cervical Cancer Patients. Biomedicines 2025, 13, 2206. https://doi.org/10.3390/biomedicines13092206
Yordanov A, Damyanova P, Vasileva-Slaveva M, Karakadieva K, Kostov S, Shivarov V. Clinical Significance of Tumor Infiltrating T-Helper and Regulatory Cells in Bulgarian Cervical Cancer Patients. Biomedicines. 2025; 13(9):2206. https://doi.org/10.3390/biomedicines13092206
Chicago/Turabian StyleYordanov, Angel, Polina Damyanova, Mariela Vasileva-Slaveva, Konstantina Karakadieva, Stoyan Kostov, and Velizar Shivarov. 2025. "Clinical Significance of Tumor Infiltrating T-Helper and Regulatory Cells in Bulgarian Cervical Cancer Patients" Biomedicines 13, no. 9: 2206. https://doi.org/10.3390/biomedicines13092206
APA StyleYordanov, A., Damyanova, P., Vasileva-Slaveva, M., Karakadieva, K., Kostov, S., & Shivarov, V. (2025). Clinical Significance of Tumor Infiltrating T-Helper and Regulatory Cells in Bulgarian Cervical Cancer Patients. Biomedicines, 13(9), 2206. https://doi.org/10.3390/biomedicines13092206









