Exploring Multidimensional Risk Factors Associated with Local Adverse Reactions to Subcutaneous Immunoglobulin Therapy: Insights from a Nationwide Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Statement
2.2. Patient Characterization and Variable Definition
2.3. Statistics
3. Results
3.1. Demographics
3.2. Global Adverse Effects of SCIg
3.3. Local Adverse Effects of SCIg: Patient Profiles by Local Tolerability
3.4. Multivariable Prediction Model for Local Adverse Effect of SCIg
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orange, J.; Hossny, E.; Weiler, C.; Ballow, M.; Berger, M.; Bonilla, F.; Buckley, R.; Chinen, J.; Elgamal, Y.; Mazer, B. Use of intravenous immunoglobulin in human disease: A review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 2006, 117 (Suppl. 4), S525–S553, Erratum in J. Allergy Clin. Immunol. 2006, 117, 1483. [Google Scholar] [CrossRef]
- Wood, P.; Stanworth, S.; Burton, J.; Jones, A.; Peckham, D.G.; Green, T.; Hyde, C.; Chapel, H. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: A systematic review. Clin. Exp. Immunol. 2007, 149, 410–423. [Google Scholar] [CrossRef]
- Abolhassani, H.; Sadaghiani, M.S.; Aghamohammadi, A.; Ochs, H.D.; Rezaei, N. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: Systematic review and meta analysis. J. Clin. Immunol. 2012, 32, 1180–1192. [Google Scholar] [CrossRef]
- Shabaninejad, H.; Asgharzadeh, A.; Rezaei, N.; Rezapoor, A. A Comparative Study of Intravenous Immunoglobulin and Subcutaneous Immunoglobulin in Adult Patients with Primary Immunodeficiency Diseases: A Systematic Review and Meta-Analysis. Expert. Rev. Clin. Immunol. 2016, 12, 595–602. [Google Scholar] [CrossRef]
- Wasserman, R.L.; Melamed, I.; Stein, M.R.; Gupta, S.; Puck, J.; Engl, W.; Leibl, H.; McCoy, B.; Empson, V.G.; Gelmont, D.; et al. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J. Allergy Clin. Immunol. 2012, 130, 951–957.e11. [Google Scholar] [CrossRef]
- Šedivá, A.; Chapel, H.; Gardulf, A. European Immunoglobulin Map Group (35 European Countries) for European Society for Immunodeficiencies (ESID) Primary Immunodeficiencies Care in Development Working Party. Europe immunoglobulin map. Clin. Exp. Immunol. 2014, 178 (Suppl. 1), 141–143. [Google Scholar] [CrossRef]
- Gardulf, A.; Nicolay, U.; Asensio, O.; Bernatowska, E.; Böck, A.; Costa-Carvalho, B.T.; Granert, C.; Haag, S.; Hernández, D.; Kiessling, P. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J. Allergy Clin. Immunol. 2004, 114, 936–942. [Google Scholar] [CrossRef]
- Cabañero-Navalon, M.D.; Garcia-Bustos, V.; Nuñez-Beltran, M.; Fernández, P.C.; Mateu, L.; Solanich, X.; Carrillo-Linares, J.L.; Robles-Marhuenda, Á.; Puchades-Gimeno, F.; Ballesta, A.P.; et al. Current clinical spectrum of common variable immunodeficiency in Spain: The multicentric nationwide GTEM-SEMI-CVID registry. Front. Immunol. 2022, 13, 1033666. [Google Scholar] [CrossRef]
- Mercader, S.M.; Garcia-Bustos, V.; Moral, P.M.; Buenaventura, C.M.; Vergara, E.E.; Bosch, M.C.M.; Balastegui-Martín, H.; Maycas, S.G.; Mur, B.P.; Palazón, M.E.; et al. Patient-centered outcomes with subcutaneous immunoglobulin use for infection control in primary and secondary immunodeficiencies: Data of a GEIE Spanish Registry. Front. Immunol. 2025, 16, 1532367. [Google Scholar] [CrossRef]
- Ghia, D.; Thota, P.; Ritchie, T.; Rana, H.; Minhas, R.; Moolji, J.; Ritchie, B.; Adatia, A.; Baysal, M. Feasibility and resource utilization of nurse-administered subcutaneous immunoglobulin therapy in antibody deficiency: A cross-sectional study. PLoS ONE 2025, 20, e0316797. [Google Scholar] [CrossRef]
- Younger, M.E.M.; Blouin, W.; Duff, C.; Epland, K.B.; Murphy, E.; Sedlak, D. Subcutaneous immunoglobulin replacement therapy: Ensuring success. J. Infus. Nurs. 2015, 38, 70–79. [Google Scholar] [CrossRef]
- Mizera, D.; Dziedzic, R.; Drynda, A.; Matyja-Bednarczyk, A.; Padjas, A.; Celińska-Löwenhoff, M.; Jakieła, B.; Bazan-Socha, S. Current Practice and Perspectives on Subcutaneous Immunoglobulin Replacement Therapy in Patients with Primary Antibody Deficiency Among Specialized Nurses in Poland. Nurs. Rep. 2024, 14, 3280–3290. [Google Scholar] [CrossRef]
- Ballow, M.; Wasserman, R.L.; Jolles, S.; Chapel, H.; Berger, M.; Misbah, S.A. Assessment of Local Adverse Reactions to Subcutaneous Immunoglobulin (SCIG) in Clinical Trials. J. Clin. Immunol. 2017, 37, 517–518, Erratum in J. Clin. Immunol. 2018, 38, 539. [Google Scholar] [CrossRef] [PubMed]
- Gonzáleza, D.C.; Caicoyaa, A.M.; Sáncheza, A.F.; Garcíaa, V.A.; Gonzáleza, J.V.G.; Palaciosa, E.D.; García, A.S. Evaluación de la fiabilidad y validez de una escala de valoración social en el anciano. Aten. Primaria 1999, 23, 434–440. [Google Scholar]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Guerola, L.d.C.; Sacristán, A.A.G.; Portolés, A.; Jasso, M.; Guerra-Galán, T.; de la Fuente-Munoz, E.; Palacios-Ortega, M.; Fernández-Arquero, M.; Cuesta-Mínguez, C.; Rodríguez-Sanz, A.; et al. Economic impact of immunoglobulin replacement therapy in secondary immunodeficiency to hematological cancer: A single center observational study. Front. Immunol. 2024, 15, 1413231. [Google Scholar] [CrossRef] [PubMed]
- Suez, D.; Kriván, G.; Jolles, S.; Stein, M.; Gupta, S.; Paris, K.; van Hagen, P.M.; Brodszki, N.; Engl, W.; Leibl, H.; et al. Safety and tolerability of subcutaneous immunoglobulin 20% in primary immunodeficiency diseases from two continents. Immunotherapy. 2019, 11, 1057–1065. [Google Scholar] [CrossRef]
- Herrscher, R.F.; Prince, T.L.; Van Anglen, L.J.; Schroeder, C.P. Efficacy, tolerability and persistence of subcutaneous immunoglobulin provided through immunology physician practices for the treatment of primary immunodeficiencies. J. Allergy Clin. Immunol. 2019, 143 (Suppl. 2), AB115. [Google Scholar] [CrossRef]
- Langford, J.; Herrscher, R.; Kim-Romo, D.; Van Anglen, L. Real-world utilization of immune globulin subcutaneous 16.5% in treatment-naïve primary immunodeficient patients. Ann. Allergy Asthma Immunol. 2022, 129 (Suppl. 5), S53. [Google Scholar] [CrossRef]
- Kobayashi, R.H.; Litzman, J.; Melamed, I.; Mandujano, J.F.; Kobayashi, A.L.; Ritchie, B.; Geng, B.; Atkinson, T.P.; Rehman, S.; Höller, S.; et al. Long-term efficacy, safety, and tolerability of a subcutaneous immunoglobulin 16.5% (cutaquig®) in the treatment of patients with primary immunodeficiencies. Clin. Exp. Immunol. 2022, 210, 91–103. [Google Scholar] [CrossRef]
- Bonilla, F.A. Intravenous and subcutaneous immunoglobulin G replacement therapy. Allergy Asthma Proc. 2016, 37, 426–431. [Google Scholar] [CrossRef]
- Borte, M.; Kriván, G.; Derfalvi, B.; Maródi, L.; Harrer, T.; Jolles, S.; Bourgeois, C.; Engl, W.; Leibl, H.; McCoy, B.; et al. Efficacy, safety, tolerability and pharmacokinetics of a novel human immune globulin subcutaneous, 20%: A Phase 2/3 study in Europe in patients with primary immunodeficiencies. Clin. Exp. Immunol. 2017, 187, 146–159. [Google Scholar] [CrossRef]
- Borte, M.; Hanitsch, L.G.; Mahlaoui, N.; Fasshauer, M.; Huscher, D.; Speletas, M.; Dimou, M.; Kamieniak, M.; Hermann, C.; Pittrow, D.; et al. Facilitated Subcutaneous Immunoglobulin Treatment in Patients with Immunodeficiencies: The FIGARO Study. J. Clin. Immunol. 2023, 43, 1259–1271. [Google Scholar] [CrossRef]
- Hansen, S.; Gustafson, R.; Smith, C.I.; Gardulf, A. Express subcutaneous IgG infusions: Decreased time of delivery with maintained safety. Clin. Immunol. 2002, 104, 237–241. [Google Scholar] [CrossRef]
- Gupta, S.; Stein, M.; Hussain, I.; Paris, K.; Engl, W.; McCoy, B.; Rabbat, C.J.; Yel, L. Tolerability of Ig20Gly during onboarding in patients with primary immunodeficiency diseases. Ann. Allergy Asthma Immunol. 2019, 123, 271–279.e1. [Google Scholar] [CrossRef]
- Gul, Y.; Kapakli, H.; Guner, S.N.; Alan, H.B.; Hazar, E.; Keles, S.; Reisli, I. Long-Term Experience of Subcutaneous Immunoglobulin Therapy in Pediatric Primary Immunodeficient Patients with Low and Normal Body Weight. J. Clin. Immunol. 2022, 42, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.R.; Koterba, A.; Rodden, L.; Berger, M. Safety and efficacy of home-based subcutaneous immunoglobulin G in elderly patients with primary immunodeficiency diseases. Postgrad. Med. 2011, 123, 186–193. [Google Scholar] [CrossRef]
- Guicheney, M.; Jullie, M.L.; Doutre, M.S. Koebner phenomenon in systemic lupus erythematosus after subcutaneous injections of methotrexate. Ann. Dermatol. Venereol. 2022, 149, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Hulme, C.; Farragher, T.; Clarke, G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open. 2016, 6, e013059. [Google Scholar] [CrossRef] [PubMed]

| Variable | n | Frequency (%) |
|---|---|---|
| Local adverse effects | 163 | 98.19% |
| Local rash | 108 | 65.06% |
| Local pain | 53 | 31.93% |
| Pruritus | 108 | 65.45% |
| Swelling | 117 | 70.48% |
| Nodules | 12 | 7.45% |
| Leak | 18 | 10.84% |
| Hematoma | 18 | 10.84% |
| Ulceration | 4 | 2.41% |
| Necrosis | 8 | 4.82% |
| Systemic adverse effects | 67 | 40.36% |
| Headache | 43 | 25.9% |
| Asthenia | 29 | 17.47% |
| Fever | 13 | 7.83% |
| Arthromyalgia | 23 | 13.86% |
| Systemic rash | 6 | 3.61% |
| Wear-off | 33 | 24.63% |
| Variable | Category | No Local Adverse Effect | Local Adverse Effect | p-Value |
|---|---|---|---|---|
| Age | 50.15 (SD 17.73) | 46.01 (SD 18.74) | 0.131 | |
| Diagnosis | PID | 42 (70%) | 103 (63.19%) | 0.4288 |
| SID | 18 (30%) | 60 (36.81%) | ||
| Sex | Men | 24 (40%) | 62 (38.04%) | 0.8769 |
| Women | 36 (60%) | 101 (61.96%) | ||
| Cytopenia | No | 51 (85%) | 147 (90.18%) | 0.3378 |
| Yes | 9 (15%) | 16 (9.82%) | ||
| Immune thrombopenia | No | 54 (90%) | 154 (94.48%) | 0.2394 |
| Yes | 6 (1%) | 9 (5.52%) | ||
| Anticoagulation | No | 55 (91.67%) | 152 (93.25%) | 0.7704 |
| Yes | 5 (8.33%) | 11 (6.75%) | ||
| Skin disease | No | 53 (89.83%) | 121 (74.69%) | 0.0155 * |
| Yes | 6 (10.17%) | 41 (25.31%) | ||
| Hematopoietic stem cell transplantation | No | 55 (93.22%) | 146 (89.57%) | 0.604 |
| Yes | 4 (6.78%) | 17 (10.43%) | ||
| Solid organ transplantation | No | 58 (98.31%) | 150 (95.54%) | 0.4514 |
| Yes | 1 (1.69%) | 7 (4.46%) | ||
| Weekly SCIg dose | 8.10 (SD 2.48) | 8.02 (SD 2.93) | 0.835 | |
| Total infusion volume (both 10% and 20%) | 159.81 (SD 145.20) | 155.88 (SD 123.05) | 0.888 | |
| Posology | Every two weeks | 7 (13.34%) | 16 (9.82%) | 0.0507 |
| Monthly | 12 (20%) | 28 (7.18%) | ||
| Other | 1 (1.67%) | 0 (0.0%) | ||
| Weekly | 30 (50%) | 65 (39.88%) | ||
| Every three weeks | 9 (15%) | 53 (32.52%) | ||
| Injection points | 1.93 (0.41) | 1.84 (0.37) | 0.124 | |
| Needle length | 6 mm | 0 (0%) | 6 (3.7%) | 0.023 * |
| 8 mm | 2 (3.3%) | 12 (7.4%) | ||
| 9 mm | 56 (93.3%) | 120 (73.6%) | ||
| 10 mm | 1 (1.7%) | 6 (3.7%) | ||
| 12 mm | 1 (1.7%) | 19 (11.7%) | ||
| SCIg formula | 10% | 23 (38.33%) | 82 (50.31%) | 0.1311 |
| 20% | 37 (61.67%) | 81 (49.69%) | ||
| Injection place | Abdomen | 49 (81.67%) | 88 (53.99%) | 0.002 * |
| Alternates abdomen and thighs | 5 (8.33%) | 44 (26.99%) | ||
| Alternates arms and thighs | 1 (1.67%) | 1 (0.61%) | ||
| Alternates abdomen, arms, and thighs | 0 (0.0%) | 3 (1.84%) | ||
| Arms | 0 (0.0%) | 2 (1.23%) | ||
| Thighs | 5 (8.33%) | 25 (15.34%) | ||
| Needle thickness | 26 G | 37 (61.67%) | 92 (56.44%) | 0.5421 |
| 27 G | 23 (38.33%) | 71 (43.56%) | ||
| Infusion time (min) | 80.75 (SD 74.88) | 60.69 (27.17) | 0.047 * | |
| Abdominal perimeter | 94.94 (SD 13.17) | 87.34 (SD 12.57) | 0.0002 * | |
| Hospital distance (km) | 20.76 (SD 28.63) | 34.96 (SD 42.47) | 0.005 * | |
| Previous IVIg | No | 19 (32.2%) | 55 (33.74%) | 0.8732 |
| Yes | 40 (67.8%) | 108 (66.26%) | ||
| Laboral situation | Disability | 8 (13.3%) | 29 (17.8%) | 0.085 |
| Employed | 29 (48.3%) | 59 (36.2%) | ||
| Unemployed | 2 (3.3%) | 8 (4.9%) | ||
| Student | 3 (5%) | 29 (17.8%) | ||
| Retired | 18 (30%) | 38 (23.3%) | ||
| Familiar situation | Lives with a spouse of similar age | 16 (27.12%) | 54 (33.33%) | 0.314 |
| Lives with family members without physical or mental dependency | 31 (52.54%) | 73 (45.06%) | ||
| Lives with family and/or spouse and shows some signs of dependency | 8 (13.56%) | 15 (9.26%) | ||
| Lives alone and has no children or they live far away | 1 (1.69%) | 13 (8.02%) | ||
| Lives alone and has children nearby | 3 (05.08%) | 7 (4.32%) | ||
| Social situation | Active social relationships | 57 (95%) | 154 (94.48%) | 0.334 |
| Social relationships only with family or neighbors | 0 (0.0%) | 5 (3.07%) | ||
| Social relationships both with family and neighbors | 3 (5%) | 4 (2.45%) | ||
| Mobility and independence | I have no problems walking | 43 (72.88%) | 132 (80.98%) | |
| I have some problems walking | 13 (22.03%) | 28 (17.18%) | ||
| I have to stay in bed | 3 (5.08%) | 3 (1.84%) | ||
| Personal care | I have no problems with personal care | 53 (89.83%) | 147 (90.18%) | 1 |
| I am unable to wash or dress myself | 2 (3.39%) | 6 (3.68%) | ||
| I have some problems washing or dressing myself | 4 (6.78%) | 10 (6.13%) | ||
| Anxiety and depression | I am moderately anxious or depressed | 2 (3.45%) | 44 (27.5%) | 2.87 × 10−5 * |
| I am very anxious or depressed | 0 (0.0%) | 3 (1.88%) | ||
| I am neither anxious nor depressed | 56 (96.55%) | 113 (70.62%) | ||
| Discomfort | I have no pain or discomfort | 49 (84.48%) | 97 (60.25%) | 0.000519 * |
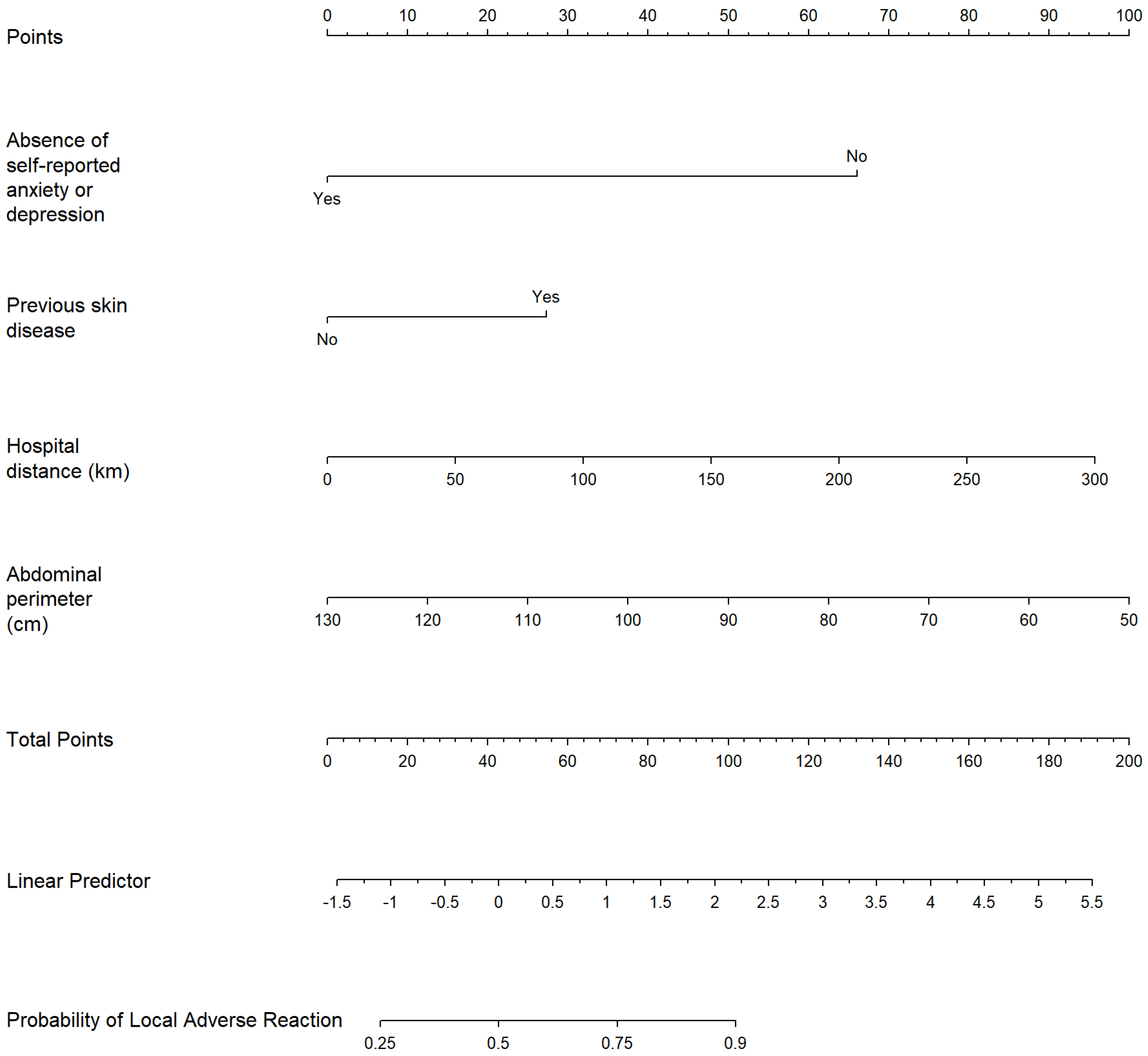
| Variable | Odds Ratio | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| Abdominal perimeter | 0.9550 | 0.9300 | 0.9800 | <0.001 |
| Previous skin disease | 2.7500 | 1.0300 | 7.3800 | 0.044 |
| Distance to hospital | 1.0100 | 1.0000 | 1.0200 | 0.0504 |
| Not feeling anxious or depressed | 0.0892 | 0.0204 | 0.3900 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Mercader, S.; Garcia-Bustos, V.; Moral Moral, P.; Martínez Buenaventura, C.; Escudero Vergara, E.; Montaner Bosch, M.C.; Balastegui-Martín, H.; Galindo Maycas, S.; González Amores, M.; Gimenez Sanz, N.; et al. Exploring Multidimensional Risk Factors Associated with Local Adverse Reactions to Subcutaneous Immunoglobulin Therapy: Insights from a Nationwide Multicenter Study. Biomedicines 2025, 13, 1991. https://doi.org/10.3390/biomedicines13081991
Martínez Mercader S, Garcia-Bustos V, Moral Moral P, Martínez Buenaventura C, Escudero Vergara E, Montaner Bosch MC, Balastegui-Martín H, Galindo Maycas S, González Amores M, Gimenez Sanz N, et al. Exploring Multidimensional Risk Factors Associated with Local Adverse Reactions to Subcutaneous Immunoglobulin Therapy: Insights from a Nationwide Multicenter Study. Biomedicines. 2025; 13(8):1991. https://doi.org/10.3390/biomedicines13081991
Chicago/Turabian StyleMartínez Mercader, Sandra, Victor Garcia-Bustos, Pedro Moral Moral, Carmen Martínez Buenaventura, Elisa Escudero Vergara, María Carmen Montaner Bosch, Héctor Balastegui-Martín, Sonia Galindo Maycas, Miriam González Amores, Noemí Gimenez Sanz, and et al. 2025. "Exploring Multidimensional Risk Factors Associated with Local Adverse Reactions to Subcutaneous Immunoglobulin Therapy: Insights from a Nationwide Multicenter Study" Biomedicines 13, no. 8: 1991. https://doi.org/10.3390/biomedicines13081991
APA StyleMartínez Mercader, S., Garcia-Bustos, V., Moral Moral, P., Martínez Buenaventura, C., Escudero Vergara, E., Montaner Bosch, M. C., Balastegui-Martín, H., Galindo Maycas, S., González Amores, M., Gimenez Sanz, N., Escobar Palazón, M., Moreno Mulet, M., Campanero Carrasco, I., López, A., Hernández Ruiz, C. D., Ruiz-López, L., Guzmán Guzmán, R., & Cabañero-Navalon, M. D. (2025). Exploring Multidimensional Risk Factors Associated with Local Adverse Reactions to Subcutaneous Immunoglobulin Therapy: Insights from a Nationwide Multicenter Study. Biomedicines, 13(8), 1991. https://doi.org/10.3390/biomedicines13081991







