The Role of Impella in Cardiogenic Shock in the Post-DanGer Shock Era
Abstract
1. Introduction
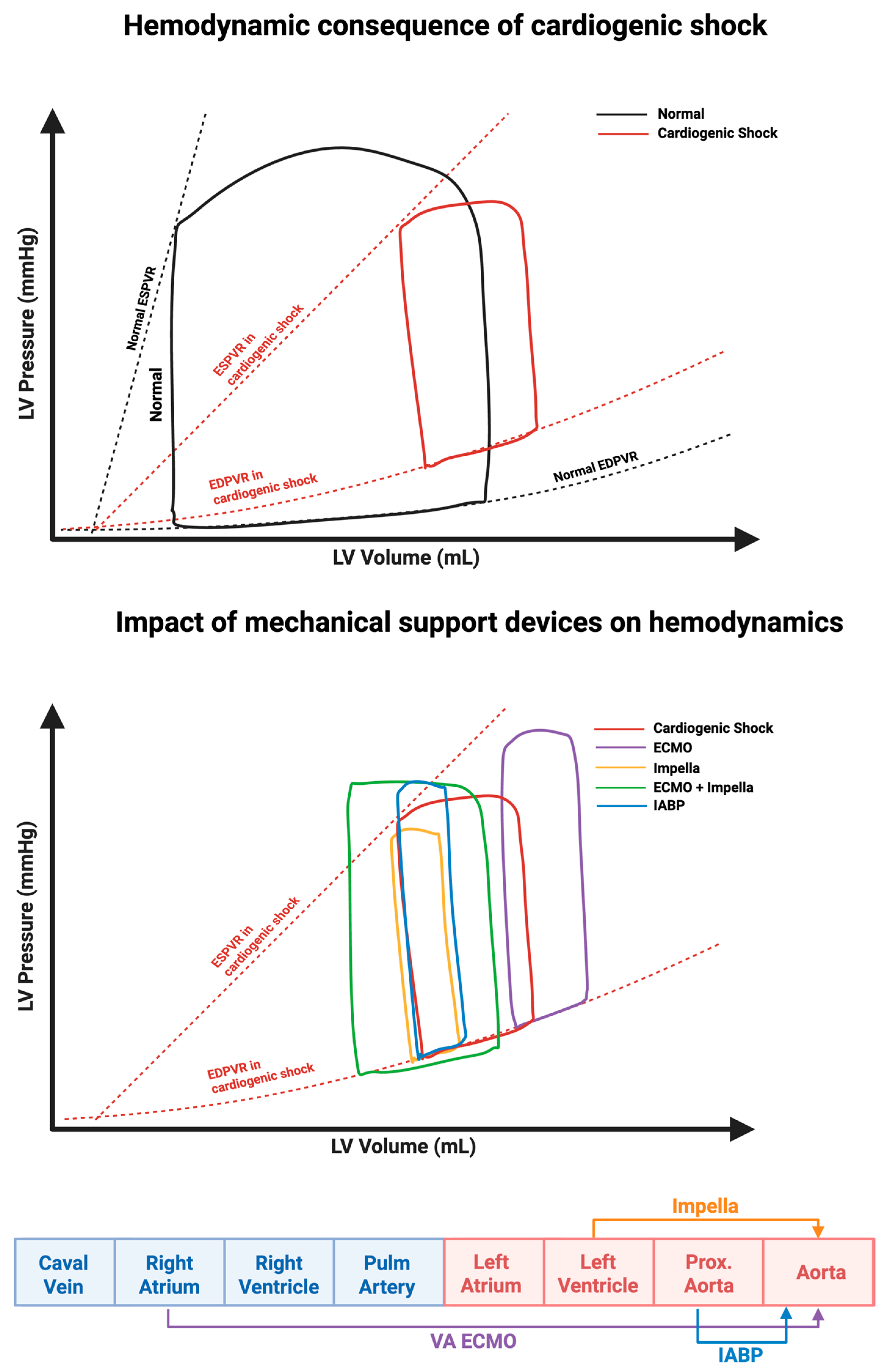
2. Technical Specifications and Mechanism of Action
2.1. Effect on the Pressure Volume Loop
2.2. Variation in Impella Models
3. Outcomes
3.1. History
3.2. Observational Studies
3.3. Randomized Trials
3.4. mAFP in the DanGer Shock Era
4. Guidelines and Indications
| Society/Region | Class/Level | Notes | |
|---|---|---|---|
| Cardiogenic Shock | High-Risk PCI | ||
| ACC/AHA (USA) [66] | Class IIa/Level B-R | Class IIb/Level B-R | “In selected * patients with STEMI and severe or refractory cardiogenic shock, insertion of a microaxial intravascular flow pump is reasonable to reduce death.” |
| ESC (Europe) [73] | Class IIa/Level C | Class IIb/Level C | “Short-term MCS should be considered in patients with cardiogenic shock as a BTR, BTD, BTB. Further indications include treatment of the cause of cardiogenic shock or long-term MCS or transplantation.” |
| NICE (UK) [71] | No formal class; cautious use in expert centers | No formal class; supported in specialized centers | “No national guidance exists in the UK for the use of hemodynamic support devices.” |
| CCS (Canada) [70] | Strong Recommendation; Moderate-Quality Evidence | Not formally recommended outside research | “We recommend that patients in cardiogenic shock be considered for temporary MCS to afford an opportunity for evaluation for long-term options.” |
| JCS (Japan) [72] | N/A | N/A | “However, all of these data are from clinical trials with small sample sizes, there is a lack of high-quality RCTs, and interestingly, to date there are no specific recommendations in guidelines from Europe and North America, where IMPELLA is more widely used than in Japan.” |
| CSC (China) [68] | Class IIa/Level B | No formal class | “Percutaneous ventricular assist devices and extracorporeal membrane oxygenation: These devices can be utilized as transitional therapies for fulminant myocarditis, acute severe HF, or cardiogenic shock, allowing for further evaluation of the need for heart transplantation or long-term MCS (IIa, B).” |
| ANZ (Australia/ New Zealand) [67] | Strength of recommendation weak with moderate certainty of evidence | N/A | “Consider left ventricular assist devices in people with STEMI and cardiogenic shock on a case-by-case basis, given the selected population enrolled and the complication rate in the DanGer Shock trial.” |
| Asia-Pacific Region [69] | Low level of evidence | N/A | “Temporary MCS (e.g., intra-aortic balloon pump [IABP], Impella or venoarterial extracorporeal membrane oxygenation [VAECMO]) may be considered in AMI patients in Stage C and Stage D CS.” |
5. Troubleshooting and Complications
5.1. Systematic Approach to Interpreting Impella Console Alarms and Waveforms
5.2. Malposition
5.2.1. Deep Malposition
5.2.2. Shallow Malposition
5.2.3. Malrotation
5.2.4. Correcting Malposition
5.3. Thrombosis
5.4. Hemolysis
5.5. Vascular Access
6. Unanswered Questions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kanwar, M.; Sinha, S.S.; Thayer, K.L.; Garan, A.R.; Hernandez-Montfort, J.; Zhang, Y.; Li, B.; Baca, P.; Dieng, F.; et al. Criteria for Defining Stages of Cardiogenic Shock Severity. J. Am. Coll. Cardiol. 2022, 80, 185–198. [Google Scholar] [CrossRef]
- Thiele, H.; de Waha-Thiele, S.; Freund, A.; Zeymer, U.; Desch, S.; Fitzgerald, S. Management of cardiogenic shock. EuroIntervention 2021, 17, 451–465. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; Waha, S.d.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef]
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Dzavik, V.; Buller, C.E.; Aylward, P.; Col, J.; White, H.D.; Investigators, S. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 2006, 295, 2511–2515. [Google Scholar] [CrossRef]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Frohlich, G.; Bott-Flugel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schomig, A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 278–287. [Google Scholar] [CrossRef]
- Griffith, B.P.; Anderson, M.B.; Samuels, L.E.; Pae, W.E., Jr.; Naka, Y.; Frazier, O.H. The RECOVER I: A multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J. Thorac. Cardiovasc. Surg. 2013, 145, 548–554. [Google Scholar] [CrossRef]
- Moller, J.E.; Engstrom, T.; Jensen, L.O.; Eiskjaer, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef]
- Saito, S.; Okubo, S.; Matsuoka, T.; Hirota, S.; Yokoyama, S.; Kanazawa, Y.; Takei, Y.; Tezuka, M.; Tsuchiya, G.; Konishi, T.; et al. Impella—Current issues and future expectations for the percutaneous, microaxial flow left ventricular assist device. J. Cardiol. 2024, 83, 228–235. [Google Scholar] [CrossRef]
- Zein, R.; Patel, C.; Mercado-Alamo, A.; Schreiber, T.; Kaki, A. A Review of the Impella Devices. Interv. Cardiol. 2022, 17, e05. [Google Scholar] [CrossRef]
- Hosseinipour, M.; Gupta, R.; Bonnell, M.; Elahinia, M. Rotary mechanical circulatory support systems. J. Rehabil. Assist. Technol. Eng. 2017, 4, 2055668317725994. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S. Cardiogenic shock complicating acute myocardial infarction: Expanding the paradigm. Circulation 2003, 107, 2998–3002. [Google Scholar] [CrossRef] [PubMed]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically mediated changes in capacitance: Redistribution of the venous reservoir as a cause of decompensation. Circulation Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Menon, V.; Lowe, A.M.; Lange, M.; Dzavik, V.; Sleeper, L.A.; Hochman, J.S.; Investigators, S. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch. Intern. Med. 2005, 165, 1643–1650. [Google Scholar] [CrossRef]
- Attinger-Toller, A.; Bossard, M.; Cioffi, G.M.; Tersalvi, G.; Madanchi, M.; Bloch, A.; Kobza, R.; Cuculi, F. Ventricular Unloading Using the Impella(TM) Device in Cardiogenic Shock. Front. Cardiovasc. Med. 2022, 9, 856870. [Google Scholar] [CrossRef]
- Nakata, J.; Yamamoto, T.; Saku, K.; Ikeda, Y.; Unoki, T.; Asai, K. Mechanical circulatory support in cardiogenic shock. J. Intensive Care 2023, 11, 64. [Google Scholar] [CrossRef]
- Saku, K.; Kakino, T.; Arimura, T.; Sunagawa, G.; Nishikawa, T.; Sakamoto, T.; Kishi, T.; Tsutsui, H.; Sunagawa, K. Left Ventricular Mechanical Unloading by Total Support of Impella in Myocardial Infarction Reduces Infarct Size, Preserves Left Ventricular Function, and Prevents Subsequent Heart Failure in Dogs. Circulation Heart Fail. 2018, 11, e004397. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, G.; Neri, G.; Macri, L.M.; Salerno, N.; De Rosa, S.; Torella, D. Use of Impella device in cardiogenic shock and its clinical outcomes: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2022, 40, 101007. [Google Scholar] [CrossRef] [PubMed]
- Glazier, J.J.; Kaki, A. The Impella Device: Historical Background, Clinical Applications and Future Directions. Int. J. Angiol. 2019, 28, 118–123. [Google Scholar] [CrossRef]
- Asleh, R.; Resar, J.R. Utilization of Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock Complicating Acute Myocardial Infarction and High-Risk Percutaneous Coronary Interventions. J. Clin. Med. 2019, 8, 1209. [Google Scholar] [CrossRef]
- Ramzy, D.; Soltesz, E.; Anderson, M. New Surgical Circulatory Support System Outcomes. ASAIO J. 2020, 66, 746–752. [Google Scholar] [CrossRef]
- Bernhardt, A.M.; Blumer, V.; Vandenbriele, C.; Schrage, B.; Mody, K.; Pappalardo, F.; Silvestry, S.; Anderson, M.; Abraham, J.; Gage, A.; et al. Clinical Management of the Impella 5.5 Pump. J. Heart Lung Transpl. 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Wampler, R.K.; Moise, J.C.; Frazier, O.H.; Olsen, D.B. In vivo evaluation of a peripheral vascular access axial flow blood pump. ASAIO Trans. 1988, 34, 450–454. [Google Scholar]
- Siess, T.; Nix, C.; Menzler, F. From a Lab Type to a Product: A Retrospective View on Impella’s Assist Technology. Artif. Organs 2001, 25, 414–421. [Google Scholar] [CrossRef]
- Meyns, B.; Stolinski, J.; Leunens, V.; Verbeken, E.; Flameng, W. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J. Am. Coll. Cardiol. 2003, 41, 1087–1095. [Google Scholar] [CrossRef]
- Meyns, B.; Sergeant, P.; Nishida, T.; Perek, B.; Zietkiewicz, M.; Flameng, W. Micropumps to support the heart during CABG. Eur. J. Cardiothorac. Surg. 2000, 17, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.P.; Spertus, J.A.; Curtis, J.P.; Desai, N.; Masoudi, F.A.; Bach, R.G.; McNeely, C.; Al-Badarin, F.; House, J.A.; Kulkarni, H.; et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation 2020, 141, 273–284. [Google Scholar] [CrossRef]
- Bjarnason, T.A.; Mentias, A.; Panaich, S.; Vaughan Sarrazin, M.; Gao, Y.; Desai, M.; Pandey, A.; Dhruva, S.S.; Desai, N.R.; Girotra, S. Diffusion of Percutaneous Ventricular Assist Devices in US Markets. Circ. Cardiovasc. Interv. 2022, 15, e011778. [Google Scholar] [CrossRef]
- Khera, R.; Cram, P.; Lu, X.; Vyas, A.; Gerke, A.; Rosenthal, G.E.; Horwitz, P.A.; Girotra, S. Trends in the use of percutaneous ventricular assist devices: Analysis of national inpatient sample data, 2007 through 2012. JAMA Intern. Med. 2015, 175, 941–950. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Schreiber, T.; Wohns, D.H.; Rihal, C.; Naidu, S.S.; Civitello, A.B.; Dixon, S.R.; Massaro, J.M.; Maini, B.; Ohman, E.M. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: Results from the USpella Registry. J. Interv. Cardiol. 2014, 27, 1–11. [Google Scholar] [CrossRef]
- Maini, B.; Naidu, S.S.; Mulukutla, S.; Kleiman, N.; Schreiber, T.; Wohns, D.; Dixon, S.; Rihal, C.; Dave, R.; O’Neill, W. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: The USpella Registry. Catheter. Cardiovasc. Interv. 2012, 80, 717–725. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.; Dixon, S.; Alaswad, K.; Patel, K.; Almany, S.; Khandelwal, A.; Hanson, I.; George, A.; Ashbrook, M.; et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter. Cardiovasc. Interv. 2018, 91, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Basir, M.B.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.L.; Grines, C.L.; Dixon, S.R.; Moses, J.W.; Maini, B.S.; Khandelwal, A.K.; Ohman, E.M.; O’Neill, W.W. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am. J. Cardiol. 2017, 119, 845–851. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ako, J.; Toda, K.; Hirayama, A.; Kinugawa, K.; Kobayashi, Y.; Ono, M.; Nishimura, T.; Sato, N.; Shindo, T.; et al. Short-Term Outcomes of Impella Support in Japanese Patients With Cardiogenic Shock Due to Acute Myocardial Infarction―Japanese Registry for Percutaneous Ventricular Assist Device (J-PVAD). Circ. J. 2023, 87, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Scherer, C.; Lusebrink, E.; Kupka, D.; Stocker, T.J.; Stark, K.; Stremmel, C.; Orban, M.; Petzold, T.; Germayer, A.; Mauthe, K.; et al. Long-Term Clinical Outcome of Cardiogenic Shock Patients Undergoing Impella CP Treatment vs. Standard of Care. J. Clin. Med. 2020, 9, 3803. [Google Scholar] [CrossRef] [PubMed]
- Lauten, A.; Engstrom, A.E.; Jung, C.; Empen, K.; Erne, P.; Cook, S.; Windecker, S.; Bergmann, M.W.; Klingenberg, R.; Luscher, T.F.; et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: Results of the Impella-EUROSHOCK-registry. Circulation Heart Fail. 2013, 6, 23–30. [Google Scholar] [CrossRef]
- Azzalini, L.; Johal, G.S.; Baber, U.; Bander, J.; Moreno, P.R.; Bazi, L.; Kapur, V.; Barman, N.; Kini, A.S.; Sharma, S.K. Outcomes of Impella-supported high-risk nonemergent percutaneous coronary intervention in a large single-center registry. Catheter. Cardiovasc. Interv. 2021, 97, E26–E33. [Google Scholar] [CrossRef] [PubMed]
- Brereton, B.; Guzman, F.N.; Karipineni, S.; Regassa, H.; Nalluri, S.D.; Ganesan, S.; Choday, S.; Veliginti, S.; Fatima, B.; Akbari, H.; et al. Contemporary Trends in Percutaneous Ventricular Assist Device Utilization and Inpatient Mortality in Elderly Hospitalizations with Stemi-Associated Cardiogenic Shock—A National Inpatient Sample Analysis (2016–2019). J. Am. Coll. Cardiol. 2023, 81, 516. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA 2021, 326, 1840–1850, Correction in JAMA 2021, 326, 2333. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Bromfield, S.G.; Ma, Q.; Crawford, G.; Whitney, J.; DeVries, A.; Desai, N.R. Clinical Outcomes and Cost Associated With an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump in Patients Presenting With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Intern. Med. 2022, 182, 926–933. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.; Dixon, S.; Massaro, J.; Palacios, I.; Maini, B.; Mulukutla, S.; Dzavik, V.; et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: The PROTECT II study. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef]
- Bochaton, T.; Huot, L.; Elbaz, M.; Delmas, C.; Aissaoui, N.; Farhat, F.; Mewton, N.; Bonnefoy, E.; IMPELLA-STIC Investigators. Mechanical circulatory support with the Impella(R) LP5.0 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: The IMPELLA-STIC randomized study. Arch. Cardiovasc. Dis. 2020, 113, 237–243. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Eriksen, E.; Seyfarth, M.; Henriques, J.P. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump for Treating Cardiogenic Shock: Meta-Analysis. J. Am. Coll. Cardiol. 2017, 69, 358–360. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.P.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Use of Mechanical Circulatory Support Devices Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Netw. Open 2021, 4, e2037748. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.M.; Smith, N.R.; Uyl-de Groot, C.A.; den Uil, C.A.; Ross, J.S.; Mohamed, M.O.; Mamas, M.A.; Banerjee, A.; Ko, D.T.; Landon, B.; et al. Variations in the Medical Device Authorization and Reimbursement Landscape: A Case Study of 2 Cardiovascular Devices Across 4 Countries. Circ. Cardiovasc. Qual. Outcomes 2025, 18, e011636. [Google Scholar] [CrossRef]
- Strom, J.B.; Zhao, Y.; Shen, C.; Chung, M.; Pinto, D.S.; Popma, J.J.; Yeh, R.W. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention 2018, 13, e2152–e2159. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Basu, T.; VanAken, G.; Aggarwal, V.; Lee, R.; Abdul-Aziz, A.; Birati, E.Y.; Basir, M.B.; Nallamothu, B.K.; Shore, S. National Trends for Temporary Mechanical Circulatory Support Utilization in Patients With Cardiogenic Shock From Decompensated Chronic Heart Failure: Incidence, Predictors, Outcomes, and Cost. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101177. [Google Scholar] [CrossRef]
- Chandrasekar, B. Mechanical circulatory support with Impella in percutaneous coronary intervention: Current status. Am. Heart J. Plus Cardiol. Res. Pract. 2021, 1, 100002. [Google Scholar] [CrossRef]
- O’Brien, C.G.; Brusca, S.B.; Barnett, C.F.; Berg, D.D.; Baird-Zars, V.M.; Park, J.G.; Bohula, E.A.; Morrow, D.A.; Investigators, C. Using Selection Criteria From the DanGer Shock Trial in a Contemporary Cohort With Cardiogenic Shock. J. Am. Coll. Cardiol. 2024, 84, 2490–2493. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Grines, C.; Schreiber, T.; Moses, J.; Maini, B.; Dixon, S.R.; Ohman, E.M. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am. Heart J. 2018, 202, 33–38. [Google Scholar] [CrossRef]
- Moller, J.E.; Beske, R.P.; Jensen, L.O.; Eiskjaer, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Schrage, B.; et al. Effect of Microaxial Flow Pump on Hemodynamics in STEMI-Related Cardiogenic Shock. J. Am. Coll. Cardiol. 2025, 85, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Kadosh, B.S.; Berg, D.D.; Bohula, E.A.; Park, J.G.; Baird-Zars, V.M.; Alviar, C.; Alzate, J.; Barnett, C.F.; Barsness, G.W.; Burke, J.; et al. Pulmonary Artery Catheter Use and Mortality in the Cardiac Intensive Care Unit. JACC Heart Fail. 2023, 11, 903–914. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Toda, K.; Ako, J.; Hirayama, A.; Kinugawa, K.; Kobayashi, Y.; Ono, M.; Nishimura, T.; Sato, N.; Shindo, T.; Takayama, M.; et al. Three-year experience of catheter-based micro-axial left ventricular assist device, Impella, in Japanese patients: The first interim analysis of Japan registry for percutaneous ventricular assist device (J-PVAD). J. Artif. Organs 2023, 26, 17–23. [Google Scholar] [CrossRef]
- Schrage, B.; Zeymer, U.; Montalescot, G.; Windecker, S.; Serpytis, P.; Vrints, C.; Stepinska, J.; Savonitto, S.; Oldroyd, K.G.; Desch, S.; et al. Impact of Center Volume on Outcomes in Myocardial Infarction Complicated by Cardiogenic Shock: A CULPRIT-SHOCK Substudy. J. Am. Heart Assoc. 2021, 10, e021150. [Google Scholar] [CrossRef]
- McGrath, P.D.; Wennberg, D.E.; Dickens, J.D., Jr.; Siewers, A.E.; Lucas, F.L.; Malenka, D.J.; Kellett, M.A., Jr.; Ryan, T.J., Jr. Relation between operator and hospital volume and outcomes following percutaneous coronary interventions in the era of the coronary stent. JAMA 2000, 284, 3139–3144. [Google Scholar] [CrossRef]
- Kontos, M.C.; Wang, Y.; Chaudhry, S.I.; Vetrovec, G.W.; Curtis, J.; Messenger, J.; NCDR. Lower hospital volume is associated with higher in-hospital mortality in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction: A report from the NCDR. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 659–667. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef] [PubMed]
- Brieger, D.; Cullen, L.; Briffa, T.; Zaman, S.; Scott, I.; Papendick, C.; Bardsley, K.; Baumann, A.; Bennett, A.S.; Clark, R.A.; et al. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Comprehensive Australian Clinical Guideline for Diagnosing and Managing Acute Coronary Syndromes 2025. Heart Lung Circ. 2025, 34, 309–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y. Chinese Guidelines for the Diagnosis and Treatment of Heart Failure 2024. J. Geriatr. Cardiol. 2025, 22, 277–331. [Google Scholar] [CrossRef]
- Tan, J.W.C.; Chew, D.P.; Lo, S.; Henry, T.D.; Lin, W.; Chia, Y.W.; Abdulaziz, S.; Seth, A.; Yap, J.; Evangelista, L.K.M.; et al. Asian Pacific Society of Cardiology Consensus Statements on the Diagnosis and Management of Acute MI-Cardiogenic Shock and Endorsed by the Asian Pacific Society of Interventional Cardiology. J. Asian Pac. Soc. Cardiol. 2024, 3, e10. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [PubMed]
- Impella 2.5 for Haemodynamic Support During High-Risk Percutaneous Coronary Interventions. Available online: https://www.nice.org.uk/advice/mib89/chapter/the-technology (accessed on 18 June 2025).
- Nishimura, T.; Hirata, Y.; Ise, T.; Iwano, H.; Izutani, H.; Kinugawa, K.; Kitai, T.; Ohno, T.; Ohtani, T.; Okumura, T.; et al. JCS/JSCVS/JCC/CVIT 2023 Guideline Focused Update on Indication and Operation of PCPS/ECMO/IMPELLA. Circ. J. 2024, 88, 1010–1046, Corrigendum in Circ. J. 2024, 88, 1347. [Google Scholar] [CrossRef]
- Authors/Task Force, M.; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Tran, T.; Mudigonda, P.; Mahr, C.; Kirkpatrick, J. Echocardiographic imaging of temporary percutaneous mechanical circulatory support devices. J. Echocardiogr. 2022, 20, 77–86. [Google Scholar] [CrossRef]
- Sacks, S.; Feinman, J. A 2024 Update From the American Society of Echocardiography: Multimodality Imaging for Patients With Left Ventricular Assist Devices and Temporary Mechanical Circulatory Support. J. Cardiothorac. Vasc. Anesth. 2025, 39, 1919–1923. [Google Scholar] [CrossRef]
- Baldetti, L.; Beneduce, A.; Romagnolo, D.; Frias, A.; Gramegna, M.; Sacchi, S.; Calvo, F.; Pazzanese, V.; Cappelletti, A.M.; Ajello, S.; et al. Impella Malrotation Within the Left Ventricle Is Associated With Adverse In-Hospital Outcomes in Cardiogenic Shock. JACC Cardiovasc. Interv. 2023, 16, 739–741. [Google Scholar] [CrossRef]
- Burzotta, F.; Trani, C.; Doshi, S.N.; Townend, J.; van Geuns, R.J.; Hunziker, P.; Schieffer, B.; Karatolios, K.; Moller, J.E.; Ribichini, F.L.; et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int. J. Cardiol. 2015, 201, 684–691. [Google Scholar] [CrossRef]
- Baldetti, L.; Beneduce, A.; Chieffo, A.; Scandroglio, A.M. Technical tips for inserting and positioning the Impella device. Card. Interv. Today 2023, 17, 60–65. [Google Scholar]
- Catena, E.; Milazzo, F.; Paino, R.; Pittella, G.; Garatti, A.; Colombo, T.; Mantero, A.; Vitali, E.; Merli, M. Transesophageal and transthoracic echocardiography in the monitoring of a new endovascular left ventricle assist device. Ital. Heart J. Suppl. 2003, 4, 428–432. [Google Scholar] [PubMed]
- Badiye, A.P.; Hernandez, G.A.; Novoa, I.; Chaparro, S.V. Incidence of Hemolysis in Patients with Cardiogenic Shock Treated with Impella Percutaneous Left Ventricular Assist Device. ASAIO J. 2016, 62, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Van Edom, C.J.; Gramegna, M.; Baldetti, L.; Beneduce, A.; Castelein, T.; Dauwe, D.; Frederiks, P.; Giustino, G.; Jacquemin, M.; Janssens, S.P.; et al. Management of Bleeding and Hemolysis During Percutaneous Microaxial Flow Pump Support: A Practical Approach. JACC Cardiovasc. Interv. 2023, 16, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Vandenbriele, C.; Arachchillage, D.J.; Frederiks, P.; Giustino, G.; Gorog, D.A.; Gramegna, M.; Janssens, S.; Meyns, B.; Polzin, A.; Scandroglio, M.; et al. Anticoagulation for Percutaneous Ventricular Assist Device-Supported Cardiogenic Shock: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 1949–1962. [Google Scholar] [CrossRef]
- Al-Ayoubi, A.M.; Bhavsar, K.; Hobbs, R.A.; Smith, K.L.; Lee, L.Y.; Ikegami, H.; Singhal, A.K. Use of Sodium Bicarbonate Purge Solution in Impella Devices for Heparin-Induced Thrombocytopenia. J. Pharm. Pract. 2023, 36, 1035–1038. [Google Scholar] [CrossRef]
- Esposito, M.L.; Morine, K.J.; Annamalai, S.K.; O’Kelly, R.; Aghili, N.; Pedicini, R.; Breton, C.; Mullin, A.; Hamadeh, A.; Kiernan, M.S.; et al. Increased Plasma-Free Hemoglobin Levels Identify Hemolysis in Patients With Cardiogenic Shock and a Trans valvular Micro-Axial Flow Pump. Artif. Organs 2019, 43, 125–131. [Google Scholar] [CrossRef]
- Pieri, M.; Sorrentino, T.; Oppizzi, M.; Melisurgo, G.; Lembo, R.; Colombo, A.; Zangrillo, A.; Pappalardo, F. The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J. Interv. Cardiol. 2018, 31, 717–724. [Google Scholar] [CrossRef]
- Roberts, N.; Chandrasekaran, U.; Das, S.; Qi, Z.; Corbett, S. Hemolysis associated with Impella heart pump positioning: In vitro hemolysis testing and computational fluid dynamics modeling. Int. J. Artif. Organs 2020, 43, 391398820909843. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, M.; Ranka, S.; Chehab, O.; Mishra, T.; Akintoye, E.; Adegbala, O.; Yassin, A.S.; Ando, T.; Thayer, K.L.; Shah, P.; et al. Incidence and clinical outcomes of bleeding complications and acute limb ischemia in STEMI and cardiogenic shock. Catheter. Cardiovasc. Interv. 2021, 97, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Lemor, A.; Hosseini Dehkordi, S.H.; Basir, M.B.; Villablanca, P.A.; Jain, T.; Koenig, G.C.; Alaswad, K.; Moses, J.W.; Kapur, N.K.; O’Neill, W. Impella Versus Extracorporeal Membrane Oxygenation for Acute Myocardial Infarction Cardiogenic Shock. Cardiovasc. Revasc. Med. 2020, 21, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Vetrovec, G.W.; Kaki, A.; Dahle, T.G. A Review of Bleeding Risk with Impella-supported High-risk Percutaneous Coronary Intervention. Heart Int. 2020, 14, 92–99. [Google Scholar] [CrossRef]
- Saito, Y.; Tateishi, K.; Toda, K.; Matsumiya, G.; Kobayashi, Y.; J-PVAD Registry Study Investigators. Complications and Outcomes of Impella Treatment in Cardiogenic Shock Patients With and Without Acute Myocardial Infarction. J. Am. Heart Assoc. 2023, 12, e030819. [Google Scholar] [CrossRef]
- Abaunza, M.; Kabbani, L.S.; Nypaver, T.; Greenbaum, A.; Balraj, P.; Qureshi, S.; Alqarqaz, M.A.; Shepard, A.D. Incidence and prognosis of vascular complications after percutaneous placement of left ventricular assist device. J. Vasc. Surg. 2015, 62, 417–423. [Google Scholar] [CrossRef]
- Tan, S.R.; Low, C.J.W.; Ng, W.L.; Ling, R.R.; Tan, C.S.; Lim, S.L.; Cherian, R.; Lin, W.; Shekar, K.; Mitra, S.; et al. Microaxial Left Ventricular Assist Device in Cardiogenic Shock: A Systematic Review and Meta-Analysis. Life 2022, 12, 1629. [Google Scholar] [CrossRef]
- Ancona, M.B.; Montorfano, M.; Masiero, G.; Burzotta, F.; Briguori, C.; Pagnesi, M.; Pazzanese, V.; Trani, C.; Piva, T.; De Marco, F.; et al. Device-related complications after Impella mechanical circulatory support implantation: An IMP-IT observational multicentre registry substudy. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 999–1006. [Google Scholar] [CrossRef]
- Sinning, J.M.; Ibrahim, K.; Schroder, J.; Sef, D.; Burzotta, F. Optimal bail-out and complication management strategies in protected high-risk percutaneous coronary intervention with the Impella. Eur. Heart J. Suppl. 2022, 24, J37–J42. [Google Scholar] [CrossRef] [PubMed]
- Richarz, S.; Siegemund, M.; d’Amico, R.; Bachofen, B.; Dobele, T.; Gurke, L.; Mujagic, E. Temporary Extracorporeal Femoro-Femoral Crossover Bypass to Treat Acute Limb Ischemia due to Occlusive Femoral Transaortic Microaxial Left Ventricular Assist Device—A Novel Technique and Case Series. Ann. Vasc. Surg. 2022, 80, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Buda, K.G.; Hryniewicz, K.; Eckman, P.M.; Basir, M.B.; Cowger, J.A.; Alaswad, K.; Mukundan, S.; Sandoval, Y.; Elliott, A.; Brilakis, E.S.; et al. Early vs. delayed mechanical circulatory support in patients with acute myocardial infarction and cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 390–397. [Google Scholar] [CrossRef]
- Iannaccone, M.; Franchin, L.; Hanson, I.D.; Boccuzzi, G.; Basir, M.B.; Truesdell, A.G.; O’Neill, W. Timing of impella placement in PCI for acute myocardial infarction complicated by cardiogenic shock: An updated meta-analysis. Int. J. Cardiol. 2022, 362, 47–54. [Google Scholar] [CrossRef]
- Delmas, C.; Laine, M.; Schurtz, G.; Roubille, F.; Coste, P.; Leurent, G.; Hraiech, S.; Pankert, M.; Gonzalo, Q.; Dabry, T.; et al. Rationale and design of the ULYSS trial: A randomized multicenter evaluation of the efficacy of early Impella CP implantation in acute coronary syndrome complicated by cardiogenic shock. Am. Heart J. 2023, 265, 203–212. [Google Scholar] [CrossRef]
- Nap, A. UNLOAD-HF Randomised Controlled Trial for Left Ventricular Unloading in Acute Heart Failure. Interv. Cardiol. 2024, 19 (Suppl. S1), 19–20. [Google Scholar] [CrossRef]
- National Library of Medicine. Early Impella® Support in Patients with ST-Segment Elevation Myocardial Infarction Complicated by Cardiogenic Shock: The RECOVER IV Trial. Available online: https://clinicaltrials.gov/study/NCT05506449 (accessed on 7 June 2025).
- Subramaniam, A.V.; Barsness, G.W.; Vallabhajosyula, S.; Vallabhajosyula, S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiol. Ther. 2019, 8, 211–228. [Google Scholar] [CrossRef]
- Feng, I.; Dardik, G.; Kaku, Y.; Zhao, Y.; Del Carmen, H.; DePaolo, J.; Cevasco, M.; Biscotti, M.; Wald, J.W.; Fried, J.A.; et al. Outcomes of prolonged support on surgically implanted microaxial left ventricular assist devices for refractory cardiogenic shock. JTCVS Open 2025, 25, 173–189. [Google Scholar] [CrossRef]
- Miller, P.E.; Huber, K.; Bohula, E.A.; Krychtiuk, K.A.; Poss, J.; Roswell, R.O.; Tavazzi, G.; Solomon, M.A.; Kristensen, S.D.; Morrow, D.A.; et al. Research Priorities in Critical Care Cardiology: JACC Expert Panel. J. Am. Coll. Cardiol. 2023, 82, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Brusca, S.B.; Ko, D.T.; Dhruva, S.S. The Potential Role of Early Left Ventricular Unloading in ST-Segment Elevation Myocardial Infarction-Related Cardiogenic Shock. J. Am. Coll. Cardiol. 2025, 85, 2469–2471. [Google Scholar] [CrossRef]
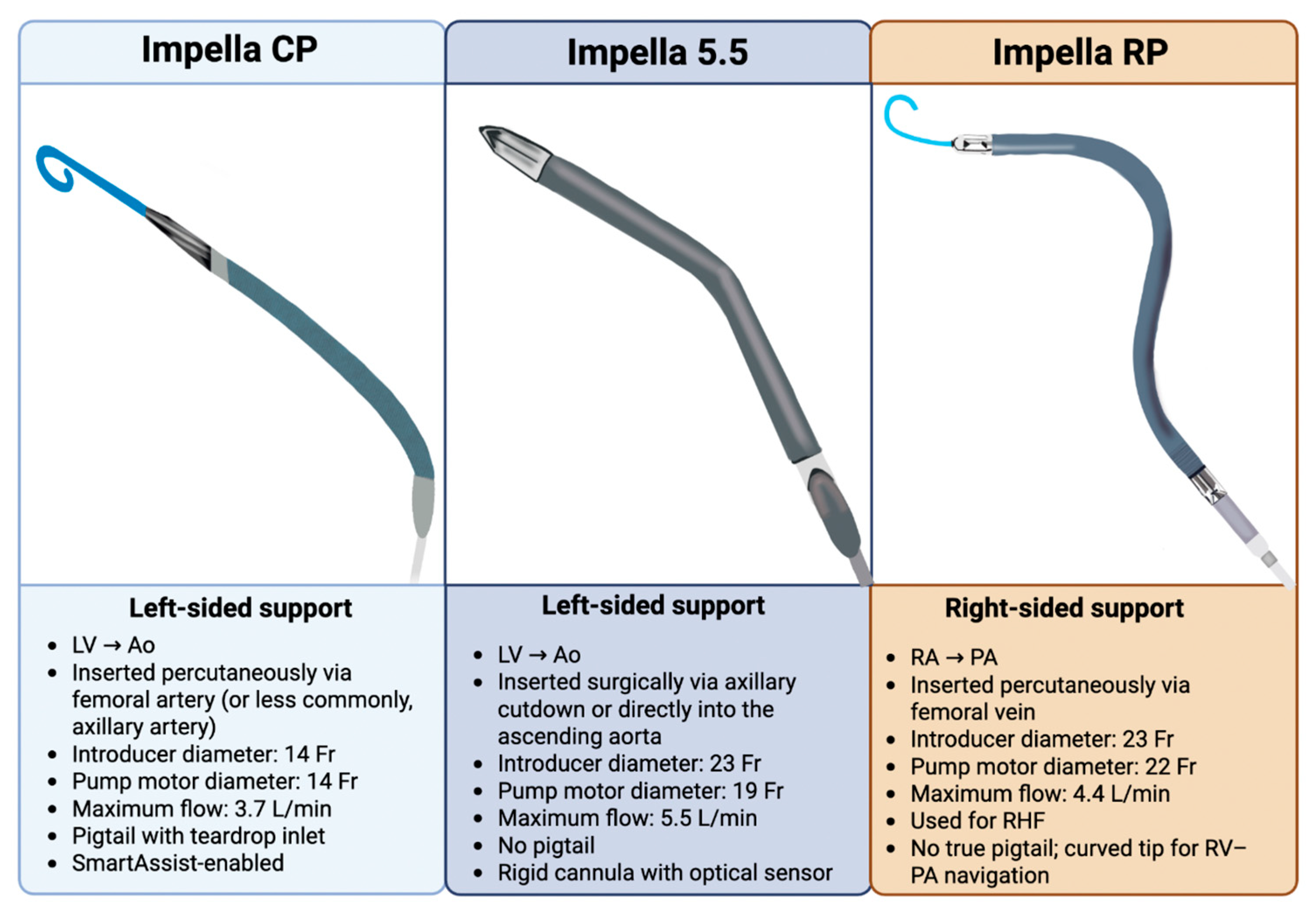




| Impella 2.5 (Historical) | Impella CP (Current) | Impella 5.0 (Historical) | Impella LD (Historical) | Impella 5.5 (Current) | |
|---|---|---|---|---|---|
| Indication | HR-PCI and CS | HR-PCI and CS | CS | CS | CS |
| Access | Percutaneous femoral or axillary 1 | Percutaneous femoral or axillary | Femoral or axillary cutdown | Direct insertion into AA | Axillary cutdown or direct insertion into AA |
| Motor Size (Fr) | 12 | 14 | 21 | 21 | 19 |
| Catheter Size (Fr) | 9 | 9 | 9 | 9 | 9 |
| Sheath Size (Fr) | 13 | 14 | 23 | 23 | 23 |
| Max Flow Rate (L/min) | 2.5 | 3.7 | 5 | 5.3 | 5.5 |
| Max Speed (rpm) | 51,000 | 51,000 | 33,000 | 33,000 | 33,000 |
| Performance Levels | P0–P9 | P0–P9 | P0–P9 | P0–P9 | P0–P9 |
| Duration of support | HR-PCI: ≤6 h/ CS: ≤4 d | HR-PCI: ≤6h/ CS: ≤4d | 14 days | 14 days | 14 days |
| SmartAssist | No | Yes | No | No | Yes |
| Pressure Sensor | Fluid transducer | Optical sensor | Fluid transducer | Fluid transducer | Optical sensor |
| Guide Wire | 0.018″ diameter × 260 cm placement guidewire | Wireless possible if direct insertion | |||
| Anticoagulation (AC) | Heparin purge + systemic AC or BBPS alone 2 | ||||
| Common Complications | Hemolysis, limb ischemia, bleeding, stroke, infection | ||||
| Contraindications (CI) | Severe AS/AR, mechanical AV, LV thrombus, CI to AC | ||||
| ISAR-SHOCK | PROTECT II | IMPRESS | DanGer Shock | |
|---|---|---|---|---|
| Enrollment Period | 2004–2007 | 2007–2010 | 2012–2015 | 2013–2023 |
| N (Patients) | 25 | 452 | 48 | 355 |
| Population | AMI-CS revascularized by PCI | HR-PCI in patients with reduced LVEF | STEMI-CS revascularized by PCI | STEMI-CS revascularized by PCI or CABG |
| Exclusion Criteria | -Age < 18 -Valvular disease (MV; severe AR) -Resuscitation > 30 min -CS from AMI-related mechanical complications -RV failure -Other conditions (HOCM, LV thrombus, cerebral disease, PE, sepsis, coagulopathy, bleeding requiring surgery, pregnancy) | -Recent MI with persistent elevated cardiac enzymes -LV thrombus -Platelet count ≤ 75,000/mm3 -Creatinine ≥ 4 mg/dL (Dialysis patients were eligible) -Severe PVD | -Severe aorto-iliac arterial disease -severe aortic valvular disease -life expectancy of <1 year -Prior study participation (within 30 days) or recent CABG (within 1 week) | -Shock > 24 h -Mechanical MI complications -Severe AV disease or MV -Already established MCS (Impella or VA-ECMO) -Life expectancy of <1 year -Other shock causes (hypovolemia, sepsis, pulmonary embolism, or anaphylaxis) -Other conditions (Severe PAD; LV thrombus; IE; RV failure; OHCA with persistent GCS < 8; HIT) |
| Inclusion Criteria | -AMI within 48 h complicated by CS | -Age ≥ 18 -nonemergent PCI (ULM or last patent vessel with a LVEF ≤ 35%) -3xVessel disease with LVEF < 30% | -STEMI-CS undergoing immediate PCI -Mechanically ventilated before randomization | -Age ≥ 18 years -STEMI-CS |
| Impella Use | After PCI | During PCI | Before/immediately after PCI | Before/after PCI |
| Control | IABP (post PCI) | IABP (during PCI) | IABP (before or immediately after PCI) | Standard care |
| Key Endpoints | -CI at 30 min post-implantation -30 d all-cause mortality | -30 and 90 d major adverse events (all-cause mortality, stroke, repeat revascularization) | -30 d and 6 mo all-cause mortality | -180 d all-cause mortality -Need for additional MCS or heart transplant |
| Main Findings | -↑ CI, -No 30 d mortality difference | -No 3 d difference -Trend toward better 90 d outcomes | -No difference in 30 d or 6 mo all-cause mortality | -↓ 180 d all-cause mortality in Impella group |
| SCAI Stage | Primarily stage C | N/A | Primarily stage C | Primarily stage E |
| STEMI (%) | N/A | No | 100% | 100% |
| Lactate (mmol/L) | 6.2 | N/A | 7.5 ± 3.2 (Impella) and 8.9 ± 6.6 (IABP) | 4.5 (3.3–7.1) |
| MCS Timing | 100% post-revascularization | 100% pre-revascularization | 80% (Impella) and 88% (IABP) post-revascularization | 46.9% pre-revascularization |
| PAC Use | N/A | No | N/A | 68% |
| Crossover | 1 patient in mAFP arm did not receive it | N/A | Crossover or upgrading 4.2% mAFP and 12.5% IABP | 1.7% crossed over to mAFP |
| Escalation | N/A | N/A | 1 bridged to surgical LVAD in IABP arm | 5.6% (Impella) and 2.3% (standard of care) bridged to LVAD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhat, K.; Pollanen, S.; Damrongwatanasuk, R.; DiChiacchio, L.; Salerno, C.; Sikand, N.; Khalife, W.I.; Hu, J.-R. The Role of Impella in Cardiogenic Shock in the Post-DanGer Shock Era. Biomedicines 2025, 13, 2198. https://doi.org/10.3390/biomedicines13092198
Farhat K, Pollanen S, Damrongwatanasuk R, DiChiacchio L, Salerno C, Sikand N, Khalife WI, Hu J-R. The Role of Impella in Cardiogenic Shock in the Post-DanGer Shock Era. Biomedicines. 2025; 13(9):2198. https://doi.org/10.3390/biomedicines13092198
Chicago/Turabian StyleFarhat, Kassem, Sara Pollanen, Rongras Damrongwatanasuk, Laura DiChiacchio, Colby Salerno, Nikhil Sikand, Wissam I. Khalife, and Jiun-Ruey Hu. 2025. "The Role of Impella in Cardiogenic Shock in the Post-DanGer Shock Era" Biomedicines 13, no. 9: 2198. https://doi.org/10.3390/biomedicines13092198
APA StyleFarhat, K., Pollanen, S., Damrongwatanasuk, R., DiChiacchio, L., Salerno, C., Sikand, N., Khalife, W. I., & Hu, J.-R. (2025). The Role of Impella in Cardiogenic Shock in the Post-DanGer Shock Era. Biomedicines, 13(9), 2198. https://doi.org/10.3390/biomedicines13092198







