Advanced Immunomodulation in Rheumatoid Arthritis: Immune Checkpoints, microRNAs, and Cell-Based Therapies
Abstract
1. Introduction
Immune Cell Activation and Loss of Tolerance in RA
2. Materials and Methods
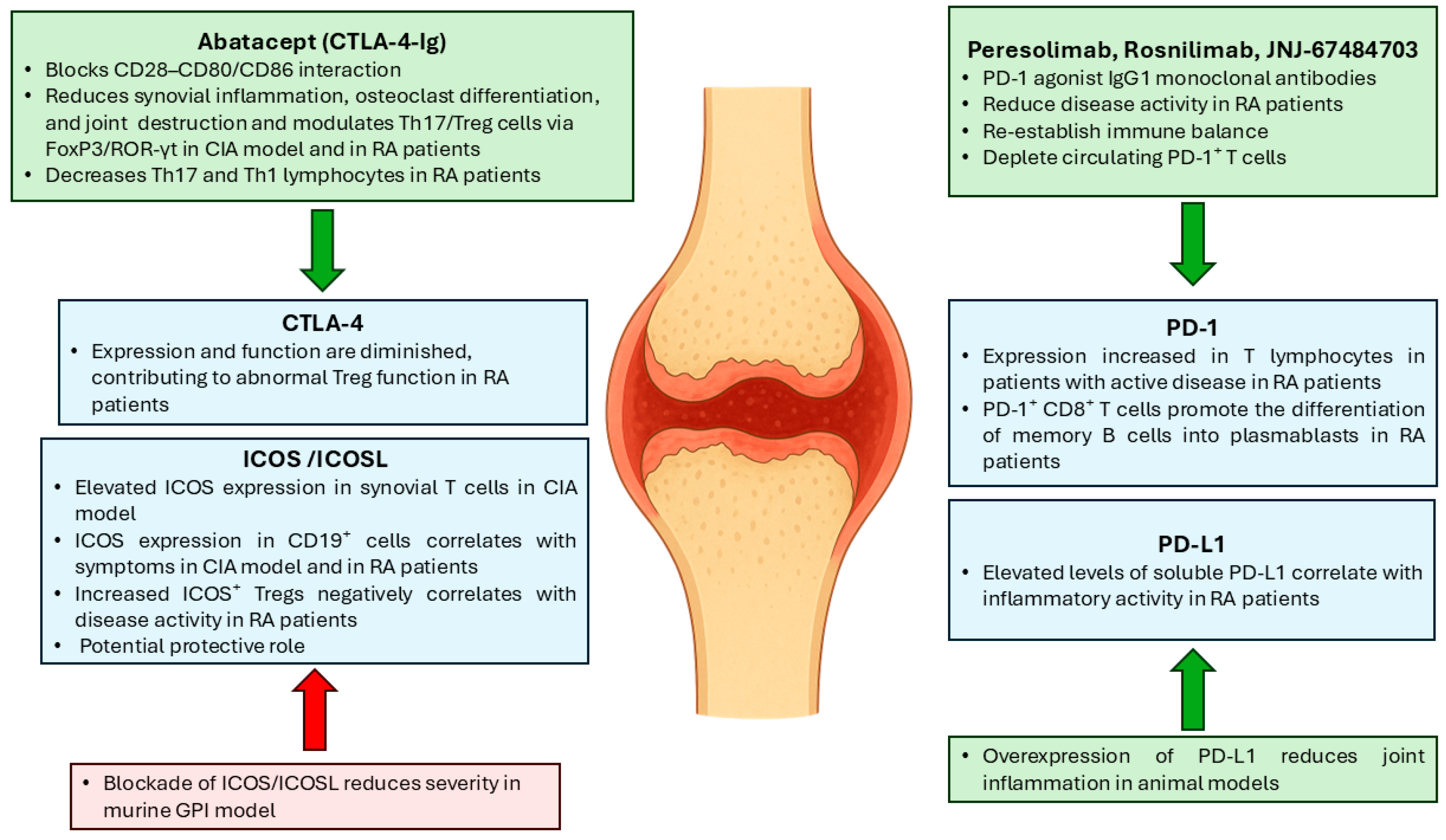
3. Immune Checkpoint Modulation in RA
3.1. CTLA-4
3.2. PD-1/PD-L1
3.3. ICOS
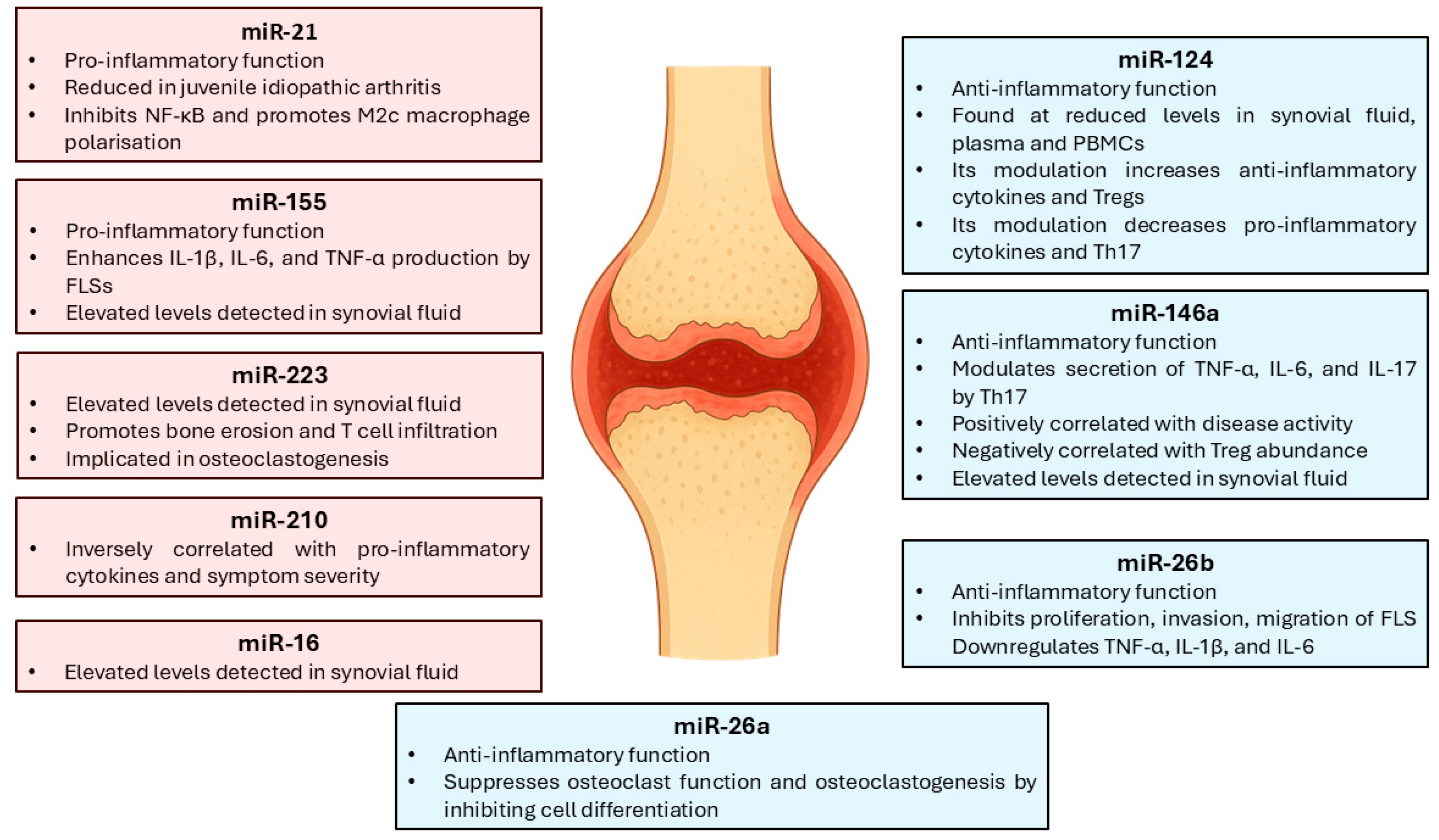
4. Immune Regulation and Clinical Applications of miRNAs in RA
4.1. Pro-Inflammatory and Anti-Inflammatory miRNAs
4.2. Diagnostic, Prognostic, and Therapeutic Response Biomarkers
5. Immunomodulatory Cell-Based Therapies
5.1. CAR-T Cells
5.2. Treg Cells
5.3. TolDCs
5.4. MSCs and Extracellular Vesicles
6. Therapeutic Perspectives and Challenges in RA Immunomodulation
6.1. Combination Strategies: Cell-Based Therapy, Immune Checkpoints, and miRNAs
6.2. Current Limitations of Traditional Therapies
6.3. Towards Personalised Immunotherapy in RA
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACPA | Anti-cyclic citrullinated peptide antibody |
| ACR | American College of Rheumatology |
| ADAb | Anti-drug antibody |
| AGO | Argonaute |
| AXL | AXL receptor tyrosine kinase |
| bDMARD | Biological DMARD |
| CAR | Chimeric antigen receptor |
| CFA | Complete Freund’s adjuvant |
| CIA | Collagen-induced arthritis |
| Col-Treg | Collagen type II-specific Tr1 cell |
| CRP | C reactive protein |
| csDMARD | Conventional synthetic DMARD |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| CXCL | CXC motif chemokine ligand |
| DAS | Disease activity score |
| DC | Dendritic cell |
| DGCR8 | DiGeorge syndrome critical region 8 |
| DMARD EMA | Disease-modifying antirheumatic drug European Medicines Agency |
| ESR | Erythrocyte sedimentation rate |
| EULAR | European League Against Rheumatism |
| FCGR3A FDA | Fc fragment of IgG receptor IIIa Food and Drug Administration |
| FLS | Fibroblast-like synoviocyte |
| FoxP3 | Forkhead box P3 |
| GPI | Glucose-6-phosphate isomerase |
| ICOS | Inducible T-cell costimulator |
| ICOSL | Inducible T-cell costimulatory ligand |
| IFITM3 | IFN-induced transmembrane 3 |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| IL-6R | Interleukin-6 receptor |
| irAEs | Immune-related adverse events |
| JAK | Janus kinase |
| JIA JNJ | Juvenile idiopathic arthritis Johnson & Johnson |
| LAG-3 | Lymphocyte-activation gene 3 |
| miRNA miRNA* | microRNA Passenger strand |
| MMP | Matrix metalloproteinase |
| MRI | Magnetic resonance imaging |
| MSC | Mesenchymal stromal cell |
| MSC-sEV | MSC-derived small extracellular vesicle |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer cell |
| ORAL Surveillance | Safety study of tofacitinib versus tumour necrosis factor (TNF) inhibitor in subjects with rheumatoid arthritis |
| PBMC | Peripheral blood mononuclear cell |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed death-ligand 1 |
| Pre-miRNA | Precursor miRNA |
| Pri-miRNA | Primary miRNA |
| RA | Rheumatoid arthritis |
| RASF | Rheumatoid arthritis synovial fibroblast |
| RF | Rheumatoid factor |
| RISC | RNA-induced silencing complex |
| RORγT | Retinoic acid receptor-related orphan receptor gamma t |
| scRNA-seq | Single-cell RNA sequencing |
| SPP1 | Secreted phosphoprotein 1 |
| Tfh | Follicular helper T cell |
| TGF | Transforming growth factor |
| Th | T helper cell |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| TIM | T-cell immunoglobulin and mucin domain-containing protein |
| TLR | Toll-like receptor |
| TNF-α | Tumour necrosis factor alpha |
| TolDC | Tolerogenic dendritic cell |
| TRBP | Transactivation RNA-binding protein |
| Treg | Regulatory T cell |
| tsDMARD | Targeted synthetic DMARD |
References
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Goemaere, S.; Ackerman, C.; Goethals, K.; De Keyser, F.; Van Der Straeten, C.; Verbruggen, G.; Mielants, H.; Veys, E.M. Onset of symptoms of rheumatoid arthritis in relation to age, sex and menopausal transition. J. Rheumatol. 1990, 17, 1620–1622. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, R.F. Sex differences in rheumatoid arthritis: More than meets the eye. BMC Med. 2009, 7, 12. [Google Scholar] [CrossRef]
- Sudoł -Szopińska, I.; Kontny, E.; Maśliński, W.; Prochorec-Sobieszek, M.; Kwiatkowska, B.; Zaniewicz -Kaniewska, K.; Warczyńska, A. The pathogenesis of rheumatoid arthritis in radiological studies. Part I: Formation of inflammatory infiltrates within the synovial membrane. J. Ultrason. 2012, 12, 202–213. [Google Scholar] [CrossRef]
- Dick, B.; Eccleston, C.; Crombez, G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Care Res. 2002, 47, 639–644. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1310–1314. [Google Scholar] [CrossRef]
- Jordan, E.; Gallicchio, V.S. Stem Cell Therapy as a Treatment Method for Rheumatoid Arthritis. Stem. Cell Regen. Med. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.; Hansen, M.; Stoltenberg, M.; Gideon, P.; Klarlund, M.; Jensen, K.E.; Lorenzen, I. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999, 42, 918–929. [Google Scholar] [CrossRef]
- Tan, Y.K.; Moorakonda, R.B.; Allen, J.C.; Chew, L.C.; Thumboo, J. Back to the basics: Understanding joint swelling and tenderness at the wrist in rheumatoid arthritis through the use of ultrasonography. Int. J. Rheum. Dis. 2019, 22, 68–72. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2022, 82, 3–18. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Cohen, S.B. Oral surveillance and JAK inhibitor safety: The theory of relativity. Nat. Rev. Rheumatol. 2022, 18, 301–304. [Google Scholar] [CrossRef]
- Balsa, A.; Cabezón, A.; Orozco, G.; Cobo, T.; Miranda-Carus, E.; López-Nevot, M.A.; Vicario, J.L.; Martín-Mola, E.; Martín, J.; Pascual-Salcedo, D. Influence of HLA DRB1 alleles in the susceptibility of rheumatoid arthritis and the regulation of antibodies against citrullinated proteins and rheumatoid factor. Arthritis Res. Ther. 2010, 12, 1–8. [Google Scholar] [CrossRef]
- Huber, L.C.; Brock, M.; Hemmatazad, H.; Giger, O.T.; Moritz, F.; Trenkmann, M.; Distler, J.H.W.; Gay, R.E.; Kolling, C.; Moch, H.; et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007, 56, 1087–1093. [Google Scholar] [CrossRef]
- Rantapää-Dahlqvist, S.; De Jong, B.A.W.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies Against Cyclic Citrullinated Peptide and IgA Rheumatoid Factor Predict the Development of Rheumatoid Arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Ortells, J.M.; Pérez-García, V.; Marín-Alberca, G.; Peris-Pertusa, A.; Benito, J.M.; Marco, F.M.; Zubcoff, J.J.; Navarro-Blasco, F.J. Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to Disease Activity Score-28. Autoimmunity 2009, 42, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, S.; Manzo, A.; Montecucco, C.; Caporali, R. The clinical value of autoantibodies in rheumatoid arthritis. Front. Med. 2018, 5, 339. [Google Scholar] [CrossRef]
- Kaur, J.; Cairns, E.; Barra, L. Restoring Balance: Immune Tolerance in Rheumatoid Arthritis. J. Rheumatol. 2023, 50, 991–1001. [Google Scholar] [CrossRef]
- Mitsdoerffer, M.; Lee, Y.; Jäger, A.; Kim, H.J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation-lessons from rheumatoid arthritis. Front. Immunol. 2019, 10, 353. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Huang, J.; Luo, D.; Lv, S.; Lu, X.; Xiao, C. Dysfunctions, Molecular Mechanisms, and Therapeutic Strategies of Regulatory T Cells in Rheumatoid Arthritis. Front. Pharmacol. 2021, 12, 716081. [Google Scholar] [CrossRef] [PubMed]
- Noss, E.H.; Brenner, M.B. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008, 223, 252–270. [Google Scholar] [CrossRef]
- Tolboom, T.C.A.; Pieterman, E.; van der Laan, W.H.; Toes, R.E.M.; Huidekoper, A.L.; Nelissen, R.G.H.H.; Breedveld, F.C.; Huizinga, T.W.J. Invasive properties of fibroblast-like synoviocytes: Correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann. Rheum. Dis. 2002, 61, 975–980. [Google Scholar] [CrossRef]
- Mahmoud, D.E.; Kaabachi, W.; Sassi, N.; Tarhouni, L.; Rekik, S.; Jemmali, S.; Sehli, H.; Kallel-Sellami, M.; Cheour, E.; Laadhar, L. The synovial fluid fibroblast-like synoviocyte: A long-neglected piece in the puzzle of rheumatoid arthritis pathogenesis. Front. Immunol. 2022, 13, 942417. [Google Scholar] [CrossRef] [PubMed]
- Sukkaew, A.; Suksatu, A.; Roytrakul, S.; Smith, D.R.; Ubol, S. Proteomic analysis of CHIKV-infected human fibroblast-like synoviocytes: Identification of host factors potentially associated with CHIKV replication and cellular pathogenesis. Microbiol. Immunol. 2020, 64, 445–457. [Google Scholar] [CrossRef]
- Tan, J.; Tan, L.; Huang, H.; Li, B. Expression of miR-146a in Th17 cells from rheumatoid arthritis patients and its correlation with inflammatory cytokines. Immunobiology 2025, 230, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.L.; Shi, G.R.; Xie, J.; Du, X.Z.; Yang, H. MicroRNA-27a inhibits cell migration and invasion of fibroblast-like synoviocytes by targeting follistatin-like protein 1 in rheumatoid arthritis. Mol. Cells 2016, 39, 611–618. [Google Scholar] [CrossRef]
- Pascual-García, S.; Martínez-Peinado, P.; Pujalte-Satorre, C.; Navarro-Sempere, A.; Esteve-Girbés, J.; López-Jaén, A.B.; Javaloyes-Antón, J.; Cobo-Velacoracho, R.; Navarro-Blasco, F.J.; Sempere-Ortells, J.M. Exosomal Osteoclast-Derived miRNA in Rheumatoid Arthritis: From Their Pathogenesis in Bone Erosion to New Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 1506. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Liu, P.C.; Ssu, C.T.; Tsao, Y.P.; Liou, T.L.; Tsai, C.Y.; Chou, C.T.; Chen, M.H.; Leu, C.M. Cytotoxic T lymphocyte-associated antigen-4-Ig (CTLA-4-Ig) suppresses Staphylococcus aureus-induced CD80, CD86, and pro-inflammatory cytokine expression in human B cells. Arthritis Res. Ther. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Hossen, M.M.; Ma, Y.; Yin, Z.; Xia, Y.; Du, J.; Huang, J.Y.; Huang, J.J.; Zou, L.; Ye, Z.; Huang, Z. Current understanding of CTLA-4: From mechanism to autoimmune diseases. Front. Immunol. 2023, 14, 1198365. [Google Scholar] [CrossRef] [PubMed]
- Flores-Borja, F.; Jury, E.C.; Mauri, C.; Ehrenstein, M.R. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2008, 105, 19396–19401. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; Giampà, E.; Chimenti, M.S.; Kroegler, B.; Perricone, R. Effects of Abatacept on T-Lymphocyte Sub-populations and Immunoglobulins in Patients Affected by Rheumatoid Arthritis. Isr. Med. Assoc. J. 2017, 19, 406–410. [Google Scholar]
- Jansen, D.T.S.L.; el Bannoudi, H.; Arens, R.; Habets, K.L.L.; Hameetman, M.; Huizinga, T.W.J.; Stoop, J.N.; Toes, R.E.M. Abatacept decreases disease activity in a absence of CD4+ T cells in a collagen-induced arthritis model. Arthritis Res. Ther. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Tada, Y.; Ono, N.; Suematsu, R.; Tashiro, S.; Sadanaga, Y.; Tokuda, Y.; Ono, Y.; Nakao, Y.; Maruyama, A.; Ohta, A.; et al. The balance between Foxp3 and Ror-γt expression in peripheral blood is altered by tocilizumab and abatacept in patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2016, 17, 290. [Google Scholar] [CrossRef]
- Goutakoli, P.; Papadaki, G.; Repa, A.; Avgoustidis, N.; Kalogiannaki, E.; Flouri, I.; Bertsias, A.; Zoidakis, J.; Samiotaki, M.; Bertsias, G.; et al. A Peripheral Blood Signature of Increased Th1 and Myeloid Cells Combined with Serum Inflammatory Mediators Is Associated with Response to Abatacept in Rheumatoid Arthritis Patients. Cells 2023, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 1–50. [Google Scholar] [CrossRef]
- Li, H.X.; Zheng, C.; Han, J.; Zhu, J.; Liu, S.; Jin, T. PD-1/PD-L1 Axis as a Potential Therapeutic Target for Multiple Sclerosis: A T Cell Perspective. Front. Cell. Neurosci. 2021, 15, 716747. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Ye, J.; Zeng, L.; Luo, Z.; Deng, Z.; Li, X.; Guo, Y.; Huang, Z.; Li, J. Elevated expression of PD-1 on T cells correlates with disease activity in rheumatoid arthritis. Mol. Med. Rep. 2018, 17, 3297–3305. [Google Scholar] [CrossRef]
- Wu, X.; Xu, L.; Cheng, Q.; Nie, L.; Zhang, S.; Du, Y.; Xue, J. Increased serum soluble programmed death ligand 1(sPD-L1) is associated with the presence of interstitial lung disease in rheumatoid arthritis: A monocentric cross-sectional study. Respir. Med. 2020, 166, 1–5. [Google Scholar] [CrossRef]
- Higashioka, K.; Yoshimura, M.; Sakuragi, T.; Ayano, M.; Kimoto, Y.; Mitoma, H.; Ono, N.; Arinobu, Y.; Kikukawa, M.; Yamada, H.; et al. Human PD-1hiCD8+ T Cells Are a Cellular Source of IL-21 in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 654623. [Google Scholar] [CrossRef]
- Li, W.; Sun, J.; Feng, S.L.; Wang, F.; Miao, M.Z.; Wu, E.Y.; Wallet, S.; Loeser, R.; Li, C. Intra-articular delivery of AAV vectors encoding PD-L1 attenuates joint inflammation and tissue damage in a mouse model of rheumatoid arthritis. Front. Immunol. 2023, 14, 1116084. [Google Scholar] [CrossRef]
- Tuttle, J.; Drescher, E.; Simón-Campos, J.A.; Emery, P.; Greenwald, M.; Kivitz, A.; Rha, H.; Yachi, P.; Kiley, C.; Nirula, A. A Phase 2 Trial of Peresolimab for Adults with Rheumatoid Arthritis. New Engl. J. Med. 2023, 388, 1853–1862. [Google Scholar] [CrossRef]
- Afshari, A.; Khorramdelazad, H.; Abbasifard, M. Toward immune tolerance in rheumatoid arthritis: Emerging immunotherapies and targets for long-term remission. Int. Immunopharmacol. 2025, 162, 1–27. [Google Scholar] [CrossRef]
- Ling, I.; Marciniak, S.; Clarke, S.; Lakshminarayanan, V.; Loza, M.J.; Liva, S.; Wang, T.; Orillion, A.; Tikhonov, I.; Noss, E. POS0597 Safety, Tolerability, and Activity of JNJ-67484703 in Participants with Active Rheumatoid Arthritis: Results of A Multicenter, Double-Blind, Placebo-Controlled, Randomized, Multiple-Dose Phase 1B Study. Ann. Rheum. Dis. 2025, 84, 794–795. [Google Scholar] [CrossRef]
- Canavan, M.; Floudas, A.; Veale, D.J.; Fearon, U. The PD-1:PD-L1 axis in Inflammatory Arthritis. BMC Rheumatol. 2021, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, M.C.; Manzano, N.; Messina, O.; Zylberman, M. Rheumatological adverse events secondary to immune checkpoint inhibitors. Rheumatol. Clin. 2023, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Paulos, C.M.; Carpenito, C.; Plesa, G.; Suhoski, M.M.; Varela-Rohena, A.; Golovina, T.N.; Carroll, R.G.; Riley, J.L.; June, C.H. The Inducible Costimulator (ICOS) Is Critical for the Development of Human TH17 Cells. Sci. Transl. Med. 2010, 2, 55–78. [Google Scholar] [CrossRef]
- Abdeladhim, M.; Karnell, J.L.; Rieder, S.A. In or out of control: Modulating regulatory T cell homeostasis and function with immune checkpoint pathways. Front. Immunol. 2022, 13, 1033705. [Google Scholar] [CrossRef]
- Frey, O.; Meisel, J.; Hutloff, A.; Bonhagen, K.; Bruns, L.; Kroczek, R.A.; Morawietz, L.; Kamradt, T. Inducible costimulator (ICOS) blockade inhibits accumulation of polyfunctional T helper 1/T helper 17 cells and mitigates autoimmune arthritis. Ann. Rheum. Dis. 2010, 69, 1495–1501. [Google Scholar] [CrossRef]
- Panneton, V.; Bagherzadeh Yazdchi, S.; Witalis, M.; Chang, J.; Suh, W.K. ICOS Signaling Controls Induction and Maintenance of Collagen-Induced Arthritis. J. Immunol. 2018, 200, 3067–3076. [Google Scholar] [CrossRef]
- Ding, S.; Sun, Z.; Jiang, J.; Chang, X.; Shen, Y.; Gu, Y.; Liu, C. Inducible costimulator ligand (ICOSL) on CD19+ B cells is involved in immunopathological damage of rheumatoid arthritis (RA). Front. Immunol. 2022, 13, 1015831. [Google Scholar] [CrossRef]
- Wang, H.X.; Kang, X.; Chu, S.; Li, H.; Li, X.; Yin, X.; Qiu, Y.R.; Lai, W. Dysregulated ICOS+ proinflammatory and suppressive regulatory T cells in patients with rheumatoid arthritis. Exp. Ther. Med. 2018, 16, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fan, W.; Ma, L.; Geng, X. MiR-708-5p promotes fibroblast-like synoviocytes’ cell apoptosis and ameliorates rheumatoid arthritis by the inhibition of Wnt3a/β-catenin pathway. Drug Des. Devel. Ther. 2018, 12, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, J.; He, H.; Li, J.; Wu, Y.; Shen, Z. MiR-525-3p mediates antiviral defense to rotavirus infection by targeting nonstructural protein 1. Biochim. Biophys Acta. Mol. Basis Dis. 2017, 1863, 3212–3225. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeon, K.; Lee, J.T.; Kim, S.; Kim, V.N. MicroRNA maturation: Stepwise processing and subcellular localization. EMBO J. 2002, 21, 4663–4670. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.H.; Kim, Y.K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004, 18, 3016–3027. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Görlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Chendrimada, T.P.; Gregory, R.I.; Kumaraswamy, E.; Norman, J.; Cooch, N.; Nishikura, K.; Shiekhattar, R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005, 436, 740–744. [Google Scholar] [CrossRef]
- Ketting, R.F.; Fischer, S.E.J.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H.A. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Boiphys Acta. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef]
- Okamura, K.; Ladewig, E.; Zhou, L.; Lai, E.C. Functional small RNAs are generated from select miRNA hairpin loops in flies and mammals. Genes Dev. 2013, 27, 778–792. [Google Scholar] [CrossRef]
- Szczyrba, J.; Jung, V.; Beitzinger, M.; Nolte, E.; Wach, S.; Hart, M.; Sapich, S.; Wiesehöfer, M.; Taubert, H.; Wennemuth, G.; et al. Analysis of Argonaute Complex Bound mRNAs in DU145 Prostate Carcinoma Cells Reveals New miRNA Target Genes. Prostate Cancer 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Quévillon Huberdeau, M.; Zeitler, D.M.; Hauptmann, J.; Bruckmann, A.; Fressigné, L.; Danner, J.; Piquet, S.; Strieder, N.; Engelmann, J.C.; Jannot, G.; et al. Phosphorylation of Argonaute proteins affects mRNA binding and is essential for micro RNA—Guided gene silencing in vivo. EMBO J. 2017, 36, 2088–2106. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Wade, S.; Floudas, A.; Orr, C.; McGarry, T.; Wade, S.; Cregan, S.; Fearon, U.; Veale, D.J. Serum miRNA Signature in Rheumatoid Arthritis and “At-Risk Individuals”. Front. Immunol. 2021, 12, 633201. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, T.; Duan, S.; Shi, Y.; Li, S.; Zhang, X.; Zhang, L. miR-155 promotes fibroblast-like synoviocyte proliferation and inflammatory cytokine secretion in rheumatoid arthritis by targeting FOXO3a. Exp. Ther. Med. 2020, 19, 1288–1296. [Google Scholar] [CrossRef]
- Roos, J.; Enlund, E.; Funcke, J.B.; Tews, D.; Holzmann, K.; Debatin, K.M.; Wabitsch, M.; Fischer-Posovszky, P. MiR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Su, L.; Duan, X.; Chen, X.; Hays, A.; Upadhyayula, S.; Shivde, J.; Wang, H.; Li, Y.; Huang, D.; et al. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol. Immunol. 2018, 101, 608–614. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef]
- Peacock, O.; Lee, A.C.; Cameron, F.; Tarbox, R.; Vafadar-Isfahani, N.; Tufarelli, C.; Lund, J.N. Inflammation and MiR-21 pathways functionally interact to downregulate PDCD4 in colorectal cancer. PLoS ONE. 2014, 9, e110267. [Google Scholar] [CrossRef]
- Shaikh, F.S.; Siegel, R.J.; Srivastava, A.; Fox, D.A.; Ahmed, S. Challenges and promise of targeting miRNA in rheumatic diseases: A computational approach to identify miRNA association with cell types, cytokines, and disease mechanisms. Front. Immunol. 2024, 14, 1322806. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Zeng, H.S. Regulation of JAK/STAT signal pathway by miR-21 in the pathogenesis of juvenile idiopathic arthritis. World J. Pediatr. 2020, 16, 502–513. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, Y.; Liang, Q.; Ge, C.; Yang, J.; Shan, B.; Liu, Y.; Zhou, X.; Yin, L. Inflammation-Instructed Hierarchical Delivery of IL-4/miR-21 Orchestrates Osteoimmune Microenvironment toward the Treatment of Rheumatoid Arthritis. Adv. Funct. Mater. 2021, 31, 1–14. [Google Scholar] [CrossRef]
- Abdul-Maksoud, R.; Sediq, A.; Kattaia, A.; Elsayed, W.; Ezzeldin, N.; Abdel Galil, S.M.; Ibrahem, R.A. Serum miR-210 and miR-155 expression levels as novel biomarkers for rheumatoid arthritis diagnosis. Br. J. Biomed. Sci. 2017, 74, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Dong, Z.S.; Zheng, S.; Guan, X.; Zhang, L.; Li, L.; Liu, Z. The effects of miR-26b-5p on fibroblast-like synovial cells in rheumatoid arthritis (RA-FLS) via targeting EZH2. Tissue Cell 2021, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, P.; Chen, Y.; Chen, Y.; Yang, J.; Xu, G.; Mao, H.; Qiu, Y. MicroRNA-26b inhibits cell proliferation and cytokine secretion in human RASF cells via the Wnt/GSK-3β/β-catenin pathway. Diagn. Pathol. 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Liu, M.; Ren, T.; Lin, Z.; Hua, M. Upregulated miR-146a Expression in Peripheral Blood Relates to Th17 and Treg Imbalance in Elder Rheumatoid Arthritis Patients. Lifestyle Genom. 2022, 15, 98–106. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, M.F.; Han, Y.F.; Liu, M.Y.; Liu, X.B.; Ma, X.N.; Feng, W.; Lin, C.S.; Liu, Q.P. Kunduan Yimu Decoction affected Th17/Treg balance through microRNA-124 to improve rheumatoid arthritis pathology. Phytomedicine 2024, 135, 1–13. [Google Scholar] [CrossRef]
- Murata, K.; Yoshitomi, H.; Tanida, S.; Ishikawa, M.; Nishitani, K.; Ito, H.; Nakamura, T. Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2010, 12, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, H.; Wang, Z.; Li, K. Associations of miRNA-146a and miRNA-223 with Rheumatoid Arthritis and Their Predictive Values. Int. J. Gen. Med. 2023, 16, 3211–3218. [Google Scholar] [CrossRef]
- Nakasa, T.; Nagata, Y.; Yamasaki, K.; Ochi, M. A mini-review: microRNA in arthritis. Physiol. Genomics. 2011, 43, 566–570. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, Z. Cell-based therapies for rheumatoid arthritis: Opportunities and challenges. Ther. Adv. Musculoskelt. Dis. 2022, 14, 1–21. [Google Scholar] [CrossRef]
- Schett, G.; Mackensen, A.; Mougiakakos, D. CAR T-cell therapy in autoimmune diseases. Lancet. 2023, 402, 2034–2044. [Google Scholar] [CrossRef]
- Whittington, K.B.; Prislovsky, A.; Beaty, J.; Albritton, L.; Radic, M.; Rosloniec, E.F. CD8+ T Cells Expressing an HLA-DR1 Chimeric Antigen Receptor Target Autoimmune CD4+ T Cells in an Antigen-Specific Manner and Inhibit the Development of Autoimmune Arthritis. J. Immunol. 2022, 208, 16–26. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhao, X.; Sheng, J.; Xue, L.; Schett, G.; Shi, C.; Hu, B.; Wang, X.; Chen, Z. Fourth-generation chimeric antigen receptor T-cell therapy is tolerable and efficacious in treatment-resistant rheumatoid arthritis. Cell Res. 2025, 35, 220–223. [Google Scholar] [CrossRef]
- Liu, A.; Cui, Q.; Yang, S. Induced regulatory T cells remain suppressive capability on effector T cells and synovial fibroblasts in collagen-induced arthritis. Immunol. Res. 2023, 71, 628–638. [Google Scholar] [CrossRef]
- Asnagli, H.; Martire, D.; Belmonte, N.; Quentin, J.; Bastian, H.; Boucard-Jourdin, M.; Fall, P.B.; Mausset-Bonnefont, A.L.; Mantello-Moreau, A.; Rouquier, S.; et al. Type 1 regulatory T cells specific for collagen type II as an efficient cell-based therapy in arthritis. Arthritis Res. Ther. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Wu, H.; Chang, X. Therapeutic Effect of Exogenous Regulatory T Cells on Collagen-induced Arthritis and Rheumatoid Arthritis. Cell Transplant. 2020, 29, 1–12. [Google Scholar] [CrossRef]
- Jansen, M.A.A.; Spiering, R.; Ludwig, I.S.; van Eden, W.; Hilkens, C.M.U.; Broere, F. Matured tolerogenic dendritic cells effectively inhibit autoantigen specific CD4+ T cells in a murine arthritis model. Front. Immunol. 2019, 10, 02068. [Google Scholar] [CrossRef]
- Bell, G.M.; Anderson, A.E.; Diboll, J.; Reece, R.; Eltherington, O.; Harry, R.A.; Fouweather, T.; MacDonald, C.; Chadwick, T.; McColl, E.; et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 2017, 76, 227–234. [Google Scholar] [CrossRef]
- Elbasha, Y.I.; Mesbah, N.M.; Abdel-Hamed, A.R.; Abo-Elmatty, D.M.; Bakry, S.; Mansour, A.M.; Elbeialy, A.A. Effect of autologous bone marrow derived mesenchymal stem cells in treatment of rheumatoid arthritis. Transpl. Immunol. 2023, 80, 1–15. [Google Scholar] [CrossRef]
- Vij, R.; Stebbings, K.A.; Kim, H.; Park, H.; Chang, D. Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: A phase I/IIa, open-label, non-randomized pilot trial. Stem. Cell Res. Ther. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Álvaro-Gracia, J.M.; Jover, J.A.; García-Vicuña, R.; Carreño, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martínez-Taboada, V.M.; Taylor, P.; Marsal, S.; et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Clin. Epidemiol. Res. 2017, 76, 196–202. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Lim, S.K. Therapeutic Efficacy of Mesenchymal Stem/Stromal Cell Small Extracellular Vesicles in Alleviating Arthritic Progression by Restoring Macrophage Balance. Biomolecules 2023, 13, 1501. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, J.; Zhang, K.; Zhang, B.; Luo, X.; Sun, H.; Zheng, Z.; Zhu, P. Th1-Like Treg Cells Are Increased But Deficient in Function in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 863753. [Google Scholar] [CrossRef]
- Sahar, A.; Nicorescu, I.; Barran, G.; Paterson, M.; Hilkens, C.M.U.; Lord, P. Tolerogenic dendritic cell reporting: Has a minimum information model made a difference? PeerJ 2023, 11, 1–16. [Google Scholar] [CrossRef]
- Robertson, H.; Li, J.; Kim, H.J.; Rhodes, J.W.; Harman, A.N.; Patrick, E.; Rogers, N.M. Transcriptomic Analysis Identifies A Tolerogenic Dendritic Cell Signature. Front. Immunol. 2021, 12, 733231. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem. Cells Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.S. Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. Dev. Reprod. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Thäte, C.; Woischwill, C.; Brandenburg, G.; Müller, M.; Böhm, S.; Baumgart, J. Non-clinical assessment of safety, biodistribution and tumorigenicity of human mesenchymal stromal cells. Toxicol. Rep. 2021, 8, 1960–1969. [Google Scholar] [CrossRef]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.; Zhao, R.; Guo, W.; Zhu, M.; Xing, W.; Jiang, D.; Liu, C.; Xu, X. Serum IFN-γ levels predict the therapeutic effect of mesenchymal stem cell transplantation in active rheumatoid arthritis. J. Transl. Med. 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Wright, G.P.; Notley, C.A.; Xue, S.A.; Bendle, G.M.; Holler, A.; Schumacher, T.N.; Ehrenstein, M.R.; Stauss, H.J. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc. Natl. Acad. Sci. USA 2009, 106, 19078–19083. [Google Scholar] [CrossRef]
- Pedersen, J.M.; Hansen, A.S.; Skejø, C.; Juul-Madsen, K.; Junker, P.; Hørslev-Petersen, K.; Hetland, M.L.; Stengaard-Pedersen, K.; Østergaard, M.; Møller, B.K.; et al. Lymphocyte activation gene 3 is increased and affects cytokine production in rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 1–13. [Google Scholar] [CrossRef]
- Nozaki, Y.; Akiba, H.; Akazawa, H.; Yamazawa, H.; Ishimura, K.; Kinoshita, K.; Matsumura, I. Inhibition of the TIM-1 and -3 signaling pathway ameliorates disease in a murine model of rheumatoid arthritis. Clin. Exp. Immunol. 2024, 218, 55–64. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, R.; Xia, C.; Feng, J.; Li, W.; Long, Y. Impact of immune checkpoint TIGIT on the activation and function of natural killer cells in rheumatoid arthritis patients. Rheumatology 2025, 305, 1–11. [Google Scholar] [CrossRef]
- Liao, H.; Hsu, P. Immunomodulatory effects of extracellular vesicles from mesenchymal stromal cells. Implication for therapeutic approach in autoimmune diseases. Kaohsiung J. Med. Sci. 2024, 40, 520–529. [Google Scholar] [CrossRef]
- Anderson, A.E.; Swan, D.J.; Wong, O.Y.; Buck, M.; Eltherington, O.; Harry, R.A.; Patterson, A.M.; Pratt, A.G.; Reynolds, G.; Doran, J.P.; et al. Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-β1. Clin. Exp. Immunol. 2017, 187, 113–123. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, J.; Meng, X.; Cui, Y.; Zhu, T.; Sun, F.; Li, Y.; Teng, L. Polyketal Nanoparticles Co-Loaded With miR-124 and Ketoprofen for Treatment of Rheumatoid Arthritis. J. Pharm. Sci. 2021, 110, 2233–2240. [Google Scholar] [CrossRef]
- Han, H.; Xing, J.; Chen, W.; Jia, J.; Li, Q. Fluorinated polyamidoamine dendrimer-mediated miR-23b delivery for the treatment of experimental rheumatoid arthritis in rats. Nat. Commun. 2023, 14, 1–20. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Zheng, X.; Jia, M.; Mei, Z.; Wang, Y.; Zhang, Z.; Zhou, M.; Li, C. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J. Control Release 2022, 341, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Bjornson-Hooper, Z.B.; Fragiadakis, G.K.; Spitzer, M.H.; Chen, H.; Madhireddy, D.; Hu, K.; Lundsten, K.; McIlwain, D.R.; Nolan, G.P. A Comprehensive Atlas of Immunological Differences Between Humans, Mice, and Non-Human Primates. Front. Immunol. 2022, 13, 867015. [Google Scholar] [CrossRef] [PubMed]
- Marco-Bonilla, M.; Fresnadillo, M.; de la Riva-Bueno, M.; Herrero-Beaumont, G.; Largo, R.; Mediero, A. Animal Models in Rheumatoid Arthritis: Is There a Correlation Between Autoantibodies in Human Pathology and Animal Models? Biology 2025, 14, 460. [Google Scholar] [CrossRef]
- Watanabe, R.; Okano, T.; Gon, T.; Yoshida, N.; Fukumoto, K.; Yamada, S.; Hashimoto, M. Difficult-to-treat rheumatoid arthritis: Current concept and unsolved problems. Front. Med. 2022, 9, 1049875. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.; David, T.; Jani, M. Impact of Adverse Events Associated With Medications in the Treatment and Prevention of Rheumatoid Arthritis. Clin. Ther. 2019, 41, 1376–1396. [Google Scholar] [CrossRef]
- Schaeverbeke, T.; Truchetet, M.E.; Kostine, M.; Barnetche, T.; Bannwarth, B.; Richez, C. Immunogenicity of biologic agents in rheumatoid arthritis patients: Lessons for clinical practice. Rheumatology 2016, 55, 210–220. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. New Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.; Cordell, H.J.; Barton, A.; Daly, A.K.; Hyrich, K.L.; Mann, D.A.; Morgan, A.W.; Wilson, A.G.; Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate (BRAGGSS); Isaacs, J.D. Association between anti-tumour necrosis factor treatment response and genetic variants within the TLR and NFκB signalling pathways. Ann. Rheum. Dis. 2010, 69, 1315–1320. [Google Scholar] [CrossRef]
- Pál, I.; Szamosi, S.; Hodosi, K.; Szekanecz, Z.; Váróczy, L. Effect of Fcγ-receptor 3a (FCGR3A) gene polymorphisms on rituximab therapy in Hungarian patients with rheumatoid arthritis. RMD Open. 2017, 3, 1–4. [Google Scholar] [CrossRef]
- Sainz, L.; Riera, P.; Moya, P.; Bernal, S.; Casademont, J.; Díaz-Torné, C.; Millán, A.M.; Park, H.S.; Lasa, A.; Corominas, H. Clinical Value of IL6R Gene Variants as Predictive Biomarkers for Toxicity to Tocilizumab in Patients with Rheumatoid Arthritis. J. Pers. Med. 2023, 13, 61. [Google Scholar] [CrossRef]
- Ciechomska, M.; Bonek, K.; Merdas, M.; Zarecki, P.; Swierkot, J.; Gluszko, P.; Bogunia-Kubik, K.; Maslinski, W. Changes in MiRNA-5196 Expression as a Potential Biomarker of Anti-TNF-α Therapy in Rheumatoid Arthritis and Ankylosing Spondylitis Patients. Arch. Immunol. Ther. Exp. 2018, 66, 389–397. [Google Scholar] [CrossRef]
- Brandt, L.L.N.; Schulze-Koops, H.; Hügle, T.; Nissen, M.J.; von Kempis, J.; Müeller, R.B. Radiographic Progression in Patients with Rheumatoid Arthritis in Clinical Remission or Low Disease Activity: Results from a Swiss National Registry (SCQM). J. Clin. Med. 2024, 13, 7424. [Google Scholar] [CrossRef] [PubMed]
- Binvignat, M.; Miao, B.Y.; Wibrand, C.; Yang, M.M.; Rychkov, D.; Flynn, E.; Nititham, J.; Tamaki, W.; Khan, U.; Carvidi, A.; et al. Single-cell RNA-Seq analysis reveals cell subsets and gene signatures associated with rheumatoid arthritis disease activity. JCI Insight. 2024, 9, 1–20. [Google Scholar] [CrossRef]
- Xia, X.; He, C.; Xue, Z.; Wang, Y.; Qin, Y.; Ren, Z.; Huang, Y.; Luo, H.; Chen, H.N.; Zhang, W.H.; et al. Single cell immunoprofile of synovial fluid in rheumatoid arthritis with TNF/JAK inhibitor treatment. Nat. Commun. 2025, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Whittaker, J.E.; Vekariya, R.; Ramirez-Perez, S.; Gangishetti, U.; Drissi, H.; Bhattaram, P. The distinct transcriptomic signature of the resolution phase fibroblast-like synoviocytes supports endothelial cell dysfunction. Commun. Biol. 2025, 8, 1–16. [Google Scholar] [CrossRef]
- Macdonald, D.; Kirk, D.; Metzler, M.; Nilges, L.M.; Schempp, P.; Wright, J. It’s all very well, in theory: Theoretical perspectives and their applications in contemporary pedagogical research. Quest 2002, 54, 133–156. [Google Scholar] [CrossRef]
- Iqbal, J.D.; Krauthammer, M.; Biller-Andorno, N. The Use and Ethics of Digital Twins in Medicine. J. Law Med. Ethics. 2022, 50, 583–596. [Google Scholar] [CrossRef]
- Niarakis, A.; Laubenbacher, R.; An, G.; Ilan, Y.; Fisher, J.; Flobak, Å.; Reiche, K.; Rodríguez Martínez, M.; Geris, L.; Ladeira, L.; et al. Immune digital twins for complex human pathologies: Applications, limitations, and challenges. npj Syst. Biol. Appl. 2024, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kearns, W.G.; Glick, J.; Baisch, L.; Benner, A.; Brough, D.; Du, L.; Germain, C.; Kearns, L.; Stamoulis, G. Biomimetic Digital Twins and Multiomics: Applications to Rheumatoid Arthritis and the Potential Reclassification of Variants of Unknown Clinical Significance. J. Mol. Diagn. 2025, 27, 256–269. [Google Scholar] [CrossRef]
- Zerrouk, N.; Augé, F.; Niarakis, A. Building a modular and multi-cellular virtual twin of the synovial joint in Rheumatoid Arthritis. npj Digit. Med. 2024, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coras, R.; Narasimhan, R.; Guma, M. Liquid biopsies to guide therapeutic decisions in rheumatoid arthritis. Transl. Res. 2018, 201, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Therapy | Mechanism in RA (Summary) | Experimental Context | References |
|---|---|---|---|
| CAR-T cells | Selectively eliminate autoreactive CD4+ T cells; suppress Th responses, inflammation, autoantibody production, and deplete pathogenic B cells without global immunosuppression. | Murine models | [86,87,88] |
| Treg cells | Attenuate inflammation and joint pathology. Suppress immune activation via anti-inflammatory cytokine release; improve clinical signs. | CIA models and in vitro assays | [89,90,91] |
| Reduce disease severity, B cell activation, and cytokine production; inhibit RASF proliferation and induce apoptosis. | |||
| TolDCs | Induce Treg cells and modulate naïve/effector CD4+ T cells; reduce joint damage. | Synovial fluid-derived cells in murine arthritis models and phase I clinical trial in RA | [92,93] |
| MSC-based therapy | Anti-inflammatory and regenerative effects; reduction in IL-6, IL-10, and TNF-α; improved bone repair and tissue survival. | CFA-induced arthritis in rats | [94,95,96] |
| Clinically reduce joint swelling and tenderness without altering systemic inflammation; well tolerated. | Phase I/IIa clinical trial in RA | ||
| MSC-derived small extracellular vesicle therapy | Alleviate clinical and histological severity; reduce cytokines and complement activation; promote anti-inflammatory macrophage phenotype. | CIA mouse model | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-García, S.; Cobo, R.; Bolinches, J.L.; Ortiz, I.; Viamonte, P.; Sempere-Ortells, J.M.; Martínez-Peinado, P. Advanced Immunomodulation in Rheumatoid Arthritis: Immune Checkpoints, microRNAs, and Cell-Based Therapies. Biomedicines 2025, 13, 2186. https://doi.org/10.3390/biomedicines13092186
Pascual-García S, Cobo R, Bolinches JL, Ortiz I, Viamonte P, Sempere-Ortells JM, Martínez-Peinado P. Advanced Immunomodulation in Rheumatoid Arthritis: Immune Checkpoints, microRNAs, and Cell-Based Therapies. Biomedicines. 2025; 13(9):2186. https://doi.org/10.3390/biomedicines13092186
Chicago/Turabian StylePascual-García, Sandra, Raúl Cobo, José Luis Bolinches, Iván Ortiz, Pedro Viamonte, José Miguel Sempere-Ortells, and Pascual Martínez-Peinado. 2025. "Advanced Immunomodulation in Rheumatoid Arthritis: Immune Checkpoints, microRNAs, and Cell-Based Therapies" Biomedicines 13, no. 9: 2186. https://doi.org/10.3390/biomedicines13092186
APA StylePascual-García, S., Cobo, R., Bolinches, J. L., Ortiz, I., Viamonte, P., Sempere-Ortells, J. M., & Martínez-Peinado, P. (2025). Advanced Immunomodulation in Rheumatoid Arthritis: Immune Checkpoints, microRNAs, and Cell-Based Therapies. Biomedicines, 13(9), 2186. https://doi.org/10.3390/biomedicines13092186







