Advanced Gene-Expression Analysis of Skeletal Muscles Focusing on Normal, Glucose-Intolerant, and Diabetic Individuals with Type 2 Diabetes
Abstract
1. Introduction
2. Methods
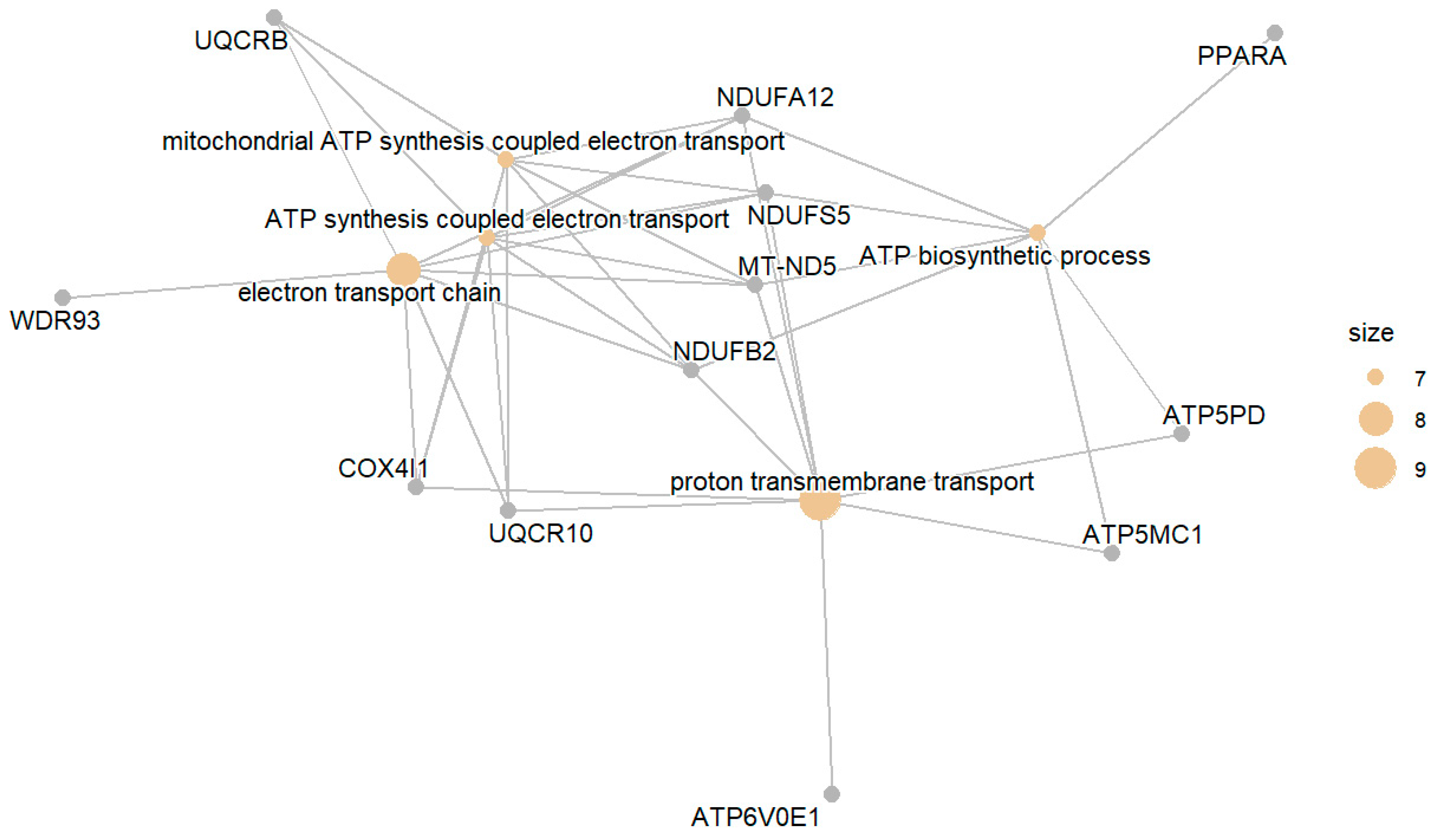
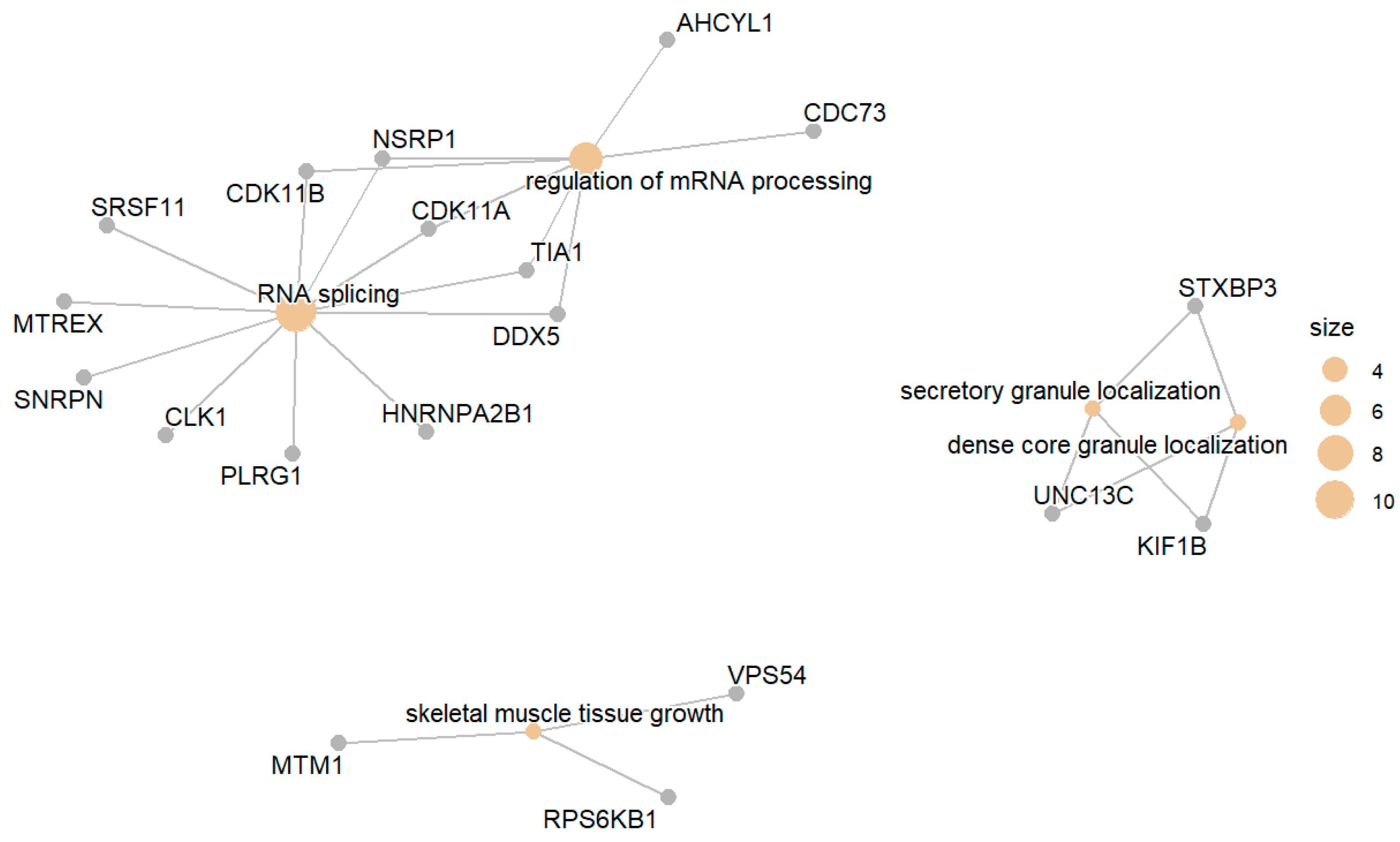
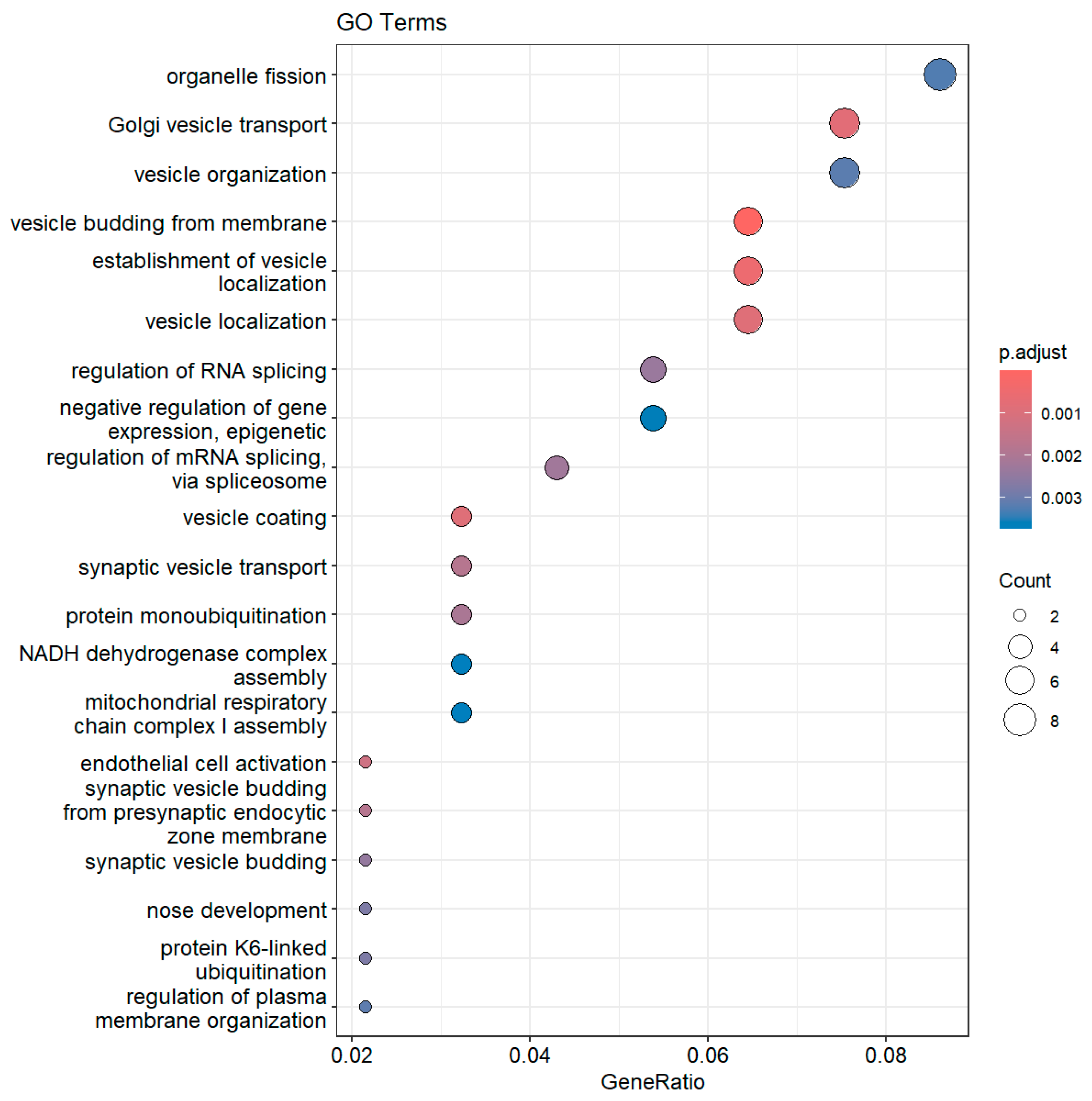
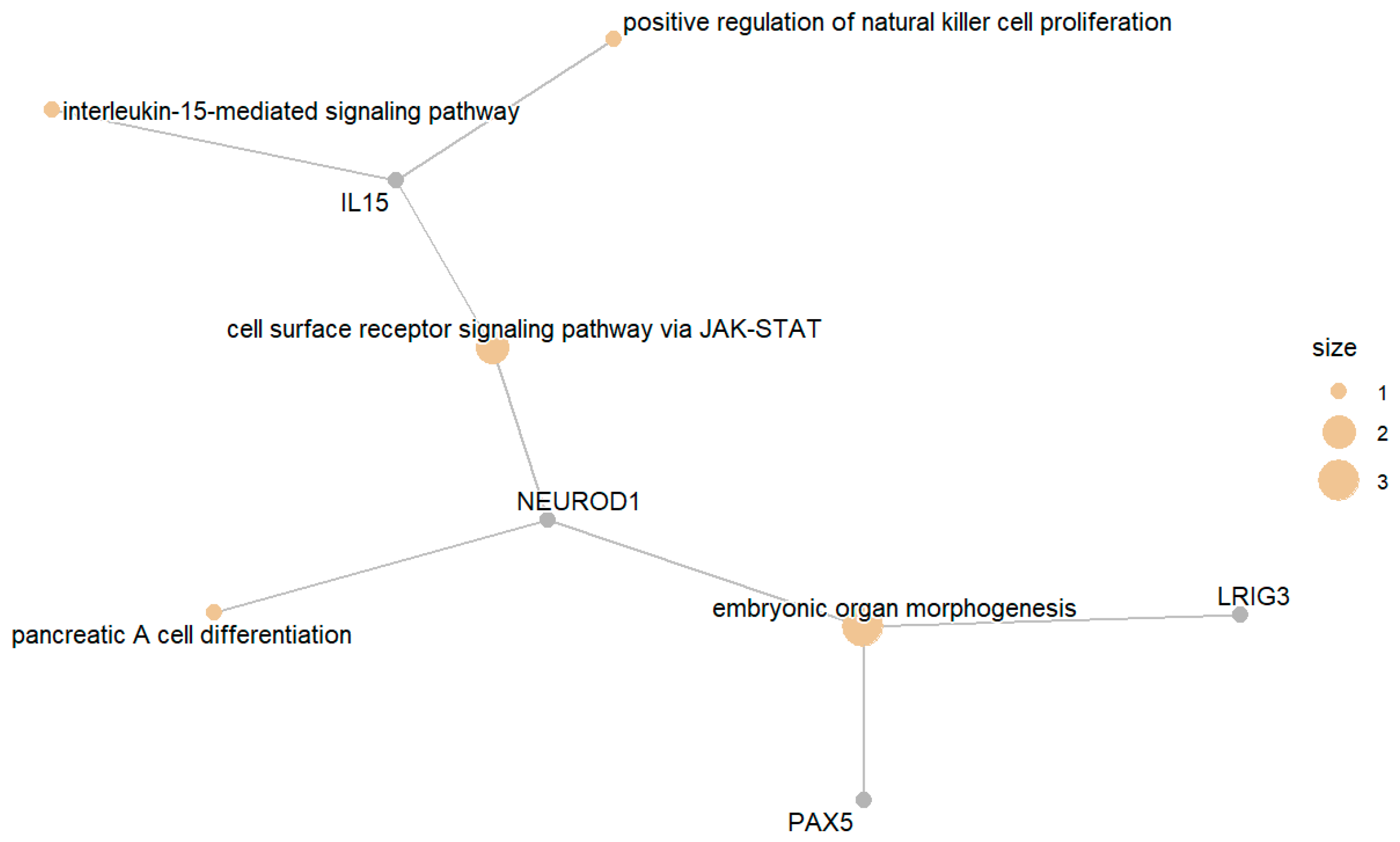
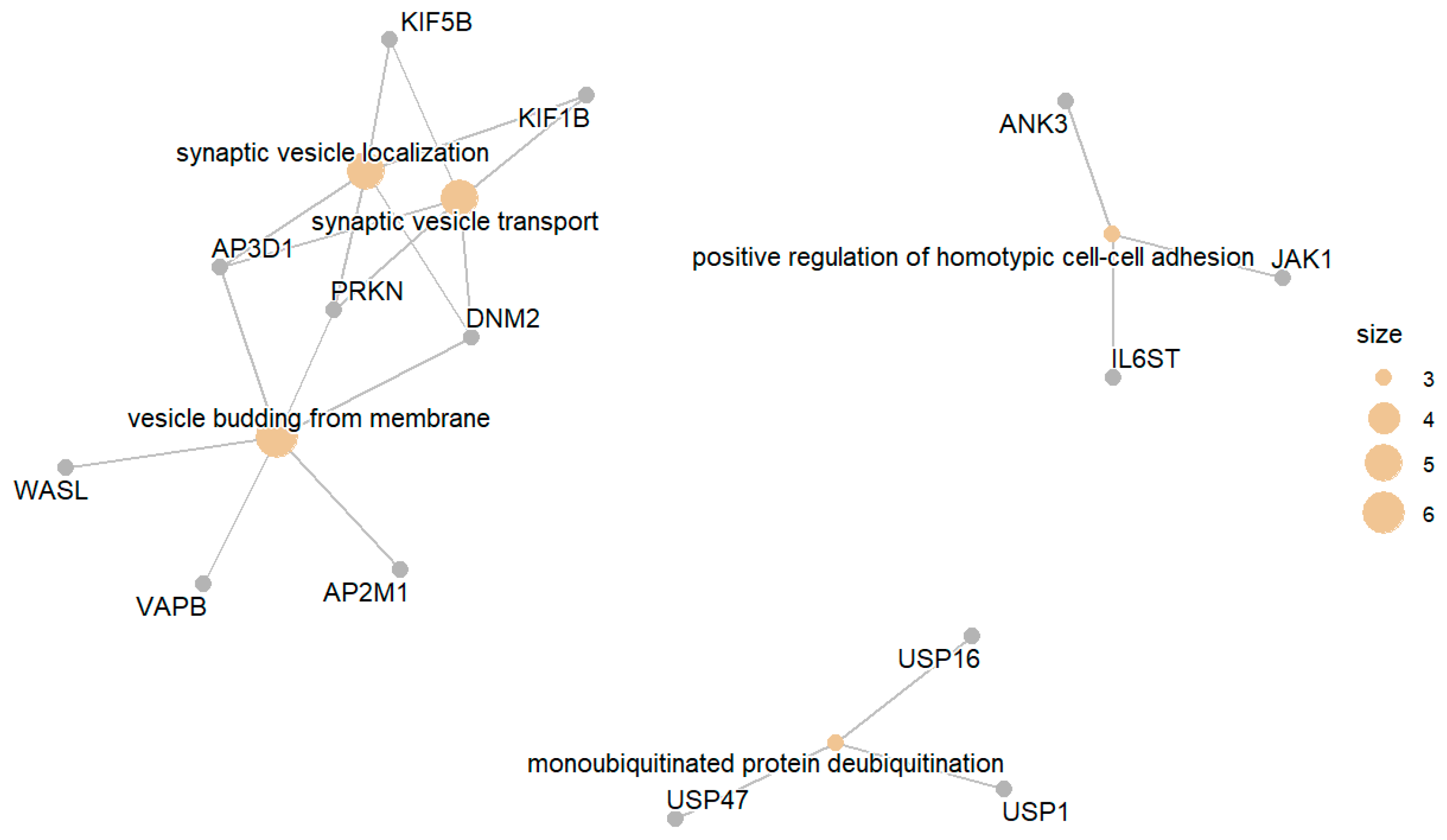
3. Results
3.1. Diabetic Group (DM) Compared with the Normal Group (NGT)
3.1.1. Downregulated DM Compared to NGT
3.1.2. Upregulated DM Compared with NGT
3.2. Glucose-Intolerant Group (IGT) Compared with the Normal Group (NGT)
3.2.1. Downregulated IGT Compared to NGT
3.2.2. Upregulated IGT Compared with NGT
3.3. Diabetic Group (DM) Compared with the Glucose-Intolerant Group (IGT)
3.3.1. Downregulated DM Compared with IGT
3.3.2. Upregulated DM Compared with IGT
3.4. Differential Expression of Protein Targets for the Treatment of Diabetes
4. Discussion
4.1. Diabetic Group (DM) Compared with the Normal Group (NGT)
4.2. Glucose-Intolerant Group (IGT) Compared with the Normal Group (NGT)
4.3. Diabetic Group (DM) Compared with the Glucose-Intolerant Group (IGT)
4.4. Differential Expression of Protein Targets for the Treatment of Diabetes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| NCBI | National Center for Biotechnology Information |
| GEO | Gene-Expression Omnibus |
| DM | Diabetic Group |
| IGT | Glucose-Intolerant Group |
| NGT | Normal Group |
References
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B. Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef]
- World Health Organization. Diabetes (Fact Sheet). WHO 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 6 May 2025).
- World Health Organization. Classification of Diabetes Mellitus; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/325182 (accessed on 6 May 2025).
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. β-cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab. 2014, 99, 1983–1992. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Hendler, R.; Felig, P.; Wahren, J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J. Clin. Investig. 1981, 68, 1468–1474. [Google Scholar] [CrossRef]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and tissue sites of non-insulin-mediated and insulin-mediated glucose uptake in humans. Am. J. Physiol. 1988, 255 Pt 1, E769–E774. [Google Scholar] [CrossRef]
- Winer, D.A.; Winer, S.; Chng, M.H.Y.; Shen, L.; Engleman, E.G. B lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell. Mol. Life Sci. 2014, 71, 1033–1043. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Cura, A.J.; Carruthers, A. Role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasis. Compr. Physiol. 2012, 2, 863–914. [Google Scholar] [CrossRef]
- O′Doherty, R.M.; Bracy, D.P.; Osawa, H.; Wasserman, D.H.; Granner, D.K. Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. Am. J. Physiol. 1994, 266 Pt 1, E171–E178. [Google Scholar] [CrossRef] [PubMed]
- Steenberg, D.E.; Jørgensen, N.B.; Birk, J.B.; Sjøberg, K.A.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F.P. Exercise training reduces the insulin-sensitizing effect of a single bout of exercise in human skeletal muscle. J. Physiol. 2019, 597, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Gollisch, K.S.C.; Holton, L.; Kim, Y.-B.; Brandauer, J.; Fujii, N.L.; Hirshman, M.F.; Goodyear, L.J. Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content. FEBS J. 2013, 280, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.R.; Burnett, M.; Hoffman-Goetz, L. Training effects in mice after long-term voluntary exercise. Med. Sci. Sports Exerc. 2006, 38, 250–255. [Google Scholar] [CrossRef]
- LeBlanc, P.J.; Howarth, K.R.; Gibala, M.J.; Heigenhauser, G.J.; Spriet, L.L. Effects of aerobic training on pyruvate dehydrogenase and pyruvate dehydrogenase kinase in human skeletal muscle. J. Physiol. 2004, 557, 559–570. [Google Scholar] [CrossRef]
- Kriti, M.; Ojha, R.; Singh, S.; Sarma, D.K.; Verma, V.; Yadav, A.K.; Nagpal, R.; Kumar, M. Implication of gut mycobiome and virome in type 2 diabetes mellitus: Uncovering the hidden players. Phenomics 2025, 5, 51–64. [Google Scholar] [CrossRef] [PubMed]
- DasNandy, A.; Virge, R.; Hegde, H.V.; Chattopadhyay, D. A review of patent literature on the regulation of glucose metabolism by six phytocompounds in the management of diabetes mellitus and its complications. J. Integr. Med. 2023, 21, 226–235. [Google Scholar] [CrossRef]
- Shanak, S.; Bassalat, N.; Barghash, A.; Kadan, S.; Ardah, M.; Zaid, H. Drug discovery of plausible lead natural compounds that target the insulin signaling pathway: Bioinformatics approaches. Evid. Based Complement. Alternat Med. 2022, 2022, 2832889. [Google Scholar] [CrossRef]
- Sreekumar, R.; Halvatsiotis, P.; Schimke, J.C.; Nair, K.S. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 2002, 51, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Mohlke, K.L.; Bonnycastle, L.L.; Willer, C.J.; Li, Y.; Duren, W.L.; Erdos, M.R.; Stringham, H.M.; Chines, P.S.; Jackson, A.U.; et al. The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun. 2016, 7, 11764. [Google Scholar] [CrossRef] [PubMed]
- Barberio, M.D.; Dohm, G.L.; Pories, W.J.; Gadaleta, N.A.; Houmard, J.A.; Nadler, E.P.; Hubal, M.J. Type 2 diabetes modifies skeletal muscle gene expression response to gastric bypass surgery. Front. Endocrinol. 2021, 12, 728593. [Google Scholar] [CrossRef]
- Suh, Y.H.; Kim, Y.; Bang, J.H.; Choi, K.S.; Lee, J.W.; Lee, K.H.; Moon, C.; Kang, H.J.; Lee, Y.J.; Kim, K.W.; et al. Analysis of gene expression profiles in insulin-sensitive tissues from pre-diabetic and diabetic Zucker diabetic fatty rats. J. Mol. Endocrinol. 2005, 34, 299–315. [Google Scholar] [CrossRef]
- Voss, M.D.; Beha, A.; Tennagels, N.; Tschank, G.; Herling, A.W.; Quint, M.; Gerl, M.; Metz-Weidmann, C.; Haun, G.; Korn, M. Gene expression profiling in skeletal muscle of Zucker diabetic fatty rats: Implications for a role of stearoyl-CoA desaturase 1 in insulin resistance. Diabetologia 2005, 48, 2622–2630. [Google Scholar] [CrossRef]
- Parikh, H.M.; Elgzyri, T.; Alibegovic, A.; Hiscock, N.; Ekström, O.; Eriksson, K.-F.; Vaag, A.; Groop, L.C.; Ström, K.; Hansson, O. Relationship between insulin sensitivity and gene expression in human skeletal muscle. BMC Endocr. Disord. 2021, 21, 32. [Google Scholar] [CrossRef]
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. GAPDH binds GLUT4 reciprocally to hexokinase-II and regulates glucose transport activity. Biochem. J. 2009, 419, 475–484. [Google Scholar] [CrossRef]
- Tuo, Y.; Xiang, M. mTOR: A double-edged sword for diabetes. J. Leukoc. Biol. 2019, 106, 385–395. [Google Scholar] [CrossRef]
- Hoeffer, C.A.; Klann, E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B.; Cariou, B. mTOR inhibitors and diabetes. Diabetes Res. Clin. Pract. 2015, 110, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.G.; Selvaraj, A.; Thomas, G. mTORC1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 2007, 13, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhang, N.; Chen, F.; Yang, C.; Ning, H.; Xiao, C.; Sun, K.; Liu, Y.; Yang, M.; Hu, T.; et al. Irisin ameliorates high glucose-induced cardiomyocyte injury via AMPK/mTOR signaling pathway. Cell Biol. Int. 2020, 44, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, M.; Ketzinel-Gilad, M.; Ariav, Y.; Pappo, O.; Karaca, M.; Castel, J.; Berthault, M.F.; Magnan, C.; Cerasi, E.; Kaiser, N.; et al. mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 2008, 57, 945–957. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Azarpira, N.; Mostafavi-Pour, Z. Role of mTOR complex 1 signaling pathway in the pathogenesis of diabetes complications: A mini review. Int. J. Mol. Cell Med. 2021, 10, 181–189. [Google Scholar] [CrossRef]
- Palsgaard, J.; Brown, A.E.; Jensen, M.; Borup, R.; Walker, M.; De Meyts, P. Gene expression in skeletal muscle biopsies from people with type 2 diabetes and relatives: Differential regulation of insulin signaling pathways. PLoS ONE 2009, 4, e6575. [Google Scholar] [CrossRef]
- Moller, A.B.; Kampmann, U.; Hedegaard, J.; Thorsen, K.; Nordentoft, I.; Vendelbo, M.H.; Møller, N.; Jessen, N. Altered gene expression and repressed markers of autophagy in skeletal muscle of insulin resistant patients with type 2 diabetes. Sci. Rep. 2017, 7, 43775. [Google Scholar] [CrossRef]
- Gallagher, I.J.; Scheele, C.; Keller, P.; Nielsen, A.R.; Remenyi, J.; Fischer, C.P.; Roder, K.; Babraj, J.; Wahlestedt, C.; Hutvagner, G.; et al. Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in type 2 diabetes. Genome Med. 2010, 2, 9. [Google Scholar] [CrossRef]
- Kessler, S.; Barghash, A.; Helms, V.; Grünewald, T.G.P. Lipid metabolism signatures in NASH-associated HCC: Letter. Cancer Res. 2014, 74, 2903–2904. [Google Scholar] [CrossRef] [PubMed]
- Barghash, A.; Helms, V.; Kessler, S. Overexpression of IGF2 mRNA-binding protein 2 (IMP2/p62) as a feature of basal-like breast cancer correlates with short survival. Scand. J. Immunol. 2015, 82, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Barghash, A.; Helms, V.; Kessler, S.M.; Bernsmeier, C.; Otto, G.; Lee, J.S.; Thorgeirsson, S.S.; Friess, H.; Schirmacher, P.; Bergmann, F.; et al. Elevated expression of the IGF2 mRNA-binding protein 2 (IGF2BP2/IMP2) is linked to short survival and metastasis in esophageal adenocarcinoma. Oncotarget 2016, 7, 49743–49750. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2024, 53, D672–D677. [Google Scholar] [CrossRef]
- Carlson, M. org.Hs.eg.db: Genome Wide Annotation for Human, R package version 3.18.0; Bioconductor: Seattle, WA, USA, 2025. [Google Scholar]
- Fröhlich, H.; Speer, N.; Poustka, A.; Beissbarth, T. GOSim: An R package for computation of information theoretic GO similarities between terms and gene products. BMC Bioinform. 2007, 8, 166. [Google Scholar] [CrossRef]
- Yu, G. Thirteen years of clusterProfiler. Innovation 2024, 5, 100722. [Google Scholar] [CrossRef]
- Xu, S.; Hu, E.; Cai, Y.; Xie, Z.; Luo, X.; Zhan, L.; Tang, W.; Wang, Q.; Liu, B.; Wang, R.; et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024, 19, 3292–3320. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. A 2011, 174, 245–246. [Google Scholar] [CrossRef]
- John, S.; Weiss, J.N.; Ribalet, B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS ONE 2011, 6, e17674. [Google Scholar] [CrossRef] [PubMed]
- Shanak, S.; Saad, B.; Zaid, H. Metabolic and epigenetic action mechanisms of antidiabetic medicinal plants. Evid. Based Complement. Alternat Med. 2019, 2019, 3583067. [Google Scholar] [CrossRef]
- Stuart, C.A.; Stone, W.L.; Howell, M.E.; Brannon, M.F.; Hall, H.K.; Gibson, A.L.; Stone, M.H. Myosin content of individual human muscle fibers isolated by laser capture microdissection. Am. J. Physiol. Cell Physiol. 2016, 310, C381–C389. [Google Scholar] [CrossRef]
- Saad, B.; Zaid, H.; Shanak, S.; Kadan, S. Anti-Diabetes and Anti-Obesity Medicinal Plants and Phytochemicals; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Wang, J.; Wen, S.; Chen, M.; Xie, J.; Lou, X.; Zhao, H.; Chen, Y.; Zhao, M.; Shi, G. Regulation of endocrine cell alternative splicing revealed by single-cell RNA sequencing in type 2 diabetes pathogenesis. Commun. Biol. 2024, 7, 778. [Google Scholar] [CrossRef]
- White, J.P.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Zhuang, L.; Liu, X.; Zhao, H.; Shan, Y.; Liu, Z.; Li, F.; Wang, Y.; Fang, J. Berberine decreases insulin resistance in PCOS rats by improving GLUT4: Dual regulation of the PI3K/AKT and MAPK pathways. Regul. Toxicol. Pharmacol. 2020, 110, 104544. [Google Scholar] [CrossRef]
- Yoon, M.S. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Kohan, F. Toll-like receptor 2 and type 2 diabetes. Cell. Mol. Biol. Lett. 2016, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Fink, L.N.; Costford, S.R.; Lee, Y.S.; Jensen, T.E.; Bilan, P.J.; Oberbach, A.; Blüher, M.; Olefsky, J.M.; Sams, A.; Klip, A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 2014, 22, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Itahana, Y.; Itahana, K. Emerging roles of p53 family members in glucose metabolism. Int. J. Mol. Sci. 2018, 19, 776. [Google Scholar] [CrossRef]
- Jin, T. The WNT signalling pathway and diabetes mellitus. Diabetologia 2008, 51, 1771–1780. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef]
- Belfiore, A.; Malaguarnera, R. Insulin receptor and cancer. Endocr. Relat. Cancer 2011, 18, R125–R147. [Google Scholar] [CrossRef]
- Hale, L.J.; Hurcombe, J.; Lay, A.C.; Santamaría, B.; Valverde, A.M.; Saleem, M.A.; Mathieson, P.W.; Welsh, G.I.; Coward, R.J.M. Insulin directly stimulates VEGF-A production in the glomerular podocyte. Am. J. Physiol. Renal Physiol. 2013, 305, F182–F188. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.D.; Jiang, W.-G.; Luo, X.; Li, R.-R.; Zhao, Y.-C.; Tian, G.; Li, Y.-N. Vascular endothelial growth factor B inhibits insulin secretion in MIN6 cells and reduces Ca2+ and cyclic adenosine monophosphate levels through PI3K/AKT pathway. World J. Diabetes 2021, 12, 480–498. [Google Scholar] [CrossRef]
- Kettner, A.; Di Matteo, M.; Santoni, A. Insulin potentiates FcεRI-mediated signaling in mouse bone marrow-derived mast cells. Mol. Immunol. 2010, 47, 1039–1046. [Google Scholar] [CrossRef]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-talk between PPARγ and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ferrat, A.; Lavandero, S.; Jaimovich, E.; Klip, A. Calcium signaling in insulin action on striated muscle. Cell Calcium 2014, 56, 390–396. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, G.; Guo, Y.; Deng, J.; Fischer, H.; Craig, L.B.; Kem, D.C.; Yu, X. Autoimmune activation of the GnRH receptor induces insulin resistance independent of obesity in a female rat model. Physiol. Rep. 2021, 8, e14672. [Google Scholar] [CrossRef]
- Ogunyemi, D.; Xu, J.; Mahesan, A.M.; Rad, S.; Kim, E.; Yano, J.; Alexander, C.; Rotter, J.I.; Chen, Y.-D.I. Differentially expressed genes in adipocytokine signaling pathway of adipose tissue in pregnancy. J. Diabetes Mellitus. 2013, 3, 86–95. [Google Scholar] [CrossRef]
- Khan, I.M.; Perrard, X.-Y.; Brunner, G.; Lui, H.; Sparks, L.M.; Smith, S.R.; Wang, X.; Shi, Z.-Z.; Lewis, D.E.; Wu, H.; et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int. J. Obes. 2015, 39, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Stanya, K.J.; Jacobi, D.; Liu, S.; Bhargava, P.; Dai, L.; Gangl, M.R.; Inouye, K.; Barlow, J.L.; Ji, Y.; Mizgerd, J.P.; et al. Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Investig. 2013, 123, 261–271. [Google Scholar] [CrossRef]
- Ghasempour, G.; Mohammadi, A.; Zamani-Garmsiri, F.; Soleimani, A.A.; Najafi, M. Upregulation of TGF-β type II receptor in high glucose-induced vascular smooth muscle cells. Mol. Biol. Rep. 2022, 49, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Chen, J.W. The role of chemokines and chemokine receptors in diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 3172. [Google Scholar] [CrossRef]
- Li, J.; Ahat, E.; Wang, Y. Golgi structure and function in health, stress, and diseases. Results Probl. Cell Differ. 2019, 67, 441–485. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Ling, C.; Del Guerra, S.; Lupi, R.; Rönn, T.; Granhall, C.; Luthman, H.; Masiello, P.; Marchetti, P.; Groop, L.; Del Prato, S.; et al. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 2008, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Volkmar, M.; Dedeurwaerder, S.; Cunha, D.A.; Ndlovu, M.N.; Defrance, M.; Deplus, R.; Calonne, E.; Volkmar, U.; Igoillo-Esteve, M.; Naamane, N.; et al. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J. 2012, 31, 1405–1426. [Google Scholar] [CrossRef]
- Hernandez, D.G.; Nalls, M.A.; Gibbs, J.R.; Arepalli, S.; van der Brug, M.; Chong, S.; Moore, M.; Longo, D.L.; Cookson, M.R.; Traynor, B.J.; et al. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum. Mol. Genet. 2011, 20, 1164–1172. [Google Scholar] [CrossRef]
- Do, H.T.T.; Shanak, S.; Barghash, A.; Helms, V. Differential exon usage of developmental genes is associated with deregulated epigenetic marks. Sci. Rep. 2023, 13, 12256. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Pappas, E.G.; Thomas, H.E.; Gough, D.J. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016, 283, 3002–3015. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Hoier, B.; Hellsten, Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation 2014, 21, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, J.; Pan, Y.; Ju, H.; Zhu, L.; Liu, Y.; Zhang, Y. The difference between steroid diabetes mellitus and type 2 diabetes mellitus: A whole-body 18F-FDG PET/CT study. Acta Diabetol. 2020, 57, 1383–1393. [Google Scholar] [CrossRef]
- Bergeron, A.; Pucci, L.; Bezzi, P.; Regazzi, R.; Meunier, F.A. Analysis of synaptic-like microvesicle exocytosis of B-cells using a live imaging technique. PLoS ONE 2014, 9, e87758. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, W.C.; Sorrenson, B.; Shepherd, P.R. The role of adherens junction proteins in the regulation of insulin secretion. Biosci. Rep. 2018, 38, BSR20170989. [Google Scholar] [CrossRef]
- Zhu, S.; Hou, S.; Lu, Y.; Sheng, W.; Cui, Z.; Dong, T.; Feng, H.; Wan, Q. USP36-mediated deubiquitination of DOCK4 contributes to the diabetic renal tubular epithelial cell injury via Wnt/β-catenin signaling pathway. Front. Cell Dev. Biol. 2021, 9, 638477. [Google Scholar] [CrossRef]
- Dif, N.; Euthine, V.; Gonnet, E.; Laville, M.; Vidal, H.; Lefai, E. Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem. J. 2006, 400, 179–188. [Google Scholar] [CrossRef]
- Silva, J.F.; Serakides, R. Intrauterine trophoblast migration: A comparative view of humans and rodents. Cell Adh. Migr. 2016, 10, 88–110. [Google Scholar] [CrossRef]
- Bonegio, R.; Susztak, K. Notch signaling in diabetic nephropathy. Exp. Cell Res. 2012, 318, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, L.; Zhang, M.; Shama; Cheng, K.K.Y.; Jia, W. The interplay between the muscle and liver in the regulation of glucolipid metabolism. J. Mol. Cell Biol. 2023, 15, mjad073. [Google Scholar] [CrossRef]
- Rahman, I.; Athar, M.T.; Islam, M. Type 2 diabetes, obesity, and cancer share some common and critical pathways. Front. Oncol. 2021, 10, 600824. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.M.; Al-Sajee, D.; Hawke, T.J. Diabetic myopathy: Impact of diabetes mellitus on skeletal muscle progenitor cells. Front. Physiol. 2013, 4, 379. [Google Scholar] [CrossRef]
- Rosen, E.D.; Kaestner, K.H.; Natarajan, R.; Patti, M.E.; Sallam, T.; Sander, M.; Susztak, K. Epigenetics and epigenomics: Implications for diabetes and obesity. Diabetes 2018, 67, 1923–1931. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barghash, A.; Shanak, S. Advanced Gene-Expression Analysis of Skeletal Muscles Focusing on Normal, Glucose-Intolerant, and Diabetic Individuals with Type 2 Diabetes. Biomedicines 2025, 13, 2181. https://doi.org/10.3390/biomedicines13092181
Barghash A, Shanak S. Advanced Gene-Expression Analysis of Skeletal Muscles Focusing on Normal, Glucose-Intolerant, and Diabetic Individuals with Type 2 Diabetes. Biomedicines. 2025; 13(9):2181. https://doi.org/10.3390/biomedicines13092181
Chicago/Turabian StyleBarghash, Ahmad, and Siba Shanak. 2025. "Advanced Gene-Expression Analysis of Skeletal Muscles Focusing on Normal, Glucose-Intolerant, and Diabetic Individuals with Type 2 Diabetes" Biomedicines 13, no. 9: 2181. https://doi.org/10.3390/biomedicines13092181
APA StyleBarghash, A., & Shanak, S. (2025). Advanced Gene-Expression Analysis of Skeletal Muscles Focusing on Normal, Glucose-Intolerant, and Diabetic Individuals with Type 2 Diabetes. Biomedicines, 13(9), 2181. https://doi.org/10.3390/biomedicines13092181




