Preclinical Safety Profile of Deg-AZM, a Clinical-Stage New Transgelin Agonist: hERG Inhibition Study In Vitro, Cardiovascular–Respiratory Pharmacology, and Single/Repeated-Dose Toxicity in Beagle Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Materials
2.2. Animals
2.3. hERG Channel Inhibition Assay
2.4. Cardiovascular and Respiratory Safety Pharmacology Study in Beagle Dogs
2.5. Single-Dose Toxicity Study of Oral Deg-AZM Administration in Beagle Dogs
2.5.1. Clinical Observations
2.5.2. Electrocardiographic Examination
2.5.3. Hematological Examination
2.5.4. Serum Biochemical Analysis
2.5.5. Toxicokinetic Analysis in Single-Dose Toxicity Study
2.6. 28-Day Repeated Dose Oral Toxicity Study of Deg-AZM in Beagle Dogs
2.6.1. Toxicological Observations
2.6.2. Electrocardiographic Examination
2.6.3. Hematological Examination and Coagulation Tests
2.6.4. Serum Biochemical Analysis
2.6.5. Toxicokinetic Analysis in 28-Day Repeated Dose Oral Toxicity Study
2.6.6. Pathological Examination
2.7. Data Statistics Method
3. Results
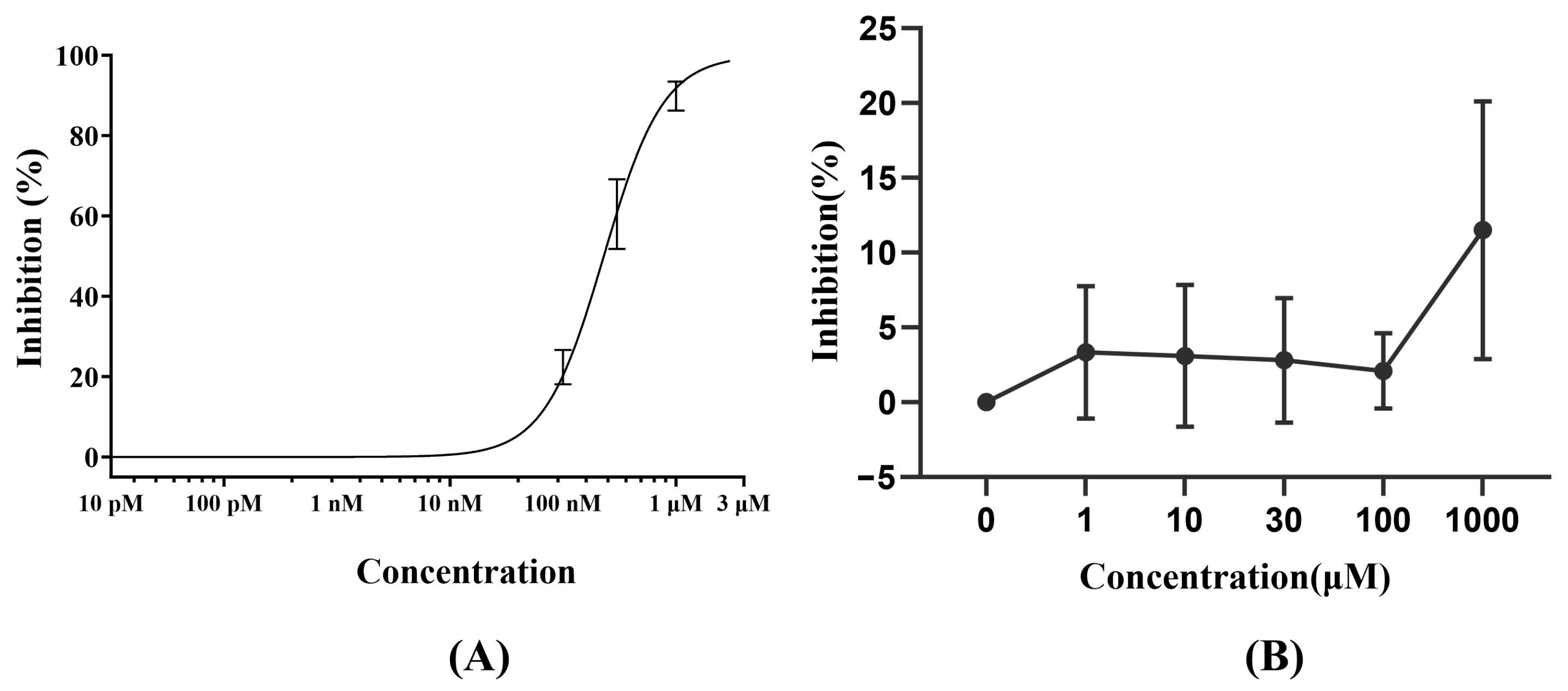
3.1. hERG Channel Inhibition Assay
3.2. Cardiovascular and Respiratory Safety Pharmacology Study of Deg-AZM
3.2.1. Blood Pressure of Beagle Dogs
3.2.2. Electrocardiographic Parameters in Beagle Dogs
3.2.3. Respiratory Function in Beagle Dogs
3.3. Single-Dose Toxicity Study of Oral Deg-AZM Administration in Beagle Dogs
3.3.1. Toxicological Observations
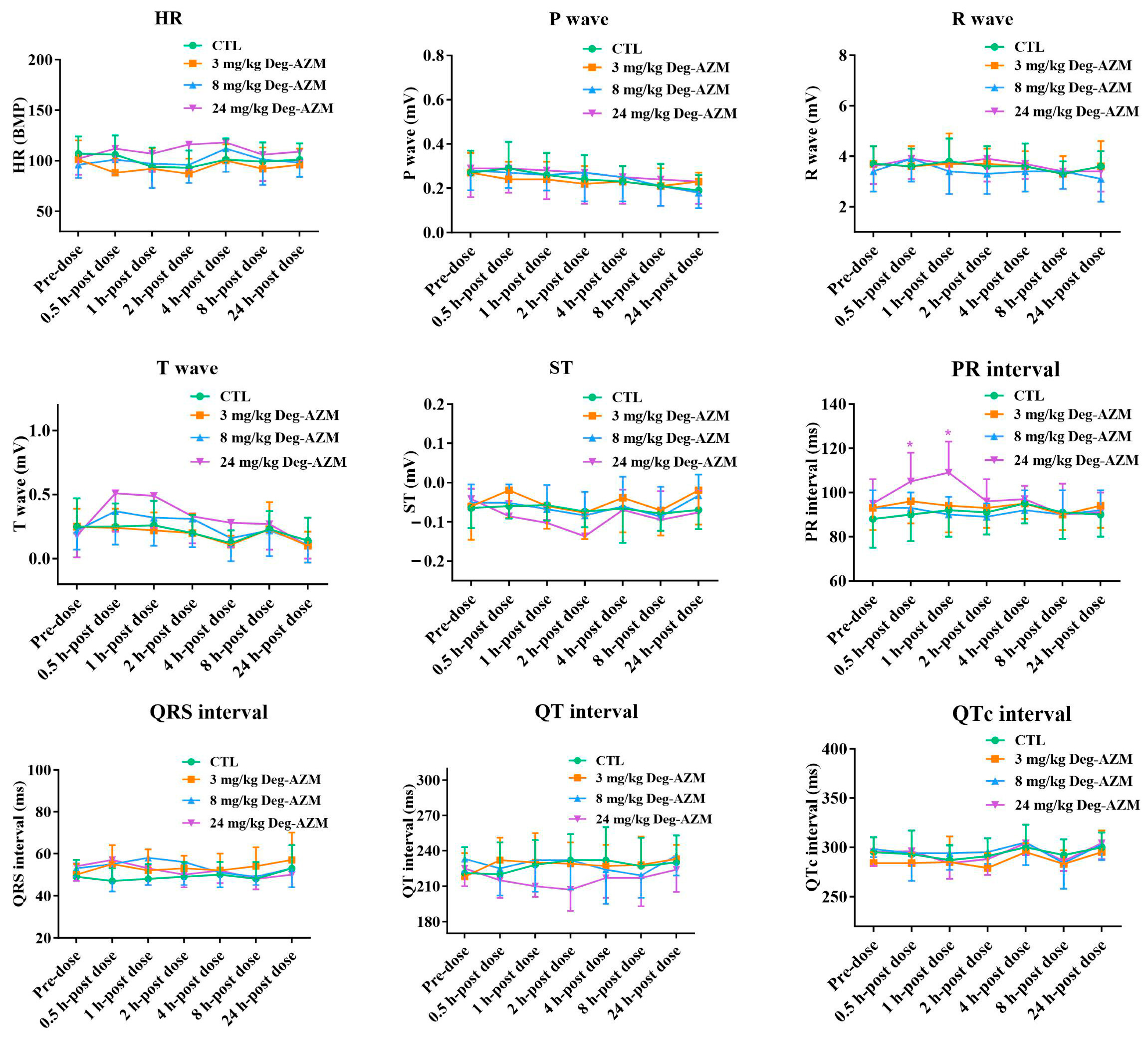
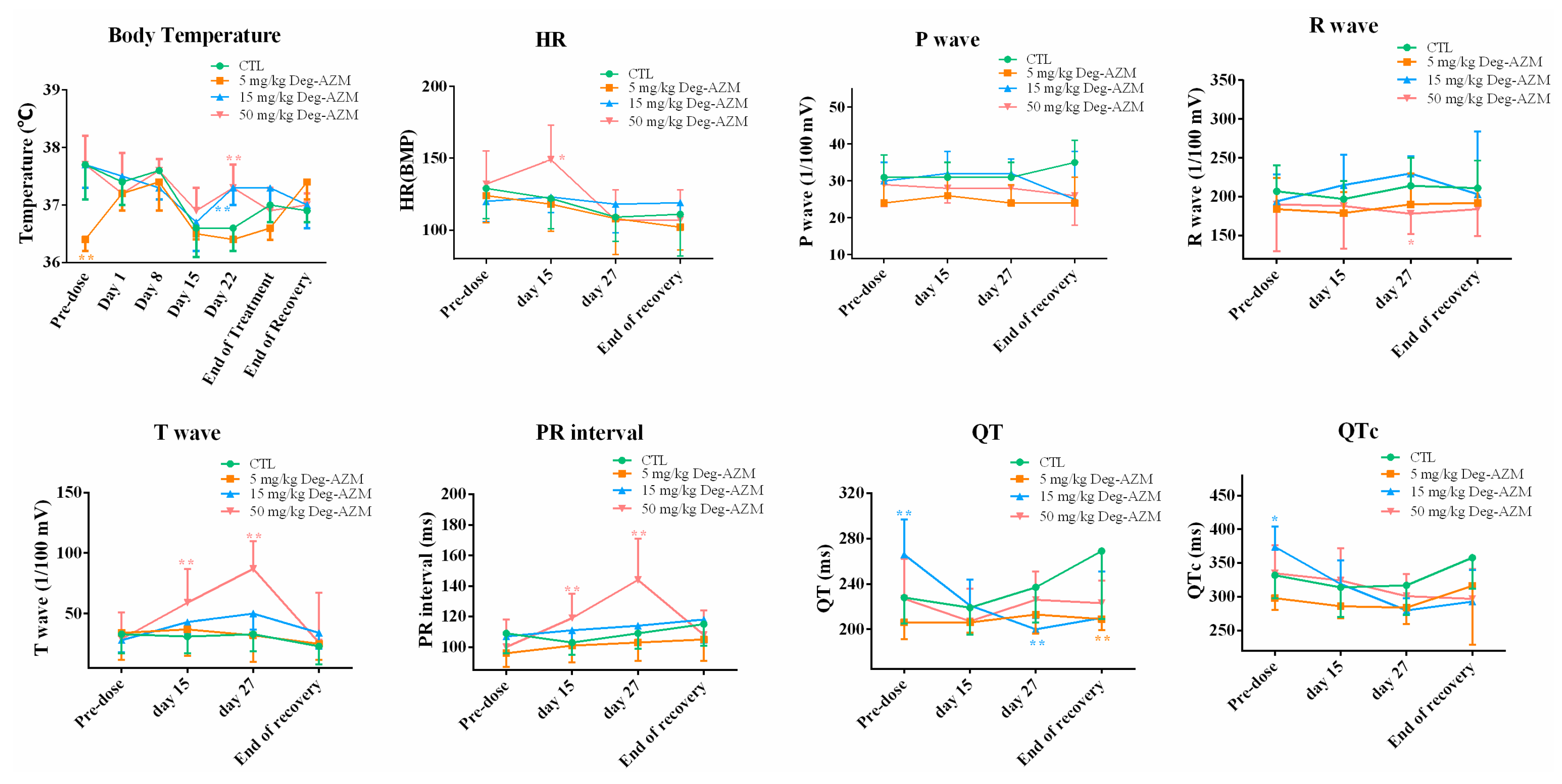
3.3.2. Electrocardiographic Examination
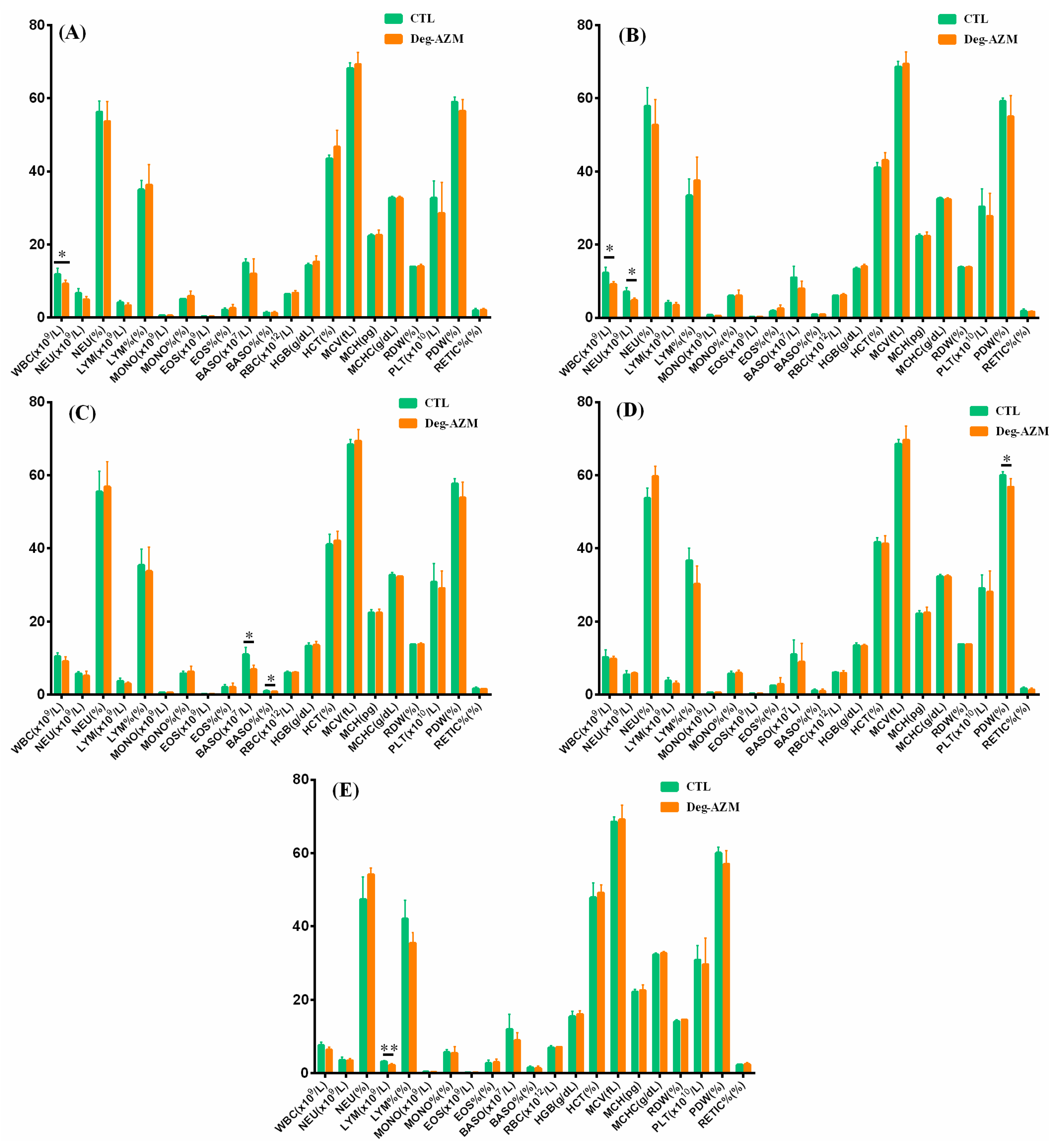
3.3.3. Hematological Examination
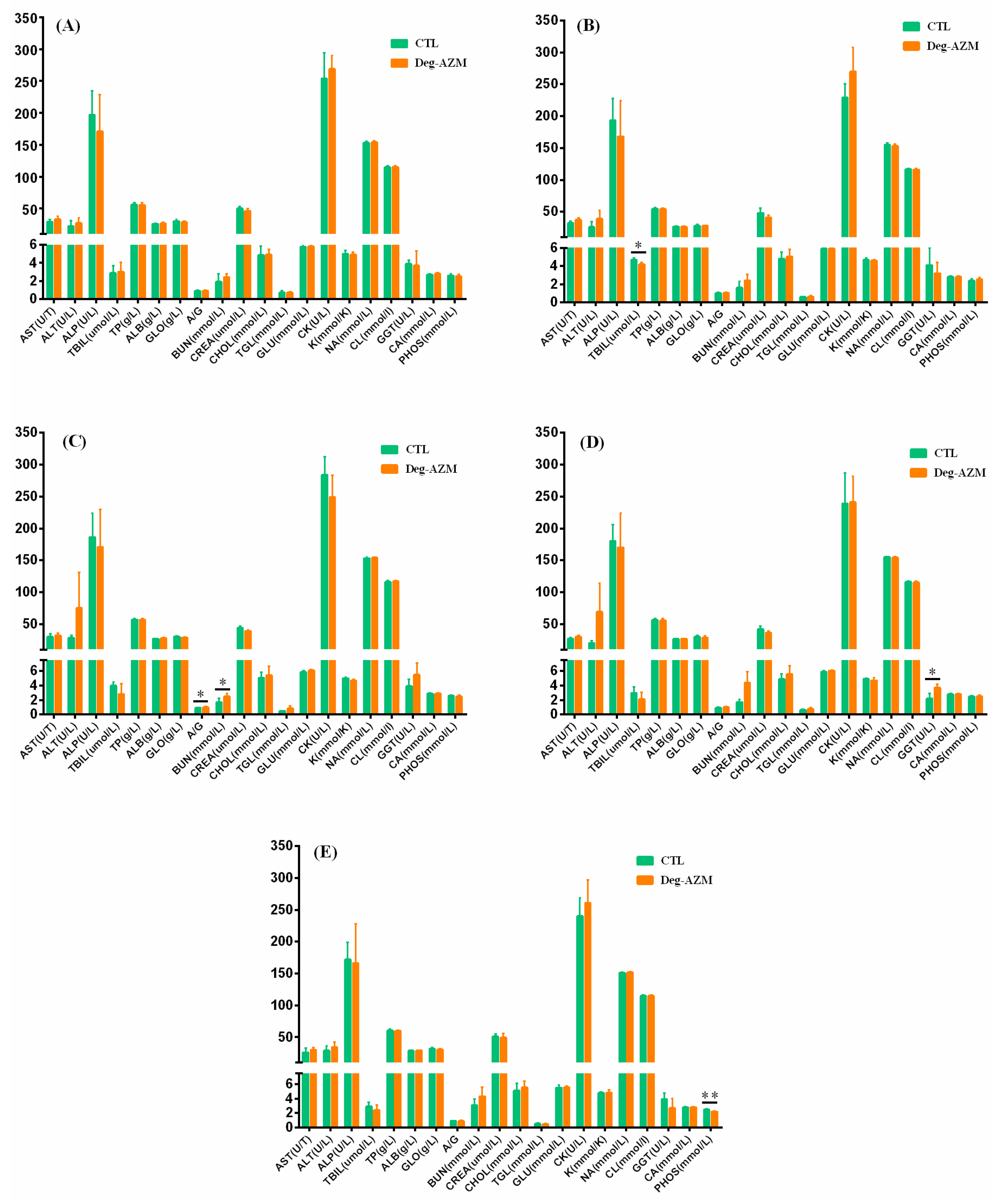
3.3.4. Serum Biochemical Analysis
3.3.5. Toxicokinetic Analysis in Single-Dose Toxicity Study
3.4. 28-Day Repeated Dose Oral Toxicity Study of Deg-AZM in Beagle Dogs
3.4.1. Toxicological Observations
3.4.2. Electrocardiographic Examination
3.4.3. Hematological Examination and Coagulation Tests
3.4.4. Serum Biochemical Analysis
3.4.5. Toxicokinetic Analysis in 28-Day Repeated Dose Oral Toxicity Study
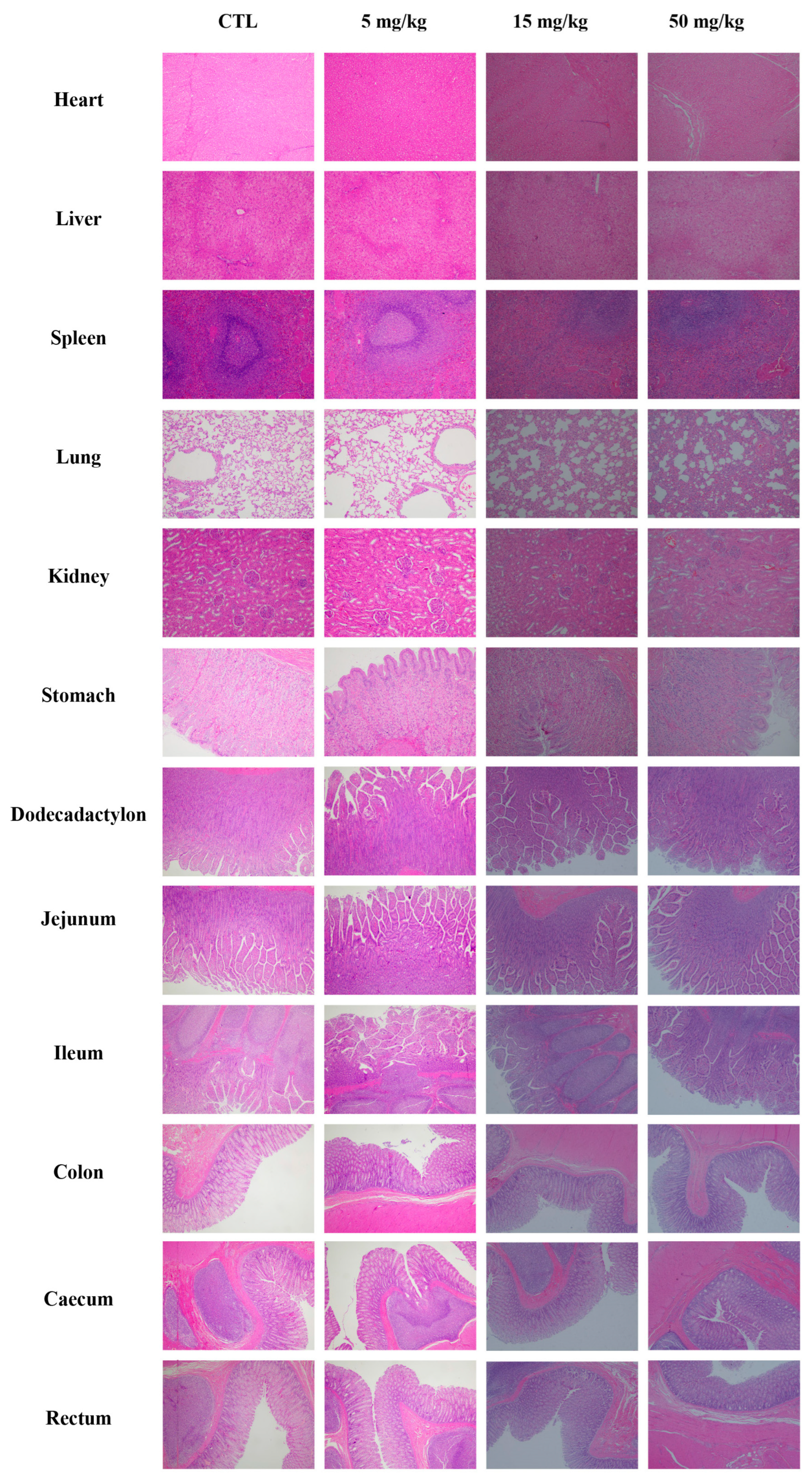
3.4.6. Pathological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| A/G | albumin-to-globulin ratio |
| ALB | albumin |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| APTT | activated partial thromboplastin time |
| AST | aspartate aminotransferase |
| AUC | area under the concentration-time curve |
| AZM | azithromycin |
| BASO | basophil count |
| BUN | blood urea nitrogen |
| cGMP | cyclic guanosine monophosphate |
| CHOL | total cholesterol |
| CK | creatine kinase |
| ClC | chloride channel activators |
| Cmax | peak concentration |
| CMC-Na | carboxymethylcellulose sodium |
| CREA | creatinine |
| Deg-AZM | deglycosylated azithromycin |
| ECG | electrocardiogram |
| EOS | eosinophil count |
| EOS% | eosinophil percentage |
| F-actin | filamentous actin |
| FIB | fibrinogen |
| G-actin | globular actin |
| GC-C | guanylate cyclase-C |
| GGT | gamma-glutamyl transferase |
| GLO | globulin |
| GLU | glucose |
| H&E | hematoxylin and eosin |
| HCT | hematocrit |
| HED | human equivalent dose |
| hERG | human ether-à-go-go-related gene |
| hERG-HEK293 | The stably transfected hERG-expressing cell line |
| HGB | hemoglobin concentration |
| HR | heart rate |
| LD50 | median lethal dose |
| LYM | lymphocyte count |
| LYM% | lymphocyte percentage |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| MCV | mean corpuscular volume |
| MLD | minimum lethal dose |
| MONO | monocyte count |
| MONO% | monocyte percentage |
| MRSD | maximum recommended starting dose |
| NBF | neutral buffered formalin |
| NEU | neutrophil count |
| NEU% | neutrophil percentage |
| NOAEL | no observed adverse effect level |
| PDW | platelet distribution width |
| PLT | platelet count |
| PT | prothrombin time |
| QTc | QT interval |
| RBC | red blood cell count |
| RDW | red cell distribution width |
| RETIC% | reticulocyte percentage |
| STC | slow transit constipation |
| T1/2 | terminal elimination half-life |
| TBIL | total bilirubin |
| TGL | triglycerides |
| Tmax | time to maximum concentration |
| TP | total protein |
| TT | thrombin time |
| WBC | white blood cell count |
References
- Park, S.-Y. Diagnosis of Chronic Constipation. Korean J. Gastroenterol. 2024, 83, 179–183. [Google Scholar] [CrossRef]
- Vlismas, L.J.; Wu, W.; Ho, V. Idiopathic Slow Transit Constipation: Pathophysiology, Diagnosis, and Management. Medicina 2024, 60, 108. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemianrad, M.; Ammari-Allahyari, M.; Rasoulpoor, S.; Shohaimi, S.; Mohammadi, M. Global prevalence of constipation in older adults: A systematic review and meta-analysis. Wien. Klin. Wochenschr. 2023, 135, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.C.; Husk, K.E. Defecatory Dysfunction. Obstet. Gynecol. Clin. North. Am. 2021, 48, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-L.; Zhu, L.-J.; Yang, X.-D. Exploration of the complex origins of primary constipation. World J. Clin. Cases 2024, 12, 5476–5482. [Google Scholar] [CrossRef]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; DiLorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef] [PubMed]
- van der Schoot, A.; Drysdale, C.; Whelan, K.; Dimidi, E. The Effect of Fiber Supplementation on Chronic Constipation in Adults: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2022, 116, 953–969. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kawamura, Y.; Yamamoto, K.; Yamaguchi, Y.; Tamura, Y.; Izawa, S.; Nakagawa, H.; Wakita, Y.; Hijikata, Y.; Ebi, M.; et al. Internet Survey of Japanese Patients With Chronic Constipation: Focus on Correlations Between Sleep Quality, Symptom Severity, and Quality of Life. J. Neurogastroenterol. Motil. 2021, 27, 602–611. [Google Scholar] [CrossRef]
- Xue, X.; Zeng, H.; Chen, D.; Zheng, B.; Liang, B.; Xu, D.; Lin, S. Comparing the short-term clinical outcomes and therapeutic effects of different colectomies in patients with refractory slow-transit constipation in eastern countries: A network meta-analysis. Updat. Surg. 2024, 76, 411–422. [Google Scholar] [CrossRef]
- Pannemans, J.; Masuy, I.; Tack, J. Functional Constipation: Individualising Assessment and Treatment. Drugs 2020, 80, 947–963. [Google Scholar] [CrossRef]
- Shakir, A.K.; Altaf, M.A. Azithromycin Induces Migrating Motor Complexes in Pediatric Patients Undergoing Antroduodenal Motility Studies. J. Pediatr. Pharmacol. Ther. 2018, 23, 390–394. [Google Scholar] [CrossRef]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xu, P.; Choonara, I.; Bo, Z.; Pan, X.; Li, W.; Ni, X.; Xiong, T.; Chen, C.; Huang, L.; et al. Safety of azithromycin in pediatrics: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; Aguirre, I.; Unemo, M.; Kong, F.Y.S.; Fairley, C.K.; Hocking, J.S.; Chow, E.P.F.; Tieosapjaroen, W.; Ly, J.; Chen, M.Y. Comparison of gastrointestinal side effects from different doses of azithromycin for the treatment of gonorrhoea. J. Antimicrob. Chemother. 2022, 77, 2011–2016. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, M.; Liu, S.; Wang, X. Efficacy and safety of reduning injection combined with azithromycin in the treatment of mycoplasma pneumonia among children: A systematic review and meta-analysis. Phytomedicine 2022, 106, 154402. [Google Scholar] [CrossRef]
- Zhong, W.; Sun, B.; Ruan, H.; Yang, G.; Qian, B.; Cao, H.; He, L.; Fan, Y.; Roberts, A.G.; Liu, X.; et al. Deglycosylated Azithromycin Targets Transgelin to Enhance Intestinal Smooth Muscle Function. iScience 2020, 23, 101464. [Google Scholar] [CrossRef]
- Tang, D.D. The Dynamic Actin Cytoskeleton in Smooth Muscle. Adv. Pharmacol. 2018, 81, 1–38. [Google Scholar] [CrossRef]
- Rasmussen, M.; Jin, J.-P. Mechanoregulation and function of calponin and transgelin. Biophys. Rev. 2024, 5, 011302. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-B.; Jin, J.-P. Evolution and function of calponin and transgelin. Front. Cell Dev. Biol. 2023, 11, 1206147. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Lepailleur, A.; Mignani, S.M.; Dallemagne, P.; Rochais, C. hERG toxicity assessment: Useful guidelines for drug design. Eur. J. Med. Chem. 2020, 195, 112290. [Google Scholar] [CrossRef]
- Delpy, E.; Bétat, A.-M.; Delaunois, A.; la Rochelle, C.D.; Martel, E.; Valentin, J.-P. A comprehensive review of 20 years of progress in nonclinical QT evaluation and proarrhythmic assessment. J. Pharmacokinet. Pharmacodyn. 2025, 52, 32. [Google Scholar] [CrossRef] [PubMed]
- Kearney, K.; Metea, M.; Gleason, T.; Edwards, T.; Atterson, P. Evaluation of respiratory function in freely moving Beagle dogs using implanted impedance technology. J. Pharmacol. Toxicol. Methods 2010, 62, 119–126. [Google Scholar] [CrossRef]
- Goineau, S.; Lemaire, M.; Froget, G. Overview of Safety Pharmacology. Curr. Protoc. Pharmacol. 2013, 63, 10.1.1–10.1.8. [Google Scholar] [CrossRef]
- Gintant, G.; Sager, P.T.; Stockbridge, N. Evolution of strategies to improve preclinical cardiac safety testing. Nat. Rev. Drug Discov. 2016, 15, 457–471. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, S.S.C.; Kearns, K.; Orleck, K.D.; Waldman, S.A. Review article: Diagnosis, management and patient perspectives of the spectrum of constipation disorders. Aliment. Pharmacol. Ther. 2021, 53, 1250–1267. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Role of prucalopride, a serotonin (5-HT4) receptor agonist, for the treatment of chronic constipation. Clin. Exp. Gastroenterol. 2010, 3, 49–56. [Google Scholar] [CrossRef]
- Diederen, K.; Mugie, S.M.; Benninga, M.A. Efficacy and safety of prucalopride in adults and children with chronic constipation. Expert. Opin. Pharmacother. 2014, 16, 407–416. [Google Scholar] [CrossRef]
- Johanson, J.F.; Ueno, R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: A double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment. Pharmacol. Ther. 2007, 25, 1351–1361. [Google Scholar] [CrossRef]
- Lembo, A.J.; Johanson, J.F.; Parkman, H.P.; Rao, S.S.; Miner, P.B.; Ueno, R. Long-Term Safety and Effectiveness of Lubiprostone, a Chloride Channel (ClC-2) Activator, in Patients with Chronic Idiopathic Constipation. Dig. Dis. Sci. 2011, 56, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Busby, R.W.; Bryant, A.P.; Bartolini, W.P.; Cordero, E.A.; Hannig, G.; Kessler, M.M.; Mahajan-Miklos, S.; Pierce, C.M.; Solinga, R.M.; Sun, L.J.; et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur. J. Pharmacol. 2010, 649, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Karle, C.A.; Kiehn, J. The Cardiac hERG/IKr Potassium Channel as Pharmacological Target: Structure, Function, Regulation, and Clinical Applications. Curr. Pharm. Des. 2006, 12, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Rampe, D.; Roy, M.-L.; Dennis, A.; Brown, A.M. A mechanism for the proarrhythmic effects of cisapride (Propulsid): High affinity blockade of the human cardiac potassium channel HERG. FEBS Lett. 1997, 417, 28–32. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Williams, J. Evaluation of the safety of macrolides. Int. J. Antimicrob. Agents 2001, 18, 77–81. [Google Scholar] [CrossRef] [PubMed]



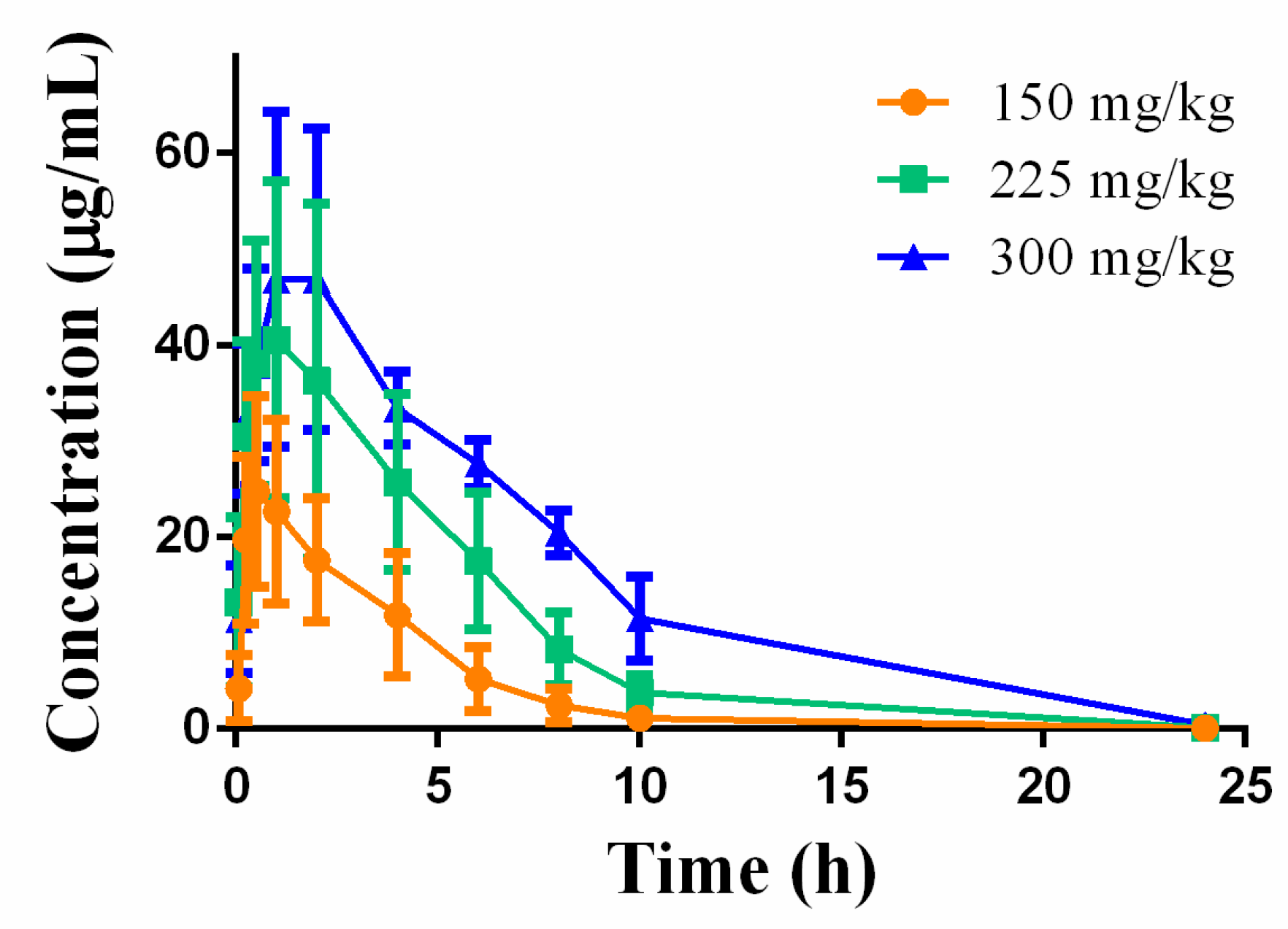
| Parameters | 150 mg/kg | 225 mg/kg | 300 mg/kg |
|---|---|---|---|
| AUC0-t (μg/mL*h) | 100.72 ± 43.51 | 231.63 ± 84.21 | 386.34 ± 43.85 |
| Cmax (μg/mL) | 25.20 ± 9.46 | 41.53 ± 17.24 | 49.27 ± 19.89 |
| T1/2 (h) | 1.74 ± 0.23 | 1.98 ± 0.32 | 2.60 ± 0.94 |
| Tmax (h) | 0.63 ± 0.25 | 1.38 ± 0.75 | 1.67 ± 0.58 |
| Parameters | Pre-Dose | Day 15 | Day 27 | End of Recovery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | |
| WBC (×109/L) | 10.7 ± 1.8 | 8.8 ± 2.4 | 10.1 ± 1.7 | 9.4 ± 1.4 | 8.4 ± 1.4 | 11.1 ± 3.5 | 8.7 ± 1.4 | 8.7 ± 1.1 | 7.9 ± 1.1 | 10.4 ± 2.1 | 8.5 ± 2.3 | 7.7 ± 1.5 | 11.3 ± 1.5 | 10.3 ± 1.2 | 9.4 ± 0.7 | 9.5 ± 1.4 |
| NEU% (%) | 5.6 ± 1.2 | 4.7 ± 1.3 | 6.2 ± 1.1 | 5.5 ± 1.0 | 4.6 ± 1.0 | 6.5 ± 3.0 | 5.0 ± 1.0 | 4.8 ± 0.9 | 4.5 ± 0.8 | 5.8 ± 1.7 | 4.6 ± 1.6 | 4.1 ± 1.0 | 7.0 ± 1.0 | 6.0 ± 1.2 | 5.4 ± 0.4 | 5.2 ± 0.9 |
| NEU% (%) | 52.4 ± 5.8 | 52.8 ± 4.2 | 61.4 ± 3.3 | 58.4 ± 3.8 | 54.9 ± 5.0 | 57.2 ± 6.7 | 56.7 ± 4.9 | 54.8 ± 4.5 | 56.2 ± 4.4 | 56.9 ± 6.4 | 54.0 ± 5.8 | 53.4 ± 3.8 | 62.2 ± 3.3 | 58.1 ± 5.9 | 57.2 ± 1.5 | 54.3 ± 5.9 |
| LYM (×109/L) | 3.6 ± 0.9 | 3.0 ± 1.0 | 3.0 ± 0.5 | 3.0 ± 0.4 | 3.0 ± 0.6 | 3.2 ± 0.5 | 3.0 ± 0.5 | 3.1 ± 0.4 | 2.7 ± 0.5 | 3.1 ± 0.6 | 3.0 ± 0.7 | 2.8 ± 0.6 | 3.0 ± 0.5 | 2.8 ± 0.1 | 3.1 ± 0.2 | 3.7 ± 0.7 |
| LYM% (%) | 33.5 ± 6.8 | 34.1 ± 4.2 | 29.4 ± 3.6 | 32.6 ± 4.4 | 36.1 ± 4.0 | 30.2 ± 6.3 | 34.1 ± 4.2 | 35.4 ± 4.8 | 34.2 ± 4.0 | 31.7 ± 5.5 | 36.0 ± 5.4 | 36.4 ± 3.8 | 26.3 ± 3.7 | 27.9 ± 3.5 | 33.4 ± 2.4 | 38.3 ± 4.9 |
| MONO (×109/L) | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.8 ± 0.3 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.0 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| MONO% (%) | 6.9 ± 1.6 | 6.8 ± 1.1 | 5.7 ± 1.0 | 6.0 ± 1.2 | 5.9 ± 1.8 | 7.3 ± 1.6 | 6.1 ± 1.2 | 6.4 ± 1.2 | 6.1 ± 1.4 | 6.1 ± 0.8 | 5.4 ± 1.5 | 6.0 ± 1.2 | 7.6 ± 1.3 | 7.6 ± 0.6 | 5.7 ± 1.1 | 4.0 ± 0.5 |
| EOS (×109/L) | 0.5 ± 0.5 | 0.4 ± 0.2 | 0.3 ± 0.3 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.5 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.10 |
| EOS% (%) | 4.9 ± 3.9 | 4.2 ± 2.5 | 2.5 ± 1.7 | 1.9 ± 1.0 | 2.2 ± 0.8 | 3.3 ± 1.5 | 2.1 ± 0.9 | 2.4 ± 1.2 | 2.5 ± 1.1 | 3.3 ± 1.8 | 3.1 ± 1.9 | 3.2 ± 2.5 | 2.9 ± 1.5 | 5.1 ± 2.1 | 2.5 ± 1.2 | 2.3 ± 1.0 |
| BASO (×109/L) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| BASO% (%) | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.9 ± 0.3 | 1.0 ± 0.8 | 0.7 ± 0.3 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.9 ± 0.4 | 0.7 ± 0.2 |
| RBC (×1012/L) | 6.3 ± 0.4 | 5.6 ± 1.4 | 6.3 ± 0.3 | 6.6 ± 0.6 | 6.5 ± 0.5 | 6.3 ± 0.5 | 6.6 ± 0.4 | 6.3 ± 0.6 | 6.7 ± 0.4 | 6.4 ± 0.7 | 6.6 ± 0.4 | 6.4 ± 0.4 | 6.7 ± 0.3 | 6.6 ± 0.1 | 6.9 ± 0.3 | 6.8 ± 0.3 |
| HGB (g/dL) | 15.3 ± 1.2 | 15.1 ± 0.9 | 13.8 ± 0.7 | 14.5 ± 1.2 | 14.5 ± 0.8 | 14.7 ± 1.2 | 14.4 ± 0.7 | 14.0 ± 1.3 | 14.9 ± 0.7 | 14.8 ± 1.5 | 14.5 ± 0.8 | 14.2 ± 1.1 | 15.3 ± 1.1 | 15.4 ± 0.2 | 15.7 ± 1.1 | 15.3 ± 1.5 |
| HCT (%) | 46.8 ± 3.4 | 40.7 ± 11.3 | 42.6 ± 1.9 | 44.2 ± 3.6 | 44.5 ± 2.6 | 45.0 ± 3.5 | 44.3 ± 2.5 | 42.6 ± 4.0 | 45.0 ± 2.1 | 45.5 ± 4.8 | 43.7 ± 2.3 | 42.8 ± 3.1 | 47.7 ± 3.0 | 47.3 ± 0.6 | 47.4 ± 2.5 | 45.1 ± 4.5 |
| MCV (fL) | 74.0 ± 1.5 | 72.4 ± 3.6 | 67.7 ± 2.0 | 67.4 ± 2.0 | 68.6 ± 1.7 | 71.8 ± 2.0 | 67.8 ± 1.7 | 67.4 ± 1.6 | 67.7 ± 1.4 | 70.9 ± 2.0 | 66.7 ± 2.0 | 66.6 ± 1.7 | 71.2 ± 1.5 | 71.4 ± 1.3 | 69.0 ± 1.1 | 66.3 ± 0.6 |
| MCH (pg) | 24.2 ± 0.6 | 29.7 ± 11.6 | 21.8 ± 0.7 | 22.0 ± 0.7 | 22.4 ± 0.5 | 23.5 ± 0.7 | 22.0 ± 0.6 | 22.2 ± 0.8 | 22.4 ± 0.5 | 23.1 ± 0.5 | 13.4 ± 0.5 | 22.1 ± 0.7 | 22.8 ± 0.7 | 23.2 ± 0.5 | 22.8 ± 0.5 | 22.5 ± 0.3 |
| MCHC (g/dL) | 32.7 ± 0.8 | 41.7 ± 18.8 | 32.3 ± 0.5 | 32.7 ± 0.3 | 32.6 ± 0.6 | 32.7 ± 0.5 | 32.5 ± 0.4 | 32.9 ± 0.5 | 33.2 ± 0.3 | 32.6 ± 0.5 | 22.1 ± 0.7 | 33.2 ± 0.5 | 32.1 ± 0.5 | 32.5 ± 0.2 | 33.1 ± 0.6 | 33.9 ± 0.4 |
| RDW (%) | 13.1 ± 0.4 | 14.8 ± 3.4 | 13.5 ± 0.5 | 13.5 ± 0.6 | 13.9 ± 0.9 | 13.3 ± 0.3 | 13.5 ± 0.5 | 13.7 ± 0.8 | 13.6 ± 0.9 | 13.1 ± 0.3 | 33.1 ± 0.3 | 13.7 ± 0.8 | 13.0 ± 0.2 | 13.0 ± 0.3 | 13.6 ± 0.3 | 14.3 ± 0.7 |
| PLT (×109/L) | 331 ± 75 | 697 ± 721 | 388 ± 35 | 444 ± 46 | 335 ± 39 | 293 ± 63 | 347 ± 36 | 402 ± 92 | 324 ± 38 | 283 ± 52 | 13.4 ± 0.5 | 365 ± 102 | 256 ± 67 | 230 ± 40 | 305 ± 43 | 380 ± 99 |
| PDW (%) | 50.2 ± 5.0 | 45.0 ± 8.8 | 57.4 ± 3.0 | 56.4 ± 4.8 | 53.8 ± 2.5 | 51.8 ± 3.5 | 53.7 ± 3.7 | 54.8 ± 3.5 | 54.0 ± 3.6 | 53.1 ± 2.0 | 342 ± 26 | 54.1 ± 5.4 | 49.4 ± 3.5 | 52.4 ± 4.9 | 56.5 ± 1.9 | 55.3 ± 2.4 |
| RETIC (%) | 1.2 ± 0.4 | 1.7 ± 0.5 * | 2.2 ± 0.6 | 1.7 ± 0.5 | 2.1 ± 0.8 | 1.7 ± 0.4 | 2.2 ± 0.5 | 1.4 ± 0.4 ** | 1.8 ± 0.8 | 1.4 ± 0.3 | 56.5 ± 3.8 | 1.2 ± 0.4 ** | 1.2 ± 0.4 | 1.6 ± 0.4 | 2.0 ± 0.2 | 1.8 ± 0.4 |
| PT (s) | 75.0 ± 25.3 | 105.6 ± 34.0 * | 8.2 ± 0.7 | 8.6 ± 1.2 | 8.1 ± 0.6 | 7.2 ± 0.4 | 8.0 ± 0.6 | 8.4 ± 1.1 | 8.5 ± 0.7 | 7.5 ± 0.4 | 1.57 ± 0.39 | 8.5 ± 1.0 | 7.5 ± 0.7 | 7.5 ± 0.2 | 7.6 ± 0.3 | 8.2 ± 0.7 |
| APTT (s) | 6.2 ± 0.5 | 6.1 ± 0.3 | 13.1 ± 1.4 | 13.6 ± 1.8 | 14.7 ± 1.1 | 16.0 ± 1.9 | 14.4 ± 1.1 | 14.3 ± 2.6 | 15.1 ± 2.0 | 16.9 ± 2.9 | 8.1 ± 0.8 | 13.8 ± 2.0 | 18.2 ± 1.2 | 19.3 ± 2.3 | 16.3 ± 2.6 | 16.9 ± 1.4 |
| FIB (mg/dL) | 15.0 ± 2.7 | 14.7 ± 2.7 | 257 ± 43 | 261 ± 41 | 239 ± 36 | 276 ± 78 | 262 ± 43 | 290 ± 106 | 231 ± 26 | 294 ± 88 | 14.3 ± 2.0 | 225 ± 49 | 265 ± 28 | 307 ± 83 | 217 ± 26 | 207 ± 39 |
| TT (s) | 240 ± 72 | - | 9.9 ± 0.4 | 9.9 ± 0.4 | 11.5 ± 1.5 | 9.2 ± 0.3 | 11.5 ± 1.8 | 11.2 ± 1.3 | 10.6 ± 0.4 | 9.1 ± 0.3 | 251 ± 40 | 10.6 ± 0.6 | 10.4 ± 0.3 | 10.0 ± 0.3 | 10.6 ± 0.6 | 11.3 ± 0.9 |
| Parameters | Pre-Dose | Day 15 | Day 27 | End of Recovery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | CTL | 5 mg/kg | 15 mg/kg | 50 mg/kg | |
| AST (U/L) | 37 ± 9 | 22 ± 4 | 43 ± 28 | 35 ± 7 | 30 ± 6 | 26 ± 5 | 32 ± 7 | 33 ± 5 | 31 ± 4 | 26 ± 5 | 71 ± 134 | 30 ± 4 | 29 ± 11 | 23 ± 5 | 31 ± 10 | 34 ± 4 |
| ALT (U/L) | 33 ± 13 | 34 ± 15 | 85 ± 196 | 33 ± 13 | 28 ± 11 | 29 ± 9 | 26 ± 8 | 30 ± 9 | 31 ± 9 | 31 ± 12 | 156 ± 413 | 27 ± 8 | 42 ± 17 | 36 ± 8 | 23 ± 9 | 27 ± 13 |
| ALP (U/L) | 180 ± 41 | 144 ± 27 | 165 ± 41 | 154 ± 32 | 187 ± 35 | 160 ± 33 | 176 ± 40 | 162 ± 33 | 177 ± 37 | 161 ± 23 | 164 ± 33 | 160 ± 33 | 192 ± 43 | 150 ± 25 | 182 ± 26 | 142 ± 11 |
| TBIL (µmol/L) | 3.0 ± 0.9 | 2.7 ± 0.2 | 3.1 ± 0.9 | 3.4 ± 0.7 | 2.7 ± 0.5 | 1.8 ± 0.5 | 3.5 ± 0.4 | 2.9 ± 0.7 | 3.5 ± 0.8 | 2.6 ± 0.4 | 3.5 ± 1.1 | 3.5 ± 0.9 | 3.7 ± 0.5 | 2.5 ± 0.7 | 3.7 ± 2.3 | 3.4 ± 1.6 |
| TP (g/L) | 59.6 ± 3.7 | 55.0 ± 3.2 | 59.4 ± 1.8 | 59.5 ± 2.1 | 58.9 ± 2.8 | 57.9 ± 2.9 | 59.0 ± 1.8 | 58.4 ± 2.1 | 58.9 ± 3.3 | 57.4 ± 2.7 | 59.8 ± 3.1 | 58.0 ± 1.4 | 60.1 ± 3.6 | 56.9 ± 3.9 | 60.6 ± 2.6 | 56.6 ± 2.3 |
| ALB (g/L) | 27.6 ± 2.0 | 27.8 ± 2.0 | 27.1 ± 0.9 | 27.5 ± 1.3 | 27.4 ± 1.5 | 29.3 ± 2.0 | 26.4 ± 1.0 | 25.6 ± 1.3 ** | 27.5 ± 1.9 | 28.5 ± 2.3 | 26.9 ± 1.6 | 26.6 ± 1.2 | 28.3 ± 1.5 | 27.6 ± 3.0 | 27.7 ± 1.8 | 26.5 ± 0.5 |
| GLO (g/L) | 32.0 ± 2.3 | 27.2 ± 3.9 ** | 32.4 ± 2.0 | 32.0 ± 2.4 | 31.5 ± 2.1 | 28.7 ± 4.3 | 32.7 ± 1.3 | 32.8 ± 2.2 | 31.4 ± 2.5 | 29.0 ± 4.0 | 32.9 ± 2.1 | 31.5 ± 1.0 | 31.7 ± 2.2 | 29.3 ± 6.3 | 32.9 ± 1.9 | 30.1 ± 2.0 |
| A/G | 0.9 ± 0.1 | 1.0 ± 0.2 * | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.0 | 0.8 ± 0.1 * | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.0 | 1.0 ± 0.3 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| BUN (mmol/L) | 2.2 ± 0.6 | 3.1 ± 0.6 | 1.9 ± 0.4 | 2.3 ± 0.5 | 3.0 ± 0.5 | 3.3 ± 1.1 | 2.7 ± 0.9 | 3.4 ± 0.7 | 2.9 ± 1.0 | 3.5 ± 0.9 | 2.8 ± 1.3 | 2.9 ± 0.7 | 3.1 ± 0.6 | 4.7 ± 1.5 | 2.4 ± 0.5 | 3.3 ± 0.5 |
| CREA (µmol/L) | 41 ± 12 | 61 ± 6 | 37 ± 7 | 44 ± 10 | 38 ± 7 | 60 ± 7 | 34 ± 8 | 37 ± 10 | 53 ± 6 | 63 ± 6 | 50 ± 9 | 59 ± 8 | 48 ± 8 | 61 ± 7 | 49 ± 8 | 53 ± 12 |
| CHOL (mmol/L) | 4.3 ± 0.7 | 5.7 ± 0.7 | 4.3 ± 0.9 | 4.7 ± 0.8 | 4.7 ± 0.8 | 5.7 ± 1.1 | 4.6 ± 0.9 | 5.4 ± 1.2 | 4.7 ± 0.9 | 5.4 ± 1.2 | 4.9 ± 1.4 | 5.3 ± 0.9 | 5.0 ± 0.1 | 5.2 ± 0.9 | 4.6 ± 0.4 | 4.4 ± 1.3 |
| TGL (mmol/L) | 0.3 ± 0.1 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.7 ± 0.3 | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| GLU (mmol/L) | 5.7 ± 1.0 | 6.3 ± 0.4 | 5.3 ± 0.4 | 5.4 ± 0.3 | 6.1 ± 0.3 | 6.5 ± 0.4 | 6.2 ± 0.2 | 6.2 ± 0.4 | 5.7 ± 0.3 | 5.9 ± 0.3 | 5.8 ± 0.4 | 5.9 ± 0.4 | 5.8 ± 0.2 | 6.0 ± 0.1 | 5.8 ± 0.3 | 5.9 ± 0.4 |
| CK (U/L) | 343 ± 117 | 258 ± 35 | 317 ± 82 | 317 ± 34 | 246 ± 32 | 209 ± 48 * | 227 ± 38 | 220 ± 43 | 226 ± 31 | 252 ± 41 | 260 ± 88 | 221 ± 39 | 219 ± 27 | 186 ± 21 | 267 ± 48 | 256 ± 57 |
| K (mmol/L) | 5.5 ± 0.3 | 4.8 ± 0.2 | 5.4 ± 0.4 | 5.4 ± 0.2 | 4.9 ± 0.4 | 4.6 ± 0.2 | 5.0 ± 0.3 | 4.9 ± 0.3 | 4.9 ± 0.3 | 4.6 ± 0.3 | 4.9 ± 0.3 | 4.8 ± 0.3 | 4.8 ± 0.2 | 4.6 ± 0.2 | 5.0 ± 0.3 | 4.9 ± 0.2 |
| NA (mmol/L) | 153 ± 2 | 149 ± 1 | 153 ± 1 | 152 ± 2 | 150 ± 1 | 150 ± 1 * | 150 ± 1 | 150 ± 1 | 150 ± 1 | 149 ± 2 | 150 ± 1 | 150 ± 2 | 150 ± 1 | 146 ± 2 | 151 ± 1 | 149 ± 3 |
| CL (mmol/L) | 112 ± 2 | 113 ± 2 | 112 ± 1 | 111 ± 1 | 114 ± 1 | 113 ± 2 | 114 ± 1 | 113 ± 2 | 114 ± 1 | 112 ± 2 | 115 ± 2 | 114 ± 2 | 114 ± 1 | 113 ± 1 | 116 ± 1 | 114 ± 3 |
| GGT (U/L) | 4.4 ± 2.0 | 4.1 ± 0.9 | 4.4 ± 1.9 | 4.1 ± 1.7 | 5.8 ± 2.5 | 3.3 ± 1.8 | 5.0 ± 1.8 | 4.9 ± 3.4 | 6.0 ± 1.7 | 5.6 ± 1.0 * | 5.6 ± 1.8 | 4.6 ± 2.1 | 3.8 ± 2.7 | 5.8 ± 2.6 | 3.9 ± 2.3 | 4.9 ± 0.9 |
| CA (mmol/L) | 2.6 ± 0.1 | 2.9 ± 0.1 | 2.6 ± 0.1 | 2.5 ± 0.1 | 2.7 ± 0.1 | 2.9 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 |
| PHOS (mmol/L) | 2.4 ± 0.2 | 2.6 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.3 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.3 | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.1 * | 2.3 ± 0.2 | 2.1 ± 0.2 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.3 |
| Groups Parameters | 5 mg/kg | 15 mg/kg | 50 mg/kg | ||||
|---|---|---|---|---|---|---|---|
| Day1 | Day28 | Day1 | Day28 | Day1 | Day28 | ||
| Male | AUC0-t (μg/mL*h) | 5.2 ± 2.1 | 7.6 ± 6.6 | 10.3 ± 3.7 | 17.0 ± 5.7 | 25.1 ± 4.5 | 42.0 ± 9.8 |
| T1/2 (h) | 1.1 ± 0.15 | 1.5 ± 0.5 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.2 | |
| Tmax (h) | 0.70 ± 0.41 | 0.65 ± 0.34 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.5 ± 0.0 | |
| Cmax (μg/mL) | 2.5 ± 0.82 | 2.6 ± 1.3 | 4.1 ± 1.2 | 6.1 ± 1.7 | 10.7 ± 2.4 | 14.7 ± 2.9 | |
| Female | AUC0-t (μg/mL*h) | 5.4 ± 2.0 | 8.0 ± 4.7 | 12.7 ± 5.6 | 16.3 ± 8.2 | 45.4 ± 11.6 | 60.6 ± 15.7 |
| T1/2 (h) | 1.1 ± 0.33 | 4.5 ± 7.0 | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.8 ± 0.2 | 1.7 ± 0.2 | |
| Tmax (h) | 0.70 ± 0.27 | 0.60 ± 0.22 | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.9 ± 0.7 | 0.4 ± 0.1 | |
| Cmax (μg/mL) | 2.6 ± 1.0 | 3.2 ± 1.9 | 5.3 ± 1.7 | 5.7 ± 2.3 | 12.3 ± 3.0 | 18.4 ± 3.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Li, X.; Li, H.; Liu, N.; Xu, Y.; Li, K.; Zhang, J.; Wang, X.; Zhang, X.; Ding, Y.; et al. Preclinical Safety Profile of Deg-AZM, a Clinical-Stage New Transgelin Agonist: hERG Inhibition Study In Vitro, Cardiovascular–Respiratory Pharmacology, and Single/Repeated-Dose Toxicity in Beagle Dogs. Biomedicines 2025, 13, 2180. https://doi.org/10.3390/biomedicines13092180
Gu X, Li X, Li H, Liu N, Xu Y, Li K, Zhang J, Wang X, Zhang X, Ding Y, et al. Preclinical Safety Profile of Deg-AZM, a Clinical-Stage New Transgelin Agonist: hERG Inhibition Study In Vitro, Cardiovascular–Respiratory Pharmacology, and Single/Repeated-Dose Toxicity in Beagle Dogs. Biomedicines. 2025; 13(9):2180. https://doi.org/10.3390/biomedicines13092180
Chicago/Turabian StyleGu, Xiaoting, Xiaohe Li, Hailong Li, Nannan Liu, Ying Xu, Keran Li, Jia Zhang, Xiaoting Wang, Xiaoting Zhang, Yanjie Ding, and et al. 2025. "Preclinical Safety Profile of Deg-AZM, a Clinical-Stage New Transgelin Agonist: hERG Inhibition Study In Vitro, Cardiovascular–Respiratory Pharmacology, and Single/Repeated-Dose Toxicity in Beagle Dogs" Biomedicines 13, no. 9: 2180. https://doi.org/10.3390/biomedicines13092180
APA StyleGu, X., Li, X., Li, H., Liu, N., Xu, Y., Li, K., Zhang, J., Wang, X., Zhang, X., Ding, Y., Zhou, H., Ai, X., & Yang, C. (2025). Preclinical Safety Profile of Deg-AZM, a Clinical-Stage New Transgelin Agonist: hERG Inhibition Study In Vitro, Cardiovascular–Respiratory Pharmacology, and Single/Repeated-Dose Toxicity in Beagle Dogs. Biomedicines, 13(9), 2180. https://doi.org/10.3390/biomedicines13092180







