Interventional Oncology for Colorectal Liver Metastases: From Local Cure to Salvage Therapy
Abstract
1. Introduction
2. Ablation Modalities
2.1. Thermal Ablation
2.2. Non-Thermal Ablation
2.2.1. Irreversible Electroporation
2.2.2. Histotripsy
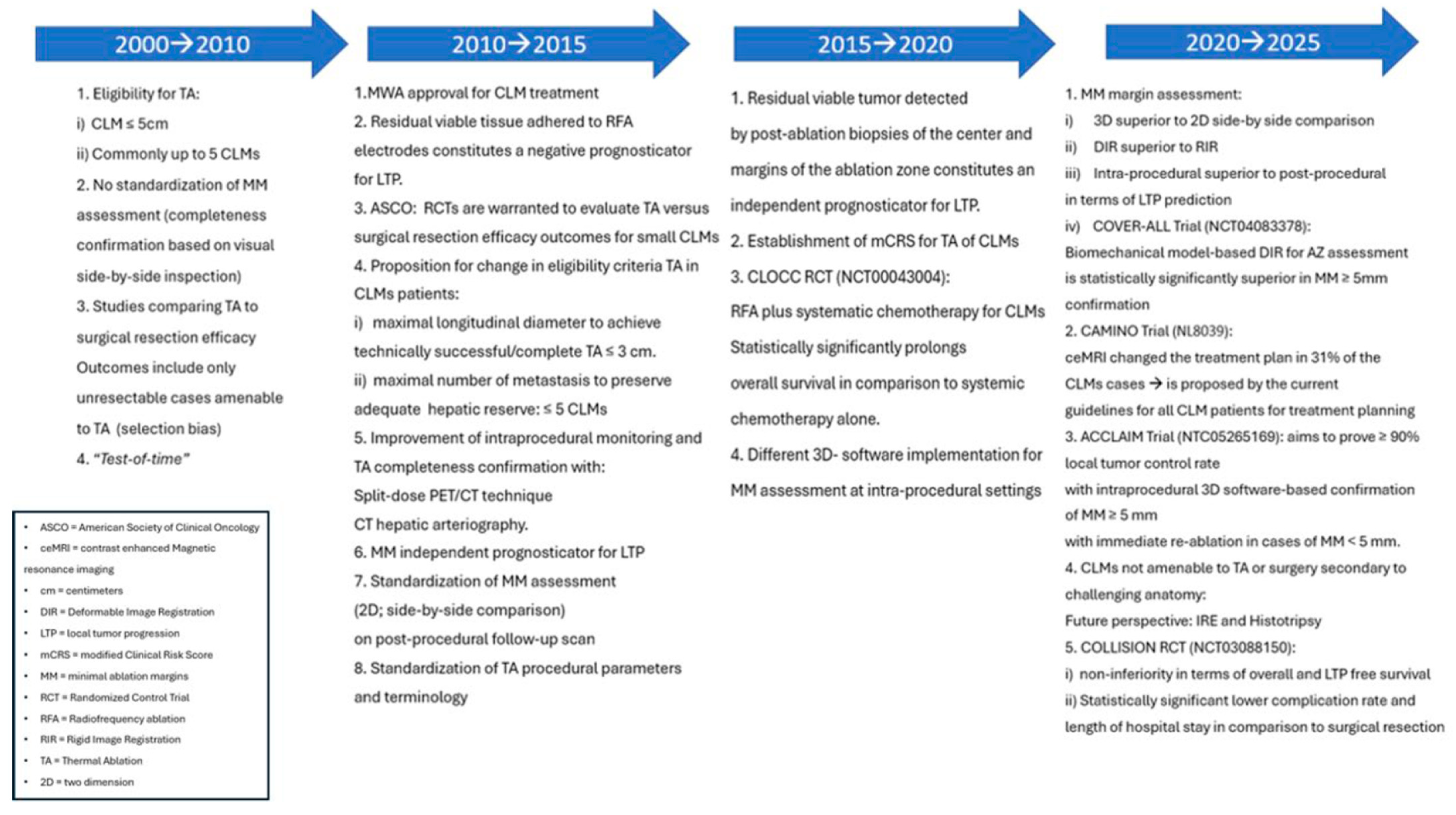
2.3. Evolution of Thermal Ablation as an Equivalent Treatment to Surgical Resection for Colorectal Liver Metastases ≤ 3 cm
2.3.1. Target Tumor Size
2.3.2. Metastases Location, Number, and Chemotherapy Synergy
2.3.3. Minimal Ablation Margins Assessment
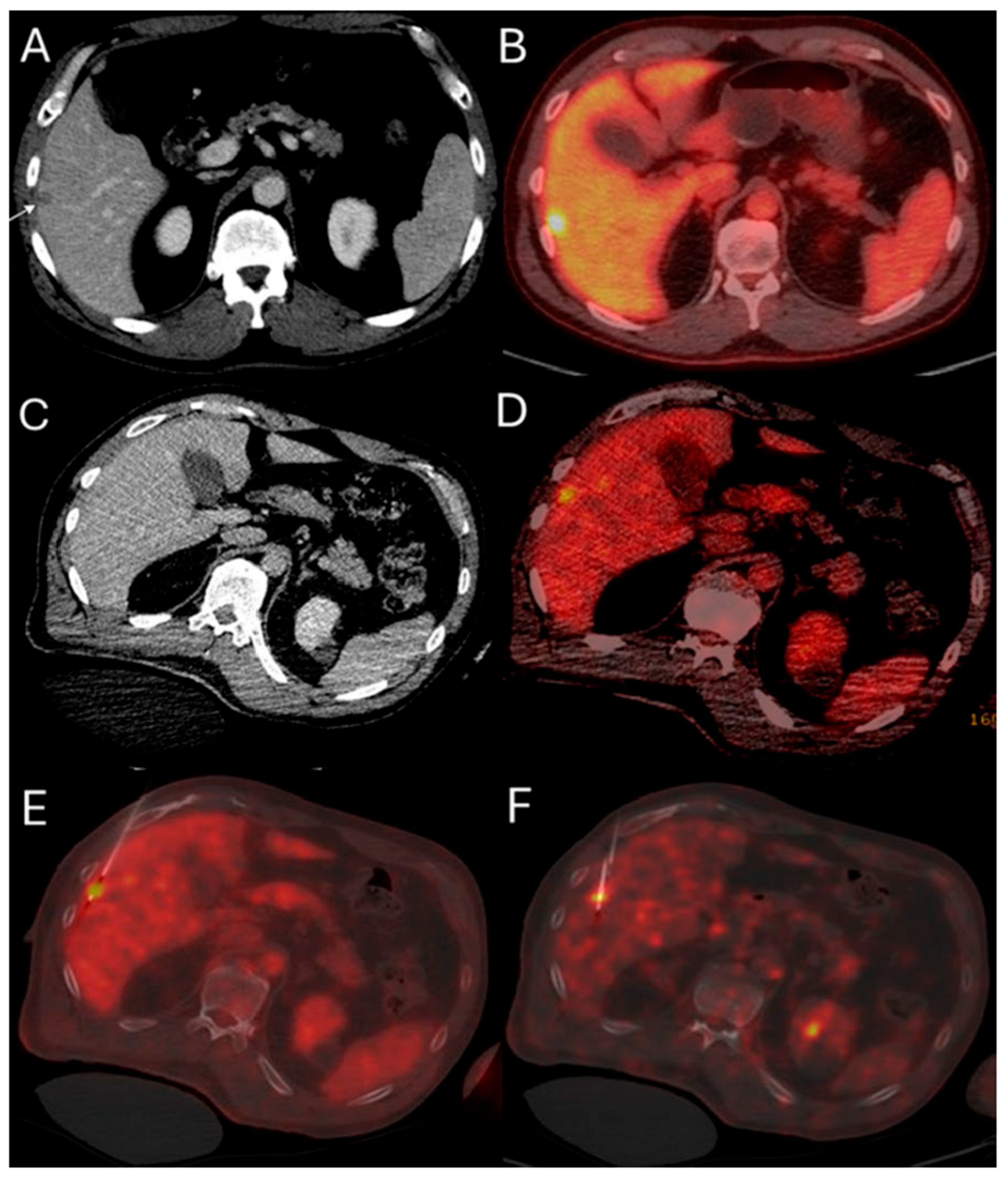
2.3.4. Optimization of Ablation Monitoring with Split-Dose PET/CT and CTHA
2.3.5. Biopsy Confirmation
2.3.6. Patient Selection Bias When Comparing Ablation to Surgery
2.3.7. Patient Eligibility for Ablation and Establishment of a Clinical Risk Score
2.3.8. Treatment Efficacy in Salvage Settings
2.3.9. Procedural Completeness, Treatment Efficacy and Disease Surveillance
2.3.10. Guideline Change
3. Radioembolization
3.1. Trans-Arterial Radioembolization
3.2. Radiation Segmentectomy
3.3. Radiation Lobectomy
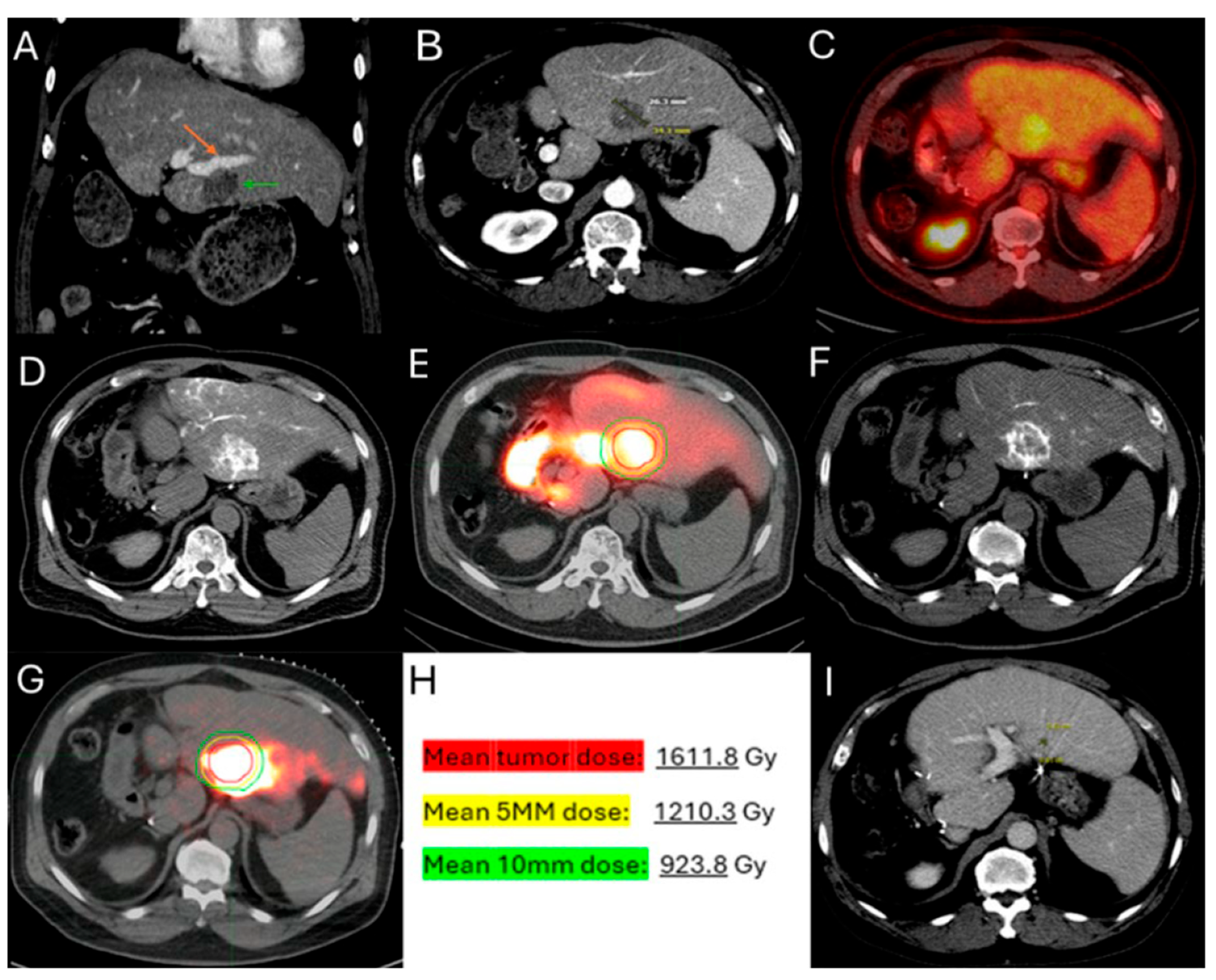
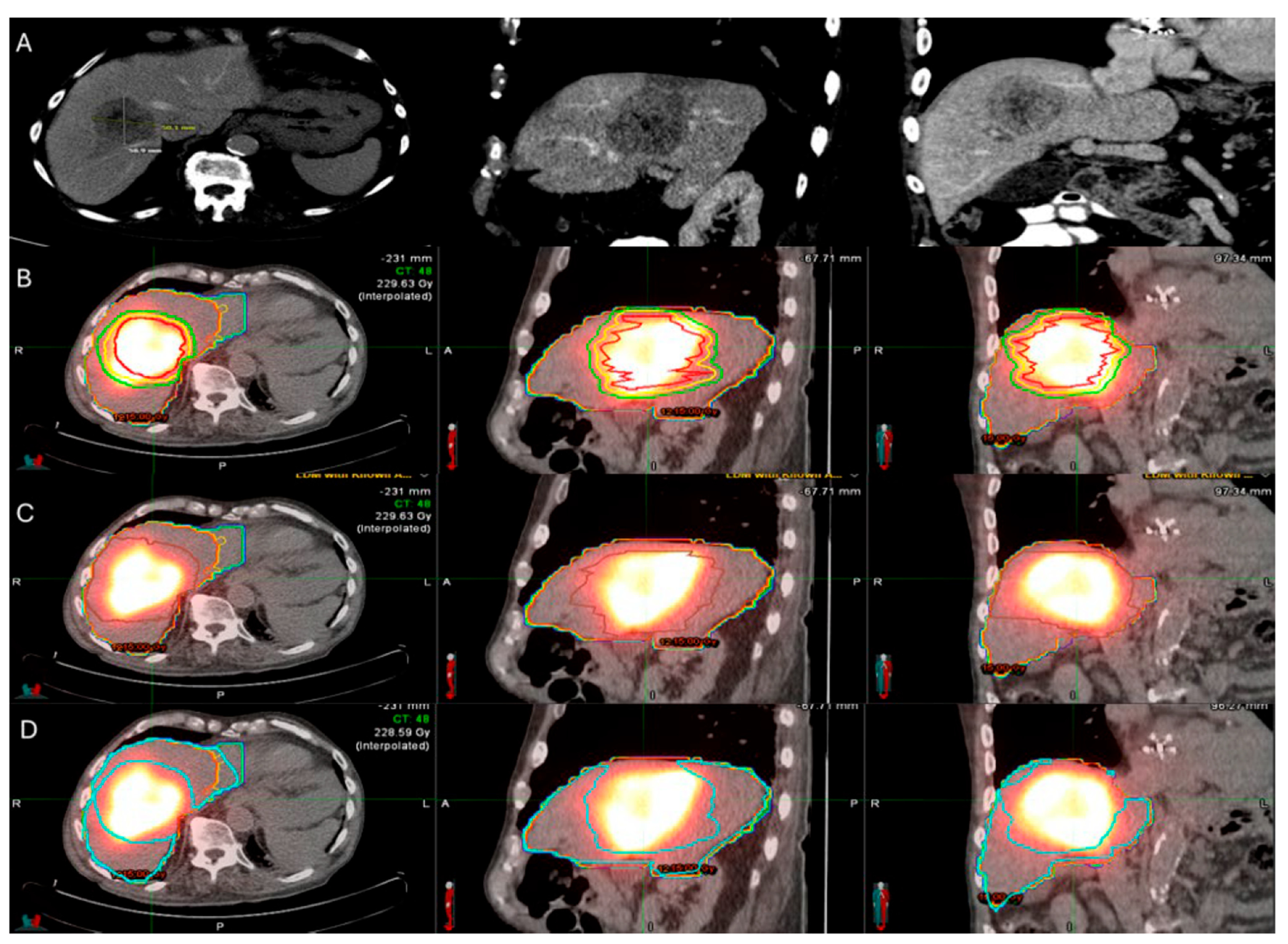
3.4. 90Y TARE Planning and Predictors of Oncologic Outcomes
3.4.1. Mapping Arteriography
3.4.2. Dosimetry
3.4.3. Pre-TARE Predictors of Treatment Response for Patient Selection Optimization, Hepatic Arterial Infusion Pump Synergy and Other Isotopes
3.4.4. Treatment Response Assessment and Disease Surveillance
4. Chemoembolization
4.1. Mitomycin-C
4.2. DEBIRI
4.3. Comparison of TACE to Other Local Treatments
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Horn, S.R.; Stoltzfus, K.C.; Lehrer, E.J.; Dawson, L.A.; Tchelebi, L.; Gusani, N.J.; Sharma, N.K.; Chen, H.; Trifiletti, D.M.; Zaorsky, N.G. Epidemiology of liver metastases. Cancer Epidemiology 2020, 67, 101760. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Simmonds, P.C.; Primrose, J.N.; Colquitt, J.L.; Garden, O.J.; Poston, G.J.; Rees, M. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br. J. Cancer 2006, 94, 982–999. [Google Scholar] [CrossRef]
- Alinia, S.; Ahmadi, S.; Mohammadi, Z.; Shirvandeh, F.R.; Asghari-Jafarabadi, M.; Mahmoudi, L.; Safari, M.; Roshanaei, G. Exploring the impact of stage and tumor site on colorectal cancer survival: Bayesian survival modeling. Sci. Rep. 2024, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suthananthan, A.E.; Bhandari, M.; Platell, C. Influence of primary site on metastatic distribution and survival in stage IV colorectal cancer. ANZ J. Surg. 2017, 88, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Gonen, M.; Erinjeri, J.P.; Brown, K.T.; Covey, A.M.; Alago, W.; Durack, J.C.; Maybody, M.; Brody, L.A.; et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes—A 10-year Experience at a Single Center. Radiology 2016, 278, 601–611. [Google Scholar] [CrossRef]
- Kamarinos, N.V.; Kaye, E.A.; Sofocleous, C.T. Image-Guided Thermal Ablation for Colorectal Liver Metastases. Tech. Vasc. Interv. Radiol. 2020, 23, 100672. [Google Scholar] [CrossRef]
- Entezari, P.; Gabr, A.; Salem, R.; Lewandowski, R.J. Yttrium-90 for colorectal liver metastasis - the promising role of radiation segmentectomy as an alternative local cure. Int. J. Hyperth. 2022, 39, 620–626. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Velonakis, G.; Kelekis, A.; Sofocleous, C.T. The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics 2021, 11, 308. [Google Scholar] [CrossRef]
- Chlorogiannis, D.-D.; Sotirchos, V.S.; Georgiades, C.; Filippiadis, D.; Arellano, R.S.; Gonen, M.; Makris, G.C.; Garg, T.; Sofocleous, C.T. The Importance of Optimal Thermal Ablation Margins in Colorectal Liver Metastases: A Systematic Review and Meta-Analysis of 21 Studies. Cancers 2023, 15, 5806. [Google Scholar] [CrossRef]
- Chlorogiannis, D.-D.; Sotirchos, V.S.; Sofocleous, C.T. Oncologic Outcomes after Percutaneous Ablation for Colorectal Liver Metastases: An Updated Comprehensive Review. Medicina 2024, 60, 1536. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Petre, E.N.; Gonen, M.; Brown, K.T.; Solomon, S.B.; Covey, A.M.; Alago, W.; Brody, L.A.; Thornton, R.H.; D’Angelica, M.; et al. CT-guided Radiofrequency Ablation as a Salvage Treatment of Colorectal Cancer Hepatic Metastases Developing After Hepatectomy. J. Vasc. Interv. Radiol. 2011, 22, 755–761. [Google Scholar] [CrossRef]
- Hitpass, L.; Distelmaier, M.; Neumann, U.P.; Schöning, W.; Isfort, P.; Keil, S.; Kuhl, C.K.; Bruners, P.; Barabasch, A. Recurrent Colorectal Liver Metastases in the Liver Remnant After Major Liver Surgery—IRE as a Salvage Local Treatment When Resection and Thermal Ablation are Unsuitable. Cardiovasc. Interv. Radiol. 2021, 45, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Violari, E.G.; Sotirchos, V.S.; Shady, W.; Gonen, M.; Pandit-Taskar, N.; Petre, E.N.; Brody, L.A.; Alago, W.; Do, R.K.; et al. Radioembolization as a Salvage Therapy for Heavily Pretreated Patients with Colorectal Cancer Liver Metastases: Factors That Affect Outcomes. Clin. Color. Cancer 2015, 14, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Kalva, S.P.; Rana, R.S.; Liu, R.; Rachamreddy, N.; Dave, B.; Sharma, A.; Ganguli, S.; Rabito, C.; Kwak, E.; Blaszkowsky, L.S. Yttrium-90 Radioembolization as Salvage Therapy for Liver Metastases from Colorectal Cancer. Am. J. Clin. Oncol. 2017, 40, 288–293. [Google Scholar] [CrossRef]
- Voizard, N.; Ni, T.; Kiss, A.; Pugash, R.; Raphael, M.J.; Coburn, N.; David, E. Small Particle DEBIRI TACE as Salvage Therapy in Patients with Liver Dominant Colorectal Cancer Metastasis: Retrospective Analysis of Safety and Outcomes. Curr. Oncol. 2022, 29, 209–220. [Google Scholar] [CrossRef]
- Boas, F.E.; Bodei, L.; Sofocleous, C.T. Radioembolization of Colorectal Liver Metastases: Indications, Technique, and Outcomes. J. Nucl. Med. 2017, 58, 104S–111S. [Google Scholar] [CrossRef]
- Mulcahy, M.F.; Mahvash, A.; Pracht, M.; Montazeri, A.H.; Bandula, S.; Martin, R.C.G.; Herrmann, K.; Brown, E.; Zuckerman, D.; Wilson, G.; et al. Radioembolization with Chemotherapy for Colorectal Liver Metastases: A Randomized, Open-Label, International, Multicenter, Phase III Trial. J. Clin. Oncol. 2021, 39, 3897–3907. [Google Scholar] [CrossRef] [PubMed]
- Wasan, H.S.; Gibbs, P.; Sharma, N.K.; Taieb, J.; Heinemann, V.; Ricke, J.; Peeters, M.; Findlay, M.; Weaver, A.; Mills, J.; et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): A combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017, 18, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Frühling, P.; Nilsson, A.; Duraj, F.; Haglund, U.; Norén, A. Single-center nonrandomized clinical trial to assess the safety and efficacy of irreversible electroporation (IRE) ablation of liver tumors in humans: Short to mid-term results. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.R.; Ruarus, A.H.; Vroomen, L.G.P.H.; Puijk, R.S.; Geboers, B.; Nieuwenhuizen, S.; Bemd, B.A.T.v.D.; Nielsen, K.; de Vries, J.J.J.; van Lienden, K.P.; et al. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology 2021, 299, 470–480. [Google Scholar] [CrossRef]
- Kamarinos, N.V.; Vakiani, E.; Gonen, M.; Kemeny, N.E.; Sigel, C.; Saltz, L.B.; Brown, K.T.; Covey, A.M.; Erinjeri, J.P.; Brody, L.A.; et al. Biopsy and Margins Optimize Outcomes after Thermal Ablation of Colorectal Liver Metastases. Cancers 2022, 14, 693. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. JNCI J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Fiorentini, G.; Aliberti, C.; Tilli, M.; Mulazzani, L.; Graziano, F.; Giordani, P.; Mambrini, A.; Montagnani, F.; Alessandroni, P.; Catalano, V.; et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: Final results of a phase III study. Anticancer Res. 2012, 32, 1387–1395. [Google Scholar]
- Mendiratta-Lala, M.; Wiggermann, P.; Pech, M.; Serres-Créixams, X.; White, S.B.; Davis, C.; Ahmed, O.; Parikh, N.D.; Planert, M.; Thormann, M.; et al. The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors. Radiology 2024, 312, e233051. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ruarus, A.H.; Vroomen, L.G.P.H.; Van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; De Jong, M.C.; De Vries, J.J.J.; Zonderhuis, B.M.; Eker, H.H.; et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer 2018, 18, 821. [Google Scholar] [CrossRef]
- Hong, K.; Georgiades, C. Radiofrequency Ablation: Mechanism of Action and Devices. J. Vasc. Interv. Radiol. 2010, 21, S179–S186. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Hahn, P.F.; Tanabe, K.K.; Mueller, P.R.; Schima, W.; Athanasoulis, C.A.; Compton, C.C.; Solbiati, L.; Gazelle, G.S. Percutaneous Radiofrequency Tissue Ablation: Does Perfusion-mediated Tissue Cooling Limit Coagulation Necrosis? J. Vasc. Interv. Radiol. 1998, 9, 101–111. [Google Scholar] [CrossRef]
- Gravante, G.; Ong, S.L.; Metcalfe, M.S.; Strickland, A.; Dennison, A.R.; Lloyd, D.M. Hepatic microwave ablation: A review of the histological changes following thermal damage. Liver Int. 2008, 28, 911–921. [Google Scholar] [CrossRef]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee, F.T. Microwave Tumor Ablation: Mechanism of Action, Clinical Results, and Devices. J. Vasc. Interv. Radiol. 2010, 21, S192–S203. [Google Scholar] [CrossRef] [PubMed]
- Tsitskari, M.; Filippiadis, D.; Kostantos, C.; Palialexis, K.; Zavridis, P.; Kelekis, N.; Brountzos, E. The role of interventional oncology in the treatment of colorectal cancer liver metastases. Ann. Gastroenterol. 2019, 32, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Erinjeri, J.P.; Clark, T.W. Cryoablation: Mechanism of Action and Devices. J. Vasc. Interv. Radiol. 2010, 21, S187–S191. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, S.; Behnoush, A.H.; Akhlaghpoor, S.; Lanza, E. Survival outcomes and quality of life after percutaneous cryoablation for liver metastasis: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0289975. [Google Scholar] [CrossRef]
- Burke, C.T.; Yu, H. Comparison of Percutaneous Ablation Technologies in the Treatment of Malignant Liver Tumors. Semin. Interv. Radiol. 2014, 31, 129–137. [Google Scholar] [CrossRef]
- Di Martino, M.; Rompianesi, G.; Mora-Guzmán, I.; Martín-Pérez, E.; Montalti, R.; Troisi, R.I. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 772–781. [Google Scholar] [CrossRef]
- Littrup, P.J.; Aoun, H.D.; Adam, B.; Krycia, M.; Prus, M.; Shields, A. Percutaneous cryoablation of hepatic tumors: Long-term experience of a large U.S. series. Abdom. Imaging 2016, 41, 767–780. [Google Scholar] [CrossRef]
- Livraghi, T.; Solbiati, L.; Meloni, F.; Ierace, T.; Goldberg, S.N.; Gazelle, G.S. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: The “test-of-time approach”. Cancer 2003, 97, 3027–3035. [Google Scholar] [CrossRef]
- Kitsel, Y.; Cooke, T.; Sotirchos, V.; Sofocleous, C.T. Colorectal Cancer Liver Metastases: Genomics and Biomarkers with Focus on Local Therapies. Cancers 2023, 15, 1679. [Google Scholar] [CrossRef]
- Silk, M.T.; Wimmer, T.; Lee, K.S.; Srimathveeravalli, G.; Brown, K.T.; Kingham, P.T.; Fong, Y.; Durack, J.C.; Sofocleous, C.T.; Solomon, S.B. Percutaneous Ablation of Peribiliary Tumors with Irreversible Electroporation. J. Vasc. Interv. Radiol. 2014, 25, 112–118. [Google Scholar] [CrossRef]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; De Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Eller, A.; Schmid, A.; Schmidt, J.; May, M.; Brand, M.; Saake, M.; Uder, M.; Lell, M. Local Control of Perivascular Malignant Liver Lesions Using Percutaneous Irreversible Electroporation: Initial Experiences. Cardiovasc. Interv. Radiol. 2014, 38, 152–159. [Google Scholar] [CrossRef]
- Hosein, P.J.; Echenique, A.; Loaiza-Bonilla, A.; Froud, T.; Barbery, K.; Lima, C.M.R.; Yrizarry, J.M.; Narayanan, G. Percutaneous Irreversible Electroporation for the Treatment of Colorectal Cancer Liver Metastases with a Proposal for a New Response Evaluation System. J. Vasc. Interv. Radiol. 2014, 25, 1233–1239.e2. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, H.J.; Vroomen, L.G.P.H.; Nielsen, K.; van Tilborg, A.A.J.M.; Comans, E.F.I.; Van Kuijk, C.; Van Der Meijs, B.B.; van den Bergh, J.; van den Tol, P.M.P.; Meijerink, M.R. Colorectal liver metastatic disease: Efficacy of irreversible electroporation—A single-arm phase II clinical trial (COLDFIRE-2 trial). BMC Cancer 2015, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Lundt, J.E.; Allen, S.P.; Shi, J.; Hall, T.L.; Cain, C.A.; Xu, Z. Non-invasive, Rapid Ablation of Tissue Volume Using Histotripsy. Ultrasound Med. Biol. 2017, 43, 2834–2847. [Google Scholar] [CrossRef] [PubMed]
- Mancia, L.; Vlaisavljevich, E.; Yousefi, N.; Rodriguez, M.; Ziemlewicz, T.J.; Lee, F.T.; Henann, D.; Franck, C.; Xu, Z.; Johnsen, E. Modeling tissue-selective cavitation damage. Phys. Med. Biol. 2019, 64, 225001. [Google Scholar] [CrossRef]
- Mancia, L.; Vlaisavljevich, E.; Xu, Z.; Johnsen, E. Predicting Tissue Susceptibility to Mechanical Cavitation Damage in Therapeutic Ultrasound. Ultrasound Med. Biol. 2017, 43, 1421–1440. [Google Scholar] [CrossRef]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Smolock, A.R.; Cristescu, M.M.; Vlaisavljevich, E.; Gendron-Fitzpatrick, A.; Green, C.; Cannata, J.; Ziemlewicz, T.J.; Lee, F.T. Robotically Assisted Sonic Therapy as a Noninvasive Nonthermal Ablation Modality: Proof of Concept in a Porcine Liver Model. Radiology 2018, 287, 485–493. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; E Felsted, A.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; A Kevelin, A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-man histotripsy of hepatic tumors: The THERESA trial, a feasibility study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Wah, T.M.; Pech, M.; Thormann, M.; Serres, X.; Littler, P.; Stenberg, B.; Lenton, J.; Smith, J.; Wiggermann, P.; Planert, M.; et al. A Multi-centre, Single Arm, Non-randomized, Prospective European Trial to Evaluate the Safety and Efficacy of the HistoSonics System in the Treatment of Primary and Metastatic Liver Cancers (#HOPE4LIVER). Cardiovasc. Interv. Radiol. 2022, 46, 259–267. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Vauthey, J.-N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A. Recurrence and Outcomes Following Hepatic Resection, Radiofrequency Ablation, and Combined Resection/Ablation for Colorectal Liver Metastases. Ann. Surg. 2004, 239, 818–827. [Google Scholar] [CrossRef]
- Gillams, A.R.; Lees, W.R. Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur. Radiol. 2004, 14, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Oshowo, A.; Gillams, A.; Harrison, E.; Lees, W.R.; Taylor, I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br. J. Surg. 2003, 90, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- White, R.R.; Avital, I.; Sofocleous, C.T.; Brown, K.T.; Brody, L.A.; Covey, A.; Getrajdman, G.I.; Jarnagin, W.R.; Dematteo, R.P.; Fong, Y.; et al. Rates and Patterns of Recurrence for Percutaneous Radiofrequency Ablation and Open Wedge Resection for Solitary Colorectal Liver Metastasis. J. Gastrointest. Surg. 2007, 11, 256–263. [Google Scholar] [CrossRef]
- Weng, M.; Zhang, Y.; Zhou, D.; Yang, Y.; Tang, Z.; Zhao, M.; Quan, Z.; Gong, W.; Cheng, J.Q. Radiofrequency Ablation versus Resection for Colorectal Cancer Liver Metastases: A Meta-Analysis. PLoS ONE 2012, 7, e45493. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Li, B.; Wang, T.; Wang, S.J.; Zhou, Y.M. Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: A meta-analysis. World J. Gastroenterol. 2011, 17, 4143–4148. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.O.; Yoo, C.H.; Son, B.H.; Park, Y.L.; Cho, Y.K.; Kim, H.; Han, W.K. Comparison of Radiofrequency Ablation and Resection for Hepatic Metastasis from Colorectal Cancer. Korean J. Gastroenterol. 2012, 59, 218–223. [Google Scholar] [CrossRef]
- Lee, W.-S.; Yun, S.H.; Chun, H.-K.; Lee, W.Y.; Kim, S.-J.; Choi, S.-H.; Heo, J.-S.; Joh, J.W.; Choi, D.; Kim, S.-H.; et al. Clinical Outcomes of Hepatic Resection and Radiofrequency Ablation in Patients with Solitary Colorectal Liver Metastasis. J. Clin. Gastroenterol. 2008, 42, 945–949. [Google Scholar] [CrossRef]
- Hur, H.; Ko, Y.T.; Min, B.S.; Kim, K.S.; Choi, J.S.; Sohn, S.K.; Cho, C.H.; Ko, H.K.; Lee, J.T.; Kim, N.K. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am. J. Surg. 2009, 197, 728–736. [Google Scholar] [CrossRef]
- Reuter, N.P.; Woodall, C.E.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C.G. Radiofrequency Ablation vs. Resection for Hepatic Colorectal Metastasis: Therapeutically Equivalent? J. Gastrointest. Surg. 2008, 13, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of Surgical Margin Status on Survival and Site of Recurrence After Hepatic Resection for Colorectal Metastases. Ann. Surg. 2005, 241, 715–722; discussion 722–714. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, G.; Giuliante, F.; Ardito, F.; Vellone, M.; Giovannini, I.; Federico, B.; Vecchio, F.M. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: A single-center experience. Surgery 2008, 143, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Are, C.M.; Gonen, M.; Zazzali, K.D.; DeMatteo, R.P.M.; Jarnagin, W.R.M.; Fong, Y.M.; Blumgart, L.H.M.; D’Angelica, M.M. The Impact of Margins on Outcome After Hepatic Resection for Colorectal Metastasis. Ann. Surg. 2007, 246, 295–300. [Google Scholar] [CrossRef]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Assoc. Française De Chir. 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Wang, X.; Sofocleous, C.T.; Erinjeri, J.P.; Petre, E.N.; Gonen, M.; Do, K.G.; Brown, K.T.; Covey, A.M.; Brody, L.A.; Alago, W.; et al. Margin Size is an Independent Predictor of Local Tumor Progression After Ablation of Colon Cancer Liver Metastases. Cardiovasc. Interv. Radiol. 2013, 36, 166–175. [Google Scholar] [CrossRef]
- Gillams, A.; Goldberg, N.; Ahmed, M.; Bale, R.; Breen, D.; Callstrom, M.; Chen, M.H.; Choi, B.I.; de Baere, T.; Dupuy, D.; et al. Thermal ablation of colorectal liver metastases: A position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur. Radiol. 2015, 25, 3438–3454. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; De Baère, T.; Dodd, G.D., III; et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef]
- Veltri, A.; Sacchetto, P.; Tosetti, I.; Pagano, E.; Fava, C.; Gandini, G. Radiofrequency Ablation of Colorectal Liver Metastases: Small Size Favorably Predicts Technique Effectiveness and Survival. Cardiovasc. Interv. Radiol. 2008, 31, 948–956. [Google Scholar] [CrossRef]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local Recurrence After Hepatic Radiofrequency Coagulation. Ann. Surg. 2005, 242, 158–171. [Google Scholar] [CrossRef] [PubMed]
- van Duijnhoven, F.H.; Jansen, M.C.; Junggeburt, J.M.C.; van Hillegersberg, R.; Rijken, A.M.; van Coevorden, F.; van der Sijp, J.R.; van Gulik, T.M.; Slooter, G.D.; Klaase, J.M.; et al. Factors Influencing the Local Failure Rate of Radiofrequency Ablation of Colorectal Liver Metastases. Ann. Surg. Oncol. 2006, 13, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yoon, Y.S.; Yu, C.S.; Kim, T.W.; Kim, H.J.; Kim, P.N.; Ha, H.K.; Kim, J.C. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J. Korean Surg. Soc. 2011, 81, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Tinguely, P.; Laurell, G.; Enander, A.; Engstrand, J.; Freedman, J. Ablation versus resection for resectable colorectal liver metastases-Health care related cost and survival analyses from a quasi-randomised study. Eur. J. Surg. Oncol. (EJSO) 2022, 49, 416–425. [Google Scholar] [CrossRef]
- Siperstein, A.E.; Berber, E.; Ballem, N.; Parikh, R.T. Survival After Radiofrequency Ablation of Colorectal Liver Metastases. Ann. Surg. 2007, 246, 559–567. [Google Scholar] [CrossRef]
- Shady, W.; Petre, E.N.; Do, K.G.; Gonen, M.; Yarmohammadi, H.; Brown, K.T.; Kemeny, N.E.; D’Angelica, M.; Kingham, P.T.; Solomon, S.B.; et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 2018, 29, 268–275.e1. [Google Scholar] [CrossRef]
- Izaaryene, J.; Drai, M.; Deniel, C.; Bridge, P.; Rico, G.; Daidj, N.; Gilabert, M.; Ewald, J.; Turrini, O.; Piana, G. Computed tomography-guided microwave ablation of perivascular liver metastases from colorectal cancer: A study of the ablation zone, feasibility, and safety. Int. J. Hyperth. 2021, 38, 887–899. [Google Scholar] [CrossRef]
- Solbiati, L.; Ahmed, M.; Cova, L.; Ierace, T.; Brioschi, M.; Goldberg, S.N. Small Liver Colorectal Metastases Treated with Percutaneous Radiofrequency Ablation: Local Response Rate and Long-term Survival with Up to 10-year Follow-up. Radiology 2012, 265, 958–968. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Puijk, R.S.; Van Tilborg, A.A.J.M.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; De Jong, K.P.; Richel, D.J.; et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef]
- Liu, C.-H.; Arellano, R.S.; Uppot, R.N.; Samir, A.E.; Gervais, D.A.; Mueller, P.R. Radiofrequency ablation of hepatic tumours: Effect of post-ablation margin on local tumour progression. Eur. Radiol. 2009, 20, 877–885. [Google Scholar] [CrossRef]
- Liu, M.; Huang, G.-L.; Xu, M.; Pan, F.-S.; Lu, M.-D.; Zheng, K.-G.; Kuang, M.; Xie, X.-Y. Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: A comparison of local therapeutic efficacy. Int. J. Hyperth. 2016, 33, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Odisio, B.C.; Yamashita, S.; Huang, S.Y.; Harmoush, S.; E Kopetz, S.; Ahrar, K.; Chun, Y.S.; Conrad, C.; A Aloia, T.; Gupta, S.; et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br. J. Surg. 2017, 104, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Vakiani, E.; Ziv, E.; Gonen, M.; Brown, K.T.; Kemeny, N.E.; Solomon, S.B.; Solit, D.B.; Sofocleous, C.T. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 2017, 8, 66117–66127. [Google Scholar] [CrossRef] [PubMed]
- Sotirchos, V.S.; Petrovic, L.M.; Gönen, M.; Klimstra, D.S.; Do, R.K.G.; Petre, E.N.; Garcia, A.R.; Barlas, A.; Erinjeri, J.P.; Brown, K.T.; et al. Colorectal Cancer Liver Metastases: Biopsy of the Ablation Zone and Margins Can Be Used to Predict Oncologic Outcome. Radiology 2016, 280, 949–959. [Google Scholar] [CrossRef]
- Kurilova, I.; Bendet, A.; Petre, E.N.; Boas, F.E.; Kaye, E.; Gonen, M.; Covey, A.; Brody, L.A.; Brown, K.T.; Kemeny, N.E.; et al. Factors Associated with Local Tumor Control and Complications After Thermal Ablation of Colorectal Cancer Liver Metastases: A 15-year Retrospective Cohort Study. Clin. Color. Cancer 2021, 20, e82–e95. [Google Scholar] [CrossRef]
- Calandri, M.; Yamashita, S.; Gazzera, C.; Fonio, P.; Veltri, A.; Bustreo, S.; Sheth, R.A.; Yevich, S.M.; Vauthey, J.-N.; Odisio, B.C. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur. Radiol. 2018, 28, 2727–2734. [Google Scholar] [CrossRef]
- Rhaiem, R.; Rached, L.; Tashkandi, A.; Bouché, O.; Kianmanesh, R. Implications of RAS Mutations on Oncological Outcomes of Surgical Resection and Thermal Ablation Techniques in the Treatment of Colorectal Liver Metastases. Cancers 2022, 14, 816. [Google Scholar] [CrossRef]
- Sotirchos, V.S.; Vakiani, E.; Sigel, C.; Imam, R.; Kunin, H.S.; Cooke, T.M.; Gönen, M.; Solomon, S.B.; Erinjeri, J.P.; Sofocleous, C.T. Evaluation of the Ki-67 labeling index on immediate pre-ablation biopsies as a predictive biomarker of local recurrence of colorectal cancer liver metastases. Cytotechnology 2024, 77, 1–12. [Google Scholar] [CrossRef]
- Zadeh, M.Z.; Sotirchos, V.S.; Kirov, A.; Lafontaine, D.; Gönen, M.; Yeh, R.; Kunin, H.; Petre, E.N.; Kitsel, Y.; Elsayed, M.; et al. Three-Dimensional Margin as a Predictor of Local Tumor Progression after Microwave Ablation: Intraprocedural versus 4–8-Week Postablation Assessment. J. Vasc. Interv. Radiol. 2024, 35, 523–532.e1. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Paolucci, I.; Silva, J.A.M.; O’COnnor, C.S.; Fellman, B.M.; Jones, A.K.; Kuban, J.D.; Huang, S.Y.; Metwalli, Z.A.; Brock, K.K.; et al. Intraprocedural Versus Initial Follow-Up Minimal Ablative Margin Assessment After Colorectal Liver Metastasis Thermal Ablation. Investig. Radiol. 2023, 59, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Laimer, G.; Jaschke, N.; Schullian, P.; Putzer, D.; Eberle, G.; Solbiati, M.; Solbiati, L.; Goldberg, S.N.; Bale, R. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur. Radiol. 2021, 31, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Mulder, B.G.S.; Hendriks, P.; Baetens, T.R.; van Erkel, A.R.; van Rijswijk, C.S.P.; van der Meer, R.W.; van de Velde, C.J.H.; Vahrmeijer, A.L.; Mieog, J.S.D.; Burgmans, M.C. Quantitative margin assessment of radiofrequency ablation of a solitary colorectal hepatic metastasis using MIRADA RTx on CT scans: A feasibility study. BMC Med Imaging 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Lin, Y.; Lin, E.Y.; Cazoulat, G.; Gupta, S.; Jones, A.K.; Odisio, B.C.; Brock, K.K. A novel use of biomechanical model-based deformable image registration (DIR) for assessing colorectal liver metastases ablation outcomes. Med Phys. 2021, 48, 6226–6236. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Paolucci, I.; O’cOnnor, C.S.; Anderson, B.M.; Rigaud, B.; Fellman, B.M.; Jones, K.A.; Brock, K.K.; Odisio, B.C. Ablative Margins of Colorectal Liver Metastases Using Deformable CT Image Registration and Autosegmentation. Radiology 2023, 307, e221373. [Google Scholar] [CrossRef]
- Paolucci, I.; Silva, J.A.M.; Lin, Y.-M.; Laimer, G.; Cignini, V.; Menchini, F.; Meira, M.; Shieh, A.; O’cOnnor, C.; A Jones, K.; et al. Identification of A0 minimum ablative margins for colorectal liver metastases: Multicentre, retrospective study using deformable CT registration and artificial intelligence-based autosegmentation. Br. J. Surg. 2024, 111. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Paolucci, I.; Silva, J.A.M.; O’cOnnor, C.S.; Hong, J.; Shah, K.Y.; Abdelsalam, M.E.; Habibollahi, P.; Jones, K.A.; Brock, K.K.; et al. Ablative margin quantification using deformable versus rigid image registration in colorectal liver metastasis thermal ablation: A retrospective single-center study. Eur. Radiol. 2024, 34, 5541–5550. [Google Scholar] [CrossRef]
- Kamarinos, N.V.; Gonen, M.; Sotirchos, V.; Kaye, E.; Petre, E.N.; Solomon, S.B.; Erinjeri, J.P.; Ziv, E.; Kirov, A.; Sofocleous, C.T. 3D margin assessment predicts local tumor progression after ablation of colorectal cancer liver metastases. Int. J. Hyperth. 2022, 39, 880–887. [Google Scholar] [CrossRef]
- Odisio, B.C.; Albuquerque, J.; Lin, Y.-M.; Anderson, B.M.; O’COnnor, C.S.; Rigaud, B.; Briones-Dimayuga, M.; Jones, A.K.; Fellman, B.M.; Huang, S.Y.; et al. Software-based versus visual assessment of the minimal ablative margin in patients with liver tumours undergoing percutaneous thermal ablation (COVER-ALL): A randomised phase 2 trial. Lancet Gastroenterol. Hepatol. 2025, 10, 442–451. [Google Scholar] [CrossRef]
- Ryan, E.R.; Sofocleous, C.T.; Schöder, H.; Carrasquillo, J.A.; Nehmeh, S.; Larson, S.M.; Thornton, R.; Siegelbaum, R.H.; Erinjeri, J.P.; Solomon, S.B. Split-Dose Technique for FDG PET/CT–guided Percutaneous Ablation: A Method to Facilitate Lesion Targeting and to Provide Immediate Assessment of Treatment Effectiveness. Radiology 2013, 268, 288–295. [Google Scholar] [CrossRef]
- Zadeh, M.Z.; Yeh, R.; Kunin, H.S.; Kirov, A.S.; Petre, E.N.; Gönen, M.; Silk, M.; Cornelis, F.H.; Soares, K.C.; Ziv, E.; et al. Real-Time Split-Dose PET/CT-Guided Ablation Improves Colorectal Liver Metastasis Detection and Ablation Zone Margin Assessments without the Need for Repeated Contrast Injection. Cancers 2022, 14, 6253. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Petre, E.N.; Vakiani, E.; Klimstra, D.; Durack, J.C.; Gonen, M.; Osborne, J.; Solomon, S.B.; Sofocleous, C.T. Immediate Postablation 18F-FDG Injection and Corresponding SUV Are Surrogate Biomarkers of Local Tumor Progression After Thermal Ablation of Colorectal Carcinoma Liver Metastases. J. Nucl. Med. 2018, 59, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.S.; Nieuwenhuizen, S.; Bemd, B.A.v.D.; Ruarus, A.H.; Geboers, B.; Vroomen, L.G.; Muglia, R.; de Jong, M.C.; de Vries, J.J.; Scheffer, H.J.; et al. Transcatheter CT Hepatic Arteriography Compared with Conventional CT Fluoroscopy Guidance in Percutaneous Thermal Ablation to Treat Colorectal Liver Metastases: A Single-Center Comparative Analysis of 2 Historical Cohorts. J. Vasc. Interv. Radiol. 2020, 31, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- van der Lei, S.; Opperman, J.; Dijkstra, M.; Kors, N.; Boon, R.; Bemd, B.A.T.v.D.; Timmer, F.E.F.; Nota, I.M.G.C.; Bergh, J.E.v.D.; de Vries, J.J.J.; et al. The Added Diagnostic Value of Transcatheter CT Hepatic Arteriography for Intraprocedural Detection of Previously Unknown Colorectal Liver Metastases During Percutaneous Ablation and Impact on the Definitive Treatment Plan. Cardiovasc. Interv. Radiol. 2023, 46, 1257–1266. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Gazelle, G.S.; Compton, C.C.; Mueller, P.R.; Tanabe, K.K. Treatment of intrahepatic malignancy with radiofrequency ablation: Radiologic-pathologic correlation. Cancer 2000, 88, 2452–2463. [Google Scholar] [CrossRef]
- Snoeren, N.; Huiskens, J.; Rijken, A.M.; van Hillegersberg, R.; van Erkel, A.R.; Slooter, G.D.; Klaase, J.M.; Tol, P.M.v.D.; Kate, F.J.W.T.; Jansen, M.C.; et al. Viable Tumor Tissue Adherent to Needle Applicators after Local Ablation: A Risk Factor for Local Tumor Progression. Ann. Surg. Oncol. 2011, 18, 3702–3710. [Google Scholar] [CrossRef]
- Sofocleous, C.T.; Nascimento, R.G.; Petrovic, L.M.; Klimstra, D.S.; Gonen, M.; Brown, K.T.; Brody, L.A.; Covey, A.M.; Thornton, R.H.; Fong, Y.; et al. Histopathologic and Immunohistochemical Features of Tissue Adherent to Multitined Electrodes after RF Ablation of Liver Malignancies Can Help Predict Local Tumor Progression: Initial Results. Radiology 2008, 249, 364–374. [Google Scholar] [CrossRef]
- Sofocleous, C.T.; Garg, S.; Petrovic, L.M.; Gonen, M.; Petre, E.N.; Klimstra, D.S.; Solomon, S.B.; Brown, K.T.; Brody, L.A.; Covey, A.M.; et al. Ki-67 is a Prognostic Biomarker of Survival after Radiofrequency Ablation of Liver Malignancies. Ann. Surg. Oncol. 2012, 19, 4262–4269. [Google Scholar] [CrossRef]
- Kamarinos, N.V.; Vakiani, E.; Fujisawa, S.; Gonen, M.; Fan, N.; Romin, Y.; Do, R.K.; Ziv, E.; Erinjeri, J.P.; Petre, E.N.; et al. Immunofluorescence Assay of Ablated Colorectal Liver Metastases: The Frozen Section of Image-Guided Tumor Ablation? J. Vasc. Interv. Radiol. 2021, 33, 308–315.e1. [Google Scholar] [CrossRef]
- Gleisner, A.L.; Choti, M.A.; Assumpcao, L.; Nathan, H.; Schulick, R.D.; Pawlik, T.M. Colorectal liver metastases: Recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch. Surg. 2008, 143, 1204–1212. [Google Scholar] [CrossRef]
- Berber, E.; Tsinberg, M.; Tellioglu, G.; Simpfendorfer, C.H.; Siperstein, A.E. Resection Versus Laparoscopic Radiofrequency Thermal Ablation Of Solitary Colorectal Liver Metastasis. J. Gastrointest. Surg. 2008, 12, 1967–1972. [Google Scholar] [CrossRef]
- McKay, A.; Fradette, K.; Lipschitz, J. Long-Term Outcomes Following Hepatic Resection and Radiofrequency Ablation of Colorectal Liver Metastases. HPB Surg. 2009, 2009, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Fischbach, R.; Teichmann, W.; Bokemeyer, C.; Braumann, D. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur. J. Cancer 2009, 45, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Cervantes, A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann. Oncol. 2010, 21, v93–v97. [Google Scholar] [CrossRef] [PubMed]
- Mulier, S.; Ni, Y.; Jamart, J.; Michel, L.; Marchal, G.; Ruers, T. Radiofrequency Ablation Versus Resection for Resectable Colorectal Liver Metastases: Time for a Randomized Trial? Ann. Surg. Oncol. 2007, 15, 144–157. [Google Scholar] [CrossRef]
- Sørensen, S.M.; Mortensen, F.V.; Nielsen, D.T. Radiofrequency ablation of colorectal liver metastases: Long-term survival. Acta Radiol. 2007, 48, 253–258. [Google Scholar] [CrossRef]
- Díez-Alonso, M.; Mendoza-Moreno, F.; Jiménez-Alvarez, L.; Nuñez, O.; Blazquez-Martín, A.; Sanchez-Gollarte, A.; Matías-García, B.; Molina, R.; San-Juan, A.; Gutierrez-Calvo, A. Prognostic factors of survival in stage IV colorectal cancer with synchronous liver metastasis: Negative effect of the KRAS mutation. Mol. Clin. Oncol. 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.S.; Timmer, F.E.; Geboers, B.; Schouten, E.A.; Opperman, J.; Scheffer, H.J.; de Vries, J.J.; Swijnenburg, R.-J.; et al. Thermal Ablation Compared to Partial Hepatectomy for Recurrent Colorectal Liver Metastases: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers 2021, 13, 2769. [Google Scholar] [CrossRef]
- Schullian, P.; Johnston, E.W.; Putzer, D.; Laimer, G.; Waroschitz, G.; Braunwarth, E.; Amann, A.; Maglione, M.; Bale, R. Stereotactic radiofrequency ablation (SRFA) for recurrent colorectal liver metastases after hepatic resection. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; De Baere, T.; Smayra, T.; Ouellet, J.F.; Roche, A.; Lasser, P. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br. J. Surg. 2002, 89, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Oliveira, J. Advanced colorectal cancer: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009, 20, iv61–iv63. [Google Scholar] [CrossRef]
- Engstrom, P.F.; Arnoletti, J.P.; Benson, A.B.; Chen, Y.-J.; Choti, M.A.; Cooper, H.S.; Covey, A.; Dilawari, R.A.; Early, D.S.; Enzinger, P.C.; et al. Colon Cancer. J. Natl. Compr. Cancer Netw. 2009, 7, 778–831. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 10–32. [Google Scholar] [CrossRef]
- van der Lei, S.; Puijk, R.S.; Dijkstra, M.; Schulz, H.H.; Vos, D.J.W.; De Vries, J.J.J.; Scheffer, H.J.; I Lissenberg-Witte, B.; Aldrighetti, L.; Arntz, M.; et al. Thermal ablation versus surgical resection of small-size colorectal liver metastases (COLLISION): An international, randomised, controlled, phase 3 non-inferiority trial. Lancet Oncol. 2025, 26, 187–199. [Google Scholar] [CrossRef]
- Donath, D.; Nori, D.; Turnbull, A.; Kaufman, N.; Fortner, J.G. Brachytherapy in the treatment of solitary colorectal metastases to the liver. J. Surg. Oncol. 1990, 44, 55–61. [Google Scholar] [CrossRef]
- Ricke, J.; Mohnike, K.; Pech, M.; Seidensticker, M.; Rühl, R.; Wieners, G.; Gaffke, G.; Kropf, S.; Felix, R.; Wust, P. Local Response and Impact on Survival After Local Ablation of Liver Metastases From Colorectal Carcinoma by Computed Tomography–Guided High-Dose-Rate Brachytherapy. Int. J. Radiat. Oncol. 2010, 78, 479–485. [Google Scholar] [CrossRef]
- Boas, F.E.; Brody, L.A.; Erinjeri, J.P.; Yarmohammadi, H.; Shady, W.; Kishore, S.; Sofocleous, C.T. Quantitative Measurements of Enhancement on Preprocedure Triphasic CT Can Predict Response of Colorectal Liver Metastases to Radioembolization. Am. J. Roentgenol. 2016, 207, 671–675. [Google Scholar] [CrossRef]
- Boas, F.E.; Kamaya, A.; Do, B.; Desser, T.S.; Beaulieu, C.F.; Vasanawala, S.S.; Hwang, G.L.; Sze, D.Y. Classification of Hypervascular Liver Lesions Based on Hepatic Artery and Portal Vein Blood Supply Coefficients Calculated from Triphasic CT Scans. J. Digit. Imaging 2014, 28, 213–223. [Google Scholar] [CrossRef]
- Ariel, I.M.; Pack, G.T. Treatment of inoperable cancer of the liver by intra-arterial radioactive isotopes and chemotherapy. Cancer 1967, 20, 793–804. [Google Scholar] [CrossRef]
- Gray, B.N.; Anderson, J.E.; Burton, M.A.; van Hazel, G.; Codde, J.; Morgan, C.; Klemp, P. REGRESSION OF LIVER METASTASES FOLLOWING TREATMENT WITH YTTRIUM-90 MICROSPHERES. ANZ J. Surg. 1992, 62, 105–110. [Google Scholar] [CrossRef]
- Anderson, J.H.; Goldberg, J.A.; Bessent, R.G.; Kerr, D.J.; McKillop, J.H.; Stewart, I.; Cooke, T.G.; McArdle, C.S. Glass yttrium-90 microspheres for patients with colorectal liver metastases. Radiother. Oncol. 1992, 25, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.F.; Lewandowski, R.J.; Ibrahim, S.M.; Sato, K.T.; Ryu, R.K.; Atassi, B.; Newman, S.; Talamonti, M.; Omary, R.A.; Benson, A.; et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 2009, 115, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, R.J.; Memon, K.; Mulcahy, M.F.; Hickey, R.; Marshall, K.; Williams, M.; Salzig, K.; Gates, V.L.; Atassi, B.; Vouche, M.; et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: Survival by era and chemotherapy. Eur. J. Nucl. Med. 2014, 41, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.S.; Cannan, R.J.; Mitchell, A.W. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J. Gastrointest. Surg. 2001, 5, 294–302. [Google Scholar] [CrossRef]
- Gray, B.; Van Hazel, G.; Hope, M.; Burton, M.; Moroz, P.; Anderson, J.; Gebski, V. Randomised trial of SIR-Spheres® plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann. Oncol. 2001, 12, 1711–1720. [Google Scholar] [CrossRef]
- Lim, L.; Gibbs, P.; Yip, D.; Shapiro, J.; Dowling, R.; Smith, D.; Little, A.; Bailey, W.; Liechtenstein, M. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer 2005, 5, 132. [Google Scholar] [CrossRef][Green Version]
- Kennedy, A.S.; Coldwell, D.; Nutting, C.; Murthy, R.; Wertman, D.E.; Loehr, S.P.; Overton, C.; Meranze, S.; Niedzwiecki, J.; Sailer, S. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: Modern USA experience. Int. J. Radiat. Oncol. 2006, 65, 412–425. [Google Scholar] [CrossRef]
- Sharma, R.A.; Van Hazel, G.A.; Morgan, B.; Berry, D.P.; Blanshard, K.; Price, D.; Bower, G.; Shannon, J.A.; Gibbs, P.; Steward, W.P. Radioembolization of Liver Metastases from Colorectal Cancer Using Yttrium-90 Microspheres with Concomitant Systemic Oxaliplatin, Fluorouracil, and Leucovorin Chemotherapy. J. Clin. Oncol. 2007, 25, 1099–1106. [Google Scholar] [CrossRef]
- Jakobs, T.F.; Hoffmann, R.-T.; Dehm, K.; Trumm, C.; Stemmler, H.-J.; Tatsch, K.; La Fougere, C.; Murthy, R.; Helmberger, T.K.; Reiser, M.F. Hepatic Yttrium-90 Radioembolization of Chemotherapy-refractory Colorectal Cancer Liver Metastases. J. Vasc. Interv. Radiol. 2008, 19, 1187–1195. [Google Scholar] [CrossRef]
- Hendlisz, A.; Eynde, M.V.D.; Peeters, M.; Maleux, G.; Lambert, B.; Vannoote, J.; De Keukeleire, K.; Verslype, C.; Defreyne, L.; Van Cutsem, E.; et al. Phase III Trial Comparing Protracted Intravenous Fluorouracil Infusion Alone or with Yttrium-90 Resin Microspheres Radioembolization for Liver-Limited Metastatic Colorectal Cancer Refractory to Standard Chemotherapy. J. Clin. Oncol. 2010, 28, 3687–3694. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Gabr, A.; Abouchaleh, N.; Ali, R.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Ganger, D.; Desai, K.; Thornburg, B.; et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018, 287, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonné, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. 2021, 48, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Kurilova, I.; Bendet, A.; Fung, E.K.; Petre, E.N.; Humm, J.L.; Boas, F.E.; Crane, C.H.; Kemeny, N.; Kingham, T.P.; Cercek, A.; et al. Radiation segmentectomy of hepatic metastases with Y-90 glass microspheres. Abdom. Imaging 2021, 46, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Meiers, C.; Taylor, A.; Geller, B.; Toskich, B. Safety and initial efficacy of radiation segmentectomy for the treatment of hepatic metastases. J. Gastrointest. Oncol. 2018, 9, 311–315. [Google Scholar] [CrossRef]
- Padia, S.A.; Johnson, G.E.; Agopian, V.G.; DiNorcia, J.; Srinivasa, R.N.; Sayre, J.; Shin, D.S. Yttrium-90 radiation segmentectomy for hepatic metastases: A multi-institutional study of safety and efficacy. J. Surg. Oncol. 2020, 123, 172–178. [Google Scholar] [CrossRef]
- Dimopoulos, P.M.; Sotirchos, V.S.; Dunne-Jaffe, C.B.; Petre, E.N.; Gonen, M.; Zhao, K.; Kirov, A.S.; Crane, C.; D’aNgelica, M.; Connell, L.C.; et al. Voxel-Based Dosimetry Predicts Local Tumor Progression Post 90Y Radiation Segmentectomy of Colorectal Liver Metastases. Clin. Nucl. Med. 2024, 50, 133–142. [Google Scholar] [CrossRef]
- Gaba, R.C.; Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Ibrahim, S.M.; Mulcahy, M.F.; Ryu, R.K.; Sato, K.T.; Gates, V.; Abecassis, M.M.; et al. Radiation Lobectomy: Preliminary Findings of Hepatic Volumetric Response to Lobar Yttrium-90 Radioembolization. Ann. Surg. Oncol. 2009, 16, 1587–1596. [Google Scholar] [CrossRef]
- Denecke, T.; Rühl, R.; Hildebrandt, B.; Stelter, L.; Grieser, C.; Stiepani, H.; Werk, M.; Podrabsky, P.; Plotkin, M.; Amthauer, H.; et al. Planning transarterial radioembolization of colorectal liver metastases with Yttrium 90 microspheres: Evaluation of a sequential diagnostic approach using radiologic and nuclear medicine imaging techniques. Eur. Radiol. 2008, 18, 892–902. [Google Scholar] [CrossRef]
- Pasciak, A.S.; McElmurray, J.H.; Bourgeois, A.C.; Heidel, R.E.; Bradley, Y.C. The Impact of an Antireflux Catheter on Target Volume Particulate Distribution in Liver-Directed Embolotherapy: A Pilot Study. J. Vasc. Interv. Radiol. 2015, 26, 660–669. [Google Scholar] [CrossRef]
- Bester, L.; Salem, R. Reduction of Arteriohepatovenous Shunting by Temporary Balloon Occlusion in Patients Undergoing Radioembolization. J. Vasc. Interv. Radiol. 2007, 18, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Garlipp, B.; de Baere, T.; Damm, R.; Irmscher, R.; van Buskirk, M.; Stübs, P.; Deschamps, F.; Meyer, F.; Seidensticker, R.; Mohnike, K.; et al. Left-Liver Hypertrophy After Therapeutic Right-Liver Radioembolization Is Substantial but Less Than After Portal Vein Embolization. Hepatology 2014, 59, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Vouche, M.; Lewandowski, R.J.; Atassi, R.; Memon, K.; Gates, V.L.; Ryu, R.K.; Gaba, R.C.; Mulcahy, M.F.; Baker, T.; Sato, K.; et al. Radiation lobectomy: Time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J. Hepatol. 2013, 59, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Andel, D.; Ramdhani, K.; Braat, A.J.A.T.; Bruijnen, R.C.G.; Bol, G.; Keane, G.; Lam, M.G.E.H.; Kranenburg, O.W.; Rinkes, I.H.M.B.; Hagendoorn, J.; et al. Radiation Lobectomy in Adjunct to Double Vein Embolization to Reach Sufficient Future Liver Remnant in Patients with Colorectal Cancer Liver Metastases: A Case Series. Cardiovasc. Interv. Radiol. 2024, 1–7. [Google Scholar] [CrossRef]
- Angeretti, M.; Lumia, D.; Canì, A.; Barresi, M.; Cardim, L.N.; Piacentino, F.; Maresca, A.; Novario, R.; Genovese, E.; Fugazzola, C. Gastroduodenal artery recanalization after transcatheter fibered coil embolization for prevention of hepaticoenteric flow: Incidence and predisposing technical factors in 142 patients. Acta Radiol. 2013, 54, 790–794. [Google Scholar] [CrossRef]
- Willowson, K.P.; Hayes, A.R.; Chan, D.L.H.; Tapner, M.; Bernard, E.J.; Maher, R.; Pavlakis, N.; Clarke, S.J.; Bailey, D.L. Clinical and imaging-based prognostic factors in radioembolisation of liver metastases from colorectal cancer: A retrospective exploratory analysis. EJNMMI Res. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Levillain, H.; Derijckere, I.D.; Marin, G.; Guiot, T.; Vouche, M.; Reynaert, N.; Hendlisz, A.; Vanderlinden, B.; Flamen, P. 90Y-PET/CT-based dosimetry after selective internal radiation therapy predicts outcome in patients with liver metastases from colorectal cancer. EJNMMI Res. 2018, 8, 60. [Google Scholar] [CrossRef]
- Hoven, A.F.v.D.; Rosenbaum, C.E.; Elias, S.G.; de Jong, H.W.; Koopman, M.; Verkooijen, H.M.; Alavi, A.; Bosch, M.A.v.D.; Lam, M.G. Insights into the Dose–Response Relationship of Radioembolization with Resin 90Y-Microspheres: A Prospective Cohort Study in Patients with Colorectal Cancer Liver Metastases. J. Nucl. Med. 2016, 57, 1014–1019. [Google Scholar] [CrossRef]
- Alsultan, A.A.; van Roekel, C.; Barentsz, M.W.; Smits, M.L.J.; Kunnen, B.; Koopman, M.; Braat, A.J.; Bruijnen, R.C.; de Keizer, B.; Lam, M.G. Dose–Response and Dose–Toxicity Relationships for Glass 90Y Radioembolization in Patients with Liver Metastases from Colorectal Cancer. J. Nucl. Med. 2021, 62, 1616–1623. [Google Scholar] [CrossRef]
- Sankhla, T.; Cheng, B.; Nezami, N.; Xing, M.; Sethi, I.; Bercu, Z.; Brandon, D.; Majdalany, B.; Schuster, D.M.; Kokabi, N. Role of Resin Microsphere Y90 Dosimetry in Predicting Objective Tumor Response, Survival and Treatment Related Toxicity in Surgically Unresectable Colorectal Liver Metastasis: A Retrospective Single Institution Study. Cancers 2021, 13, 4908. [Google Scholar] [CrossRef]
- Kao, Y.H.; Tan, E.H.; Ng, C.E.; Goh, S.W. Clinical implications of the body surface area method versus partition model dosimetry for yttrium-90 radioembolization using resin microspheres: A technical review. Ann. Nucl. Med. 2011, 25, 455–461. [Google Scholar] [CrossRef]
- Grosser, O.S.; Ulrich, G.; Furth, C.; Pech, M.; Ricke, J.; Amthauer, H.; Ruf, J. Intrahepatic Activity Distribution in Radioembolization with Yttrium-90–Labeled Resin Microspheres Using the Body Surface Area Method—A Less than Perfect Model. J. Vasc. Interv. Radiol. 2015, 26, 1615–1621. [Google Scholar] [CrossRef]
- Herba, M.J.; Illescas, F.F.; Thirlwell, M.P.; Boos, G.J.; Rosenthall, L.; Atri, M.; Bret, P.M. Hepatic malignancies: Improved treatment with intraarterial Y-90. Radiology 1988, 169, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Mahvash, A.; Abdelsalam, M.; Kaseb, A.O.; Kappadath, S.C. Planning dosimetry for 90Y radioembolization with glass microspheres: Evaluating the fidelity of 99mTc-MAA and partition model predictions. Med Phys. 2020, 47, 5333–5342. [Google Scholar] [CrossRef] [PubMed]
- Spahr, N.; Schilling, P.; Thoduka, S.; Abolmaali, N.; Schenk, A. Predictive SIRT dosimetry based on a territorial model. EJNMMI Phys. 2017, 4, 25. [Google Scholar] [CrossRef]
- Kurilova, I.; Beets-Tan, R.G.; Flynn, J.; Gönen, M.; Ulaner, G.; Petre, E.N.; Boas, F.E.; Ziv, E.; Yarmohammadi, H.; Klompenhouwer, E.G.; et al. Factors Affecting Oncologic Outcomes of 90Y Radioembolization of Heavily Pre-Treated Patients with Colon Cancer Liver Metastases. Clin. Color. Cancer 2019, 18, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Ilhan, H.; Paprottka, P.M.; Jakobs, T.F.; Heinemann, V.; Bartenstein, P.; Khalaf, F.; Ezziddin, S.; Hacker, M.; Haug, A.R. Nomogram including pretherapeutic parameters for prediction of survival after SIRT of hepatic metastases from colorectal cancer. Eur. Radiol. 2015, 25, 2693–2700. [Google Scholar] [CrossRef]
- Damm, R.; Seidensticker, R.; Ulrich, G.; Breier, L.; Steffen, I.G.; Seidensticker, M.; Garlipp, B.; Mohnike, K.; Pech, M.; Amthauer, H.; et al. Y90 Radioembolization in chemo-refractory metastastic, liver dominant colorectal cancer patients: Outcome assessment applying a predictive scoring system. BMC Cancer 2016, 16, 509. [Google Scholar] [CrossRef]
- Weiner, A.A.; Gui, B.; Newman, N.B.; Nosher, J.L.; Yousseff, F.; Lu, S.-E.; Foltz, G.M.; Carpizo, D.; Lowenthal, J.; Zuckerman, D.A.; et al. Predictors of Survival after Yttrium-90 Radioembolization for Colorectal Cancer Liver Metastases. J. Vasc. Interv. Radiol. 2018, 29, 1094–1100. [Google Scholar] [CrossRef]
- Shady, W.; Sotirchos, V.S.; Do, R.K.; Pandit-Taskar, N.; Carrasquillo, J.A.; Gonen, M.; Sofocleous, C.T. Surrogate Imaging Biomarkers of Response of Colorectal Liver Metastases After Salvage Radioembolization Using 90Y-Loaded Resin Microspheres. Am. J. Roentgenol. 2016, 207, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Alis, D.; Durmaz, E.S.M.; Gulsen, F.; Bas, A.; Kabasakal, L.; Sager, S.; Numan, F. Prognostic value of ADC measurements in predicting overall survival in patients undergoing 90Y radioembolization for colorectal cancer liver metastases. Clin. Imaging 2019, 57, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, F.C.; Simon, B.; Luetkens, J.A.; Träber, F.; Meyer, C.; Schmeel, L.C.; Sabet, A.; Ezziddin, S.; Schild, H.H.; Hadizadeh, D.R. Prognostic value of pretreatment diffusion-weighted magnetic resonance imaging for outcome prediction of colorectal cancer liver metastases undergoing 90Y-microsphere radioembolization. J. Cancer Res. Clin. Oncol. 2017, 143, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Garcia, A.R.; Pandit-Taskar, N.; Do, K.G.; Brody, L.A.; Petre, E.N.; Capanu, M.; Longing, A.P.; Chou, J.F.; Carrasquillo, J.A.; et al. Phase I Trial of Selective Internal Radiation Therapy for Chemorefractory Colorectal Cancer Liver Metastases Progressing After Hepatic Arterial Pump and Systemic Chemotherapy. Clin. Color. Cancer 2014, 13, 27–36. [Google Scholar] [CrossRef]
- Stella, M.; Braat, A.J.A.T.; van Rooij, R.; de Jong, H.W.A.M.; Lam, M.G.E.H. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc. Interv. Radiol. 2022, 45, 1634–1645. [Google Scholar] [CrossRef]
- Nowicki, M.L.; Ćwikla, J.B.; Sankowski, A.J.; Shcherbinin, S.; Grimmes, J.; Celler, A.; Buscombe, J.R.; Bator, A.; Pech, M.; Pawlak, D. Initial Study of Radiological and Clinical Efficacy Radioembolization Using 188Re-Human Serum Albumin (HSA) Microspheres in Patients with Progressive, Unresectable Primary or Secondary Liver Cancers. Med Sci. Monit. 2014, 20, 1353–1362. [Google Scholar] [CrossRef]
- Singh, P.; Anil, G. Yttrium-90 radioembolization of liver tumors: What do the images tell us? Cancer Imaging 2013, 13, 645–657. [Google Scholar] [CrossRef]
- Wong, C.-Y.; Salem, R.; Raman, S.; Gates, V.L.; Dworkin, H.J. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: A comparison with CT or MRI. Eur. J. Nucl. Med. 2002, 29, 815–820. [Google Scholar] [CrossRef]
- Shady, W.; Kishore, S.; Gavane, S.; Do, R.K.; Osborne, J.R.; Ulaner, G.A.; Gonen, M.; Ziv, E.; Boas, F.E.; Sofocleous, C.T. Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after 90Y radioembolization of colorectal liver metastases: A comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur. J. Radiol. 2016, 85, 1224–1231. [Google Scholar] [CrossRef]
- Sabet, A.; Meyer, C.; Aouf, A.; Sabet, A.; Ghamari, S.; Pieper, C.C.; Mayer, K.; Biersack, H.-J.; Ezziddin, S. Early post-treatment FDG PET predicts survival after 90Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur. J. Nucl. Med. 2014, 42, 370–376. [Google Scholar] [CrossRef]
- Kato, T.; Nemoto, R.; Mori, H.; Takahashi, M.; Tamakawa, Y.; Harada, M. Arterial Chemoembolization With Microencapsulated Anticancer Drug: An Approach to Selective Cancer Chemotherapy with Sustained Effects. JAMA 1981, 245, 1123–1127. [Google Scholar] [CrossRef]
- Lang, E.K.; Brown, C.L. Colorectal metastases to the liver: Selective chemoembolization. Radiology 1993, 189, 417–422. [Google Scholar] [CrossRef]
- Sanz-Altamira, P.M.; Spence, L.D.; Huberman, M.S.; Posner, M.R.; Steele, G.; Perry, L.J.; Stuart, K.E. Selective chemoembolization in the management of hepatic metastases in refractory colorectal carcinoma. Dis. Colon Rectum 1997, 40, 770–775. [Google Scholar] [CrossRef]
- Albert, M.; Kiefer, M.V.; Sun, W.; Haller, D.; Fraker, D.L.; Tuite, C.M.; Stavropoulos, S.W.; Mondschein, J.I.; Soulen, M.C. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer 2010, 117, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Voigt, W.; Behrmann, C.; Schlueter, A.; Kegel, T.; Grothey, A.; Schmoll, H.-J. A New Chemoembolization Protocol in Refractory Liver Metastasis of Colorectal Cancer—A Feasibility Study. Oncol. Res. Treat. 2002, 25, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Gruber, T.; Balzer, J.O.; Eichler, K.; Hammerstingl, R.; Zangos, S. Repeated Transarterial Chemoembolization in the Treatment of Liver Metastases of Colorectal Cancer: Prospective Study. Radiology 2009, 250, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Tellez, C.; Benson, A.B.; Lyster, M.T.; Talamonti, M.; Shaw, J.; Braun, M.A.; Nemcek, A.A.; Vogelzang, R.L. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 1998, 82, 1250–1259. [Google Scholar] [CrossRef]
- Popov, I.; Lavrnic, S.; Jelic, S.; Jezdic, S.; Jasovic, A. Chemoembolization for liver metastases from colorectal carcinoma: Risk or a benefit. Neoplasma 2002, 49, 43–48. [Google Scholar]
- Sotirchos, V.S.; Silk, M.T.; Camacho, J.C.; Schatoff, E.M.; Kunin, H.S.; Alexander, E.S.; Zhao, K.; Connell, L.C.; Sofocleous, C.T.; Kemeny, N.E. Selective intra-arterial mitomycin-C infusions for treatment-refractory colorectal liver metastases. J. Gastrointest. Oncol. 2025, 16, 92–105. [Google Scholar] [CrossRef]
- Aliberti, C.; Tilli, M.; Benea, G.; Fiorentini, G. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: Preliminary results. Anticancer Res. 2006, 26, 3793–3795. [Google Scholar] [PubMed]
- Fiorentini, G.; Aliberti, C.; Turrisi, G.; Del Conte, A.; Rossi, S.; Benea, G.; Giovanis, P. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: Results of a phase II clinical study. In Vivo 2008, 21, 1085–1091. [Google Scholar]
- Aliberti, C.; Fiorentini, G.; Muzzio, P.C.; Pomerri, F.; Tilli, M.; Dallara, S.; Benea, G. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: Results of a phase II clinical study. Anticancer Res. 2011, 31, 4581–4587. [Google Scholar]
- Huppert, P.; Wenzel, T.; Wietholtz, H. Transcatheter Arterial Chemoembolization (TACE) of Colorectal Cancer Liver Metastases by Irinotecan-Eluting Microspheres in a Salvage Patient Population. Cardiovasc. Interv. Radiol. 2013, 37, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Barbery, K.; Suthar, R.; Guerrero, G.; Arora, G. Transarterial chemoembolization using DEBIRI for treatment of hepatic metastases from colorectal cancer. Anticancer Res. 2013, 33, 2077–2083. [Google Scholar] [CrossRef]
- Iezzi, R.; Marsico, V.A.; Guerra, A.; Cerchiaro, E.; Cassano, A.; Basso, M.; Devicienti, E.; Rodolfino, E.; Barone, C.; Bonomo, L. Trans-Arterial Chemoembolization with Irinotecan-Loaded Drug-Eluting Beads (DEBIRI) and Capecitabine in Refractory Liver Prevalent Colorectal Metastases: A Phase II Single-Center Study. Cardiovasc. Interv. Radiol. 2015, 38, 1523–1531. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, S.; Zhang, Y.; Zhao, T.; Wang, R.; Bian, J.; Wu, J.; Zhou, J. Irinotecan eluting beads-transarterial chemoembolization using Callispheres® microspheres is an effective and safe approach in treating unresectable colorectal cancer liver metastases. Ir. J. Med Sci. (1971-) 2021, 191, 1139–1145. [Google Scholar] [CrossRef]
- Malagari, K.; Kiakidis, T.; Moschouris, H.; Charokopakis, A.; Vergadis, C.; Alevisopoulos, N.; Kartsouni, V.; Panagiotou, I.; Pellerin, O.; Glantzounis, G.; et al. Prospective Series of Transarterial Chemoembolization of Metastatic Colorectal Cancer to the Liver with 30–60 μm Microspheres Loaded with Irinotecan. Cardiovasc. Interv. Radiol. 2023, 46, 880–890. [Google Scholar] [CrossRef]
- Richardson, A.J.; Laurence, J.M.; Lam, V.W. Transarterial Chemoembolization with Irinotecan Beads in the Treatment of Colorectal Liver Metastases: Systematic Review. J. Vasc. Interv. Radiol. 2013, 24, 1209–1217. [Google Scholar] [CrossRef]
- Martin, R.C.; Robbins, K.; Tomalty, D.; O’HAra, R.; Bosnjakovic, P.; Padr, R.; Rocek, M.; Slauf, F.; Scupchenko, A.; Tatum, C. Transarterial chemoembolisation (TACE) using irinotecan-loaded beads for the treatment of unresectable metastases to the liver in patients with colorectal cancer: An interim report. World J. Surg. Oncol. 2009, 7, 80. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, C.; Chen, M.; Sun, Y.; Han, J. Drug-eluting bead transarterial chemoembolization (DEB-TACE) versus conventional transarterial chemoembolization (cTACE) in colorectal liver metastasis: Efficacy, safety, and prognostic factors. J. Cancer Res. Ther. 2023, 19, 1525–1532. [Google Scholar] [CrossRef]
- Hong, K.; McBride, J.D.; Georgiades, C.S.; Reyes, D.K.; Herman, J.M.; Kamel, I.R.; Geschwind, J.-F.H. Salvage Therapy for Liver-dominant Colorectal Metastatic Adenocarcinoma: Comparison between Transcatheter Arterial Chemoembolization versus Yttrium-90 Radioembolization. J. Vasc. Interv. Radiol. 2009, 20, 360–367. [Google Scholar] [CrossRef]
- Martin, R.C.G., II; Scoggins, C.R.; Schreeder, M.; Rilling, W.S.; Laing, C.J.; Tatum, C.M.; Kelly, L.R.; Garcia-Monaco, R.D.; Sharma, V.R.; Crocenzi, T.S.; et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 2015, 121, 3649–3658. [Google Scholar] [CrossRef]
- Vogl, T.J.; Stefan, H.; Gruber-Rouh, T.; Trojan, J.; Bechstein, W.O.; Bielfeldt, J.; Adwan, H. The combination of transarterial chemoembolization and microwave ablation is superior to microwave ablation alone for liver metastases from colorectal cancer. J. Cancer Res. Clin. Oncol. 2024, 150, 1–9. [Google Scholar] [CrossRef]
- Arnold, D.; Pereira, P.; Iezzi, R.; Gjoreski, A.; Spiliopoulos, S.; Helmberger, T.; Gomez, F.; de Baère, T.; Pellerin, O.; Maleux, G.; et al. Transarterial chemoembolisation with irinotecan (irinotecan-TACE) as salvage or post-inductive therapy for colorectal cancer liver metastases: Effectiveness results from the CIREL study. ESMO Open 2025, 10, 104292. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xenos, D.; Sotirchos, V.S.; Dimopoulos, P.M.; Sofocleous, C.T. Interventional Oncology for Colorectal Liver Metastases: From Local Cure to Salvage Therapy. Biomedicines 2025, 13, 2182. https://doi.org/10.3390/biomedicines13092182
Xenos D, Sotirchos VS, Dimopoulos PM, Sofocleous CT. Interventional Oncology for Colorectal Liver Metastases: From Local Cure to Salvage Therapy. Biomedicines. 2025; 13(9):2182. https://doi.org/10.3390/biomedicines13092182
Chicago/Turabian StyleXenos, Dimitrios, Vlasios S. Sotirchos, Platon M. Dimopoulos, and Constantinos T. Sofocleous. 2025. "Interventional Oncology for Colorectal Liver Metastases: From Local Cure to Salvage Therapy" Biomedicines 13, no. 9: 2182. https://doi.org/10.3390/biomedicines13092182
APA StyleXenos, D., Sotirchos, V. S., Dimopoulos, P. M., & Sofocleous, C. T. (2025). Interventional Oncology for Colorectal Liver Metastases: From Local Cure to Salvage Therapy. Biomedicines, 13(9), 2182. https://doi.org/10.3390/biomedicines13092182






