Dysregulated Intracellular Signaling in the Pathogenesis of Vitiligo: An Update on Emerging Therapeutic Strategies
Abstract
1. Introduction
2. Targeting Vitiligo-Impaired Redox Defense Network
2.1. Clinical and Experimental Evidence Supporting Oxidative Stress Targeting in Vitiligo
2.1.1. Melanocytes Are Intrinsically Prone to Lose Oxidative Equilibrium
2.1.2. Oxidative Stress-Related Intracellular Damage
2.2. Clinical Trials Supporting the Relevance of Impaired Redox Equilibrium in Vitiligo
2.2.1. Clinical Trials Involving Vitamin Supplementation
2.2.2. Clinical Trials Involving Antioxidant Supplementation
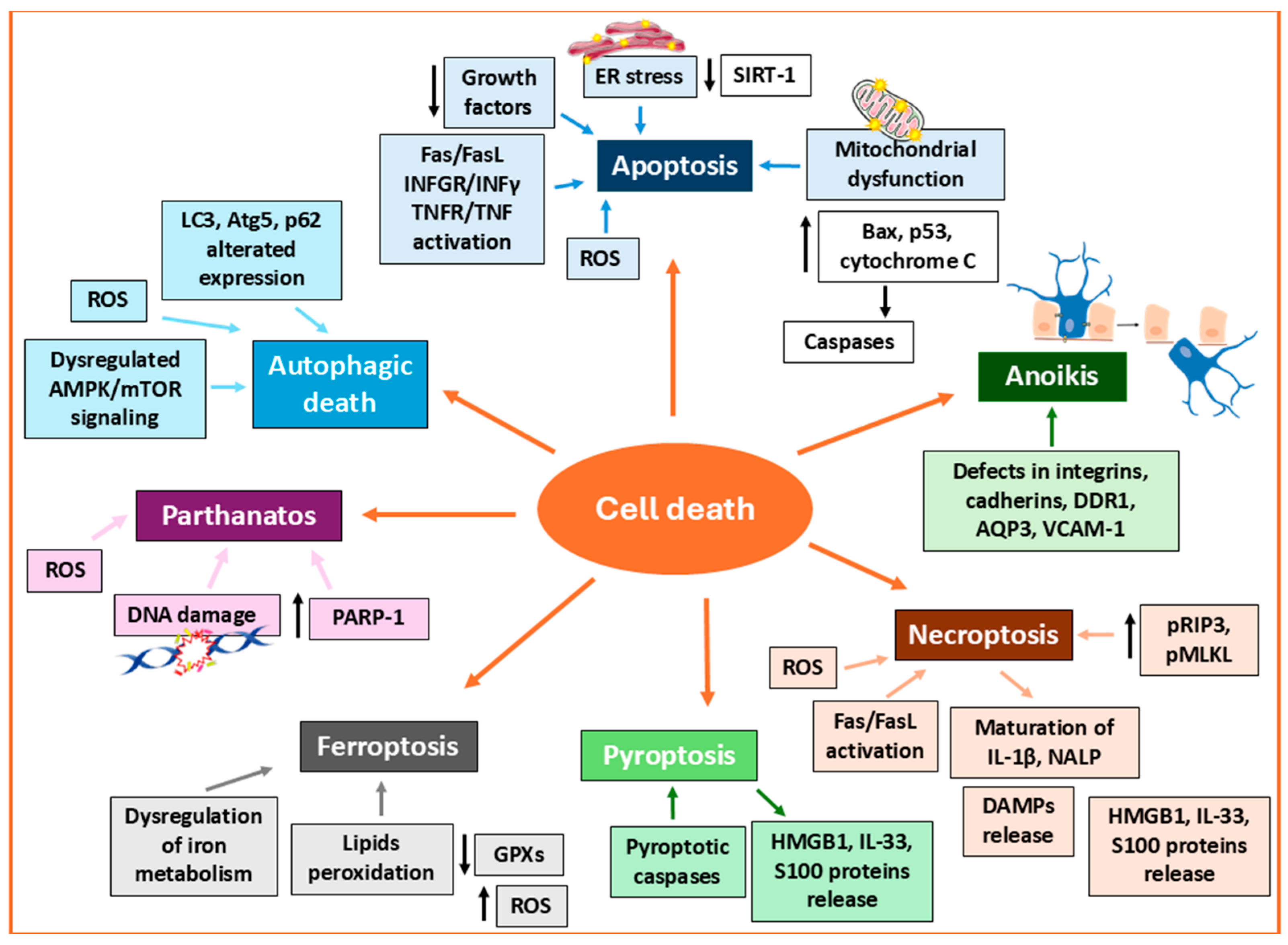
3. Proliferation, Cell Death, and Senescence
3.1. Stimulating Precursor Melanocyte Mobilization, Proliferation, and Differentiation
3.2. Targeting Melanocyte Cell Death
3.2.1. Apoptosis
3.2.2. Necroptosis
3.2.3. Pyroptosis
3.2.4. Ferroptosis
3.2.5. Parthanatos
3.2.6. Autophagy-Related Death
3.3. Targeting Premature Senescence Pathways in Vitiligo
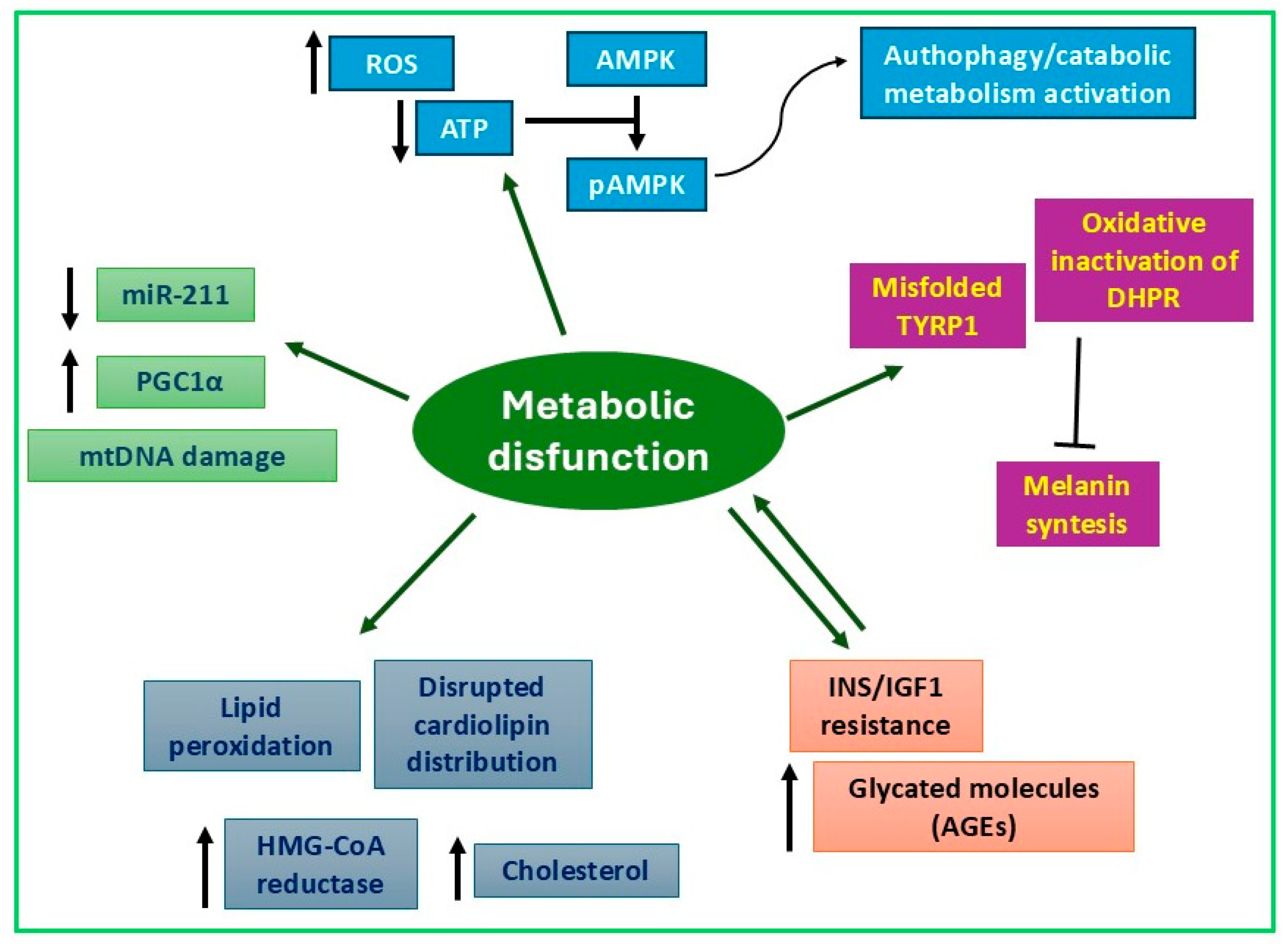
4. Alterations of Metabolism in Vitiligo Melanocytes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nordlund, J.J. The melanocyte and the epidermal melanin unit: An expanded concept. Dermatol. Clin. 2007, 25, 271–281. [Google Scholar] [CrossRef]
- Li, A. The biology of melanocyte and melanocyte stem cell. Acta Biochim. Biophys. Sin. 2014, 46, 255–260. [Google Scholar] [CrossRef]
- Slominski, A. Neuroendocrine activity of the melanocyte. Exp. Dermatol. 2009, 18, 760–763. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Fuchs, E. Building and Maintaining the Skin. Cold Spring Harb. Perspect. Biol. 2022, 14, a040840. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Ko, D.; Friedman, B.J.; Lim, H.W.; Mohammad, T.F. Disorders of hyperpigmentation. Part I. Pathogenesis and clinical features of common pigmentary disorders. J. Am. Acad. Dermatol. 2023, 88, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primers 2015, 1, 15011. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef]
- Mastacouris, N.; Strunk, A.; Garg, A. Incidence and Prevalence of Diagnosed Vitiligo According to Race and Ethnicity, Age, and Sex in the US. JAMA Dermatol. 2023, 159, 986–990. [Google Scholar] [CrossRef]
- Joge, R.R.; Kathane, P.U.; Joshi, S.H. Vitiligo: A Narrative Review. Cureus 2022, 14, e29307. [Google Scholar] [CrossRef]
- Dabas, G.; Vinay, K.; Parsad, D.; Kumar, A.; Kumaran, M.S. Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 392–399. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A focus on pathogenesis and its therapeutic implications. J. Dermatol. 2021, 48, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Pathak, G.N.; Tan, I.J.; Bai, G.; Dhillon, J.; Rao, B.K. Vitiligo: From mechanisms of disease to treatable pathways. Ski. Health Dis. 2024, 4, e460. [Google Scholar] [CrossRef]
- Pala, V.; Ribero, S.; Quaglino, P.; Mastorino, L. Updates on Potential Therapeutic Approaches for Vitiligo: Janus Kinase Inhibitors and Biologics. J. Clin. Med. 2023, 12, 7486. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Ruxolitinib Cream 1.5%: A Review in Non-Segmental Vitiligo. Drugs 2024, 84, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, L.; Shi, M.; Cong, T.; Yang, X.; Zhou, X.; Cheng, M.; Ma, C.; Yao, S.; Ying, P.; et al. JAK inhibitors in immune regulation and treatment of vitiligo. Cytokine Growth Factor. Rev. 2024, 80, 87–96. [Google Scholar] [CrossRef]
- Halliwell, B. Free Radicals and other Reactive Species in Disease. Encycl. Life Sci. 2005, 7, 381–387. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Junior, J.F.; Goncalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Klotz, L.-O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Qian, H.; Shan, Y.; Gong, R.; Lin, D.; Zhang, M.; Wang, C.; Wang, L. Mechanism of action and therapeutic effects of oxidative stress and stem cell-based materials in skin aging: Current evidence and future perspectives. Front. Bioeng. Biotechnol. 2023, 10, 1082403. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; D'Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Glassman, S.J. Vitiligo, reactive oxygen species and T-cells. Clin. Sci. 2011, 120, 99–120. [Google Scholar] [CrossRef]
- Chang, W.-L.; Ko, C.-H. The Role of Oxidative Stress in Vitiligo: An Update on Its Pathogenesis and Therapeutic Implications. Cells 2023, 12, 936. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Gaze, D.C.; Tobin, D.J.; Marshall, H.S.; Panske, A.; Panzig, E.; Hibberts, N.A. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Investig. Dermatol. Symp. Proc. 1999, 4, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Peters, E.M.; Marles, L.K.; Behrens-Williams, S.C.; Dummer, R.; Blau, N.; Thony, B. Epidermal H2O2 accumulation alters tetrahydrobiopterin (6BH4) recycling in vitiligo: Identification of a general mechanism in regulation of all 6BH4-dependent processes? J. Investig. Dermatol. 2001, 116, 167–174. [Google Scholar] [CrossRef]
- Spencer, J.D.; Gibbons, N.C.J.; Rokos, H.; Peters, E.M.J.; Wood, J.M.; Schallreuter, K.U. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J. Investig. Dermatol. 2007, 127, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.L.; Ottaviani, M.; Bellei, B.; Albanesi, V.; Cossarizza, A.; Rossi, L.; Picardo, M. Membrane lipid defects are responsible for the generation of reactive oxygen species in peripheral blood mononuclear cells from vitiligo patients. J. Cell Physiol. 2010, 223, 187–193. [Google Scholar] [CrossRef]
- Briganti, S.; Caron-Schreinemachers, A.D.B.; Picardo, M.; Westerhof, W. Anti-oxidant defence mechanism in vitiliginous skin increases with skin type. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1212–1219. [Google Scholar] [CrossRef]
- Mulayim, M.K.; Kurutas, E.B.; Nazik, H.; Ozturk, P. Assessment of Oxidative/Nitrosative Stress and Raftlin in Vitiligo. Indian J. Dermatol. 2022, 67, 624. [Google Scholar] [CrossRef]
- Mathachan, S.R.; Khurana, A.; Gautam, R.K.; Kulhari, A.; Sharma, L.; Sardana, K. Does oxidative stress correlate with disease activity and severity in vitiligo? An analytical study. J. Cosmet. Dermatol. 2021, 20, 352–359. [Google Scholar] [CrossRef]
- Iraji, F.; Seyedyousefi, S.; Heidari, A. Serum vitamins and trace elements in vitiligo patients: A systematic review and meta-analysis of observational studies. JEADV Clin. Pract. 2024, 3, 1430–1446. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Ottaviani, M.; Ludovici, M.; Cota, C.; Luzi, F.; Dell’Anna, M.L.; Picardo, M. Vitiligo: A possible model of degenerative diseases. PLoS ONE 2013, 8, e59782. [Google Scholar] [CrossRef]
- Akterin, S.; Cowburn, R.F.; Miranda-Vizuete, A.; Jimenez, A.; Bogdanovic, N.; Winblad, B.; Cedazo-Minguez, A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006, 13, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Li, K.; Song, P.; Zhu, G.; Zhu, L.; Cui, T.; Liu, B.; Tang, L.; Wang, X.; Wang, G.; et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: A possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014, 134, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Geng, M.-M.; Dong, B.-Q.; Luo, L.-F.; Jiang, S.; Le Poole, I.C.; Lei, T.-C. Increased splicing of CXCR3 isoform B (CXCR3B) by impaired NRF2 signaling leads to melanocyte apoptosis in active vitiligo. Free Radic. Biol. Med. 2024, 225, 687–698. [Google Scholar] [CrossRef]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.-W.; Zhou, Y.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med. 2014, 6, 223ra23. [Google Scholar] [CrossRef]
- Boniface, K.; Jacquemin, C.; Darrigade, A.-S.; Dessarthe, B.; Martins, C.; Boukhedouni, N.; Vernisse, C.; Grasseau, A.; Thiolat, D.; Rambert, J.; et al. Vitiligo Skin Is Imprinted with Resident Memory CD8 T Cells Expressing CXCR3. J. Investig. Dermatol. 2018, 138, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Patel, S.; Begum, R.; Dwivedi, M. Emerging role of Tissue Resident Memory T cells in vitiligo: From pathogenesis to therapeutics. Autoimmun. Rev. 2021, 20, 102868. [Google Scholar] [CrossRef]
- Richmond, J.M.; Masterjohn, E.; Chu, R.; Tedstone, J.; Youd, M.E.; Harris, J.E. CXCR3 Depleting Antibodies Prevent and Reverse Vitiligo in Mice. J. Investig. Dermatol. 2017, 137, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Le, Q.; Tong, J.; Wang, H. The IFN-γ-CXCL9/CXCL10-CXCR3 axis in vitiligo: Pathological mechanism and treatment. Eur. J. Immunol. 2024, 54, e2250281. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Nakano, M.; Tero-Kubota, S. Generation of superoxide during the enzymatic action of tyrosinase. Arch. Biochem. Biophys. 1992, 292, 570–575. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, L.; Wakamatsu, K.; Ito, S.; Adhyaru, B.; Cheng, C.-Y.; Bowers, C.R.; Simon, J.D. Comparison of Structural and Chemical Properties of Black and Red Human Hair Melanosomes. Photochem. Photobiol. 2005, 81, 135–144. [Google Scholar] [CrossRef]
- Parsad, D.; Wakamatsu, K.; Kanwar, A.J.; Kumar, B.; Ito, S. Eumelanin and phaeomelanin contents of depigmented and repigmented skin in vitiligo patients. Br. J. Dermatol. 2003, 149, 624–626. [Google Scholar] [CrossRef]
- Yonemoto, K.; Gellin, G.A.; Epstein, W.L.; Fukuyama, K. Reduction in eumelanin by the activation of glutathione reductase and γ-glutamyl transpeptidase after exposure to a depigmenting chemical. Biochem. Pharmacol. 1983, 32, 1379–1382. [Google Scholar] [CrossRef]
- Bibeau, K.; Pandya, A.G.; Ezzedine, K.; Jones, H.; Gao, J.; Lindley, A.; Harris, J.E. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1831–1844. [Google Scholar] [CrossRef]
- Maresca, V.; Flori, E.; Briganti, S.; Mastrofrancesco, A.; Fabbri, C.; Mileo, A.M.; Paggi, M.G.; Picardo, M. Correlation between melanogenic and catalase activity in in vitro human melanocytes: A synergic strategy against oxidative stress. Pigment. Cell Melanoma Res. 2008, 21, 200–205. [Google Scholar] [CrossRef]
- Smit, N.P.M.; van Nieuwpoort, F.A.; Marrot, L.; Out, C.; Poorthuis, B.; van Pelt, H.; Meunier, J.; Pavel, S. Increased melanogenesis is a risk factor for oxidative DNA damage--study on cultured melanocytes and atypical nevus cells. Photochem. Photobiol. 2008, 84, 550–555. [Google Scholar] [CrossRef]
- Maresca, V.; Flori, E.; Bellei, B.; Aspite, N.; Kovacs, D.; Picardo, M. MC1R stimulation by α-MSH induces catalase and promotes its re-distribution to the cell periphery and dendrites. Pigment. Cell Melanoma Res. 2010, 23, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, M.; Khanna, S.; Subramaniam, Y.; Rengaraju, J.; Sultan, F.; Gupta, I.; Sharma, K.; Chandna, S.; Gokhale, R.S.; Natarajan, V.T. Sustained pigmentation causes DNA damage and invokes translesion polymerase Polκ for repair in melanocytes. Nucleic Acids Res. 2023, 51, 10451–10466. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Guntas, G.; Engin, B.; Ekmekci, O.B.; Kutlubay, Z.; Ekmekci, H.; Songur, A.; Uzuncakmak, T.K.U.; Vehid, H.E.; Serdaroglu, S.; Tuzun, Y.; et al. Evaluation of advanced oxidation protein products, prooxidant-antioxidant balance, and total antioxidant capacity in untreated vitiligo patients. Ann. Dermatol. 2015, 27, 178–183. [Google Scholar] [CrossRef]
- Li, S.; Dai, W.; Wang, S.; Kang, P.; Ye, Z.; Han, P.; Zeng, K.; Li, C. Clinical Significance of Serum Oxidative Stress Markers to Assess Disease Activity and Severity in Patients with Non-Segmental Vitiligo. Front. Cell Dev. Biol. 2021, 9, 739413. [Google Scholar] [CrossRef]
- Salem, M.M.A.E.L.; Shalbaf, M.; Gibbons, N.C.J.; Chavan, B.; Thornton, J.M.; Schallreuter, K.U. Enhanced DNA binding capacity on up-regulated epidermal wild-type p53 in vitiligo by H2O2-mediated oxidation: A possible repair mechanism for DNA damage. FASEB J. 2009, 23, 3790–3807. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De Sarkar, S.; Pradhan, A.; Pati, A.K.; Pradhan, R.; Mondal, D.; Sen, S.; Ghosh, A.; Chatterjee, S.; Chatterjee, M. Levels of oxidative damage and proinflammatory cytokines are enhanced in patients with active vitiligo. Free Radic. Res. 2017, 51, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Bellandi, S.; Pitozzi, V.; Fabbri, P.; Dolara, P.; Moretti, S. Increased oxidative DNA damage in mononuclear leukocytes in vitiligo. Mutat. Res. Mol. Mech. Mutagen. 2004, 556, 101–106. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Tobin, D.J.; Panske, A. Decreased photodamage and low incidence of non-melanoma skin cancer in 136 sun-exposed caucasian patients with vitiligo. Dermatology 2002, 204, 194–201. [Google Scholar] [CrossRef]
- Sant’Anna-Silva, A.C.B.; Botton, T.; Rossi, A.; Dobner, J.; Bzioueche, H.; Thach, N.; Blot, L.; Pagnotta, S.; Kleszczynski, K.; Steinbrink, K.; et al. Vitiligo auto-immune response upon oxidative stress-related mitochondrial DNA release opens up new therapeutic strategies. Clin. Transl. Med. 2024, 14, e1810. [Google Scholar] [CrossRef]
- Hu, M.-M.; Shu, H.-B. Mitochondrial DNA-triggered innate immune response: Mechanisms and diseases. Cell Mol. Immunol. 2023, 20, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Chiuchiarelli, G.; Cemeli, E.; Elwary, S.M.; Gillbro, J.M.; Spencer, J.D.; Rokos, H.; Panske, A.; Chavan, B.; Wood, J.M.; et al. Estrogens can contribute to hydrogen peroxide generation and quinone-mediated DNA damage in peripheral blood lymphocytes from patients with vitiligo. J. Investig. Dermatol. 2006, 126, 1036–1042. [Google Scholar] [CrossRef]
- Sastry, K.S.; Naeem, H.; Mokrab, Y.; Chouchane, A.I. RNA-seq Reveals Dysregulation of Novel Melanocyte Genes upon Oxidative Stress: Implications in Vitiligo Pathogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 2841814. [Google Scholar] [CrossRef]
- Hou, X.; Shi, J.; Sun, L.; Song, L.; Zhao, W.; Xiong, X.; Lu, Y. The involvement of ERK1/2 and p38 MAPK in the premature senescence of melanocytes induced by H2O2 through a p53-independent p21 pathway. J. Dermatol. Sci. 2022, 105, 88–97. [Google Scholar] [CrossRef]
- Tobin, D.J.; Swanson, N.N.; Pittelkow, M.R.; Peters, E.M.; Schallreuter, K.U. Melanocytes are not absent in lesional skin of long duration vitiligo. J. Pathol. 2000, 191, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G.M.; Kidson, S.H. Molecular analysis of vitiligo lesions reveals sporadic melanocyte survival. Int. J. Dermatol. 2007, 46, 268–272. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, T.H.; Park, T.J.; Kang, H.Y. p16(ink4a) Positivity of Melanocytes in Non-Segmental Vitiligo. Diagnostics 2020, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D.; Kurz, D.J. Cellular senescence in vivo: Its relevance in ageing and cardiovascular disease. Exp. Gerontol. 2005, 40, 634–642. [Google Scholar] [CrossRef]
- Kritsilis, M.; Rizou, S.V.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Hinton, D.R.; Kannan, R. The Emerging Role of Senescence in Ocular Disease. Oxidative Med. Cell. Longev. 2020, 2020, 2583601. [Google Scholar] [CrossRef]
- Bellei, B.; Picardo, M. Premature cell senescence in human skin: Dual face in chronic acquired pigmentary disorders. Ageing Res. Rev. 2020, 57, 100981. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Hu, Y.; Li, W.; Eisinger, M.; Seiberg, M.; Lin, C.B. The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp. Dermatol. 2010, 19, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Hwang, J.S.; Lee, J.Y.; Ahn, J.H.; Kim, J.-Y.; Lee, E.-S.; Kang, W.H. The dermal stem cell factor and c-kit are overexpressed in melasma. Br. J. Dermatol. 2006, 154, 1094–1099. [Google Scholar] [CrossRef]
- Moretti, S.; Spallanzani, A.; Amato, L.; Hautmann, G.; Gallerani, I.; Fabbri, P. Vitiligo and epidermal microenvironment: Possible involvement of keratinocyte-derived cytokines. Arch. Dermatol. 2002, 138, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-Y. Role of keratinocytes in the development of vitiligo. Ann. Dermatol. 2012, 24, 115–125. [Google Scholar] [CrossRef]
- Burton, D.G.A.; Stolzing, A. Cellular senescence: Immunosurveillance and future immunotherapy. Ageing Res. Rev. 2018, 43, 17–25. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Dell’Anna, M.L.; Ottaviani, M.; Kovacs, D.; Mirabilii, S.; Brown, D.A.; Cota, C.; Migliano, E.; Bastonini, E.; Bellei, B.; Cardinali, G.; et al. Energetic mitochondrial failing in vitiligo and possible rescue by cardiolipin. Sci. Rep. 2017, 7, 13663. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, X.; Zheng, Y.; Xie, Y.; Lai, W. Cytosolic mtDNA-cGAS-STING axis mediates melanocytes pyroptosis to promote CD8(+) T-cell activation in vitiligo. J. Dermatol. Sci. 2025, 117, 61–70. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, X.; Fan, J.; Ye, P.; Zhang, J.; Wang, Z.; Zhou, Y.; Wang, B.; Jin, X.; Xiong, S.; et al. Oxidative stress-induced release of mitochondrial DNA (mtDNA) promotes the progression of vitiligo by activating the cGAS-STING signaling pathway in monocytes. Free Radic. Biol. Med. 2025, 235, 43–55. [Google Scholar] [CrossRef]
- Toosi, S.; Orlow, S.J.; Manga, P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J. Investig. Dermatol. 2012, 132, 2601–2609. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, X.; Xiang, L.; Zhang, C. Dysfunction of Autophagy: A Possible Mechanism Involved in the Pathogenesis of Vitiligo by Breaking the Redox Balance of Melanocytes. Oxidative Med. Cell. Longev. 2016, 2016, 3401570. [Google Scholar] [CrossRef]
- Liu, L.-Y.; He, S.-J.; Chen, Z.; Ge, M.; Lyu, C.-Y.; Gao, D.; Yu, J.-P.; Cai, M.-H.; Yuan, J.-X.; Zhang, J.-L. The Role of Regulatory Cell Death in Vitiligo. DNA Cell Biol. 2024, 43, 61–73. [Google Scholar] [CrossRef]
- Saheki, Y.; De Camilli, P. Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annu. Rev. Biochem. 2017, 86, 659–684. [Google Scholar] [CrossRef]
- Li, H.; Lismont, C.; Revenco, I.; Hussein, M.A.F.; Costa, C.F.; Fransen, M. The Peroxisome-Autophagy Redox Connection: A Double-Edged Sword? Front. Cell Dev. Biol. 2021, 9, 814047. [Google Scholar] [CrossRef]
- Chen, J.; Zhuang, T.; Chen, J.; Tian, Y.; Yi, X.; Ni, Q.; Zhang, W.; Song, P.; Jian, Z.; Liu, L.; et al. Homocysteine induces melanocytes apoptosis via PERK–eIF2α–CHOP pathway in vitiligo. Clin. Sci. 2020, 134, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Woodward, A.M.; Di Zazzo, A.; Bonini, S.; Argueso, P. Endoplasmic reticulum stress promotes inflammation-mediated proteolytic activity at the ocular surface. Sci. Rep. 2020, 10, 2216. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.; Wang, P.; Ding, Y.; Wang, H.; Kang, X. Research Progress on Targeted Antioxidant Therapy and Vitiligo. Oxidative Med. Cell. Longev. 2022, 2022, 1821780. [Google Scholar] [CrossRef]
- Jung, H.M.; Jung, Y.S.; Lee, J.H.; Kim, G.M.; Bae, J.M. Antioxidant supplements in combination with phototherapy for vitiligo: A systematic review and metaanalysis of randomized controlled trials. J. Am. Acad. Dermatol. 2021, 85, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.M.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Derm. Endocrinol. 2013, 5, 222–234. [Google Scholar] [CrossRef]
- Khurrum, H.; AlGhamdi, K.M. The Relationship Between the Serum Level of Vitamin D and Vitiligo: A Controlled Study on 300 Subjects. J. Cutan. Med. Surg. 2016, 20, 139–145. [Google Scholar] [CrossRef]
- Diaz, M.J.; Tran, J.T.; Rose, D.; Wei, A.; Lakshmipathy, D.; Lipner, S.R. Dietary Interventions, Supplements, and Plant-Derived Compounds for Adjunct Vitiligo Management: A Review of the Literature. Nutrients 2025, 17, 357. [Google Scholar] [CrossRef]
- Li, K.; Shi, Q.; Yang, L.; Li, X.; Liu, L.; Wang, L.; Li, Q.; Wang, G.; Li, C.-Y.; Gao, T.-W. The association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with generalized vitiligo. Br. J. Dermatol. 2012, 167, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Fallah, M.; Abedini, R.; Mahiabadi, S.A.; Montazeri, S.; Hosseinzadeh-Attar, M.J.; Ebrahimpour-Koujan, S. The effect of vitamin C on oxidative stress indices and skin regimentation of vitiligo patients. Arch. Dermatol. Res. 2023, 315, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Sanadi, R.M.; Deshmukh, R.S. The effect of Vitamin C on melanin pigmentation—A systematic review. J. Oral. Maxillofac. Pathol. 2020, 24, 374–382. [Google Scholar] [CrossRef]
- Adil, M.; Amin, S.S.; Mohtashim, M. N-acetylcysteine in dermatology. Indian. J. Dermatol. Venereol. Leprol. 2018, 84, 652–659. [Google Scholar] [CrossRef]
- Montes, L.F.; Diaz, M.L.; Lajous, J.; Garcia, N.J. Folic acid and vitamin B12 in vitiligo: A nutritional approach. Cutis 1992, 50, 39–42. [Google Scholar]
- Middelkamp-Hup, M.A.; Bos, J.D.; Rius-Diaz, F.; Gonzalez, S.; Westerhof, W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: A randomized double-blind placebo-controlled study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 942–950. [Google Scholar] [CrossRef]
- Gonzalez, S.; Gilaberte, Y.; Philips, N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem. Photobiol. Sci. 2010, 9, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Reyes, E.; Jaén, P.; Heras, E.d.L.; de Eusebio, E.; Carrión, F.; Cuevas, J.; González, S.; Villarrubia, V.G.; Álvarez-Mon, M. Systemic immunomodulatory effects of Polypodium leucotomos as an adjuvant to PUVA therapy in generalized vitiligo: A pilot study. J. Dermatol. Sci. 2006, 41, 213–216. [Google Scholar] [CrossRef]
- Naini, F.F.; Shooshtari, A.V.; Ebrahimi, B.; Molaei, R. The effect of pseudocatalase/superoxide dismutase in the treatment of vitiligo: A pilot study. J. Res. Pharm. Pract. 2012, 1, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Sanclemente, G.; Garcia, J.J.; Zuleta, J.J.; Diehl, C.; Correa, C.; Falabella, R. A double-blind, randomized trial of 0.05% betamethasone vs. topical catalase/dismutase superoxide in vitiligo. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Bakis-Petsoglou, S.; Le Guay, J.L.; Wittal, R. A randomized, double-blinded, placebo-controlled trial of pseudocatalase cream and narrowband ultraviolet B in the treatment of vitiligo. Br. J. Dermatol. 2009, 161, 910–917. [Google Scholar] [CrossRef]
- Yuksel, E.P.; Aydin, F.; Senturk, N.; Canturk, T.; Turanli, A.Y. Comparison of the efficacy of narrow band ultraviolet B and narrow band ultraviolet B plus topical catalase-superoxide dismutase treatment in vitiligo patients. Eur. J. Dermatol. 2009, 19, 341–344. [Google Scholar] [CrossRef]
- Gawkrodger, D.J. Pseudocatalase and narrowband ultraviolet B for vitiligo: Clearing the picture. Br. J. Dermatol. 2009, 161, 721–722. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Salem, M.A.E.L.; Holtz, S.; Panske, A. Basic evidence for epidermal H2O2/ONOO(-)-mediated oxidation/nitration in segmental vitiligo is supported by repigmentation of skin and eyelashes after reduction of epidermal H2O2 with topical NB-UVB-activated pseudocatalase PC-KUS. FASEB J. 2013, 27, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Liu, T.; Huan, Y.; Li, F.; Wang, R. Serum level of antioxidant vitamins and minerals in patients with vitiligo, a systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2020, 62, 126570. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Aoto, T.; Binh, N.T.; Okamoto, N.; Tanimura, S.; Wakayama, T.; Iseki, S.; Hara, E.; Masunaga, T.; Shimizu, H.; et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009, 137, 1088–1099. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Granter, S.R.; Fisher, D.E. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science 2005, 307, 720–724. [Google Scholar] [CrossRef]
- Bertrand, J.U.; Steingrimsson, E.; Jouenne, F.; Bressac-de Paillerets, B.; Larue, L. Melanoma Risk and Melanocyte Biology. Acta Derm. Venereol. 2020, 100, 272–283. [Google Scholar] [CrossRef]
- Birlea, S.A.; Costin, G.; Roop, D.R.; Norris, D.A. Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization. Med. Res. Rev. 2017, 37, 907–935. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Itami, S.; Watabe, H.; Yasumoto, K.; Abdel-Malek, Z.A.; Kubo, T.; Rouzaud, F.; Tanemura, A.; Yoshikawa, K.; Hearing, V.J. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J. Cell Biol. 2004, 165, 275–285. [Google Scholar] [CrossRef]
- Abdel-Naser, M.B.; Seltmann, H.; Zouboulis, C.C. SZ95 sebocytes induce epidermal melanocyte dendricity and proliferation in vitro. Exp. Dermatol. 2012, 21, 393–395. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Kim, N.-H.; Choi, W.-I.; Youm, Y.-H. Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J. Investig. Dermatol. 2005, 124, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Spallanzani, A.; Amato, L.; Hautmann, G.; Gallerani, I.; Fabiani, M.; Fabbri, P. New insights into the pathogenesis of vitiligo: Imbalance of epidermal cytokines at sites of lesions. Pigment. Cell Res. 2002, 15, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Meng, D.; Cao, P.; Sun, L.; Pang, Y.; Li, Y.; Wang, X.; Luo, Z.; Zhang, L.; Liu, G. Identification of pathogenic genes and transcription factors in vitiligo. Dermatol. Ther. 2019, 32, e13025. [Google Scholar] [CrossRef] [PubMed]
- Kingo, K.; Aunin, E.; Karelson, M.; Philips, M.-A.; Ratsep, R.; Silm, H.; Vasar, E.; Soomets, U.; Koks, S. Gene expression analysis of melanocortin system in vitiligo. J. Dermatol. Sci. 2007, 48, 113–122. [Google Scholar] [CrossRef]
- Toh, J.J.H.; Chuah, S.Y.; Jhingan, A.; Chong, W.-S.; Thng, S.T.G. Afamelanotide implants and narrow-band ultraviolet B phototherapy for the treatment of nonsegmental vitiligo in Asians. J. Am. Acad. Dermatol. 2020, 82, 1517–1519. [Google Scholar] [CrossRef]
- Liang, J.; Yu, Y.; Li, C.; Li, Q.; Chen, P.; Li, W.; Liu, W.; Li, Z.; Liu, Y.; Zhang, S.; et al. Tofacitinib combined with melanocyte protector α-MSH to treat vitiligo through dextran-based hydrogel microneedles. Carbohydr. Polym. 2023, 305, 120549. [Google Scholar] [CrossRef]
- Khan, R.; Sharma, A.; Bhushan, A.; Basnet, B.; Sharma, V.K.; Gupta, S. Relationship between α-melanocyte stimulating hormone levels and therapeutic outcome of melanocyte transplantation and phototherapy in non-segmental patients with vitiligo: A prospective study. Australas. J. Dermatol. 2018, 59, e315–e318. [Google Scholar] [CrossRef]
- Lim, H.W.; Grimes, P.E.; Agbai, O.; Hamzavi, I.; Henderson, M.; Haddican, M.; Linkner, R.V.; Lebwohl, M. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: A randomized multicenter trial. JAMA Dermatol. 2015, 151, 42–50. [Google Scholar] [CrossRef]
- Minder, E.I.; Barman-Aksoezen, J.; Schneider-Yin, X. Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders. Clin. Pharmacokinet. 2017, 56, 815–823. [Google Scholar] [CrossRef]
- Shah, B.; Godse, K.; Mahajan, S.; Grandhi, S.; Shendkar, S.; Sharma, A.; Teli, C.; Pathak, R.; Parsad, D. Efficacy and safety of basic fibroblast growth factor (bFGF) related decapeptide solution plus Tacrolimus 0.1% ointment versus Tacrolimus 0.1% ointment in the treatment of stable vitiligo. Dermatol. Ther. 2019, 32, e13109. [Google Scholar] [CrossRef]
- Caputo, S.; Papaccio, F.; Marrapodi, R.; Lopez, G.; Iacovelli, P.; Pacifico, A.; Migliano, E.; Cota, C.; Di Nardo, A.; Picardo, M.; et al. Defective Intracellular Insulin/IGF-1 Signaling Elucidates the Link Between Metabolic Defect and Autoimmunity in Vitiligo. Cells 2025, 14, 565. [Google Scholar] [CrossRef] [PubMed]
- El-Komy, M.H.M.; Sayed, K.S.; Mostafa, W.Z.; Shaker, O.; Nasser, N.; Hassan, M. Insulin-like growth factor 1: A new prime mover in the pathogenesis of vitiligo. Australas. J. Dermatol. 2023, 64, e188–e191. [Google Scholar] [CrossRef]
- Ding, X.; Liu, S.-X. Progress in the Use of Platelet-Rich Plasma to Treat Vitiligo and Melasma. Int. J. Dermatol. Venereol. 2021, 4, 236–241. [Google Scholar] [CrossRef]
- Bellei, B.; Papaccio, F.; Filoni, A.; Caputo, S.; Lopez, G.; Migliano, E.; Picardo, M. Extracellular fraction of adipose tissue as an innovative regenerative approach for vitiligo treatment. Exp. Dermatol. 2019, 28, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.L.; Maresca, V.; Briganti, S.; Camera, E.; Falchi, M.; Picardo, M. Mitochondrial impairment in peripheral blood mononuclear cells during the active phase of vitiligo. J. Investig. Dermatol. 2001, 117, 908–913. [Google Scholar] [CrossRef]
- Seneschal, J.; Boniface, K.; D’Arino, A.; Picardo, M. An update on Vitiligo pathogenesis. Pigment. Cell Melanoma Res. 2021, 34, 236–243. [Google Scholar] [CrossRef]
- Kumar, R.; Parsad, D.; Kanwar, A.J.; Kaul, D. Altered levels of LXR-α: Crucial implications in the pathogenesis of vitiligo. Exp. Dermatol. 2012, 21, 853–858. [Google Scholar] [CrossRef]
- Kang, P.; Zhang, W.; Chen, X.; Yi, X.; Song, P.; Chang, Y.; Zhang, S.; Gao, T.; Li, C.; Li, S. TRPM2 mediates mitochondria-dependent apoptosis of melanocytes under oxidative stress. Free Radic. Biol. Med. 2018, 126, 259–268. [Google Scholar] [CrossRef]
- Yi, X.; Guo, W.; Shi, Q.; Yang, Y.; Zhang, W.; Chen, X.; Kang, P.; Chen, J.; Cui, T.; Ma, J.; et al. SIRT3-Dependent Mitochondrial Dynamics Remodeling Contributes to Oxidative Stress-Induced Melanocyte Degeneration in Vitiligo. Theranostics 2019, 9, 1614–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Jin, L.; Yi, X.; Dang, E.; Yang, Y.; Li, C.; Gao, T. Oxidative stress-induced calreticulin expression and translocation: New insights into the destruction of melanocytes. J. Investig. Dermatol. 2014, 134, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, Y.; Luo, L.; Huang, X.; Wei, T.; Zuo, B.; Liu, G.; Bu, W.; Li, C. Sirtuin1 Deficiency Could Exacerbate Melanocyte Apoptosis Under Endoplasmic Reticulum Stress. Inflammation 2025. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Fiorillo, C.; Barygina, V.; Cecchi, C.; Lotti, T.; Prignano, F.; Silvestro, A.; Nassi, P.; Taddei, N. SIRT1 regulates MAPK pathways in vitiligo skin: Insight into the molecular pathways of cell survival. J. Cell Mol. Med. 2014, 18, 514–529. [Google Scholar] [CrossRef]
- Veis, D.J.; Sorenson, C.M.; Shutter, J.R.; Korsmeyer, S.J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993, 75, 229–240. [Google Scholar] [CrossRef]
- Halaban, R.; Tyrrell, L.; Longley, J.; Yarden, Y.; Rubin, J. Pigmentation and proliferation of human melanocytes and the effects of melanocyte-stimulating hormone and ultraviolet B light. Ann. N. Y. Acad. Sci. 1993, 680, 290–301. [Google Scholar] [CrossRef]
- Dong, Y.; Kawakami, T.; Komatsu, T. Regulation of adhesion molecules and basic fibroblast growth factor 2 in non-segmental vitiligo-derived primary melanocytes. J. Dermatol. Sci. 2022, 108, 109–111. [Google Scholar] [CrossRef]
- Haass, N.K.; Smalley, K.S.M.; Li, L.; Herlyn, M. Adhesion, migration and communication in melanocytes and melanoma. Pigment. Cell Res. 2005, 18, 150–159. [Google Scholar] [CrossRef]
- Kim, N.-H.; Lee, A.-Y. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J. Investig. Dermatol. 2010, 130, 2231–2239. [Google Scholar] [CrossRef]
- Reichert-Faria, A.; Jung, J.E.; Neto, V.M.; de Castro, C.C.S.; Mira, M.T. Reduced immunohistochemical expression of Discoidin Domain Receptor 1 (DDR1) in vitiligo skin. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1057–1059. [Google Scholar] [CrossRef]
- Faria, A.R.; Jung, J.E.; de Castro, C.C.S.; de Noronha, L. Reduced immunohistochemical expression of adhesion molecules in vitiligo skin biopsies. Pathol. Res. Pract. 2017, 213, 199–204. [Google Scholar] [CrossRef]
- Kumar, R.; Parsad, D.; Kanwar, A.J. Role of apoptosis and melanocytorrhagy: A comparative study of melanocyte adhesion in stable and unstable vitiligo. Br. J. Dermatol. 2011, 164, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.Y.; Luciani, F.; Cario-Andre, M.; Rubod, A.; Petit, V.; Benzekri, L.; Ezzedine, K.; Lepreux, S.; Steingrimsson, E.; Taieb, A.; et al. Altered E-Cadherin Levels and Distribution in Melanocytes Precede Clinical Manifestations of Vitiligo. J. Investig. Dermatol. 2015, 135, 1810–1819. [Google Scholar] [CrossRef]
- Willemsen, M.; Krebbers, G.; Tjin, E.P.M.; Willemsen, K.J.; Louis, A.; Konijn, V.A.L.; Narayan, V.S.; Post, N.F.; Bakker, W.J.; Melief, C.J.M.; et al. IFN-γ-induced PD-L1 expression on human melanocytes is impaired in vitiligo. Exp. Dermatol. 2022, 31, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Puglisi, R.; Mattia, G.; Samela, T.; Abeni, D.; Malorni, W. An Overview of the Biological Complexity of Vitiligo. Oxidative Med. Cell. Longev. 2024, 2024, 3193670. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.V.; Mhlaba, J.M.; Rangel, S.M.; Le Poole, I.C. The convergence theory for vitiligo: A reappraisal. Exp. Dermatol. 2019, 28, 647–655. [Google Scholar] [CrossRef]
- Jimbo, H.; Nagai, H.; Fujiwara, S.; Shimoura, N.; Nishigori, C. Fas-FasL interaction in cytotoxic T cell-mediated vitiligo: The role of lesional expression of tumor necrosis factor-α and interferon-γ in Fas-mediated melanocyte apoptosis. Exp. Dermatol. 2020, 29, 61–70. [Google Scholar] [CrossRef]
- Kim, N.-H.; Jeon, S.; Lee, H.-J.; Lee, A.-Y. Impaired PI3K/Akt activation-mediated NF-κB inactivation under elevated TNF-α is more vulnerable to apoptosis in vitiliginous keratinocytes. J. Investig. Dermatol. 2007, 127, 2612–2617. [Google Scholar] [CrossRef]
- Li, M.; Sun, D.; Li, C.; Zhang, Z.; Gao, L.; Li, K.; Li, H.; Gao, T. Functional polymorphisms of the FAS gene associated with risk of vitiligo in Chinese populations: A case-control analysis. J. Investig. Dermatol. 2008, 128, 2820–2824. [Google Scholar] [CrossRef]
- Birol, A.; Kisa, U.; Kurtipek, G.S.; Kara, F.; Kocak, M.; Erkek, E.; Caglayan, O. Increased tumor necrosis factor alpha (TNF-α) and interleukin 1 alpha (IL1-α) levels in the lesional skin of patients with nonsegmental vitiligo. Int. J. Dermatol. 2006, 45, 992–993. [Google Scholar] [CrossRef]
- Li, B.; Yi, X.; Zhuang, T.; Zhang, S.; Li, S.; Yang, Y.; Cui, T.; Chen, J.; Chang, Y.; Gao, T.; et al. RIP1-Mediated Necroptosis Facilitates Oxidative Stress-Induced Melanocyte Death, Offering Insight into Vitiligo. J. Investig. Dermatol. 2021, 141, 2921–2931.e6. [Google Scholar] [CrossRef]
- Kearney, C.J.; Martin, S.J. An Inflammatory Perspective on Necroptosis. Mol. Cell 2017, 65, 965–973. [Google Scholar] [CrossRef]
- Anderton, H.; Alqudah, S. Cell death in skin function, inflammation, and disease. Biochem. J. 2022, 479, 1621–1651. [Google Scholar] [CrossRef]
- Martin, S.J.; Henry, C.M.; Cullen, S.P. A perspective on mammalian caspases as positive and negative regulators of inflammation. Mol. Cell 2012, 46, 387–397. [Google Scholar] [CrossRef]
- He, K.; Wu, W.; Wang, X.; Dai, W.; Wang, S.; Li, C.; Li, S. Circulatory levels of alarmins in patients with non-segmental vitiligo: Potential biomarkers for disease diagnosis and activity/severity assessment. Front. Immunol. 2022, 13, 1069196. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.; Wei, G.; Mao, H.; Liu, R.; He, Y. Damage-associated molecular patterns in vitiligo: Igniter fuse from oxidative stress to melanocyte loss. Redox Rep. 2022, 27, 193–199. [Google Scholar] [CrossRef]
- Herbette, S.; Roeckel-Drevet, P.; Drevet, J.R. Seleno-independent glutathione peroxidases. More than simple antioxidant scavengers. FEBS J. 2007, 274, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.-H.; Shi, M.; Chen, H.; Cui, S.; Wu, Y.; Gao, X.-H.; Chen, H.-D. Glutathione Peroxidase Level in Patients with Vitiligo: A Meta-Analysis. Biomed. Res. Int. 2016, 2016, 3029810. [Google Scholar] [CrossRef] [PubMed]
- Em, S.; Laddha, N.C.; Chatterjee, S.; Gani, A.R.A.G.A.; Malek, R.A.; Shah, B.J.; Begum, R. Association of catalase T/C exon 9 and glutathione peroxidase codon 200 polymorphisms in relation to their activities and oxidative stress with vitiligo susceptibility in Gujarat population. Pigment. Cell Res. 2007, 20, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Ottaviani, M.; Truglio, M.; D’Arino, A.; Caputo, S.; Pacifico, A.; Iacovelli, P.; Di Nardo, A.; Picardo, M.; Bellei, B. Markers of Metabolic Abnormalities in Vitiligo Patients. Int. J. Mol. Sci. 2024, 25, 10201. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Du, C.; Sang, L.; Liu, L.; Li, Y.; Wang, F.; Fan, W.; Tang, P.; Zhang, S.; et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J. Transl. Med. 2022, 20, 363. [Google Scholar] [CrossRef]
- David, K.K.; Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Parthanatos, a messenger of death. Front. Biosci. 2009, 14, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chen, G.; Jin, W.; Mao, K.; Wan, H.; He, Y. Molecular Mechanisms of Parthanatos and Its Role in Diverse Diseases. Int. J. Mol. Sci. 2022, 23, 7292. [Google Scholar] [CrossRef]
- Kist, M.; Vucic, D. Cell death pathways: Intricate connections and disease implications. EMBO J. 2021, 40, e106700. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Li, C. Mechanisms of melanocyte death in vitiligo. Med. Res. Rev. 2021, 41, 1138–1166. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, C.; Zhang, J.; Zhong, X.; Wang, R.; Zeng, X.; Ba, X. The Role of PARPs in Inflammation-and Metabolic-Related Diseases: Molecular Mechanisms and Beyond. Cells 2019, 8, 1047. [Google Scholar] [CrossRef]
- Tulic, M.K.; Cavazza, E.; Cheli, Y.; Jacquel, A.; Luci, C.; Cardot-Leccia, N.; Hadhiri-Bzioueche, H.; Abbe, P.; Gesson, M.; Sormani, L.; et al. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat. Commun. 2019, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Pedro, J.M.B.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef]
- Boya, P.; Gonzalez-Polo, R.-A.; Casares, N.; Perfettini, J.-L.; Dessen, P.; Larochette, N.; Metivier, D.; Meley, D.; Souquere, S.; Yoshimori, T.; et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 2005, 25, 1025–1040. [Google Scholar] [CrossRef]
- Denton, D.; Xu, T.; Kumar, S. Autophagy as a pro-death pathway. Immunol. Cell Biol. 2015, 93, 35–42. [Google Scholar] [CrossRef]
- Zhang, C.-F.; Gruber, F.; Ni, C.; Mildner, M.; Koenig, U.; Karner, S.; Barresi, C.; Rossiter, H.; Narzt, M.-S.; Nagelreiter, I.M.; et al. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J. Investig. Dermatol. 2015, 135, 1348–1357. [Google Scholar] [CrossRef]
- Qiao, Z.; Xu, Z.; Xiao, Q.; Yang, Y.; Ying, J.; Xiang, L.; Zhang, C. Dysfunction of ATG7-dependent autophagy dysregulates the antioxidant response and contributes to oxidative stress-induced biological impairments in human epidermal melanocytes. Cell Death Discov. 2020, 6, 31. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Abudu, Y.P.; Claude-Taupin, A.; Gu, Y.; Kumar, S.; Choi, S.W.; Peters, R.; Mudd, M.H.; Allers, L.; Salemi, M.; et al. Galectins control MTOR and AMPK in response to lysosomal damage to induce autophagy. Autophagy 2019, 15, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Bastonini, E.; Kovacs, D.; Raffa, S.; Macchie, M.D.; Pacifico, A.; Iacovelli, P.; Torrisi, M.R.; Picardo, M. A protective role for autophagy in vitiligo. Cell Death Dis. 2021, 12, 318. [Google Scholar] [CrossRef]
- Yu, H.; Lin, X.; Huang, Y.; Cheng, H.; Seifert, O. The Difference in Expression of Autophagy-Related Proteins in Lesional and Perilesional Skin in Adult Patients with Active and Stable Generalized Vitiligo-A Cross-Sectional Pilot Study. Indian J. Dermatol. 2021, 66, 331–336. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, X.; Lu, X.; Wang, C.; Xiang, L.; Zhang, C. Identification and Validation of Autophagy-Related Genes in Vitiligo. Cells 2022, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ge, K.; Cheng, Y.; Zhang, R. Bioinformatic Analysis of Genes Associated with Autophagy in Vitiligo. Indian J. Dermatol. 2024, 69, 123–131. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Huang, Y.; Tao, X.; Fan, Y.; Li, Z.; Ding, X. Identification of the role of autophagy-related TNFSF10/ hsa-let-7a-5p axis in vitiligo development and potential herbs exploring based on a bioinformatics analysis. Heliyon 2023, 9, e23220. [Google Scholar] [CrossRef]
- Coppe, J.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Dong, B.-Q.; Liao, Z.-K.; Le, Y.; Jiang, S.; Luo, L.-F.; Miao, F.; Le Poole, I.C.; Lei, T.-C. Acceleration of melanocyte senescence by the proinflammatory cytokines IFNγ and TNFα impairs the repigmentation response of vitiligo patients to narrowband ultraviolet B (NBUVB) phototherapy. Mech. Ageing Dev. 2023, 211, 111779. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, M.; Lin, F.; Liu, D.; Hong, W.; Lu, L.; Zhu, Y.; Xu, A. Interferon-γ induces senescence in normal human melanocytes. PLoS ONE 2014, 9, e93232. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, J.C.; Kim, Y.; Kim, Y.H.; Park, S.S.; Muther, C.; Tessier, A.; Lee, G.; Gendronneau, G.; Forestier, S.; et al. Senescent melanocytes driven by glycolytic changes are characterized by melanosome transport dysfunction. Theranostics 2023, 13, 3914–3924. [Google Scholar] [CrossRef]
- Bondanza, S.; Maurelli, R.; Paterna, P.; Migliore, E.; Giacomo, F.D.; Primavera, G.; Paionni, E.; Dellambra, E.; Guerra, L. Keratinocyte cultures from involved skin in vitiligo patients show an impaired in vitro behaviour. Pigment. Cell Res. 2007, 20, 288–300. [Google Scholar] [CrossRef]
- Kovacs, D.; Bastonini, E.; Briganti, S.; Ottaviani, M.; D’Arino, A.; Truglio, M.; Sciuto, L.; Zaccarini, M.; Pacifico, A.; Cota, C.; et al. Altered epidermal proliferation, differentiation, and lipid composition: Novel key elements in the vitiligo puzzle. Sci. Adv. 2022, 8, eabn9299. [Google Scholar] [CrossRef]
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef] [PubMed]
- de Magalhaes, J.P. Cellular senescence in normal physiology. Science 2024, 384, 1300–1301. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Prignano, F.; Fiorillo, C.; Pescitelli, L.; Nassi, P.; Lotti, T.; Taddei, N. The involvement of Smac/DIABLO, p53, NF-kB, and MAPK pathways in apoptosis of keratinocytes from perilesional vitiligo skin: Protective effects of curcumin and capsaicin. Antioxid. Redox Signal 2010, 13, 1309–1321. [Google Scholar] [CrossRef]
- Kovacs, D.; Bastonini, E.; Ottaviani, M.; Cota, C.; Migliano, E.; Dell’Anna, M.L.; Picardo, M. Vitiligo Skin: Exploring the Dermal Compartment. J. Investig. Dermatol. 2018, 138, 394–404. [Google Scholar] [CrossRef]
- Cat, B.; Stuhlmann, D.; Steinbrenner, H.; Alili, L.; Holtkotter, O.; Sies, H.; Brenneisen, P. Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J. Cell Sci. 2006, 119, 2727–2738. [Google Scholar] [CrossRef]
- Taddei, M.L.; Cavallini, L.; Comito, G.; Giannoni, E.; Folini, M.; Marini, A.; Gandellini, P.; Morandi, A.; Pintus, G.; Raspollini, M.R.; et al. Senescent stroma promotes prostate cancer progression: The role of miR-210. Mol. Oncol. 2014, 8, 1729–1746. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Man, W.Y.; Lv, C.Z.; Song, S.P.; Shi, Y.J.; Elias, P.M.; Man, M.Q. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Ski. Pharmacol. Physiol. 2010, 23, 193–200. [Google Scholar] [CrossRef]
- Wen, Y.; Issa, I.A.; Lei, L.; Fu, C.; Zhou, S.; Zhang, K.; Huang, J.; Chen, J.; Zeng, Q.; Jiang, L. Impacts of Topical Treatments and Phototherapy on Stratum Corneum Hydration, Sebum and Elasticity in Vitiligo Skin. Dermatol. Ther. 2025, 15, 1163–1171. [Google Scholar] [CrossRef]
- Singh, A.; Gotherwal, V.; Junni, P.; Vijayan, V.; Tiwari, M.; Ganju, P.; Kumar, A.; Sharma, P.; Fatima, T.; Gupta, A.; et al. Mapping architectural and transcriptional alterations in non-lesional and lesional epidermis in vitiligo. Sci. Rep. 2017, 7, 9860. [Google Scholar] [CrossRef]
- Jung, S.-E.; Kang, H.Y.; Lee, E.-S.; Kim, Y.C. Changes of epidermal thickness in vitiligo. Am. J. Dermatopathol. 2015, 37, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Bastonini, E.; Kovacs, D.; Briganti, S.; Ottaviani, M.; D’Arino, A.; Migliano, E.; Pacifico, A.; Iacovelli, P.; Picardo, M. Effects of pioglitazone on the differentiation and inflammation in vitiligo keratinocytes. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e573–e575. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Lu, L.; He, H.; Feng, J.; Hu, Z.; Zhang, S.; Yang, L.; Liu, Y.; Wang, T. Post-translational modification in the pathogenesis of vitiligo. Immunol. Res. 2024, 72, 1229–1237. [Google Scholar] [CrossRef]
- Sahoo, A.; Lee, B.; Boniface, K.; Seneschal, J.; Sahoo, S.K.; Seki, T.; Wang, C.; Das, S.; Han, X.; Steppie, M.; et al. MicroRNA-211 Regulates Oxidative Phosphorylation and Energy Metabolism in Human Vitiligo. J. Investig. Dermatol. 2017, 137, 1965–1974. [Google Scholar] [CrossRef]
- Mazar, J.; Qi, F.; Lee, B.; Marchica, J.; Govindarajan, S.; Shelley, J.; Li, J.-L.; Ray, A.; Perera, R.J. MicroRNA 211 Functions as a Metabolic Switch in Human Melanoma Cells. Mol. Cell Biol. 2016, 36, 1090–1108. [Google Scholar] [CrossRef]
- Shoag, J.; Haq, R.; Zhang, M.; Liu, L.; Rowe, G.C.; Jiang, A.; Koulisis, N.; Farrel, C.; Amos, C.I.; Wei, Q.; et al. PGC-1 coactivators regulate MITF and the tanning response. Mol. Cell 2013, 49, 145–157. [Google Scholar] [CrossRef]
- Kim, E.S.; Park, S.J.; Goh, M.; Na, Y.; Jo, D.S.; Jo, Y.K.; Shin, J.H.; Choi, E.S.; Lee, H.; Kim, J.; et al. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigment. Cell Melanoma Res. 2014, 27, 1051–1062. [Google Scholar] [CrossRef]
- Tanwar, J.; Saurav, S.; Basu, R.; Singh, J.B.; Priya, A.; Dutta, M.; Santhanam, U.; Joshi, M.; Madison, S.; Singh, A.; et al. Mitofusin-2 Negatively Regulates Melanogenesis by Modulating Mitochondrial ROS Generation. Cells 2022, 11, 701. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Papaccio, F.; Bellei, B.; Ottaviani, M.; D’Arino, A.; Truglio, M.; Caputo, S.; Cigliana, G.; Sciuto, L.; Migliano, E.; Pacifico, A.; et al. A Possible Modulator of Vitiligo Metabolic Impairment: Rethinking a PPARγ Agonist. Cells 2022, 11, 3583. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.L.; Ottaviani, M.; Albanesi, V.; Vidolin, A.P.; Leone, G.; Ferraro, C.; Cossarizza, A.; Rossi, L.; Picardo, M. Membrane lipid alterations as a possible basis for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2007, 127, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rashighi, M.; Essien, K.I.; Richmond, J.M.; Randall, L.; Pazoki-Toroudi, H.; Hunter, C.A.; Harris, J.E. Simvastatin Prevents and Reverses Depigmentation in a Mouse Model of Vitiligo. J. Investig. Dermatol. 2015, 135, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Gagne, C.; Bergeron, J.; Jobin, J.; Poirier, P. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004, 3, 7. [Google Scholar] [CrossRef]
- Sadagurski, M.; Yakar, S.; Weingarten, G.; Holzenberger, M.; Rhodes, C.J.; Breitkreutz, D.; Leroith, D.; Wertheimer, E. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol. Cell Biol. 2006, 26, 2675–2687. [Google Scholar] [CrossRef]
- Sharma, Y.K.; Bansal, P.; Menon, S.; Prakash, N. Metabolic syndrome in vitiligo patients among a semi-urban Maharashtrian population: A case control study. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11 (Suppl. 1), S77–S80. [Google Scholar] [CrossRef]
- Farag, A.G.A.; Badr, E.A.E.; El-Shafey, A.E.-S.S.; Elshaib, M.E. Fatty acid-binding protein 4 circulating levels in non-segmental vitiligo. An. Bras. Dermatol. 2022, 97, 28–36. [Google Scholar] [CrossRef]
- D’Arino, A.; Picardo, M.; Truglio, M.; Pacifico, A.; Iacovelli, P. Metabolic Comorbidities in Vitiligo: A Brief Review and Report of New Data from a Single-Center Experience. Int. J. Mol. Sci. 2021, 22, 8820. [Google Scholar] [CrossRef]
- Atas, H.; Gonul, M. Increased Risk of Metabolic Syndrome in Patients with Vitiligo. Balk. Med. J. 2017, 34, 219–225. [Google Scholar] [CrossRef]
- Chang, H.-C.; Lin, M.-H.; Huang, Y.-C.; Hou, T.-Y. The association between vitiligo and diabetes mellitus: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 81, 1442–1445. [Google Scholar] [CrossRef]
- Tanacan, E.; Atakan, N. Higher incidence of metabolic syndrome components in vitiligo patients: A prospective cross-sectional study. An. Bras. Dermatol. 2020, 95, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, M.; Li, L. Identifying the Genetic Associations Between Diabetes Mellitus and the Risk of Vitiligo. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qiu, Z.; Li, C.; Wang, Y.; Lin, M.; Liu, Y.; Wen, Y.; Zheng, W. Recurrence and risk factors in cured patients with vitiligo: A real-life single-center retrospective study. J. Cosmet. Dermatol. 2023, 22, 1680–1684. [Google Scholar] [CrossRef]
- Majid, I.; Imran, S. Relapse after methylprednisolone oral minipulse therapy in childhood vitiligo: A 12-month follow-up study. Indian J. Dermatol. 2013, 58, 113–116. [Google Scholar] [CrossRef] [PubMed]


|
Pathogenic
Mechanism |
Experimental
Evidence | Proposed Target |
Clinical-Level
Observational Trials |
Clinical-Level
Interventional Trials | |
|---|---|---|---|---|---|
| Oxidative Stress | Chronic impairment of redox equilibrium and diminished capacity to activate cellular defense mechanisms after injury | In vivo and in vivo ROS accumulation, reduced activity of antioxidant enzymes, low level of small scavenger molecules | Topical and orally supplemented enzymatic and nonenzymatic antioxidant Nrf-2 activation | Stress response in vitiligo (NCT02797574) Investigating IGF-1 against oxidative stress in vitiligo (NCT05812079) | Oral Ginkgo biloba (NCT00907062, NCT01006421 *) Investigating IGF-1 against oxidative stress in vitiligo (NCT05812079) Apremilast (NCT03036995) Oral GLISODIN–SOD (NCT03941808 *) Total Glucosides of Peony (NCT03608917 *) |
| Senescence | Loss of fully functional melanocytes, possible recruitment of immune system | Presence of senescence markers (p16, p21, GADD45) and SASPs (MMPs, IGFBPs, cytokines) | Proposed use of senolytic/antioxidant repurposed compounds | No data at clinical level, preclinical evidence | Topical Ramamycin (NCT05342519 #) |
| Melanocyte Cell Death | High melanocyte turnover, likely release of DAMPs and amplification of immune response | Presence of apoptosis-, necroptosis-, ferroptosis-, pyroptosis-, and autophagy-related cell death markers | Blockage of intrinsic and extrinsic processes implicated in cell death | Evaluation of pyroptosis (NCT06261086) Histochemical study of apoptosis markers (NCT05869942) | Oral Afamelanotide (NCT06109649 *) Subcutaneous Afamelanotide (NCT05210582, NCT04525157 *, NCT01430195 *, NCT01382589 *) |
| Metabolism | Abnormal lipid profile, dysfunctional mitochondria, accumulation of glycated molecules | In vitro and in vivo increased cholesterol, lipid peroxidation, AGEs Systemic dyslipidemia and hyperglycemia | mTOR pathway inhibition, correction of lipid profile, inhibition of HMG-CoA reductase | Metabolic syndrome comorbidities (NCT03622320) Glucose and lipid metabolism (NCT05968235) Association with other autoimmune diseases (NCT04789993) Circulating mitochondria (NCT05525741) | Oral Sinvastatin (NCT01517893), Oral Torvastatin (NCT02432534), Oral Atorvastatin (NCT03247400, NCT02432534) Oral vitamin D (NCT04872257 *, NCT05364567 *) Topical Ramamycin (NCT05342519 #) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrapodi, R.; Marini, A.; Bellei, B. Dysregulated Intracellular Signaling in the Pathogenesis of Vitiligo: An Update on Emerging Therapeutic Strategies. Biomedicines 2025, 13, 2177. https://doi.org/10.3390/biomedicines13092177
Marrapodi R, Marini A, Bellei B. Dysregulated Intracellular Signaling in the Pathogenesis of Vitiligo: An Update on Emerging Therapeutic Strategies. Biomedicines. 2025; 13(9):2177. https://doi.org/10.3390/biomedicines13092177
Chicago/Turabian StyleMarrapodi, Ramona, Alberto Marini, and Barbara Bellei. 2025. "Dysregulated Intracellular Signaling in the Pathogenesis of Vitiligo: An Update on Emerging Therapeutic Strategies" Biomedicines 13, no. 9: 2177. https://doi.org/10.3390/biomedicines13092177
APA StyleMarrapodi, R., Marini, A., & Bellei, B. (2025). Dysregulated Intracellular Signaling in the Pathogenesis of Vitiligo: An Update on Emerging Therapeutic Strategies. Biomedicines, 13(9), 2177. https://doi.org/10.3390/biomedicines13092177





