Vitamin D Status in Patients at the Department of Internal, Autoimmune, and Metabolic Diseases—A Descriptive Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Methods
2.3. Statistical Analysis
2.4. Bioethics Committee
3. Results
4. Discussion
4.1. Vitamin D Deficiency
4.2. Length of Hospital Stay (LOS)
4.3. Vitamin D Deficiency and BMI
4.4. Comorbidities
4.5. Vitamin D Levels and the Month of Hospitalization
4.6. Vitamin D Supplementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LOS | length of hospital stay |
| 25(OH)D | 25-hydroxyvitamin D |
| T2DM | type 2 diabetes |
| AH | arterial hypertension |
References
- Nowak, J.; Jabczyk, M.; Jagielski, P.; Hudzik, B.; Brukało, K.; Borszcz, J.; Zubelewicz-Szkodzińska, B. Could vitamin D concentration be a marker of a long hospital stay in older adults patients? Front. Nutr. 2023, 10, 1277350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karki, A.; Vaidhya, S.; Kunwar, D.; Kunwar, R. Vitamin, D Deficiency among Patients Presenting to Outpatient Department of Medicine of a Tertiary Care Centre: A Descriptive Cross-sectional Study. JNMA J. Nepal Med. Assoc. 2022, 60, 465–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hélard, L.; Mateus-Hamdan, L.; Beauchet, O.; Annweiler, C.; Hypovitaminosis, D. in geriatric acute care unit: A biomarker of longer length of stay. Dis. Markers. 2013, 35, 525–529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boccardi, V.; Lapenna, M.; Gaggi, L.; Garaffa, F.M.; Croce, M.F.; Baroni, M.; Ercolani, S.; Mecocci, P.; Ruggiero, C. Hypovitaminosis D: A Disease Marker in Hospitalized Very Old Persons at Risk of Malnutrition. Nutrients 2019, 11, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blay, B.; Thomas, S.; Coffey, R.; Jones, L.; Murphy, C.V. Low Vitamin D Level on Admission for Burn Injury Is Associated With Increased Length of Stay. J. Burn Care Res. 2017, 38, e8–e13. [Google Scholar] [CrossRef] [PubMed]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maier, G.S.; Maus, U.; Lazovic, D.; Horas, K.; Roth, K.E.; Kurth, A.A. Is there an association between low serum 25-OH-D levels and the length of hospital stay in orthopaedic patients after arthroplasty? J. Orthop. Traumatol. 2016, 17, 297–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Othman, A.; Al-Musharaf, S.; Al-Daghri, N.M.; Krishnaswamy, S.; Yusuf, D.S.; Alkharfy, K.M.; Al-Saleh, Y.; Al-Attas, O.S.; Alokail, M.S.; Moharram, O.; et al. Effect of physical activity and sun exposure on vitamin D status of Saudi children and adolescents. BMC Pediatr. 2012, 12, 92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khattri, J.B.; Godar, S.T.; Subedi, A. A Study of Vitamin D among Patients Presenting in the Psychiatric OPD of Manipal Teaching Hospital, Pokhara. Kathmandu Univ. Med. J. (KUMJ) 2019, 17, 293–297. [Google Scholar] [PubMed]
- Bhatta, M.P.; Pandey, B.R.; Gurung, K.M.; Nakarmi, R.; Gurung, K.; Gurung, L.B.; Rana Magar, S. Prevalence of vitamin D deficiency among adult population of Western Region of Nepal. Int. J. Med. Biomed. Sci. 2016, 1, 7–12. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Łupińska, A.; Michałus, I.; Zygmunt, A.; Stawerska, R.; Lewiński, A. Leczenie niedoboru witaminy D w gabinecie lekarza POZ. Lekarz POZ 2022, 8, 413–421. [Google Scholar]
- Beauchet, O.; Launay, C.; de Decker, L.; Fantino, B.; Kabeshova, A.; Annweiler, C. Who is at risk of long hospital stay among patients admitted to geriatric acute care unit? Results from a prospective cohort study. J. Nutr. Health Aging 2013, 17, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Botros, R.M.; AbdElsalam Besibes, M.M.; Bahaaeldin, A.M.; Abo Elyazed, S. Vitamin D Status in Hospitalized Chronically Ill Patients. J. Am. Coll. Nutr. 2018, 37, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Mc Williams, C.; Golestany, K.; Sharma, R.; Nejati, G.; Cyrus-Murden, A.; Kripichnikov, D. Correlation of admitted nursing home residents’ hospital length of stay and vitamin D levels. J. Community Hosp. Intern. Med. Perspect. 2011, 1, 6313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowak, J.; Hudzik, B.; Jagielski, P.; Kulik-Kupka, K.; Danikiewicz, A.; Zubelewicz-Szkodzińska, B. Lack of Seasonal Variations in Vitamin D Concentrations among Hospitalized Elderly Patients. Int. J. Environ. Res. Public Health 2021, 18, 1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

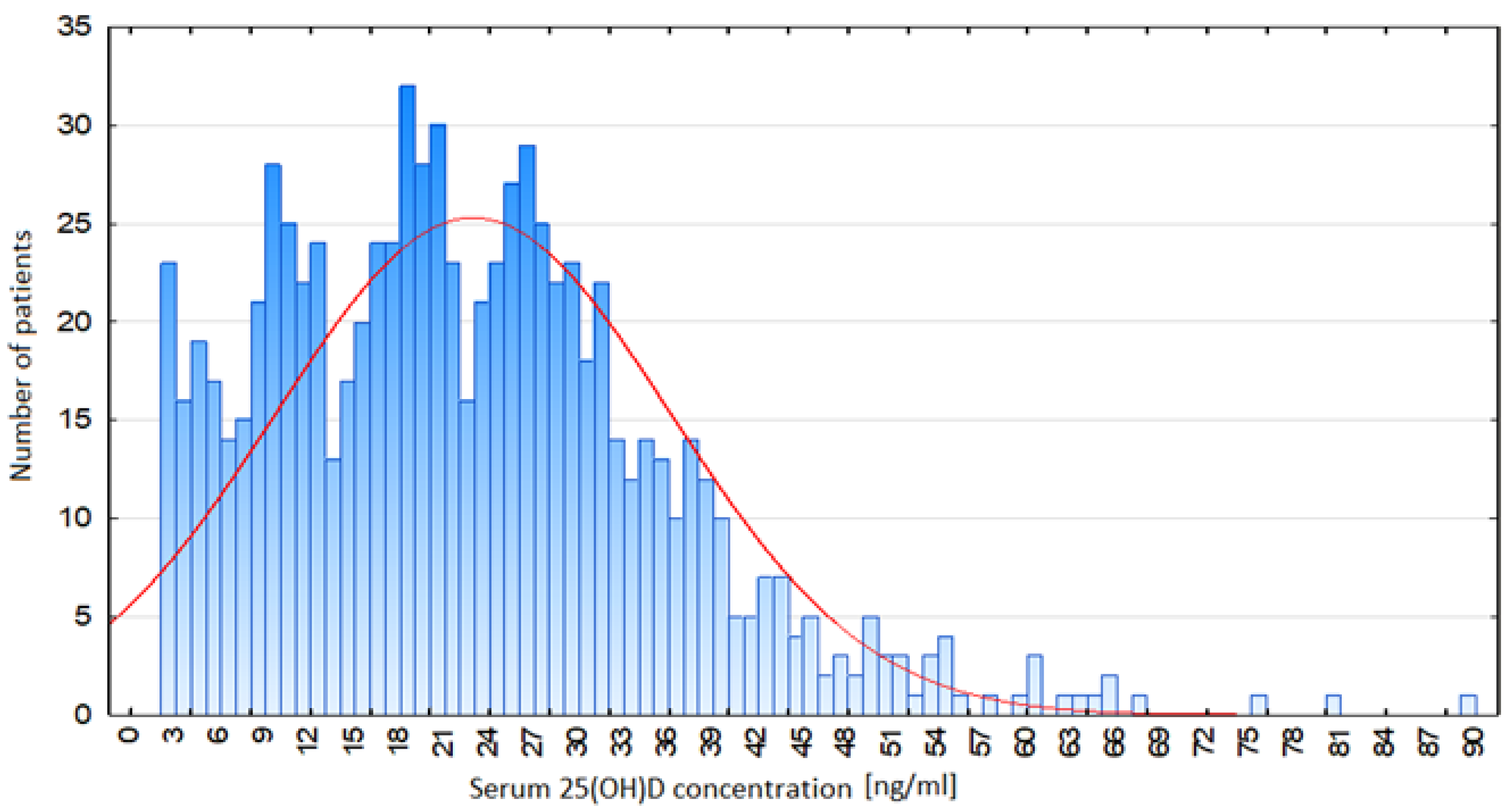

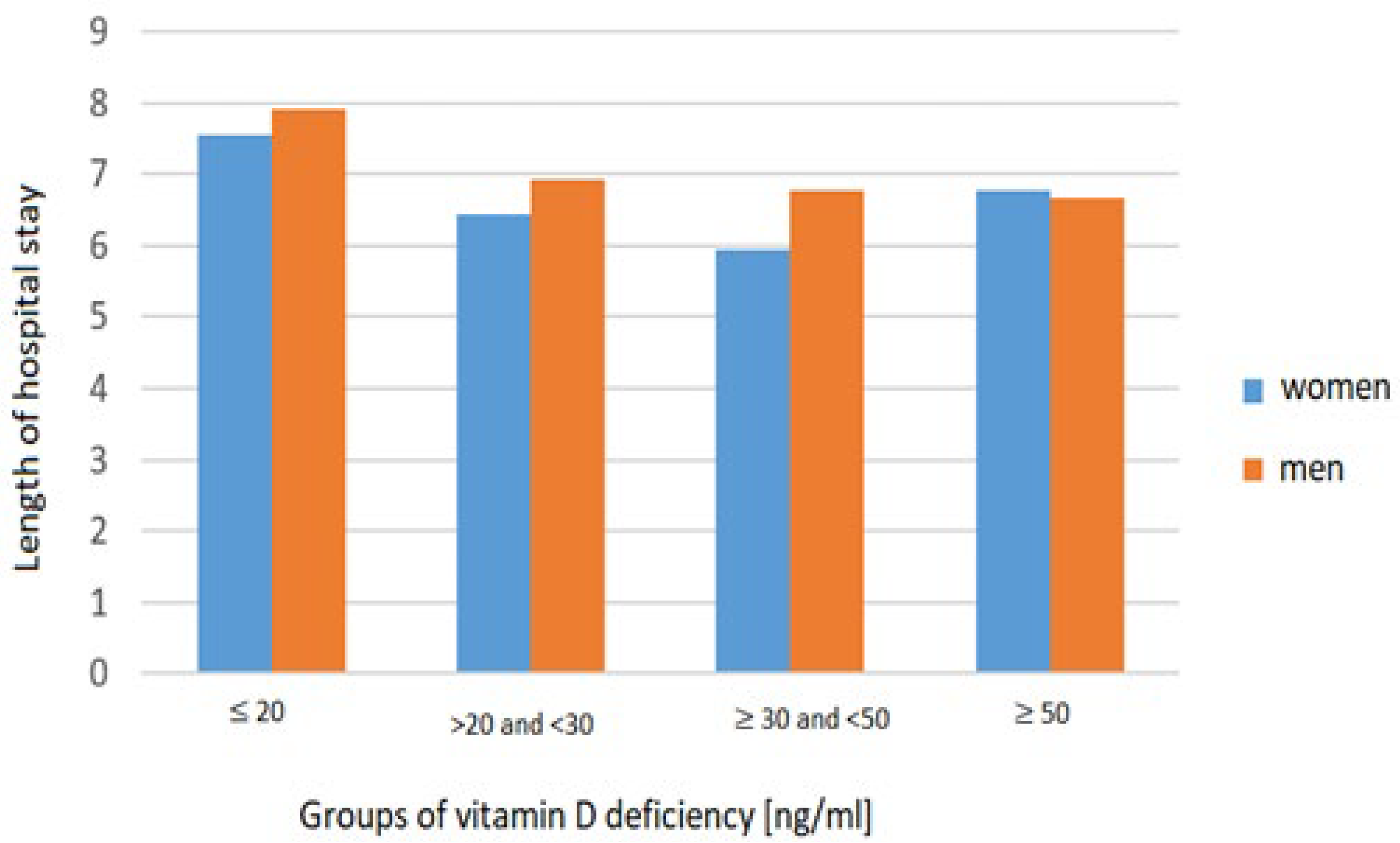
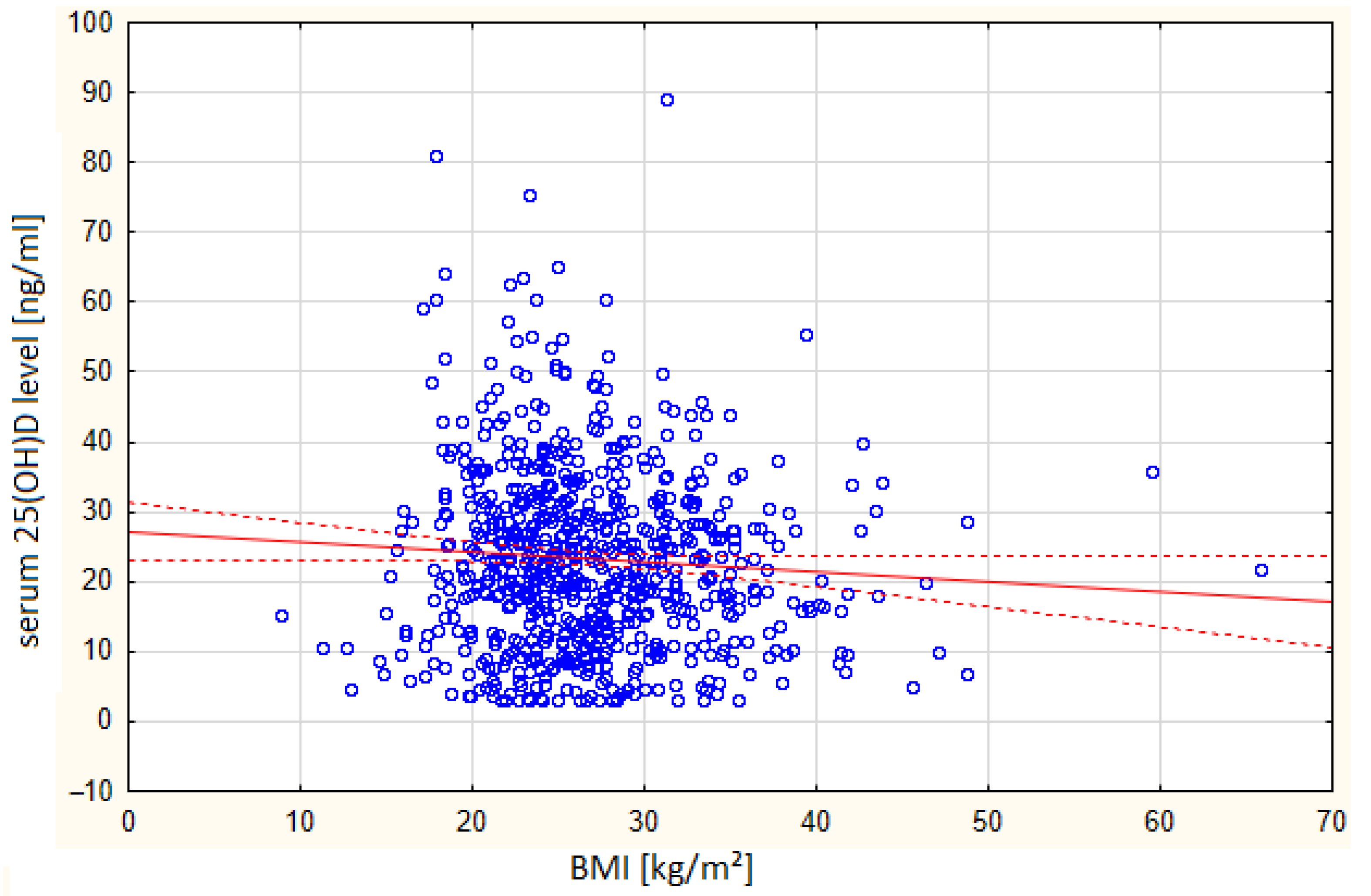

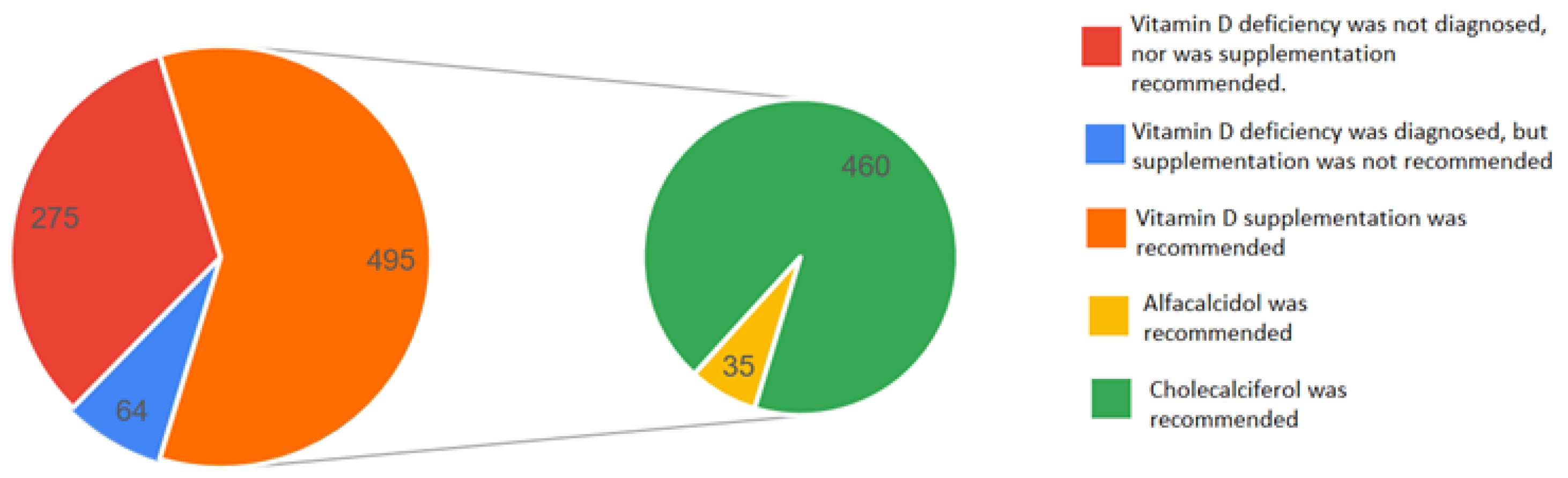
| Group Parameter [Units] | Total | Vitamin D ≤ 20 ng/mL | Vitamin D >20 and <30 ng/mL | Vitamin D ≥ 30 and <50 ng/mL | Vitamin D ≥ 50 ng/mL | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean Value | N | Mean Value | N | Mean Value | N | Mean Value | N | Mean Value | ||
| Age [years] | 834 | 63.03 | 382 | 63.48 | 236 | 62.24 | 186 | 62.84 | 30 | 64.56 | 0.78 |
| LOS [days] | 834 | 7.06 | 382 | 7.74 | 236 | 6.64 | 186 | 6.23 | 30 | 6.73 | <0.01 |
| Body weight [kg] | 733 | 75.74 | 325 | 77.60 | 211 | 75.19 | 173 | 73.87 | 24 | 68.97 | 0.90 |
| Height [cm] | 734 | 167.68 | 325 | 168.63 | 212 | 166.90 | 172 | 166.91 | 25 | 167.44 | 0.30 |
| BMI index [kg/ m2] | 726 | 26.80 | 321 | 27.17 | 210 | 26.95 | 171 | 26.36 | 24 | 23.70 | 0.05 |
| HbA1c [%] | 213 | 7.06 | 116 | 7.36 | 51 | 6.70 | 40 | 6.77 | 6 | 6.06 | 0.12 |
| Albumin [g/dL] | 243 | 3.55 | 122 | 3.21 | 65 | 4.11 | 48 | 3.72 | 8 | 3.22 | <0.01 |
| Total protein [g/dL] | 728 | 6.37 | 333 | 6.07 | 203 | 6.56 | 169 | 6.68 | 23 | 6.80 | <0.01 |
| Ionized calcium [mmol/L] | 236 | 1.34 | 107 | 1.37 | 69 | 1.38 | 52 | 1.26 | 8 | 1.27 | 0.37 |
| Total calcium [mg/dL] | 747 | 9.20 | 343 | 9.05 | 208 | 9.36 | 170 | 9.31 | 26 | 9.36 | <0.01 |
| Alanine aminotransferase (ALT) [IU/L] | 797 | 34.70 | 361 | 38.28 | 231 | 30.28 | 178 | 33.01 | 27 | 35.70 | 0.06 |
| Aspartate aminotransferase (AST) [IU/L] | 796 | 35.82 | 361 | 40.72 | 230 | 31.83 | 178 | 31.32 | 27 | 34.11 | <0.01 |
| Creatinine [mg/dL] | 814 | 1.03 | 373 | 1.04 | 227 | 0.99 | 184 | 1.05 | 30 | 1.03 | <0.01 |
| Natrium (Na+) [mmol/L] | 834 | 138.44 | 382 | 138.01 | 236 | 138.26 | 186 | 138.41 | 30 | 138.56 | 0.54 |
| Potassium (K+) [mmol/L] | 832 | 4.35 | 380 | 4.34 | 236 | 4.38 | 186 | 4.34 | 30 | 4.34 | 0.16 |
| Hemoglobin [g/dL] | 824 | 12.24 | 378 | 11.79 | 233 | 12.50 | 183 | 12.88 | 30 | 12.11 | <0.01 |
| Red blood cells (RBCs) [mln/μL] | 823 | 4.29 | 378 | 4.18 | 233 | 4.57 | 182 | 4.19 | 30 | 4.07 | <0.01 |
| Iron [ug/dL] | 217 | 66.54 | 106 | 66.56 | 61 | 74.71 | 43 | 58.23 | 7 | 46.07 | 0.43 |
| Mean corpuscular volume (MCV) [fl] | 824 | 90.70 | 378 | 91.38 | 233 | 89.90 | 183 | 90.68 | 30 | 88.50 | 0.27 |
| Vitamin B12 [pg/mL] | 196 | 510.88 | 101 | 492.99 | 51 | 494.33 | 41 | 576.58 | 3 | 497.00 | 0.93 |
| Folic acid [ng/mL] | 135 | 10.35 | 69 | 8.08 | 38 | 14.82 | 27 | 10.21 | 1 | 5.89 | <0.01 |
| Total cholesterol [mg/dL] | 805 | 159.87 | 366 | 150.47 | 230 | 166.46 | 179 | 170.83 | 30 | 158.67 | <0.01 |
| Triglycerides [mg/dL] | 791 | 121.81 | 358 | 129.06 | 228 | 119.06 | 176 | 112.27 | 29 | 111.71 | 0.05 |
| HDL cholesterol [mg/dL] | 709 | 48.40 | 319 | 42.79 | 204 | 51.60 | 160 | 55.47 | 26 | 48.47 | <0.01 |
| LDL cholesterol [mg/dL] | 697 | 90.89 | 309 | 84.09 | 202 | 93.04 | 159 | 100.90 | 27 | 93.35 | 0.03 |
| Creatine kinase-MB (CK-MB) [IU/L] | 422 | 129.31 | 198 | 147.88 | 117 | 124.14 | 92 | 106.47 | 15 | 64.40 | 0.02 |
| Group Disease | Total | Vitamin D ≤ 20 ng/mL | Vitamin D > 20 and <30 ng/mL | Vitamin D ≥ 30 and <50 ng/mL | Vitamin D ≥ 50 ng/mL | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | N | F [%] | N | F [%] | N | F [%] | N | F [%] | ||
| T2DM | 255 | 134 | 53 | 68 | 27 | 44 | 17 | 9 | 4 | 0.42 |
| AH | 512 | 234 | 46 | 137 | 27 | 124 | 24 | 17 | 3 | 0.31 |
| Osteoporosis | 42 | 13 | 31 | 16 | 38 | 10 | 24 | 3 | 7 | 0.17 |
| Group Management | Total | Vitamin D ≤ 20 ng/mL | Vitamin D > 20 and <30 ng/mL | Vitamin D ≥ 30 and <50 ng/mL | Vitamin D ≥ 50 ng/mL | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | N | F [%] | N | F [%] | N | F [%] | N | F [%] | |
| Diagnosis of vitamin D deficiency by physicians | 415 | 293 | 71 | 115 | 28 | 6 | 1 | 1 | 0 |
| Taking vitamin D before hospitalization | 118 | 31 | 26 | 37 | 31 | 38 | 32 | 12 | 10 |
| Recommendation of vitamin D at discharge | 495 | 302 | 61 | 140 | 28 | 45 | 9 | 8 | 2 |
| Recommendation of cholecalciferol | 460 | 276 | 60 | 133 | 29 | 43 | 9 | 8 | 2 |
| Recommendation of alfacalcidol | 35 | 26 | 74 | 7 | 20 | 2 | 6 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlus, Z.; Mosiołek, P.; Bierć, K.; Pilśniak, A.; Janoska-Gawrońska, A.; Holecki, M. Vitamin D Status in Patients at the Department of Internal, Autoimmune, and Metabolic Diseases—A Descriptive Cross-Sectional Study. Biomedicines 2025, 13, 2170. https://doi.org/10.3390/biomedicines13092170
Pawlus Z, Mosiołek P, Bierć K, Pilśniak A, Janoska-Gawrońska A, Holecki M. Vitamin D Status in Patients at the Department of Internal, Autoimmune, and Metabolic Diseases—A Descriptive Cross-Sectional Study. Biomedicines. 2025; 13(9):2170. https://doi.org/10.3390/biomedicines13092170
Chicago/Turabian StylePawlus, Zuzanna, Patryk Mosiołek, Karolina Bierć, Aleksandra Pilśniak, Agata Janoska-Gawrońska, and Michał Holecki. 2025. "Vitamin D Status in Patients at the Department of Internal, Autoimmune, and Metabolic Diseases—A Descriptive Cross-Sectional Study" Biomedicines 13, no. 9: 2170. https://doi.org/10.3390/biomedicines13092170
APA StylePawlus, Z., Mosiołek, P., Bierć, K., Pilśniak, A., Janoska-Gawrońska, A., & Holecki, M. (2025). Vitamin D Status in Patients at the Department of Internal, Autoimmune, and Metabolic Diseases—A Descriptive Cross-Sectional Study. Biomedicines, 13(9), 2170. https://doi.org/10.3390/biomedicines13092170





