Characterization of the Anticholinesterase and Antioxidant Properties of Phytochemicals from Moringa oleifera as a Potential Treatment for Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Liquid Chromatography–Mass Spectrometry Analysis
2.3. Assessment of Cholinesterase Inhibitory Activity
2.4. In Silico Molecular Docking
2.5. Antioxidant Assays
2.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical-Scavenging Assay
2.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.3. Lipid Peroxidation Inhibition (LPI) Assay
2.5.4. Hydroxyl Radical-Scavenging Assay
2.5.5. 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) Radical-Scavenging Assay
2.5.6. Nitric Oxide (NO) Radical-Scavenging Assay
2.6. Statistical Analysis
3. Results
3.1. Liquid Chromatography–Mass Spectrometry (LC-MS)
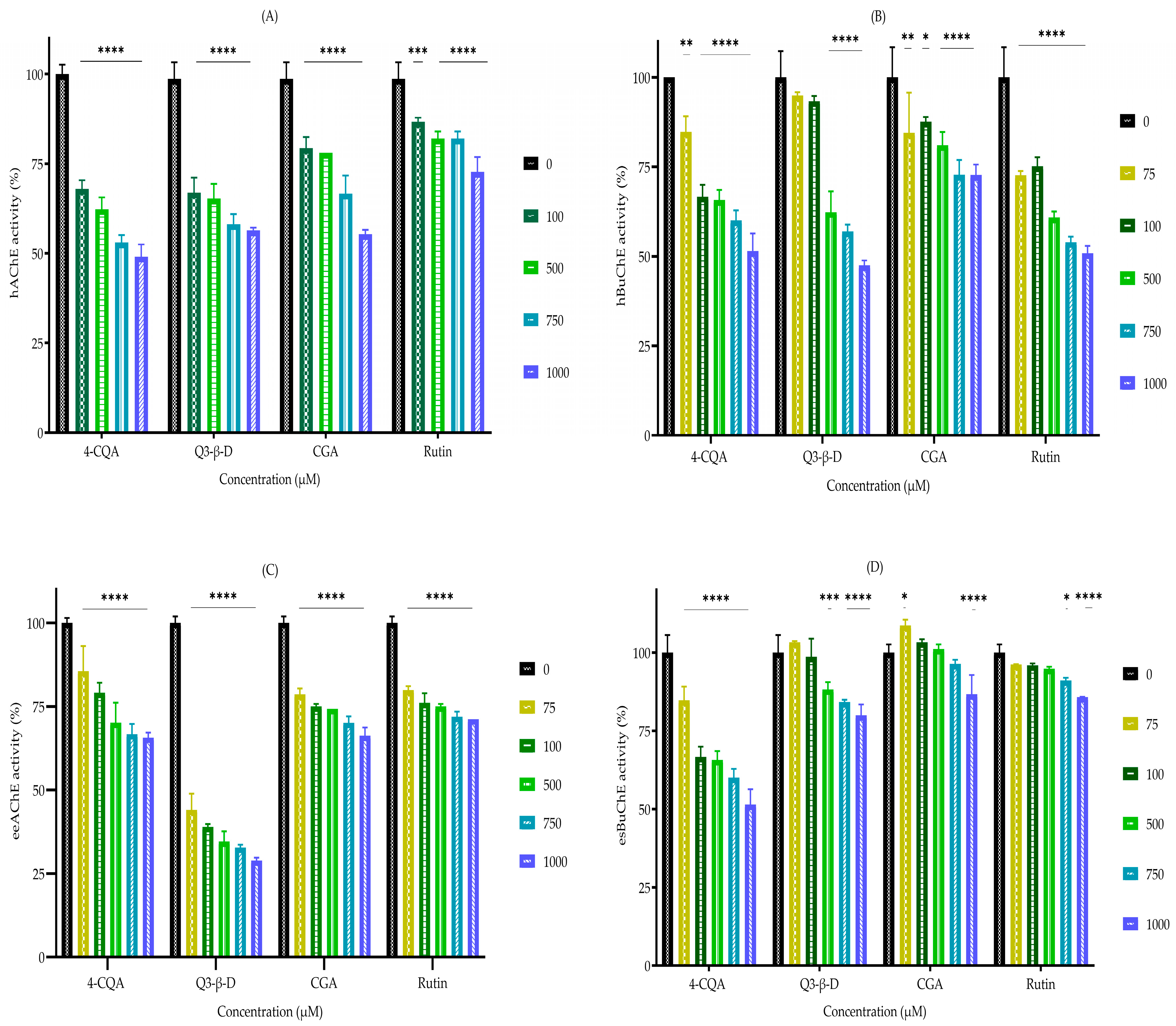
3.2. In Vitro Cholinesterase Inhibition by Phytochemicals
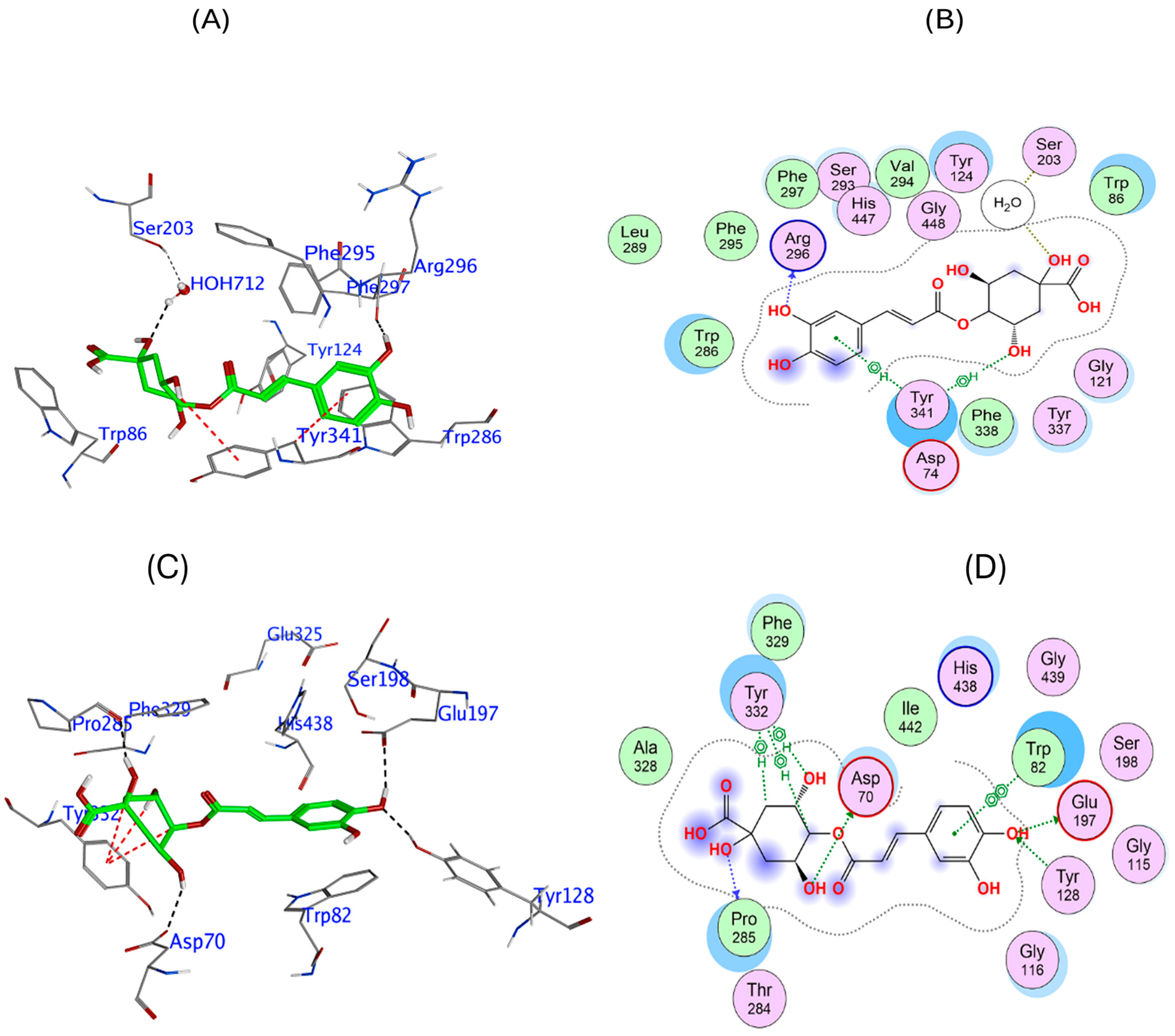
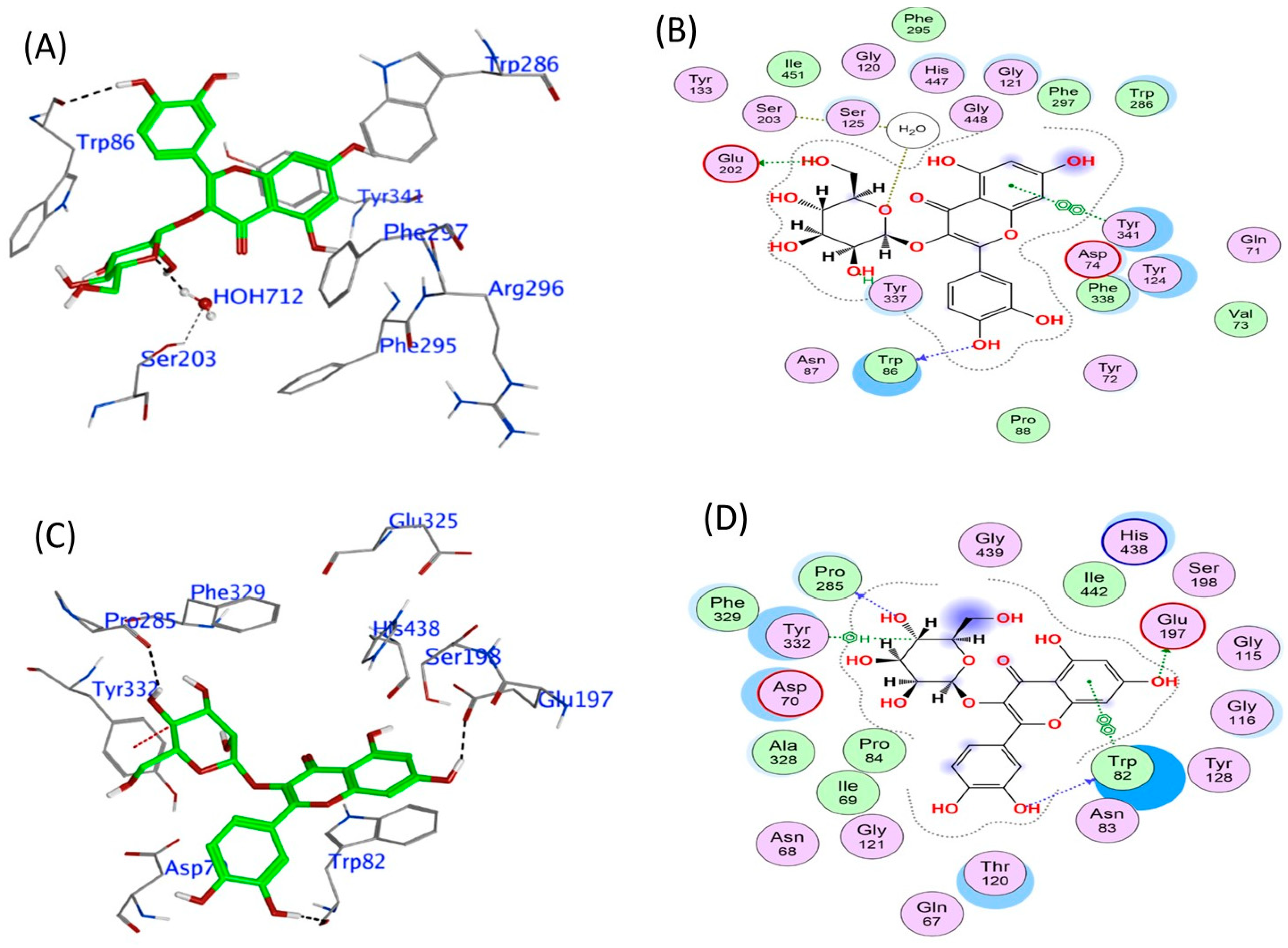
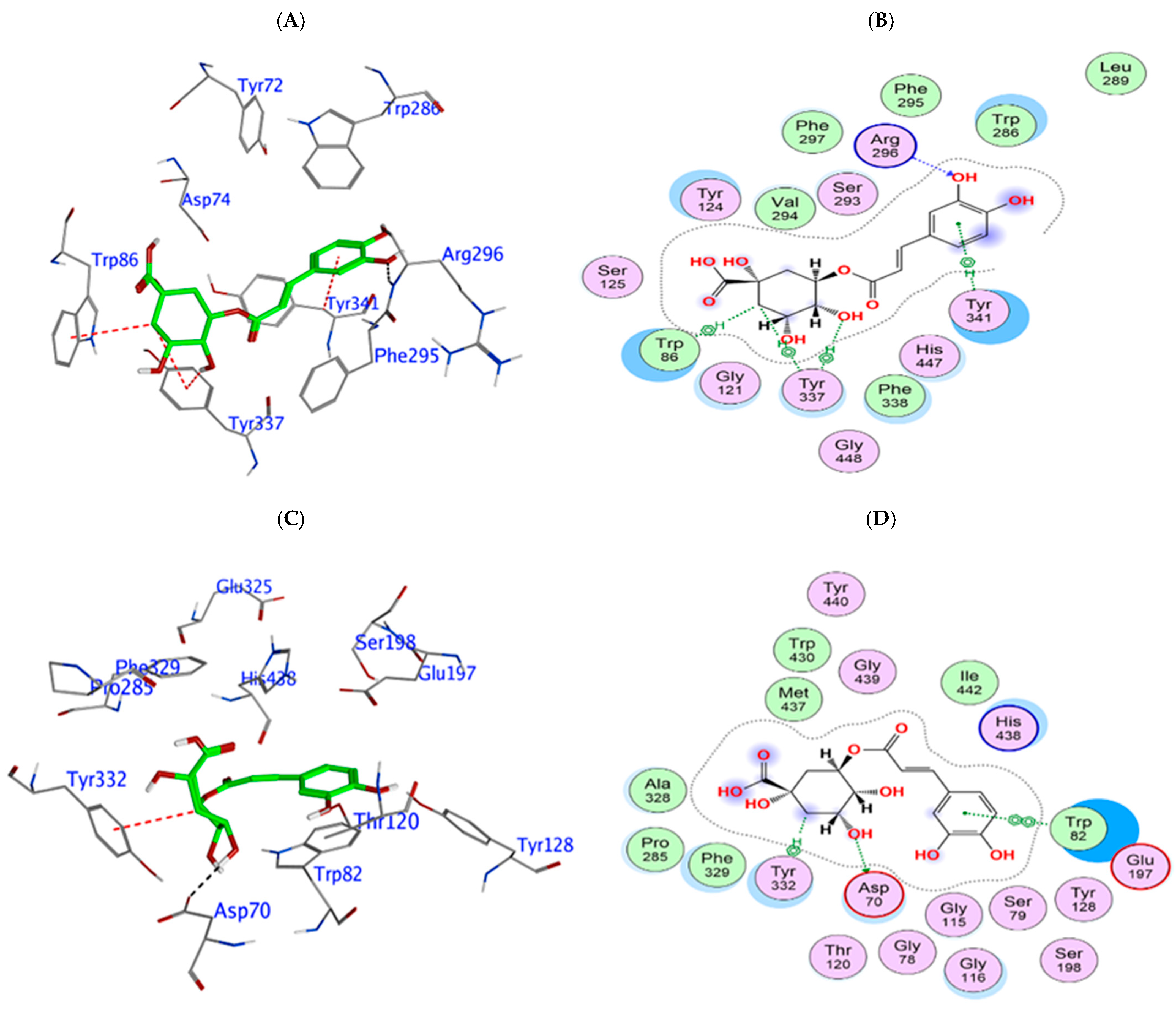
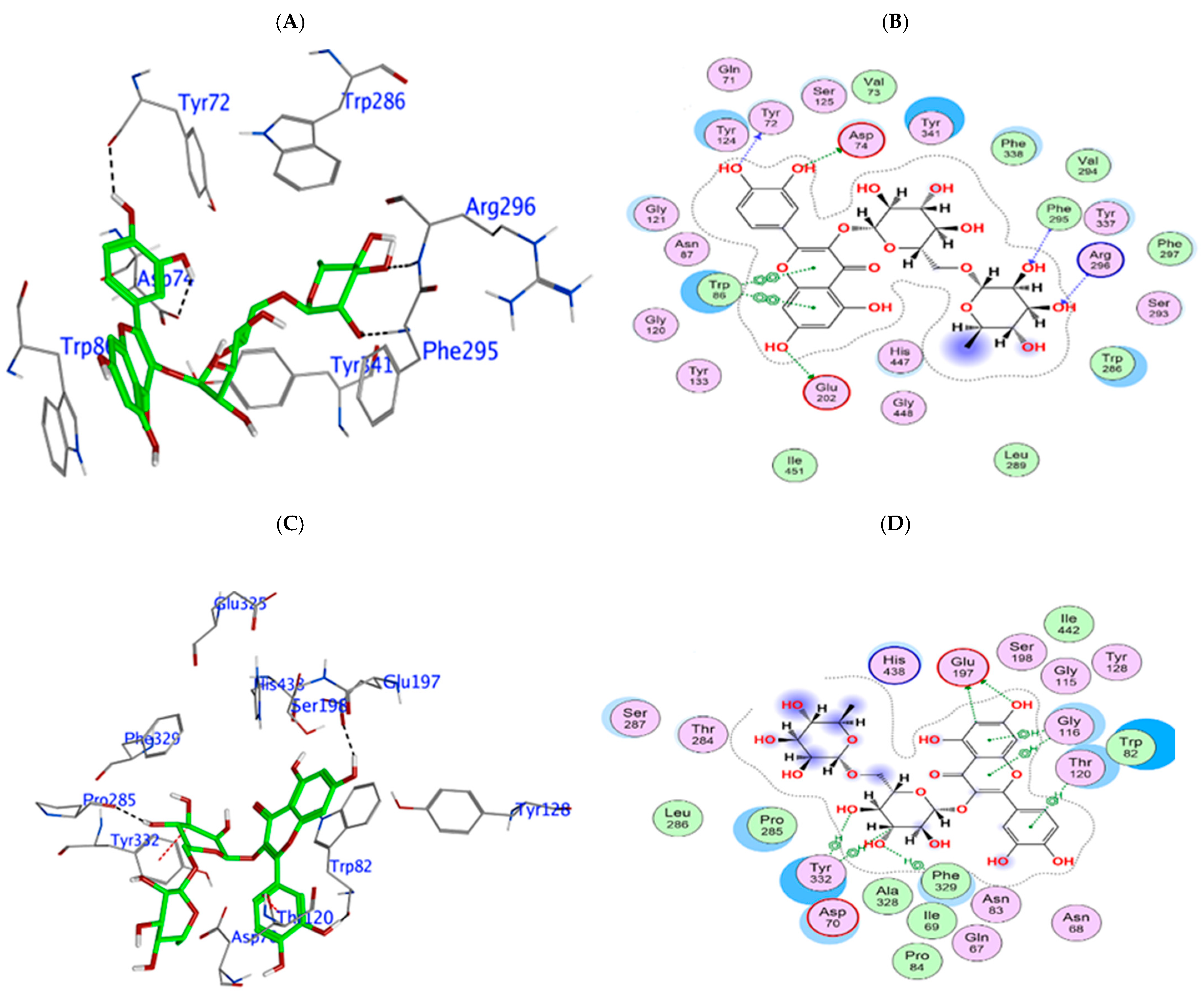
3.3. In Silico Phytochemical Docking to Cholinesterases
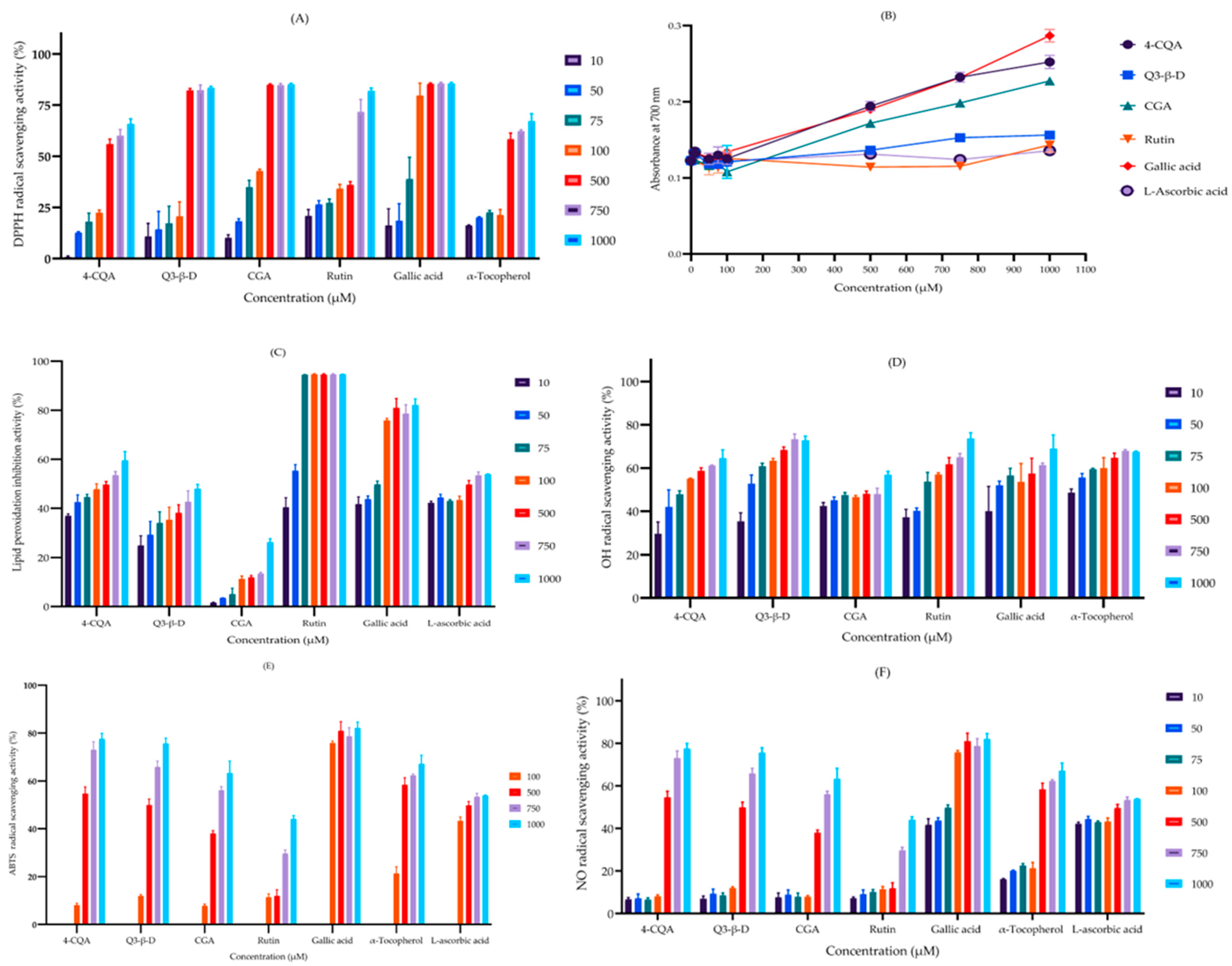
3.4. Radical-Scavenging and Antioxidant Properties of the Phytochemicals
3.4.1. 2,2-Diphenyl-1-picrylhydrazyl Radical-Scavenging Assay
3.4.2. Ferric Reducing Antioxidant Power Assay
3.4.3. Lipid Peroxidation Inhibition Assay
3.4.4. Hydroxyl Radical-Scavenging Assay
3.4.5. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Radical-Scavenging Assay
3.4.6. Nitric Oxide Radical-Scavenging Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-CQA | 4-O-caffeoylquinic acid |
| Q3-β-D | Quercetin 3-β-D-glucoside |
| CGA | Chlorogenic acid |
References
- WHO. 2021. Available online: https://www.who.int/news/item/02-09-2021-world-failing-to-address-dementia-challenge (accessed on 14 June 2025).
- WHO. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 14 June 2025).
- Bature, F.; Guinn, B.A.; Pang, D.; Pappas, Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: A systematic scoping review of literature from 1937 to 2016. BMJ Open 2017, 7, e015746. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Liang, C.S.; Li, D.J.; Yang, F.C.; Tseng, P.T.; Carvalho, A.F.; Stubbs, B.; Thompson, T.; Mueller, C.; Shin, J.I.; Radua, J.; et al. Mortality rates in Alzheimer’s disease and non-Alzheimer’s dementias: A systematic review and meta-analysis. Lancet Healthy Longev. 2021, 2, e479–e488. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Sharma, P.; Kumari, S.; Bhatti, J.S.; HariKrishnaReddy, D. Environmental Toxins and Alzheimer’s Disease: A Comprehensive Analysis of Pathogenic Mechanisms and Therapeutic Modulation. Mol. Neurobiol. 2024, 61, 3657–3677. [Google Scholar] [CrossRef]
- Igarashi, K.M. Entorhinal cortex dysfunction in Alzheimer’s disease. Trends Neurosci. 2023, 46, 124–136. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Kaufman, S.K.; Del Tredici, K.; Thomas, T.L.; Braak, H.; Diamond, M.I. Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol. 2018, 136, 57–67. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative damage in neurodegeneration: Roles in the pathogenesis and progression of Alzheimer disease. Physiol. Rev. 2024, 104, 103–197. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Day, C.M.; Abdella, S.; Garg, S. Alzheimer’s disease current therapies, novel drug delivery systems and future directions for better disease management. J. Control. Release 2024, 367, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Thangwaritorn, S.; Lee, C.; Metchikoff, E.; Razdan, V.; Ghafary, S.; Rivera, D.; Pinto, A.; Pemminati, S. A Review of Recent Advances in the Management of Alzheimer’s Disease. Cureus 2024, 16, e58416. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.; Kandala, N.B.; Chilton, P.J.; Lilford, R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef]
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Liu, R.; Liu, J.; Huang, Q.; Liu, S.; Jiang, Y. Moringa oleifera: A systematic review of its botany, traditional uses, phytochemistry, pharmacology and toxicity. J. Pharm. Pharmacol. 2022, 74, 296–320. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Elmorsy, E.; Aprioku, J.S.; Siminialayi, I.; Carter, W.G. In vitro anti-cholinesterase and antioxidant activity of extracts of Moringa oleifera plants from Rivers State, Niger Delta, Nigeria. Medicines 2018, 5, 71. [Google Scholar] [CrossRef]
- Sutalangka, C.; Wattanathorn, J.; Muchimapura, S.; Thukham-mee, W. Moringa oleifera mitigates memory impairment and neurodegeneration in animal model of age-related dementia. Oxid. Med. Cell Longev. 2013, 2013, 695936. [Google Scholar] [CrossRef]
- Srivastava, G.; Ganjewala, D. An update on the emerging neuroprotective potential of Moringa oleifera and its prospects in complimentary neurotherapy. Phytomed. Plus 2024, 4, 100532. [Google Scholar] [CrossRef]
- Afrin, S.; Hossain, A.; Begum, S. Effects of Moringa oleifera on working memory: An experimental study with memory-impaired Wistar rats tested in radial arm maze. BMC Res. Notes 2022, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Onasanwo, S.A.; Adamaigbo, V.O.; Adebayo, O.G.; Eleazer, S.E. Moringa oleifera-supplemented diet protect against cortico-hippocampal neuronal degeneration in scopolamine-induced spatial memory deficit in mice: Role of oxido-inflammatory and cholinergic neurotransmission pathway. Metab. Brain Dis. 2021, 36, 2445–2460. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Subedi, L.; Acharya, N.; Gaire, B.P. Moringa oleifera: A tree of life as a promising medicinal plant for neurodegenerative diseases. J. Agric. Food Chem. 2021, 69, 14358–14371. [Google Scholar] [CrossRef]
- ALNasser, M.N.; AlSaadi, A.M.; Whitby, A.; Kim, D.-H.; Mellor, I.R.; Carter, W.G. Acai berry (Euterpe sp.) extracts are neuroprotective against l-glutamate-induced toxicity by limiting mitochondrial dysfunction and cellular redox stress. Life 2023, 13, 1019. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Elmorsy, E.; Thornton, J.; Wijamunige, B.; Wijesekara, A.; Tarbox, R.; Warren, A.; Carter, W.G. Anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea. Pharm. Biol. 2017, 55, 1875–1883. [Google Scholar] [CrossRef]
- ALNasser, M.N.; Mellor, I.R.; Carter, W.G. A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease. Molecules 2022, 27, 4891. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant effects of protocatechuic acid and protocatechuic aldehyde: Old wine in a new bottle. Evid.-Based Complement. Altern. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical Properties of Syringic Acid in Civilization Diseases-Review. Nutrients 2023, 16, 10. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. Rsc Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids–ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Boumendjel, A.; Seteyen, A.S.; Boina, C.; Gasque, P.; Guiraud, P.; Sélambarom, J. Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomed. Plus 2022, 2, 100220. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Sampei, M.; Arai, M.A.; Ishibashi, M. Total syntheses of schizandriside, saracoside and (±)-isolariciresinol with antioxidant activities. J. Nat. Med. 2018, 72, 651–654. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.R.; Ju, M.K.; Choi, H.J.; Lee, J.S.; Yoon, J.I.; Majumder, R.; Rather, I.A.; et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Yang, X.; Kang, M.-C.; Lee, K.-W.; Kang, S.-M.; Lee, W.-W.; Jeon, Y.-J. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. coreanum. Algae 2011, 26, 201–208. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Amat-ur-Rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.-N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential nutraceutical properties of leaves from several commonly cultivated plants. Biomolecules 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.L.; Clayton, D.J.; Jones, J.; Carter, W.G.; Thackray, A.E.; King, J.A.; Pucci, A.; Batterham, R.L.; Stensel, D.J. A randomized crossover trial assessing the effects of acute exercise on appetite, circulating ghrelin concentrations, and butyrylcholinesterase activity in normal-weight males with variants of the obesity-linked FTO rs9939609 polymorphism. Am. J. Clin. Nutr. 2019, 110, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran. J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforsch. C J. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Jung, M.; Park, M. Acetylcholinesterase Inhibition by Flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Marya Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Işık, M.; Beydemir, Ş. The impact of some phenolic compounds on serum Acetylcholinesterase: Kinetic analysis of an enzyme/inhibitor interaction and molecular docking study. J. Biomol. Struct. Dyn. 2020, 39, 6515–6523. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Chen, R.; Hassan, H.; Rawlinson, C.; Morgan, D. Pharmacological properties of rutin and its potential uses for Alzheimer’s disease. J. Exp. Stroke Transl. Med. 2020, 13, 1–12. [Google Scholar]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complem. Altern. Med. 2016, 21, NP11-7. [Google Scholar] [CrossRef]
- Amat-ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Ahmed, A.; Carter, W.G. In Silico Design of Dual-Binding Site Anti-Cholinesterase Phytochemical Heterodimers as Treatment Options for Alzheimer’s Disease. Curr. Issues Mol. Biol. 2022, 44, 152–175. [Google Scholar] [CrossRef]
- Akhtar Muhammad, A.M.; Tel-Cayan, G.; Öztürk, M.; Nadeem, S.; Duru, M.E.; Itrat Anis, I.A.; Ng, S.W.; Shah, M.R. Biologically active flavonoids from Dodonaea viscosa and their structure–activity relationships. Ind. Crops Prod. 2015, 78, 66–72. [Google Scholar] [CrossRef]
- Amat-ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Carter, W.G. Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease. Brain Sci. 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Mahaman, Y.A.R.; Feng, J.; Huang, F.; Salissou, M.T.M.; Wang, J.; Liu, R.; Zhang, B.; Li, H.; Zhu, F.; Wang, X. Moringa oleifera Alleviates Aβ Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice. Nutrients 2022, 14, 4284. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Saitou, K.; Suzukamo, C.; Osaki, N.; Asada, T. Effect of Chlorogenic Acids on Cognitive Function in Mild Cognitive Impairment: A Randomized Controlled Crossover Trial. J. Alzheimers Dis. 2019, 72, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef]
- Azlan, U.K.; Mediani, A.; Rohani, E.R.; Tong, X.; Han, R.; Misnan, N.M.; Jam, F.A.; Bunawan, H.; Sarian, M.N.; Hamezah, H.S. A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera. Molecules 2022, 27, 5765. [Google Scholar] [CrossRef]

| Name of Compound | Formula | Exact Mass | Moringa oleifera Plant Extracts | Antioxidant Activity | Reference(s) | |
|---|---|---|---|---|---|---|
| PBS | Ethanol | |||||
| Phenolic compounds and phenolic acids | ||||||
| Protocatechuic acid | C7H6O4 | 154.0268 | Detected | Detected | ✓ | [31] |
| Syringic acid | C9H10O5 | 198.0530 | Detected | Detected | ✓ | [32] |
| Vanillic acid | C8H8O4 | 168.0425 | Detected | Detected | ✓ | [33] |
| Gallic acid | C7H6O5 | 170.0217 | Detected | Not Detected | ✓ | [34,35,36] |
| 4-Hydroxybenzoic acid | C7H6O3 | 138.0319 | Detected | Detected | ✓ | [36,37] |
| Benzoic acid | C7H6O2 | 122.0371 | Detected | Not Detected | ||
| 2,5-Dihydroxybenzoic acid | C7H6O4 | 154.0268 | Detected | Detected | ✓ | [36,37] |
| Chlorogenic acid | C16H18O9 | 354.0951 | Detected | Detected | ✓ | [38] |
| Methyl 4-caffeoylquinic acid | C17H20O9 | 368.1108 | Detected | Detected | - | |
| Flavonoids | ||||||
| Dihydrokaempferol | C15H12O6 | 288.0634 | Detected | Detected | - | |
| Luteolin | C15H10O6 | 286.0478 | Not Detected | Detected | ✓ | [39] |
| Quercetin | C15H10O7 | 302.0426 | Not Detected | Detected | ✓ | [40,41,42] |
| Taxifolin deoxyhexose or taxifolin | C15H12O7 | 304.0584 | Detected | Detected | ✓ | [43] |
| Quercetin-3-O- rutinoside (rutin) | C27H30O16 | 610.1519 | Not Detected | Detected | ✓ | [44,45] |
| Quercetin 3-O-glucoside (isoquercitrin) | C21H20O12 | 464.0950 | Not Detected | Detected | - | |
| Kaempferol rhamnoside | C21H20O10 | 432.1048 | Detected | Not Detected | - | |
| Isoorientin | C21H20O11 | 448.0996 | Not Detected | Detected | - | |
| Lignans | ||||||
| (+)-Isolariciresinol | C20H24O6 | 360.1575 | Detected | Detected | ✓ | [46] |
| (+)-lariciresinol | C20H24O6 | 360.1575 | Detected | Detected | ✓ | [47] |
| Dihydroconiferyl alcohol | C10H14O3 | 182.0945 | Detected | Detected | - | |
| Proanthocyanidins | ||||||
| (+)-Catechin | C15H14O6 | 290.0785 | Not Detected | Detected | ✓ | [39,48] |
| Monoterpenoids | ||||||
| (+)-Menthiafolic acid | C10H16O3 | 184.1100 | Detected | Not Detected | - | |
| (E,Z)−2,6-dimethyl−2,6-octadiene−1,8-diol | C10H18O2 | 170.1309 | Not Detected | Detected | - | |
| Norisoprenoids | ||||||
| (−)-Loliolide | C11H16O3 | 196.1099 | Not Detected | Detected | ✓ | [49] |
| Major fatty acids | ||||||
| Monounsaturated fatty acids | ||||||
| Oleic acid | C18H34O2 | 282.2558 | Detected | Detected | - | |
| Palmitoleic acid | C16H30O2 | 254.2247 | Not Detected | Detected | - | |
| Polyunsaturated fatty acids | ||||||
| Linoleic acid | C18H32O2 | 280.2401 | Not Detected | Detected | - | |
| Linolenic acid | C18H30O2 | 278.2245 | Not Detected | Detected | - | |
| Saturated fatty acids | ||||||
| Palmitic acid | C16H32O2 | 256.2403 | Not Detected | Detected | - | |
| Stearic acid | C18H36O2 | 284.2714 | Not Detected | Detected | - | |
| Amino acids | ||||||
| Alanine | C3H7NO2 | 89.0477 | Detected | Detected | - | |
| Lysine | C6H14N2O2 | 146.1055 | Detected | Not Detected | - | |
| Arginine | C6H14N4O2 | 174.1116 | Not Detected | Detected | - | |
| Methionine | C5H11NO2S | 149.0511 | Detected | Not Detected | - | |
| Phenylalanine | C9H11NO2 | 165.0790 | Detected | Detected | - | |
| Proline | C5H9NO2 | 115.0632 | Detected | Detected | - | |
| Glutamic acid | C5H9NO4 | 147.0532 | Detected | Detected | - | |
| Serine | C3H7NO3 | 105.0426 | Detected | Detected | - | |
| Glycine | C2H5NO2 | 75.0320 | Detected | Detected | - | |
| Threonine | C4H9NO3 | 119.0582 | Detected | Detected | - | |
| Histidine | C6H9N3O2 | 155.0694 | Detected | Detected | - | |
| Tryptophan | C11H12N2O2 | 204.0901 | Detected | Detected | - | |
| Tyrosine | C9H11NO3 | 181.0741 | Detected | Detected | - | |
| Isoleucine | C6H13NO2 | 131.0946 | Detected | Detected | - | |
| Valine | C5H11NO2 | 117.0788 | Detected | Detected | - | |
| Leucine | C6H13NO2 | 131.0948 | Not Detected | Detected | - | |
| Other compounds | ||||||
| Cellotetraose | C24H42O21 | 666.2225 | Not Detected | Detected | - | |
| Sucrose | C12H22O11 | 342.1155 | Detected | Detected | - | |
| Quinic acid isomer 1 | C7H12O6 | 192.0635 | Detected | Detected | - | |
| Vitamin C | C6H8O6 | 176.0322 | Detected | Detected | ✓ | [50] |
| Agent | Enzyme IC50 (µM) | |||
|---|---|---|---|---|
| hAChE | hBuChE | eeAChE | esBuChE | |
| 4-O-caffeoylquinic acid | 782 ± 106 | 770 ± 116 | 688 ± 58 | 903 ± 139 |
| Quercetin 3-β-D-glucoside | 971 ± 157 | 931 ± 49 | 868 ± 74 | 4029 ± 388 |
| Chlorogenic acid | 1362 ± 151 | 2049 ± 234 | 1034 ± 137 | 12,389 ± 4512 |
| Rutin | 2677 ± 296 | 761.4 ± 106 | 759 ± 104 | 6734 ± 523 |
| Rivastigmine | 8.7 ± 13 | 3.6 ± 0.3 | 6.7 ± 1.6 | 3 × 10−4 ± 1 × 10−4 |
| Donepezil | 1.6 ± 0.5 | 561 ± 132 | 3.5 ± 1.1 | 13.8 ± 0.8 |
| Galantamine | 8.7 ± 2.2 | 334 ± 23 | 6.5 ± 0.7 | 0.1 ± 0.05 |
| Eserine | 2 ± 0.4 | - | 0.1 ± 0.02 | - |
| Ethopropazine | - | 13.8 ± 0.8 | - | 1 × 10−2 ± 3 × 10−4 |
| Phytochemical | hAChE Binding Energy (kcal/mol) | Key Interactions | hBuChE Binding Energy (kcal/mol) | Key Interactions |
|---|---|---|---|---|
| 4-CQA | −9.12 | Arg296 (H bond), Tyr341 (π interaction), HOH 712 (H bond) | −7.97 | Glu197, Asp70 (H bond), Pro285, Tyr128 (H bond), Tyr332 (π interaction) |
| Q3-β-D | −9.96 | Glu202 (H bond), Trp86 (π interaction), Tyr337 (π-π stacking), water (H bond) | −10.27 | Pro285, Trp82, Glu197 (H bond), Tyr332 (π interaction) |
| CGA | −9.69 | Arg296 (H bond), Trp86, Tyr337 (H-π interaction) | −9.69 | Glu197, Trp82 (H bond), Tyr332 (π interaction) |
| Rutin | −14.81 | Glu202, Asp74 (H bond), Tyr72, Arg296 (H bond), Trp86 (π-π stacking) | −12.03 | Glu197, Pro285, Trp82, Asn68, Asp70 (H bond), Tyr332 (π stacking) |
| Agent | EC50 (µM) | |||||
|---|---|---|---|---|---|---|
| DPPH• | FRAP | LPI | •OH | ATBS•+ | •NO | |
| 4-CQA | 431 ± 28 | 1287 ± 45 | 207 ± 63 | 120 ± 30 | 382 ± 44 | 125 ± 30 |
| Q3-β-D | 224 ± 27 | 1785 ± 107 | 673 ± 165 | 55 ± 12 | 436 ± 32 | 261 ± 77 |
| CGA | 141 ± 8 | 1450 ± 54 | 3332 ± 319 | 231 ± 80 | 673 ± 44 | 383 ± 99 |
| Rutin | 305 ± 55 | 1941 ± 131 | 26 ± 3 | 90 ± 21 | 1717 ± 206 | 383 ± 87 |
| Gallic acid | 86 ± 15 | 1243 ± 49 | 372 ± 33 | 80 ± 23 | 53 ± 15 | 972 ± 107 |
| α-Tocopherol | 369 ± 34 | 1770 ± 100 | - | 53 ± 15 | 414 ± 22 | 368 ± 34 |
| L-Ascorbic acid | - | 182 ± 24 | - | 526 ± 110 | 720 ± 127 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljadaan, A.M.; AlSaadi, A.M.; Shaikh, I.A.; Whitby, A.; Ray, A.; Kim, D.-H.; Carter, W.G. Characterization of the Anticholinesterase and Antioxidant Properties of Phytochemicals from Moringa oleifera as a Potential Treatment for Alzheimer’s Disease. Biomedicines 2025, 13, 2148. https://doi.org/10.3390/biomedicines13092148
Aljadaan AM, AlSaadi AM, Shaikh IA, Whitby A, Ray A, Kim D-H, Carter WG. Characterization of the Anticholinesterase and Antioxidant Properties of Phytochemicals from Moringa oleifera as a Potential Treatment for Alzheimer’s Disease. Biomedicines. 2025; 13(9):2148. https://doi.org/10.3390/biomedicines13092148
Chicago/Turabian StyleAljadaan, Adel M., Ayman M. AlSaadi, Ibrahim A. Shaikh, Alison Whitby, Arundhati Ray, Dong-Hyun Kim, and Wayne G. Carter. 2025. "Characterization of the Anticholinesterase and Antioxidant Properties of Phytochemicals from Moringa oleifera as a Potential Treatment for Alzheimer’s Disease" Biomedicines 13, no. 9: 2148. https://doi.org/10.3390/biomedicines13092148
APA StyleAljadaan, A. M., AlSaadi, A. M., Shaikh, I. A., Whitby, A., Ray, A., Kim, D.-H., & Carter, W. G. (2025). Characterization of the Anticholinesterase and Antioxidant Properties of Phytochemicals from Moringa oleifera as a Potential Treatment for Alzheimer’s Disease. Biomedicines, 13(9), 2148. https://doi.org/10.3390/biomedicines13092148








