CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning
Abstract
1. Introduction
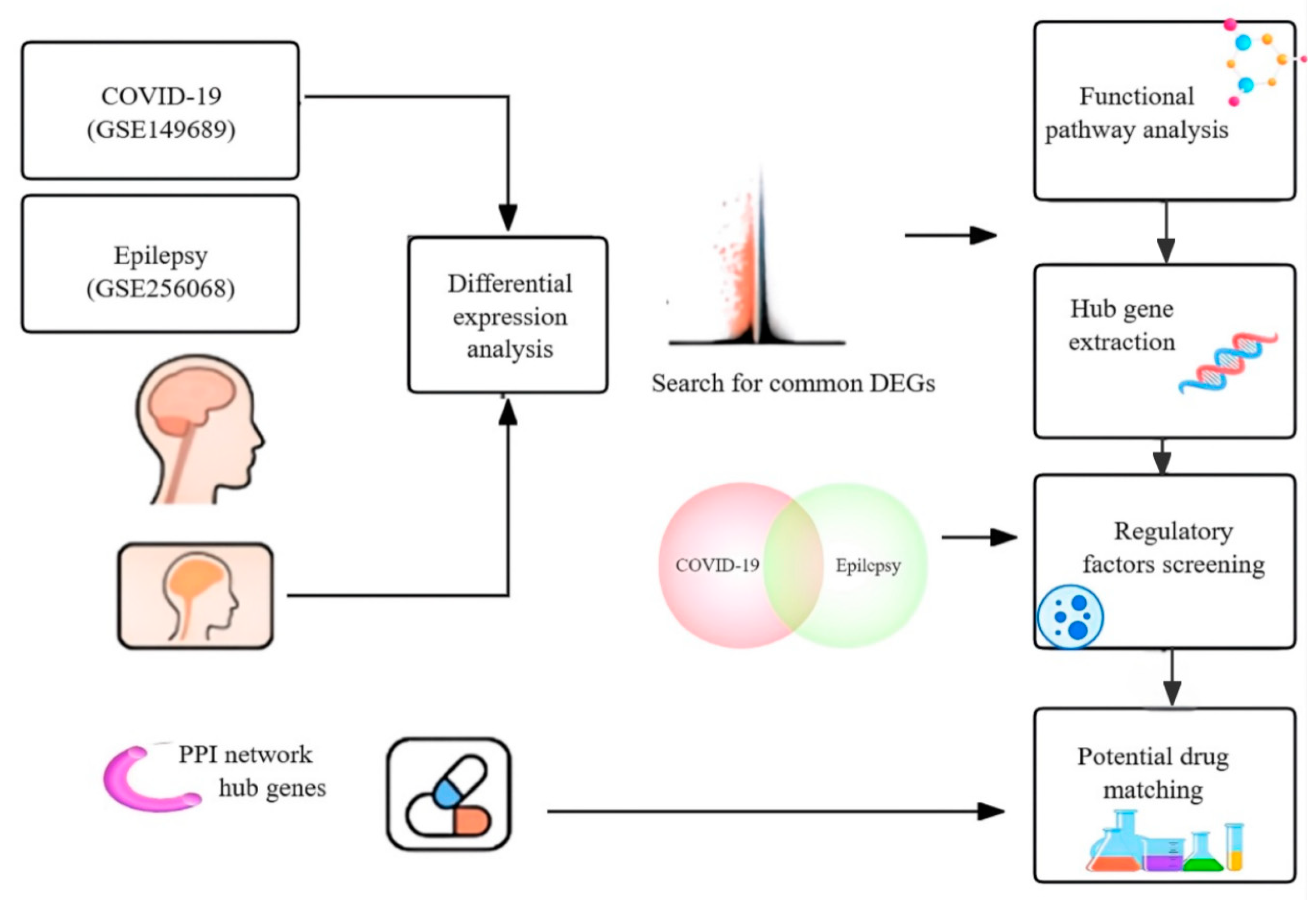
2. Materials and Methods
2.1. Patient Inclusion Criteria and Sample Characteristics
2.2. Transcriptomic Datasets and DEG Analysis
2.3. Functional Enrichment Analysis (GO and KEGG)
2.4. Protein–Protein Interaction (PPI) Network and Hub Gene Identification
2.5. Pathway-Level Immune Convergence
2.6. Regulatory Network Construction: TF and TF-miRNA
2.7. Drug Repositioning Analysis and Target Validation
2.8. Literature-Based Validation of Gabapentin Targets
3. Results
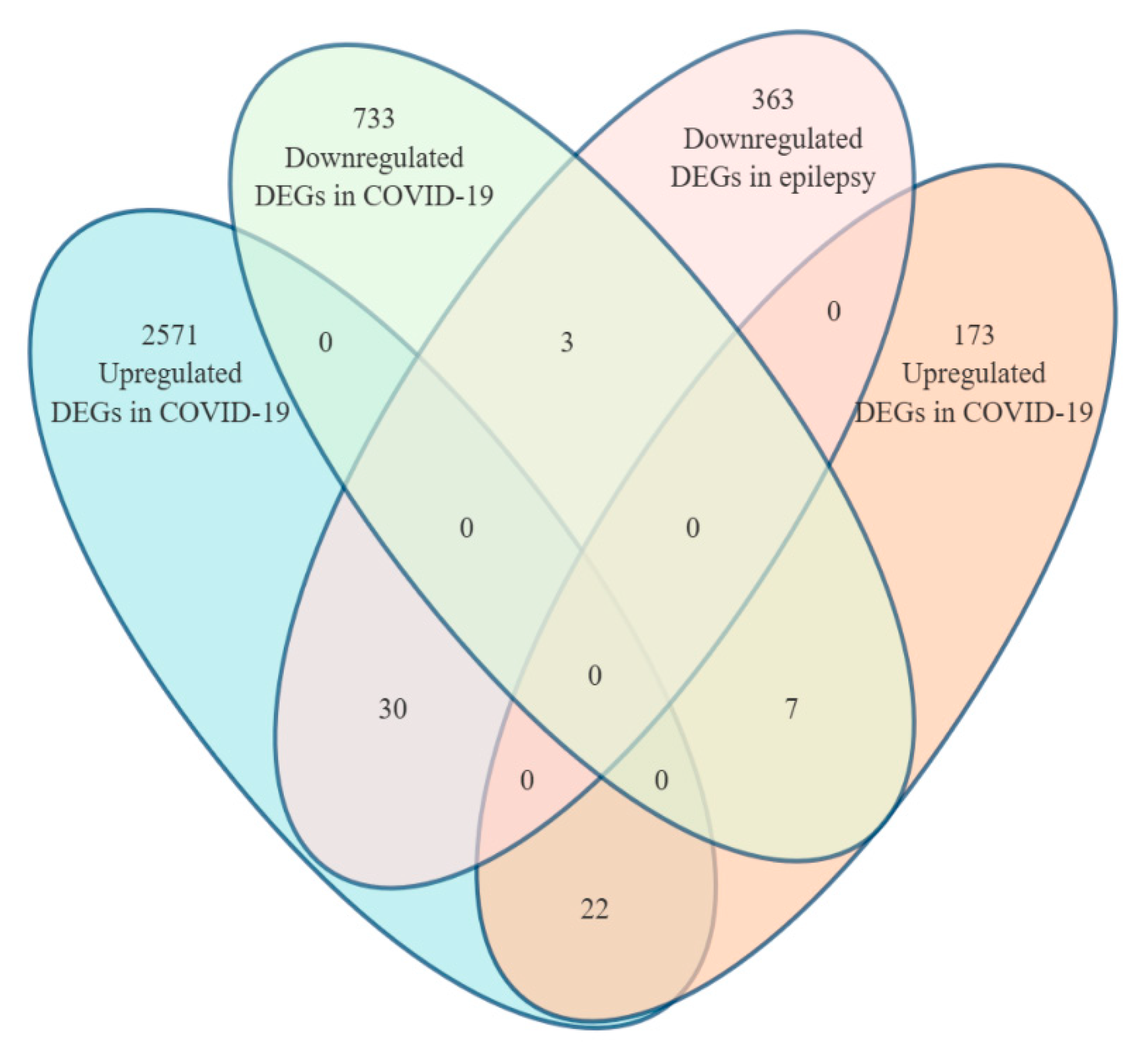
3.1. Identification of Shared Differentially Expressed Genes Between COVID-19 and Epilepsy
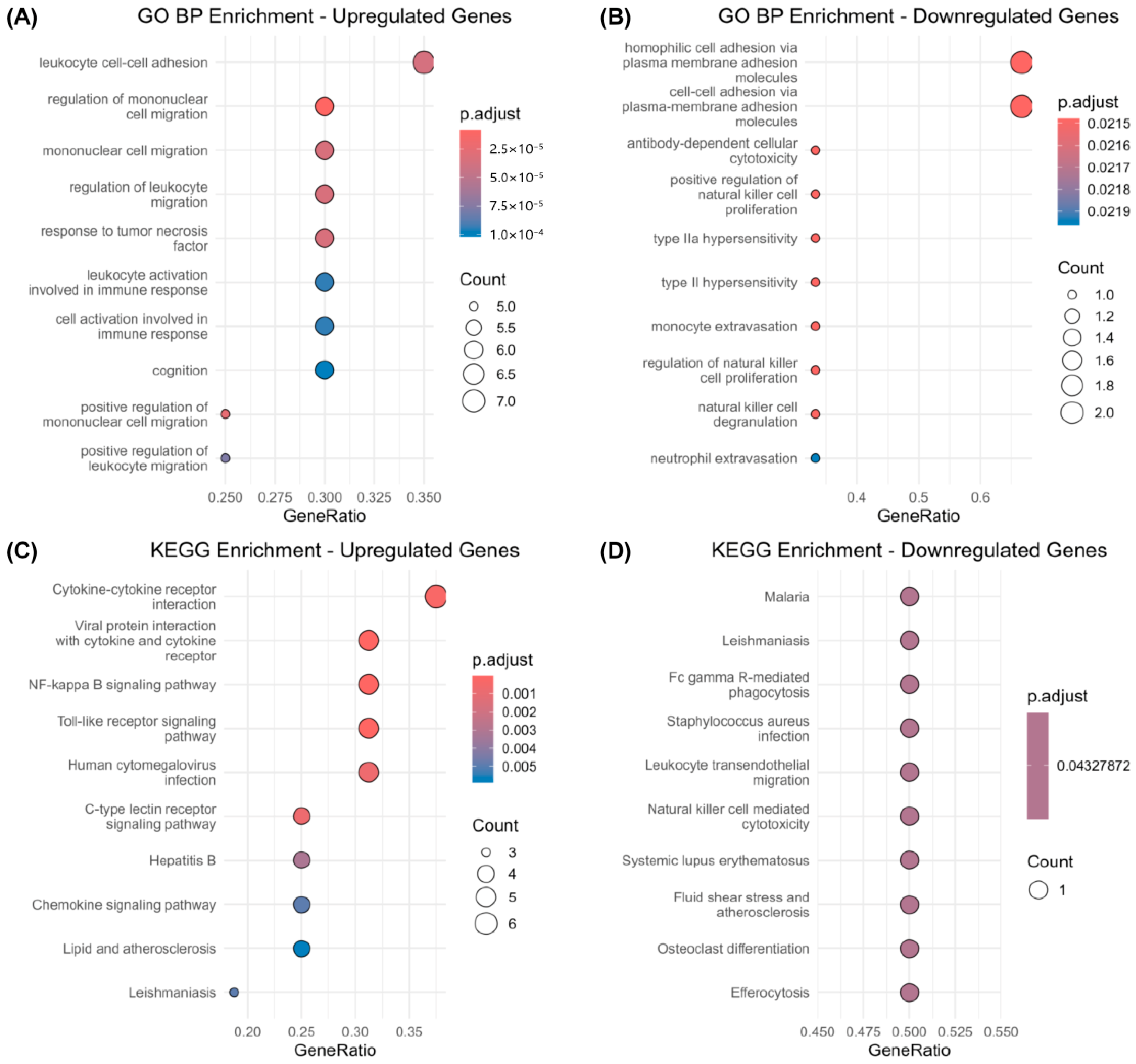
3.2. Functional Enrichment Analysis of Common DEGs Between COVID-19 and Epilepsy
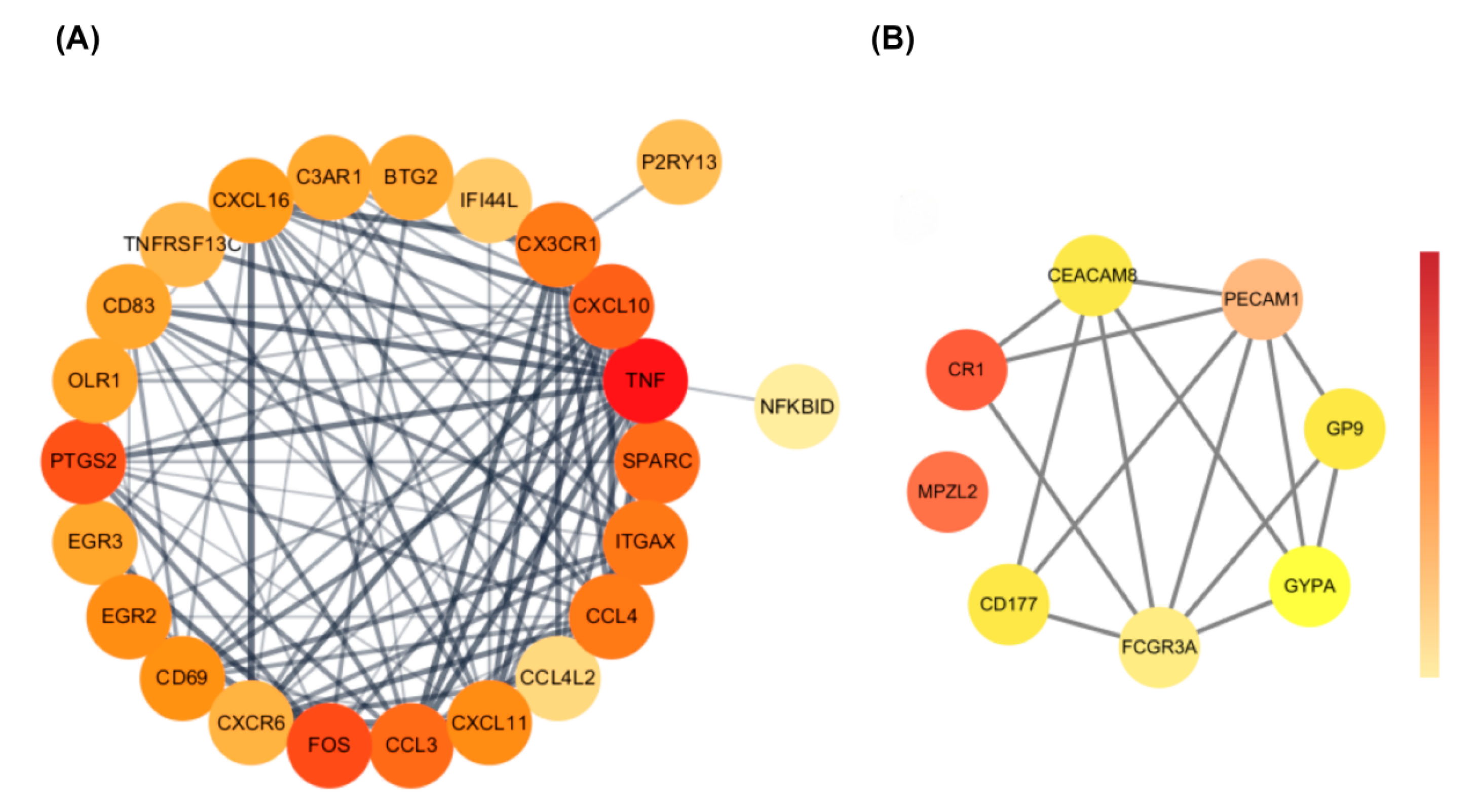
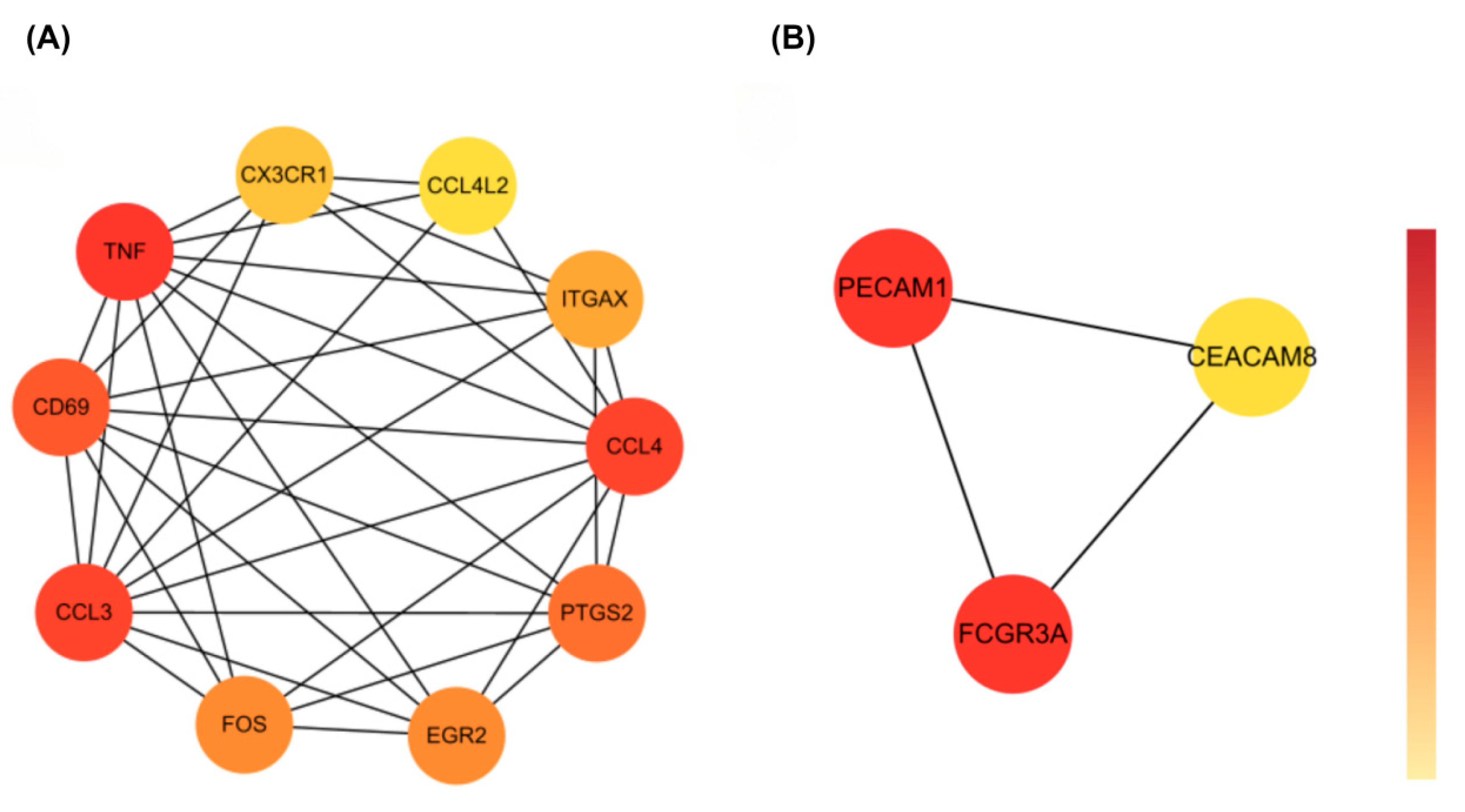
3.3. Protein–Protein Interaction (PPI) Network Analysis of Common DEGs
3.4. Identification of Hub Genes
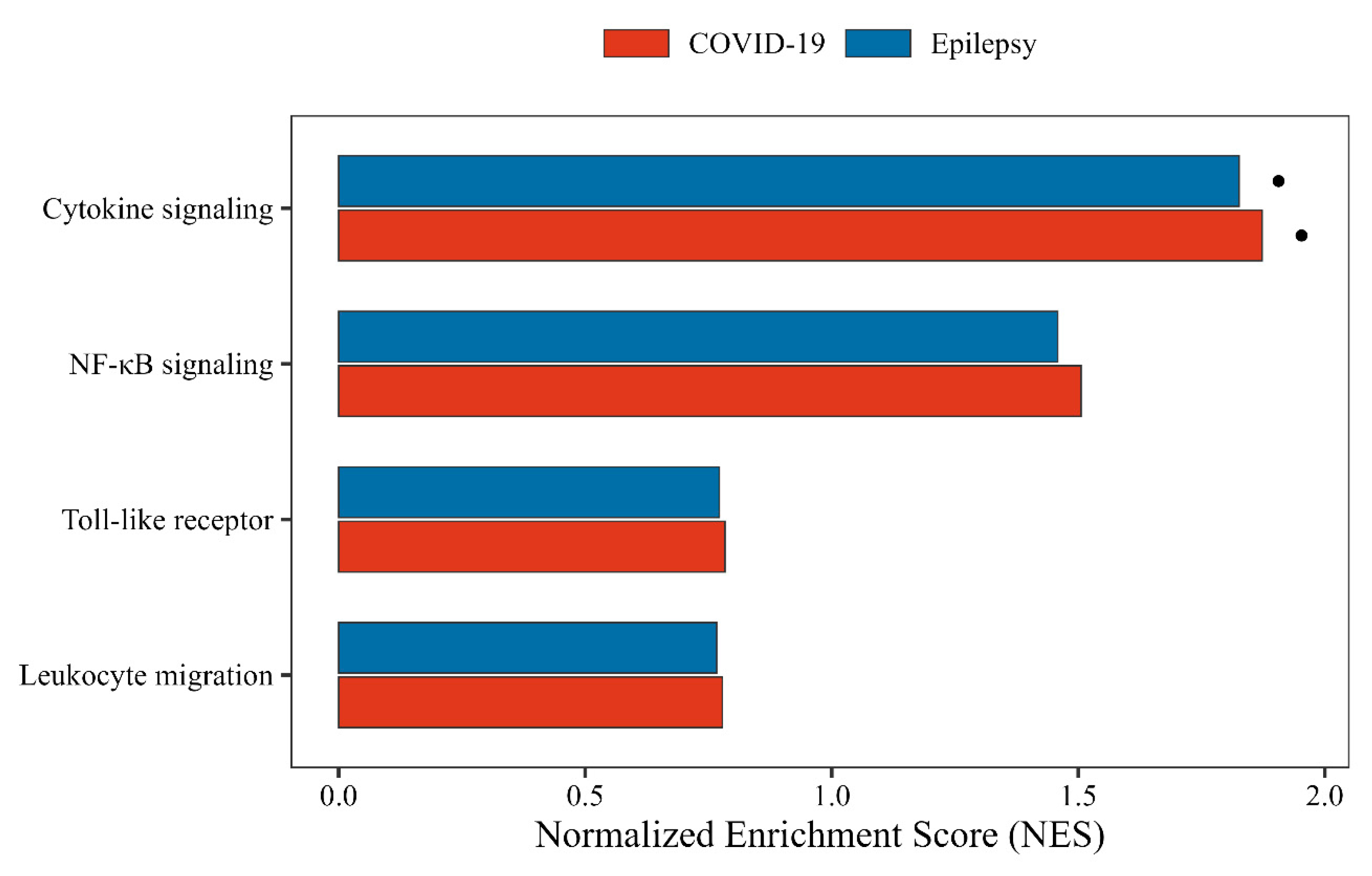
3.5. Gene Set Enrichment Analysis Reveals Immune Convergence in Cytokine Signaling
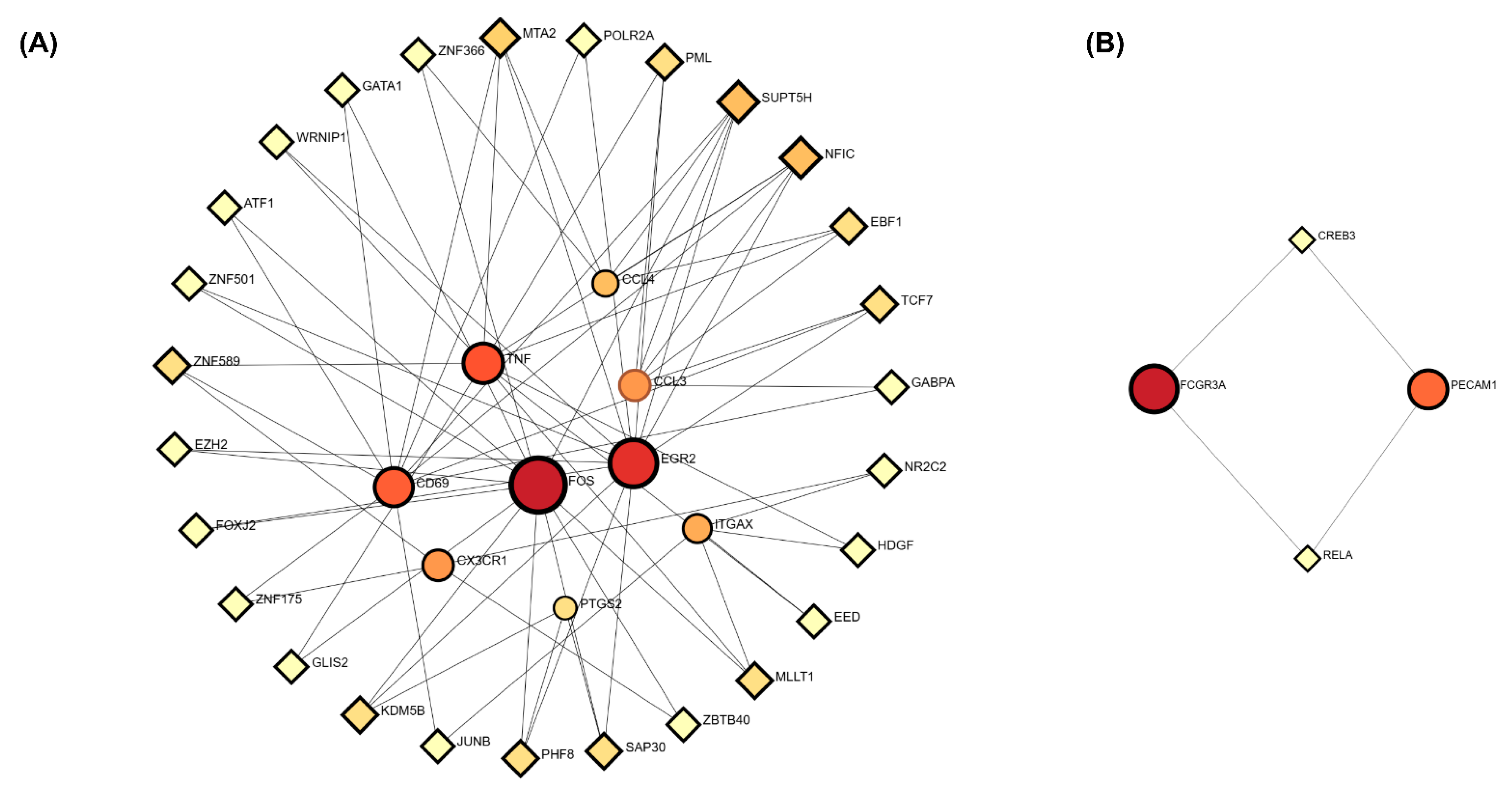
3.6. Transcriptional Regulatory Network Analysis
3.7. Drug Repositioning Prediction via LINCS L1000
3.8. Validation of Drug Efficacy and Mechanism
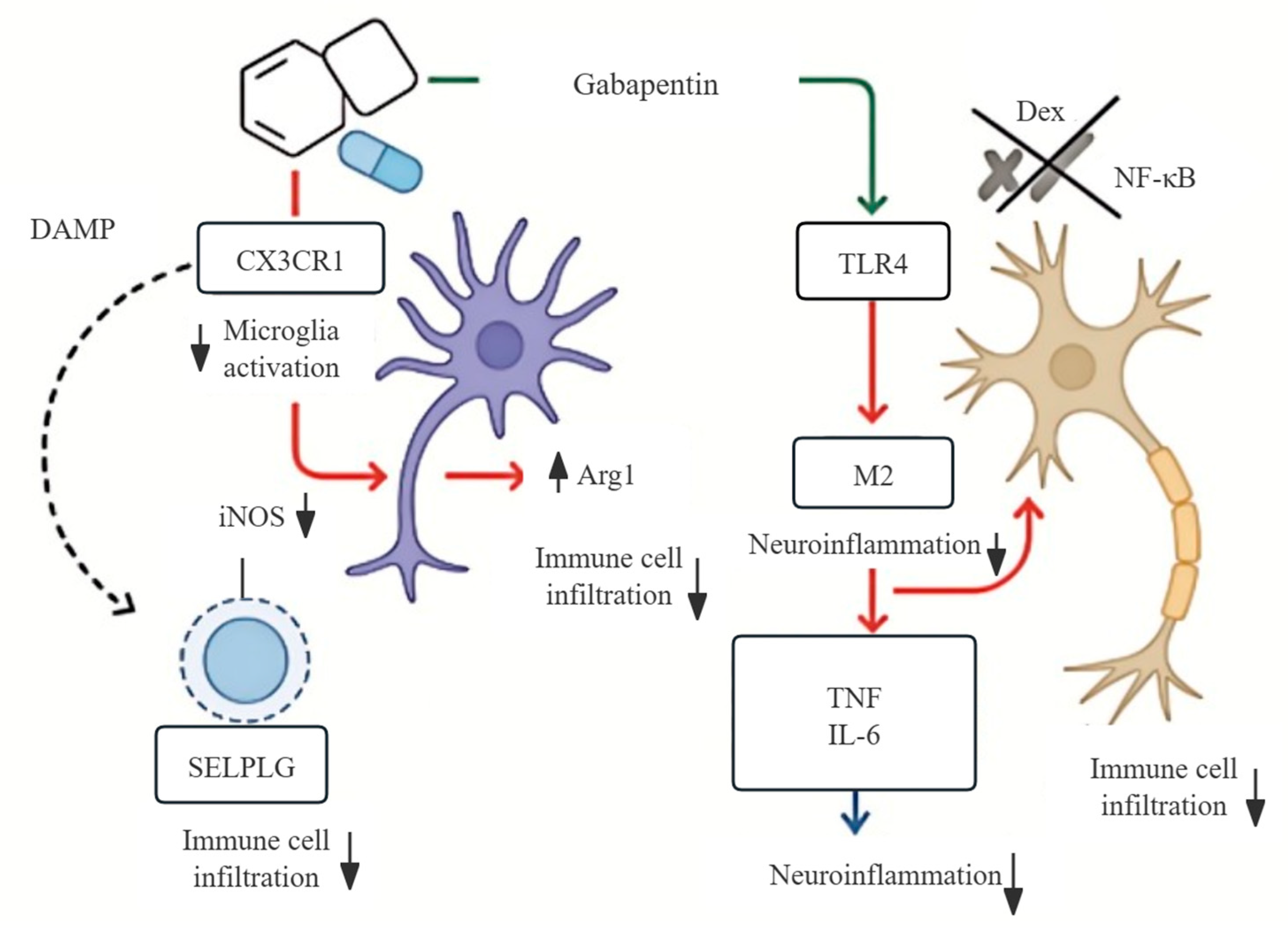
3.9. Mechanistic Model of Gabapentin’s Dual Action in Neuroinflammation
| Study (Reference) | Model/System | Gabapentin Target/Pathway | Findings | Relevance |
|---|---|---|---|---|
| Yang et al. [17] | Arthritis model + microglia | CX3CL1–CX3CR1 | Reduced CX3CL1 expression and microglial activation | Supports immune modulation |
| Rossi et al. [19] | Pilocarpine epilepsy model | α2δ voltage-gated calcium channel (VGCC), microglial response | Reduced microgliosis, increased seizure threshold | Confirms antiseizure role |
| Deng et al. [20] | Meta-analysis of 53 RCTs | — | RR = 2.30 for seizure reduction | High clinical relevance |
| Lee et al. [21] | Neuropathic pain model | IL-10 ↑, TNF/IL-6 ↓ | Suppressed proinflammatory cytokines | Aligns with transcriptomic targets |
| Mcwilliam et al. [22] | Post-COVID-19 neuropathic pain | — | Pain relief and sensory recovery | Clinical support in post-COVID-19 |
| Soltani et al. [23] | COVID-19 cough RCT | — | Reduced cough severity with Gabapentin + Montelukast | Functional neuromodulation |
| Tharakan et al. [24] | Long COVID cognitive symptoms | — | Mixed effects on sensory/cognitive outcomes | Risk–benefit insights |
| Garcia et al. [25] | RCT for COVID-19-related parosmia | — | 67% benefit in early series; RCT neutral | Suggests limited benefit |
4. Discussion
4.1. Translational Potential and Clinical Implications
4.2. Limitations and Future Directions
- (1)
- Applying longitudinal transcriptomic profiling in early-stage COVID-19 and epilepsy models to capture time-resolved immune activity;
- (2)
- Conducting mechanistic studies using CRISPR/Cas9-mediated knockout of CX3CR1 and TLR4 in microglia to assess Gabapentin’s specificity;
- (3)
- Testing Gabapentin in preclinical models of virus-triggered or immune-mediated seizures;
- (4)
- Designing stratified clinical trials in patients with neuroinflammatory signatures (e.g., elevated CX3CL1 or TSPO-PET hypermetabolism).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP-1 | activator protein-1 |
| BBB | blood–brain barrier |
| DAMP | damage-associated molecular pattern |
| DEG | differentially expressed gene |
| DSigDB | Drug Signatures Database |
| GEO | Gene Expression Omnibus |
| HMGB1 | high-mobility group box 1 |
| ITGAX | integrin subunit alpha X (CD11c) |
| MCC | Maximal Clique Centrality |
| SELPLG | selectin P ligand (PSGL-1) |
| TLE-HS | temporal-lobe epilepsy with hippocampal sclerosis |
| TSPO-PET | translocator protein positron emission tomography |
| VGCC | voltage-gated calcium channel |
References
- Majeed, C.N.; Rudnick, S.R.; Bonkovsky, H.L. Review of Antiseizure Medications for Adults With Epilepsy. JAMA 2022, 328, 680. [Google Scholar] [CrossRef]
- Klein, P.; Kaminski, R.M.; Koepp, M.; Loscher, W. New epilepsy therapies in development. Nat. Rev. Drug Discov. 2024, 23, 682–708. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef]
- Feng, S.; Qiao, W.; Xia, L.; Yu, L.; Lang, Y.; Jin, J.; Liu, Y.; Chen, F.; Feng, W.; Chen, Y. Nanoengineered, ultrasmall and catalytic potassium calcium hexacyanoferrate for neuroprotection and temporal lobe epilepsy treatment. Sci. Bull. 2025, 70, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Librizzi, L.; Noe, F.; Vezzani, A.; de Curtis, M.; Ravizza, T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann. Neurol. 2012, 72, 82–90. [Google Scholar] [CrossRef]
- Umpierre, A.D.; Li, B.; Ayasoufi, K.; Simon, W.L.; Zhao, S.; Xie, M.; Thyen, G.; Hur, B.; Zheng, J.; Liang, Y.; et al. Microglial P2Y(6) calcium signaling promotes phagocytosis and shapes neuroimmune responses in epileptogenesis. Neuron 2024, 112, 1959–1977. [Google Scholar] [CrossRef]
- Liang, L.P.; Sri Hari, A.; Day, B.J.; Patel, M. Pharmacological elevation of glutathione inhibits status epilepticus-induced neuroinflammation and oxidative injury. Redox Biol. 2024, 73, 103168. [Google Scholar] [CrossRef]
- Villasana-Salazar, B.; Vezzani, A. Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol. Dis. 2023, 178, 106027. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, F.; Mohammadkhanizadeh, A.; Mohammadi, E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult. Scler. Relat. Disord. 2020, 46, 102535. [Google Scholar] [CrossRef]
- Lu, L.; Xiong, W.; Liu, D.; Liu, J.; Yang, D.; Li, N.; Mu, J.; Guo, J.; Li, W.; Wang, G.; et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia 2020, 61, e49–e53. [Google Scholar] [CrossRef] [PubMed]
- Cothran, T.P.; Kellman, S.; Singh, S.; Beck, J.S.; Powell, K.J.; Bolton, C.J.; Tam, J.W. A brewing storm: The neuropsychological sequelae of hyperinflammation due to COVID-19. Brain Behav. Immun. 2020, 88, 957–958. [Google Scholar] [CrossRef]
- Wang, F.; Han, H.; Wang, C.; Wang, J.; Peng, Y.; Chen, Y.; He, Y.; Deng, Z.; Li, F.; Rong, Y.; et al. SARS-CoV-2 membrane protein induces neurodegeneration via affecting Golgi-mitochondria interaction. Transl. Neurodegener. 2024, 13, 68. [Google Scholar] [CrossRef]
- Santos de Lima, F.; Issa, N.; Seibert, K.; Davis, J.; Wlodarski, R.; Klein, S.; El Ammar, F.; Wu, S.; Rose, S.; Warnke, P.; et al. Epileptiform activity and seizures in patients with COVID-19. J. Neurol. Neurosurg. Psychiatry 2021, 92, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Bensken, W.P.; O’Brien, T.J. The Significance of the Increased Incidence of New Onset Seizures and Epilepsy After a COVID-19 Infection. Neurology 2023, 100, 359–360. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, J.H.; Jeon, J.; Kim, J.; Song, T.J. Risk of COVID-19 Infection and of Severe Complications Among People With Epilepsy: A Nationwide Cohort Study. Neurology 2022, 98, e1886–e1892. [Google Scholar] [CrossRef] [PubMed]
- Chagas, L.D.S.; Serfaty, C.A. The Influence of Microglia on Neuroplasticity and Long-Term Cognitive Sequelae in Long COVID: Impacts on Brain Development and Beyond. Int. J. Mol. Sci. 2024, 25, 3819. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Xu, B.; Li, S.S.; Zhang, W.S.; Xu, H.; Deng, X.M.; Zhang, Y.Q. Gabapentin reduces CX3CL1 signaling and blocks spinal microglial activation in monoarthritic rats. Mol. Brain 2012, 5, 18. [Google Scholar] [CrossRef]
- Abdelnaser, M.; Alaaeldin, R.; Attya, M.E.; Fathy, M. Hepatoprotective potential of gabapentin in cecal ligation and puncture-induced sepsis; targeting oxidative stress, apoptosis, and NF-kB/MAPK signaling pathways. Life Sci. 2023, 320, 121562. [Google Scholar] [CrossRef]
- Rossi, A.; Murta, V.; Auzmendi, J.; Ramos, A.J. Early Gabapentin Treatment during the Latency Period Increases Convulsive Threshold, Reduces Microglial Activation and Macrophage Infiltration in the Lithium-Pilocarpine Model of Epilepsy. Pharm. 2017, 10, 93. [Google Scholar] [CrossRef]
- Deng, N.J.; Li, X.Y.; Zhang, Z.X.; Xian-Yu, C.Y.; Tao, Y.T.; Ma, Y.T.; Li, H.J.; Gao, T.Y.; Liu, X.; Luo, J.; et al. Effectiveness and safety of single anti-seizure medication as adjunctive therapy for drug-resistant focal epilepsy based on network meta-analysis. Front. Pharmacol. 2025, 16, 1500475. [Google Scholar] [CrossRef]
- Lee, B.S.; Jun, I.G.; Kim, S.H.; Park, J.Y. Intrathecal gabapentin increases interleukin-10 expression and inhibits pro-inflammatory cytokine in a rat model of neuropathic pain. J. Korean Med. Sci. 2013, 28, 308–314. [Google Scholar] [CrossRef]
- McWilliam, M.; Samuel, M.; Alkufri, F.H. Neuropathic pain post-COVID-19: A case report. BMJ Case Rep. 2021, 14, e243459. [Google Scholar] [CrossRef]
- Soltani, R.; Nasirharandi, S.; Khorvash, F.; Nasirian, M.; Dolatshahi, K.; Hakamifard, A. The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID-19- related cough: A randomized, controlled clinical trial. Clin. Respir. J. 2022, 16, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, T.; Kallogjeri, D.; Piccirillo, J.F. Clinical studies in COVID-related olfactory disorders: Review of an institutional experience. World J. Otorhinolaryngol. Head. Neck Surg. 2024, 10, 129–136. [Google Scholar] [CrossRef]
- Garcia, J.A.P.; Miller, E.; Norwood, T.G.; Dorin, N.A.; Grayson, J.; Woodworth, B.; Cho, D.Y. Gabapentin improves parosmia after COVID-19 infection. Int. Forum Allergy Rhinol. 2023, 13, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Morales-Chacon, L.M. [Vertical transmission of SARS-CoV-2. Impact on the nervous system]. Rev. Med. Inst. Mex. Seguro Soc. 2020, 58, S215–S223. [Google Scholar] [CrossRef] [PubMed]
- Egervari, K.; Thomas, C.; Lobrinus, J.A.; Kuhlmann, T.; Bruck, W.; Love, S.; Crary, J.F.; Stadelmann, C.; Paulus, W.; Merkler, D. Neuropathology associated with SARS-CoV-2 infection. Lancet 2021, 397, 276–277. [Google Scholar] [CrossRef]
- Lempriere, S. High rate of epilepsy in young individuals who died with COVID-19. Nat. Rev. Neurol. 2022, 18, 65. [Google Scholar] [CrossRef]
- Berg, A.T.; Jobst, B. Epilepsy and COVID-19’s Double-Edged Sword: More Severe Disease and Delayed Epilepsy Care. Neurology 2022, 98, 779–780. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Kim, T.H.; Park, H.; Kang, T.C. Distinct Roles of CK2- and AKT-Mediated NF-kappaB Phosphorylations in Clasmatodendrosis (Autophagic Astroglial Death) within the Hippocampus of Chronic Epilepsy Rats. Antioxidants 2023, 12, 1020. [Google Scholar] [CrossRef]
- Mowry, F.E.; Peaden, S.C.; Stern, J.E.; Biancardi, V.C. TLR4 and AT1R mediate blood-brain barrier disruption, neuroinflammation, and autonomic dysfunction in spontaneously hypertensive rats. Pharmacol. Res. 2021, 174, 105877. [Google Scholar] [CrossRef]
- Croce, K.; Gao, H.; Wang, Y.; Mooroka, T.; Sakuma, M.; Shi, C.; Sukhova, G.K.; Packard, R.R.; Hogg, N.; Libby, P.; et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation 2009, 120, 427–436. [Google Scholar] [CrossRef]
- Yli-Karjanmaa, M.; Larsen, K.S.; Fenger, C.D.; Kristensen, L.K.; Martin, N.A.; Jensen, P.T.; Breton, A.; Nathanson, L.; Nielsen, P.V.; Lund, M.C.; et al. TNF deficiency causes alterations in the spatial organization of neurogenic zones and alters the number of microglia and neurons in the cerebral cortex. Brain Behav. Immun. 2019, 82, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Gil, B.; Villegas-Salmeron, J.; Salari, V.; Martins-Ferreira, R.; Arribas Blazquez, M.; Menendez Mendez, A.; Da Rosa Gerbatin, R.; Smith, J.; de Diego-Garcia, L.; et al. Opposing effects of the purinergic P2X7 receptor on seizures in neurons and microglia in male mice. Brain Behav. Immun. 2024, 120, 121–140. [Google Scholar] [CrossRef]
- Owens, G.C.; Garcia, A.J.; Mochizuki, A.Y.; Chang, J.W.; Reyes, S.D.; Salamon, N.; Prins, R.M.; Mathern, G.W.; Fallah, A. Evidence for Innate and Adaptive Immune Responses in a Cohort of Intractable Pediatric Epilepsy Surgery Patients. Front. Immunol. 2019, 10, 121. [Google Scholar] [CrossRef]
- Goonewardena, S.N.; Chen, Q.; Tate, A.M.; Grushko, O.G.; Damodaran, D.; Blakely, P.K.; Hayek, S.S.; Pinsky, D.J.; Rosenson, R.S. Monocyte-Mediated Thrombosis Linked to Circulating Tissue Factor and Immune Paralysis in COVID-19. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Winneberger, J.; Schols, S.; Lessmann, K.; Randez-Garbayo, J.; Bauer, A.T.; Mohamud Yusuf, A.; Hermann, D.M.; Gunzer, M.; Schneider, S.W.; Fiehler, J.; et al. Platelet endothelial cell adhesion molecule-1 is a gatekeeper of neutrophil transendothelial migration in ischemic stroke. Brain Behav. Immun. 2021, 93, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Gonzalez, J.; Edwards, K.; Mallajosyula, V.; Buzzanco, A.S.; Sherwood, R.; Buffone, C.; Kathale, N.; Providenza, S.; Xie, M.M.; et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat. Immunol. 2021, 22, 67–73. [Google Scholar] [CrossRef]
- Ali, I.; Chugh, D.; Ekdahl, C.T. Role of fractalkine-CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain. Neurobiol. Dis. 2015, 74, 194–203. [Google Scholar] [CrossRef]
- Yeo, S.I.; Kim, J.E.; Ryu, H.J.; Seo, C.H.; Lee, B.C.; Choi, I.G.; Kim, D.S.; Kang, T.C. The roles of fractalkine/CX3CR1 system in neuronal death following pilocarpine-induced status epilepticus. J. Neuroimmunol. 2011, 234, 93–102. [Google Scholar] [CrossRef]
- Dress, R.J.; Ginhoux, F. Monocytes and macrophages in severe COVID-19—Friend, foe or both? Immunol. Cell Biol. 2021, 99, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jia, Z.; Xiao, Z.; Zhao, J.; Lu, Y.; Chu, L.; Shao, H.; Pei, L.; Zhang, S.; Chen, Y. Baicalin Rescues Cognitive Dysfunction, Mitigates Neurodegeneration, and Exerts Anti-Epileptic Effects Through Activating TLR4/MYD88/Caspase-3 Pathway in Rats. Drug Des. Devel Ther. 2021, 15, 3163–3180. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, Y.; Dai, H.; Tan, S.; Mao, X.; Chen, Z. Dynorphin activation of kappa opioid receptor promotes microglial polarization toward M2 phenotype via TLR4/NF-kappaB pathway. Cell Biosci. 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Bhandari, R.; Kuhad, A. TLR4 as a therapeutic target for respiratory and neurological complications of SARS-CoV-2. Expert. Opin. Ther. Targets 2021, 25, 491–508. [Google Scholar] [CrossRef]
- Kyriatzis, G.; Bernard, A.; Bole, A.; Khrestchatisky, M.; Ferhat, L. In the Rat Hippocampus, Pilocarpine-Induced Status Epilepticus Is Associated with Reactive Glia and Concomitant Increased Expression of CD31, PDGFRbeta, and Collagen IV in Endothelial Cells and Pericytes of the Blood-Brain Barrier. Int. J. Mol. Sci. 2024, 25, 1693. [Google Scholar] [CrossRef]
- Diebold, M.; Vietzen, H.; Heinzel, A.; Haindl, S.; Herz, C.T.; Mayer, K.; Doberer, K.; Kainz, A.; Fae, I.; Wenda, S.; et al. Natural killer cell functional genetics and donor-specific antibody-triggered microvascular inflammation. Am. J. Transplant. 2024, 24, 743–754. [Google Scholar] [CrossRef]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Epigenetics and therapeutic targets mediating neuroprotection. Brain Res. 2015, 1628, 265–272. [Google Scholar] [CrossRef]
- Kohler, U.A.; Bohm, F.; Rolfs, F.; Egger, M.; Hornemann, T.; Pasparakis, M.; Weber, A.; Werner, S. NF-kappaB/RelA and Nrf2 cooperate to maintain hepatocyte integrity and to prevent development of hepatocellular adenoma. J. Hepatol. 2016, 64, 94–102. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Chang, S.; Zhuang, Z.; Waimei, S.; Li, X.; Chen, Z.; Jing, B.; Zhang, D.; Zhao, G. beta-Sitosterol Alleviates Neuropathic Pain by Affect Microglia Polarization through Inhibiting TLR4/NF-kappaB Signaling Pathway. J. Neuroimmune Pharmacol. 2023, 18, 690–703. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Shoaib, R.M.; Ahsan, M.Z.; Deng, M.Y.; Ma, L.; Apryani, E.; Li, X.Y.; Wang, Y.X. Microglial IL-10 and beta-endorphin expression mediates gabapentinoids antineuropathic pain. Brain Behav. Immun. 2021, 95, 344–361. [Google Scholar] [CrossRef]
- Subbarayan, M.S.; Joly-Amado, A.; Bickford, P.C.; Nash, K.R. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol. Ther. 2022, 231, 107989. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Growing Role of Gabapentin in Opioid-Related Overdoses Highlights Misuse Potential and Off-label Prescribing Practices. JAMA 2022, 328, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Medeiros Araujo de Matos, U.; Fornari Caprara, A.L. Gabapentin-Associated Movement Disorders: A Literature Review. Medicines 2023, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- El-Kattan, M.A.; Saeed, E.; Khattab, M.A.; Maksoud, F.; Eldein, M.E.; Abdel-Roaf, N.E.; Awad, W.; Elshatory, A. Gabapentin-Induced Sub-Chronic Neurotoxicity in Rats and the Protective Role of Alpha-Tocopherol. Turk. Patoloji Derg. 2025. Online ahead of print. [Google Scholar] [CrossRef]
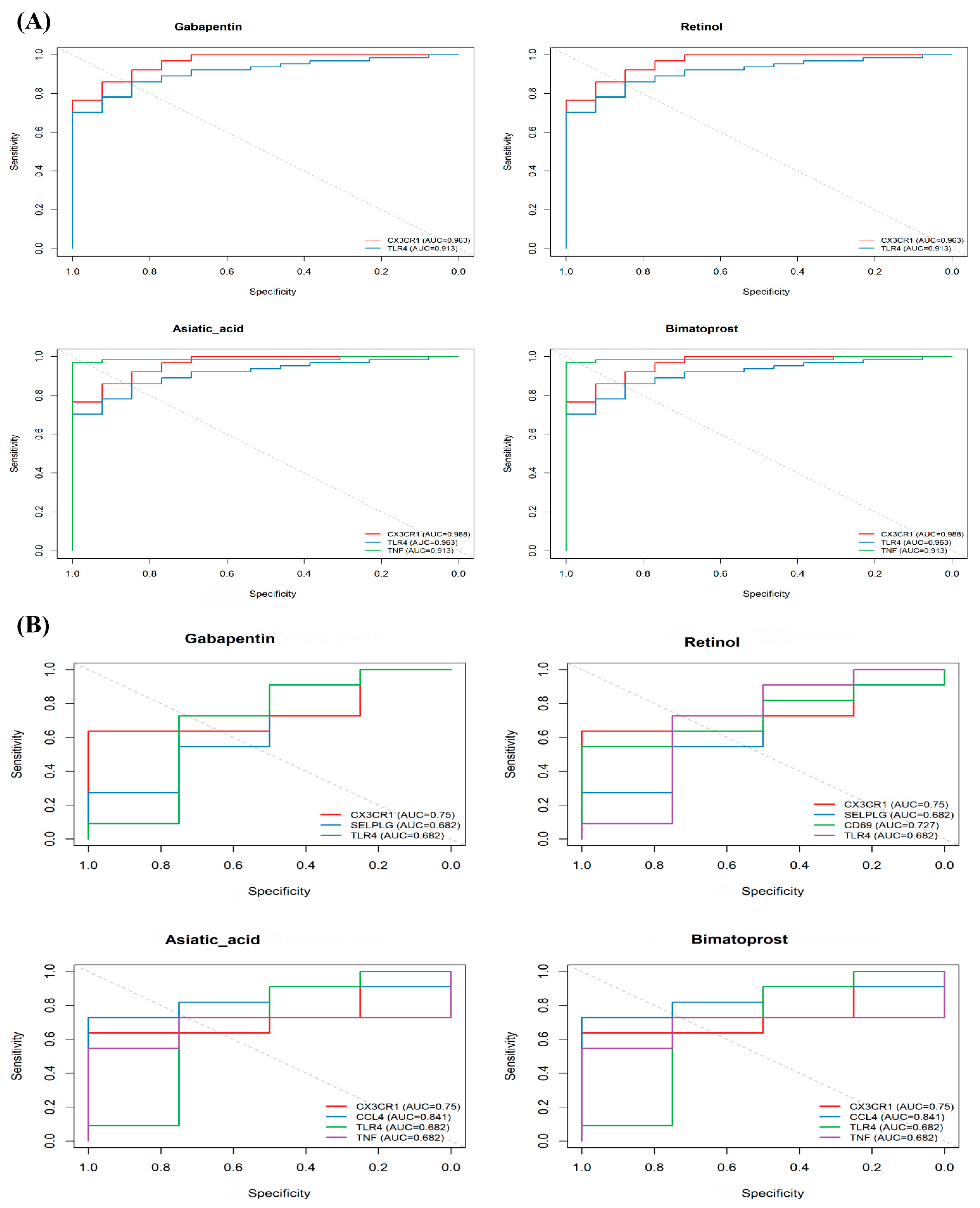
| Compound | Adjusted p-Value | Combined Score | Targeted Genes |
|---|---|---|---|
| Bimatoprost | 0.0024 | 670.68 | CX3CR1, CCL4, TLR4, TNF |
| Retinol | 0.0024 | 670.68 | CX3CR1, SELPLG, CD69, TLR4 |
| Betamethasone | 0.0024 | 667.01 | CX3CR1, CCL4, CD69, TLR4 |
| Gabapentin | 0.0082 | 295.44 | CX3CR1, SELPLG, TLR4 |
| Asiatic acid | 0.0024 | 663.38 | CX3CR1, CCL4, TLR4, TNF |
| N-benzylnaltrindole | 0.037 | 139.42 | MPZL2 |
| Nitazoxanide | 0.037 | 138.66 | MPZL2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, N.; Cao, P.; Chen, B.; Chen, L.; Liao, X.; Ning, Y. CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning. Biomedicines 2025, 13, 2133. https://doi.org/10.3390/biomedicines13092133
Pan N, Cao P, Chen B, Chen L, Liao X, Ning Y. CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning. Biomedicines. 2025; 13(9):2133. https://doi.org/10.3390/biomedicines13092133
Chicago/Turabian StylePan, Nannan, Penghui Cao, Ben Chen, Li Chen, Xuezhen Liao, and Yuping Ning. 2025. "CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning" Biomedicines 13, no. 9: 2133. https://doi.org/10.3390/biomedicines13092133
APA StylePan, N., Cao, P., Chen, B., Chen, L., Liao, X., & Ning, Y. (2025). CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning. Biomedicines, 13(9), 2133. https://doi.org/10.3390/biomedicines13092133







