Identification of Key Biomarkers Related to Lipid Metabolism in Acute Pancreatitis and Their Regulatory Mechanisms Based on Bioinformatics and Machine Learning
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Data Preprocessing
2.3. DEGs Identification and Functional Enrichment Analysis
2.4. Screening of Lipid Metabolism Genes Strongly Associated with APs
2.5. Further Screening of Genes Using Machine Learning Algorithms
2.6. Further Analysis of the Characterized Genes
2.7. Establishment of an AP Mouse Model
2.8. Pancreas Tissue Sampling and HE Staining
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Data Processing
3. Result
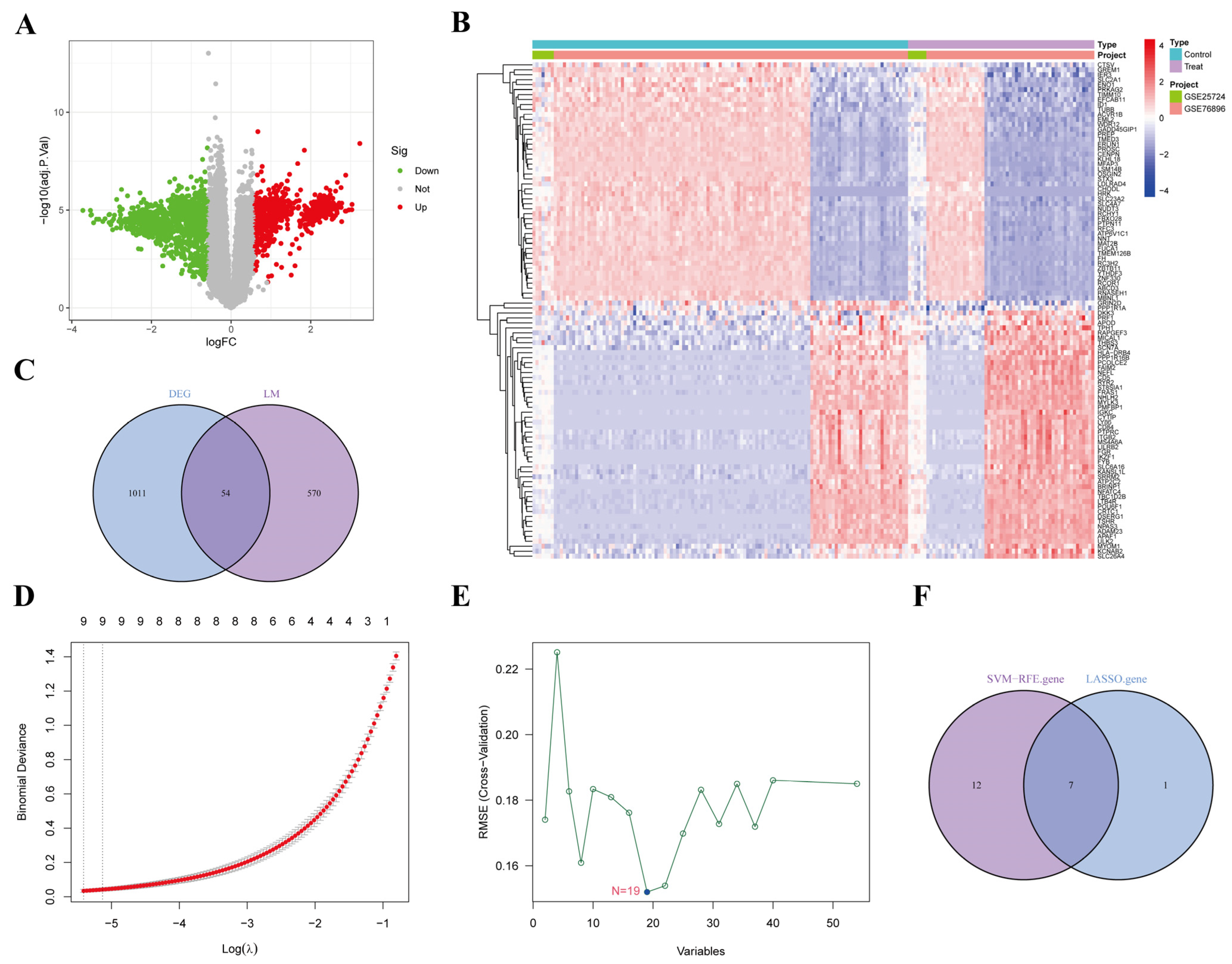
3.1. Data Standardization and Elimination of Batch Differences
3.2. Screening of DEGs
3.3. Gene Enrichment Analysis
3.4. Screening of AP-Related Lipid Metabolism Labeled Genes
3.5. Screening AP-Related Lipid Metabolism Signature Genes Using Machine Learning
3.6. Validation of Efficacy of Characterized Genes and Gene Interactions
3.7. GSEA Enrichment Analysis of Characterized Genes
3.8. Protein Structure Prediction and Interaction Analysis of Characterized Genes
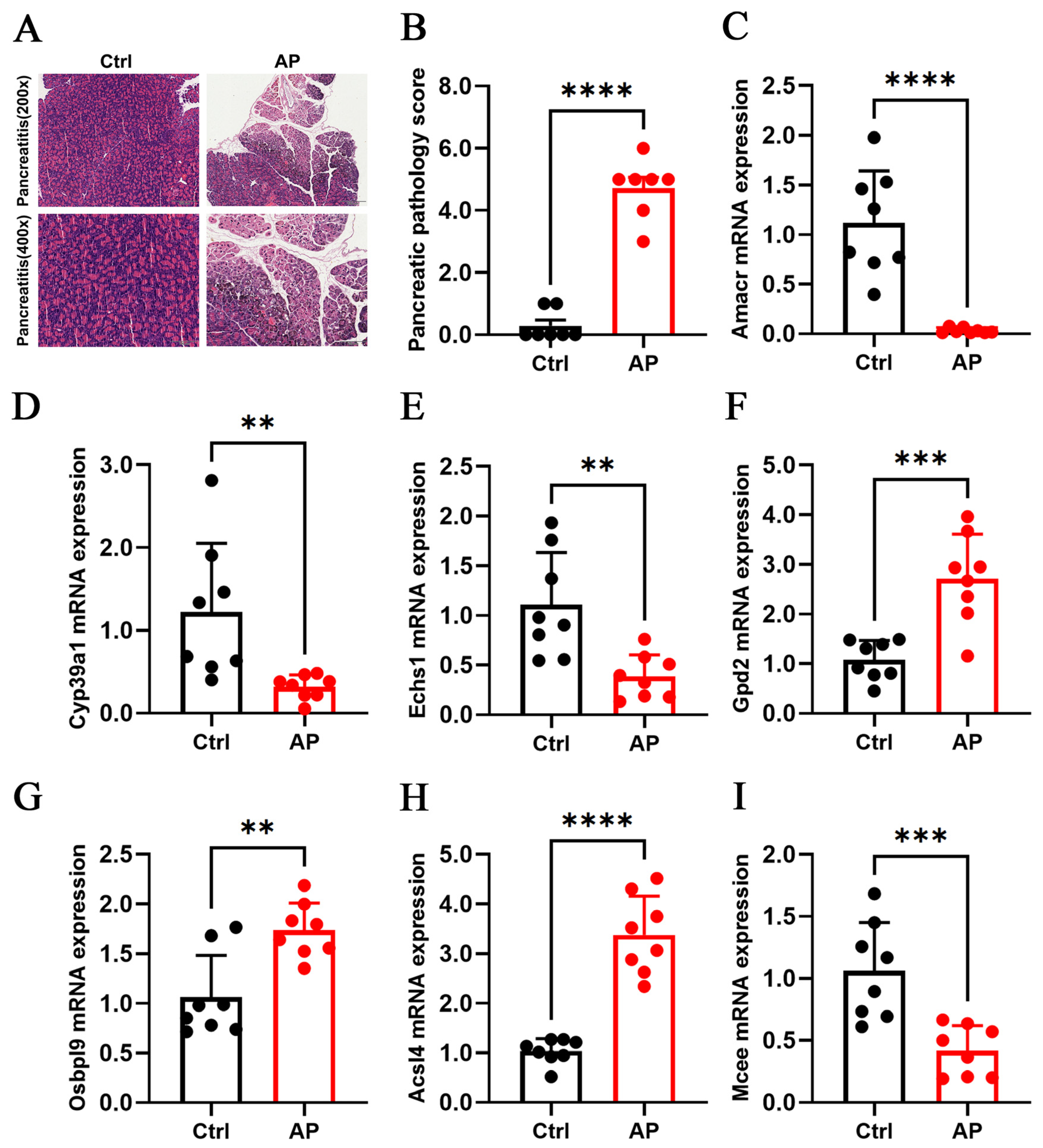
3.9. Animal Experimental Validation of Core Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSL4 | Acyl-CoA Synthetase Long Chain Family Member 4 |
| Amacr | Alpha-Methylacyl-CoA Racemase |
| AP | Acute Pancreatitis |
| AUC | Area Under the Curve |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| DEGs | Differential Expression Genes |
| DHAP | Dihydroxyacetone Phosphate |
| Echs1 | Enoyl-CoA Hydratase, Short Chain 1 |
| FDR | False Discovery Rate |
| FFA | Free Fatty Acids |
| FAO | Mitochondrial Fatty Acid β-Oxidation |
| G3P | Glycerol-3-Phosphate |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| Gpd2 | Glycerol-3-Phosphate Dehydrogenase 2 |
| GSEA | Gene Set Enrichment Analysis |
| H&E | Hematoxylin and Eosin |
| HC | Hydroxycholesterol |
| HTG | Hypertriglyceridemia |
| IL | Interleukin |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| Mcee | Methylmalonyl-CoA Epimerase |
| MCP | Monocyte Chemoattractant Protein |
| MODS | Multiple Organ Dysfunction Syndrome |
| NF-κB | Nuclear Factor Kappa B |
| Osbpl9 | Oxysterol Binding Protein Like 9 |
| PCA | Principal Component Analysis |
| RT-qPCR | Real-Time Quantitative Polymerase Chain Reaction |
| SAP | Severe Acute Pancreatitis |
| SFAs | Saturated Fatty Acids |
| SIRS | Systemic Inflammatory Response Syndrome |
| SVM-RFE | Support Vector Machine Recursive Feature Elimination |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor Necrosis Factor |
| UFAs | Unsaturated Fatty Acids |
References
- Hao, M.; Sebag, S.C.; Qian, Q.; Yang, L. Lysosomal physiology and pancreatic lysosomal stress in diabetes mellitus. eGastroenterology 2024, 2, e100096. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Ma, X.; Kim, S.; Wang, S.; Ni, H.M. Recent insights about autophagy in pancreatitis. eGastroenterology 2024, 2, e100057. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B. Acute Pancreatitis. Ann. Intern. Med. 2021, 174, ITC17–ITC32. [Google Scholar] [CrossRef]
- Nawaz, H.; Koutroumpakis, E.; Easler, J.; Slivka, A.; Whitcomb, D.C.; Singh, V.P.; Yadav, D.; Papachristou, G.I. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am. J. Gastroenterol. 2015, 110, 1497–1503. [Google Scholar] [CrossRef]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, F.; Zhen, Y.M.; Li, A.; Fang, Y. Clinical Study of 224 Patients with Hypertriglyceridemia Pancreatitis. Chin. Med. J. 2015, 128, 2045–2049. [Google Scholar] [CrossRef]
- Yang, A.L.; McNabb-Baltar, J. Hypertriglyceridemia and acute pancreatitis. Pancreatology 2020, 20, 795–800. [Google Scholar] [CrossRef]
- de Oliveira, C.; Khatua, B.; Noel, P.; Kostenko, S.; Bag, A.; Balakrishnan, B.; Patel, K.S.; Guerra, A.A.; Martinez, M.N.; Trivedi, S.; et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J. Clin. Investig. 2020, 130, 1931–1947. [Google Scholar] [CrossRef]
- Valdivielso, P.; Ramírez-Bueno, A.; Ewald, N. Current knowledge of hypertriglyceridemic pancreatitis. Eur. J. Intern. Med. 2014, 25, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Navina, S.; Acharya, C.; DeLany, J.P.; Orlichenko, L.S.; Baty, C.J.; Shiva, S.S.; Durgampudi, C.; Karlsson, J.M.; Lee, K.; Bae, K.T.; et al. Lipotoxicity causes multisystem organ failureand exacerbates acute pancreatitis in obesity. Sci. Transl. Med. 2011, 3, 107ra110. [Google Scholar] [CrossRef]
- Durgampudi, C.; Noel, P.; Patel, K.; Cline, R.; Trivedi, R.N.; DeLany, J.P.; Yadav, D.; Papachristou, G.I.; Lee, K.; Acharya, C.; et al. Acute lipotoxicity regulates severity of biliary acute pancreatitis without affecting its initiation. Am. J. Pathol. 2014, 184, 1773–1784. [Google Scholar] [CrossRef]
- Patel, K.; Trivedi, R.N.; Durgampudi, C.; Noel, P.; Cline, R.A.; DeLany, J.P.; Navina, S.; Singh, V.P. Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. Am. J. Pathol. 2015, 185, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lu, Y.; Du, L.; Guo, X.; Xie, J.; Cai, X. ACLS4 could be a potential therapeutic target for severe acute pancreatitis. Sci. Rep. 2024, 14, 13457. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.K.; Narasimhan, B.; Hastie, T. Elastic Net Regularization Paths for All Generalized Linear Models. J. Stat. Softw. 2023, 106, 1–31. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Clough, E.; Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; et al. NCBI GEO: Archive for gene expression and epigenomics data sets: 23-year update. Nucleic Acids Res. 2024, 52, D138–D144. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Zang, L.; Xiao, T.; Zhang, X.; Li, Z.; Zhu, H.; Gao, W.; Yu, X. Integrated Machine Learning and Bioinformatic Analyses Constructed a Novel Stemness-Related Classifier to Predict Prognosis and Immunotherapy Responses for Hepatocellular Carcinoma Patients. Int. J. Biol. Sci. 2022, 18, 360–373. [Google Scholar] [CrossRef]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Perides, G.; van Acker, G.J.; Laukkarinen, J.M.; Steer, M.L. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct. Nat. Protoc. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jia, F.; Zhao, Z.; Steer, M.L. Dachaihu decoction inhibits hypernutrition-induced liver metastasis from colorectal cancer by maintaining the gut vascular barrier. Cancer Pathog. Ther. 2023, 1, 98–110. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, P.; Liu, J.; Wang, R.; Kaufman, R.J.; Yaden, B.C.; Karin, M. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J. Hepatol. 2023, 79, 362–377. [Google Scholar] [CrossRef]
- Schmidt, J.; Rattner, D.W.; Lewandrowski, K.; Compton, C.C.; Mandavilli, U.; Knoefel, W.T.; Warshaw, A.L. A better model of acute pancreatitis for evaluating therapy. Ann. Surg. 1992, 215, 44–56. [Google Scholar] [CrossRef]
- Jia, F.; Yu, Q.; Zhao, L.; Shen, Y.; Guo, H.; He, F. Sodium New Houttuyfonate Inhibits Cancer-Promoting Fusobacterium nucleatum (Fn) to Reduce Colorectal Cancer Progression. Cancers 2022, 14, 6111. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Yu, Q.; Wang, R.; Shen, Y.; Guo, H.; He, F. Optimized Antimicrobial Peptide Jelleine-I Derivative Br-J-I Inhibits Fusobacterium Nucleatum to Suppress Colorectal Cancer Progression. Int. J. Mol. Sci. 2023, 24, 1469. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.; Passi, N.; Modarressi, T.; Kulkarni, V.; Soni, M.; Burke, F.; Bajaj, A.; Soffer, D. Clinical Management of Hypertriglyceridemia in the Prevention of Cardiovascular Disease and Pancreatitis. Curr. Atheroscler. Rep. 2021, 23, 72. [Google Scholar] [CrossRef]
- Tsuang, W.; Navaneethan, U.; Ruiz, L.; Palascak, J.B.; Gelrud, A. Hypertriglyceridemic pancreatitis: Presentation and management. Am. J. Gastroenterol. 2009, 104, 984–991. [Google Scholar] [CrossRef]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Šrámek, J.; Němcová-Fürstová, V.; Kovář, J. Molecular Mechanisms of Apoptosis Induction and Its Regulation by Fatty Acids in Pancreatic β-Cells. Int. J. Mol. Sci. 2021, 22, 4285. [Google Scholar] [CrossRef]
- Yang, L.; He, C.; Wang, W. Association between neutrophil to high-density lipoprotein cholesterol ratio and disease severity in patients with acute biliary pancreatitis. Ann. Med. 2024, 56, 2315225. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Y.; Peng, Y.; Xia, W.; Chen, L.; Yu, H.; Shi, L.; Yang, Y.; Su, N. Triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) ratio is an independent predictor of the severity of hyperlipidaemic acute pancreatitis. J. Hepatobiliary Pancreat. Sci. 2023, 30, 784–791. [Google Scholar] [CrossRef]
- Lloyd, M.D.; Yevglevskis, M.; Lee, G.L.; Wood, P.J.; Threadgill, M.D.; Woodman, T.J. α-Methylacyl-CoA racemase (AMACR): Metabolic enzyme, drug metabolizer and cancer marker P504S. Prog. Lipid Res. 2013, 52, 220–230. [Google Scholar] [CrossRef]
- Lloyd, M.D.; Darley, D.J.; Wierzbicki, A.S.; Threadgill, M.D. Alpha-methylacyl-CoA racemase—An ‘obscure’ metabolic enzyme takes centre stage. FEBS J. 2008, 275, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Ueda, M.; Ikeda, M.; Kobayashi, H.; Honda, Y. Oxysterol 7alpha-hydroxylase (CYP39A1) in the ciliary nonpigmented epithelium of bovine eye. Lab. Investig. 2003, 83, 349–355. [Google Scholar] [CrossRef]
- Wang, Y.; Yutuc, E.; Griffiths, W.J. Cholesterol metabolism pathways—Are the intermediates more important than the products? FEBS J. 2021, 288, 3727–3745. [Google Scholar] [CrossRef]
- Khenjanta, C.; Thanan, R.; Jusakul, A.; Techasen, A.; Jamnongkan, W.; Namwat, N.; Loilome, W.; Pairojkul, C.; Yongvanit, P. Association of CYP39A1, RUNX2 and oxidized alpha-1 antitrypsin expression in relation to cholangiocarcinoma progression. Asian Pac. J. Cancer Prev. 2014, 15, 10187–10192. [Google Scholar] [CrossRef]
- Sharpe, A.J.; McKenzie, M. Mitochondrial Fatty Acid Oxidation Disorders Associated with Short-Chain Enoyl-CoA Hydratase (ECHS1) Deficiency. Cells 2018, 7, 46. [Google Scholar] [CrossRef]
- Burgin, H.J.; Crameri, J.J.; Stojanovski, D.; Sanchez, M.I.G.L.; Ziemann, M.; McKenzie, M. Stimulating Mitochondrial Biogenesis with Deoxyribonucleosides Increases Functional Capacity in ECHS1-Deficient Cells. Int. J. Mol. Sci. 2022, 23, 12610. [Google Scholar] [CrossRef]
- Oh, S.; Mai, X.L.; Kim, J.; de Guzman, A.C.V.; Lee, J.Y.; Park, S. Glycerol 3-phosphate dehydrogenases (1 and 2) in cancer and other diseases. Exp. Mol. Med. 2024, 56, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ridgway, N.D. Substrate channeling in the glycerol-3-phosphate pathway regulates the synthesis, storage and secretion of glycerolipids. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158438. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Li, S. Oxysterol-binding proteins: Sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Prog. Lipid Res. 2013, 52, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, C.; Li, L.; Yang, M.; Jiang, N.; Luo, S.; Sun, L. Acyl-CoA synthase ACSL4: An essential target in ferroptosis and fatty acid metabolism. Chin. Med. J. 2023, 136, 2521–2537. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Bi, R.; Su, Y.; Quan, F.; Lin, Y.; Yue, C.; Cui, X.; Zhao, Q.; Liu, S.; et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022, 51, 102262. [Google Scholar] [CrossRef] [PubMed]
- Farrera, D.O.; Galligan, J.J. The Human Glyoxalase Gene Family in Health and Disease. Chem. Res. Toxicol. 2022, 35, 1766–1776. [Google Scholar] [CrossRef]
- Makarov, D.V.; Loeb, S.; Getzenberg, R.H.; Partin, A.W. Biomarkers for prostate cancer. Annu. Rev. Med. 2009, 60, 139–151. [Google Scholar] [CrossRef]
- Ryabov, V.M.; Baryshev, M.M.; Voskresenskiy, M.A.; Popov, B.V. Early Cell Cultures from Prostate Cancer Tissue Express Tissue Specific Epithelial and Cancer Markers. Int. J. Mol. Sci. 2023, 24, 2830. [Google Scholar] [CrossRef]
- Savolainen, K.; Kotti, T.J.; Schmitz, W.; Savolainen, T.I.; Sormunen, R.T.; Ilves, M.; Vainio, S.J.; Conzelmann, E.; Hiltunen, J.K. A mouse model for alpha-methylacyl-CoA racemase deficiency: Adjustment of bile acid synthesis and intolerance to dietary methyl-branched lipids. Hum. Mol. Genet. 2004, 13, 955–965. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Dacremont, G.; Wanders, R.J. Toxicity of peroxisomal C27-bile acid intermediates. Mol. Genet. Metab. 2009, 96, 121–128. [Google Scholar] [CrossRef]
- Ji, F.; Zhang, J.; Liu, N.; Gu, Y.; Gu, Y.; Zhang, Y.; Huang, P.; Zhang, N.; Lin, S.; Pan, R.; et al. Blocking hepatocarcinogenesis by a cytochrome P450 family member with female-preferential expression. Gut 2022, 71, 2313–2324. [Google Scholar] [CrossRef]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef]
- Han, H.; Wang, M.; Zhong, R.; Yi, B.; Schroyen, M.; Zhang, H. Depletion of Gut Microbiota Inhibits Hepatic Lipid Accumulation in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 9350. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.Y.; Zhao, R.; Zhang, H.L.; Zhou, Q.; Xu, F.J.; Zhang, X.; Xu, W.H.; Shao, N.; Zhou, S.X.; Dai, B.; et al. Inactivation of the AMPK-GATA3-ECHS1 Pathway Induces Fatty Acid Synthesis That Promotes Clear Cell Renal Cell Carcinoma Growth. Cancer Res. 2020, 80, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Haack, T.B.; Jackson, C.B.; Murayama, K.; Kremer, L.S.; Schaller, A.; Kotzaeridou, U.; de Vries, M.C.; Schottmann, G.; Santra, S.; Büchner, B.; et al. Deficiency of ECHS1 causes mitochondrial encephalopathy with cardiac involvement. Ann. Clin. Transl. Neurol. 2015, 2, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Burgin, H.; Sharpe, A.J.; Nie, S.; Ziemann, M.; Crameri, J.J.; Stojanovski, D.; Pitt, J.; Ohtake, A.; Murayama, K.; McKenzie, M. Loss of mitochondrial fatty acid β-oxidation protein short-chain Enoyl-CoA hydratase disrupts oxidative phosphorylation protein complex stability and function. FEBS J. 2023, 290, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Langston, P.K.; Nambu, A.; Jung, J.; Shibata, M.; Aksoylar, H.I.; Lei, J.; Xu, P.; Doan, M.T.; Jiang, H.; MacArthur, M.R.; et al. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat. Immunol. 2019, 20, 1186–1195. [Google Scholar] [CrossRef]
- Oh, S.; Jo, S.; Bajzikova, M.; Kim, H.S.; Dao, T.T.P.; Rohlena, J.; Kim, J.M.; Neuzil, J.; Park, S. Non-bioenergetic roles of mitochondrial GPD2 promote tumor progression. Theranostics 2023, 13, 438–457. [Google Scholar] [CrossRef]
- Yao, C.H.; Park, J.S.; Kurmi, K.; Hu, S.H.; Notarangelo, G.; Crowley, J.; Jacobson, H.; Hui, S.; Sharpe, A.H.; Haigis, M.C. Uncoupled glycerol-3-phosphate shuttle in kidney cancer reveals that cytosolic GPD is essential to support lipid synthesis. Mol. Cell. 2023, 83, 1340–1349.e7. [Google Scholar] [CrossRef]
- Sun, Z.; Jing, C.; Zhan, H.; Guo, X.; Suo, N.; Kong, F.; Tao, W.; Xiao, C.; Hu, D.; Wang, H.; et al. Identification of tumor antigens and immune landscapes for bladder urothelial carcinoma mRNA vaccine. Front. Immunol. 2023, 14, 1097472. [Google Scholar] [CrossRef]
- Yu, X.; Xu, C.; Zou, Y.; Liu, W.; Xie, Y.; Wu, C. A prognostic metabolism-related gene signature associated with the tumor immune microenvironment in neuroblastoma. Am. J. Cancer Res. 2024, 14, 253–273. [Google Scholar] [CrossRef]
- Yan, D.; Lehto, M.; Rasilainen, L.; Metso, J.; Ehnholm, C.; Ylä-Herttuala, S.; Jauhiainen, M.; Olkkonen, V.M. Oxysterol binding protein induces upregulation of SREBP-1c and enhances hepatic lipogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, D.; Xiao, T.; Gu, W.; Yang, H.; Yang, M.; Wang, H. ACSL4 promotes ferroptosis and M1 macrophage polarization to regulate the tumorigenesis of nasopharyngeal carcinoma. Int. Immunopharmacol. 2023, 122, 110629. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. The ACSL4 Network Regulates Cell Death and Autophagy in Diseases. Biology 2023, 12, 864. [Google Scholar] [CrossRef]
- Li, F.J.; Hu, H.; Wu, L.; Luo, B.; Zhou, Y.; Ren, J.; Lin, J.; Reiter, R.J.; Wang, S.; Dong, M.; et al. Ablation of mitophagy receptor FUNDC1 accentuates septic cardiomyopathy through ACSL4-dependent regulation of ferroptosis and mitochondrial integrity. Free Radic Biol. Med. 2024, 225, 75–86. [Google Scholar] [CrossRef]
- Heuberger, K.; Bailey, H.J.; Burda, P.; Chaikuad, A.; Krysztofinska, E.; Suormala, T.; Bürer, C.; Lutz, S.; Fowler, B.; Froese, D.S.; et al. Genetic, structural, and functional analysis of pathogenic variations causing methylmalonyl-CoA epimerase deficiency. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1265–1272. [Google Scholar] [CrossRef]
- Tejero, J.; Lazure, F.; Gomes, A.P. Methylmalonic acid in aging and disease. Trends Endocrinol. Metab. 2024, 35, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Ilter, D.; Low, V.; Drapela, S.; Schild, T.; Mullarky, E.; Han, J.; Elia, I.; Broekaert, D.; Rosenzweig, A.; et al. Altered propionate metabolism contributes to tumour progression and aggressiveness. Nat. Metab. 2022, 4, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Rickes, S.; Uhle, C. Advances in the diagnosis of acute pancreatitis. Postgrad. Med. J. 2009, 85, 208–212. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef]
- Prendeville, H.; Lynch, L. Diet, lipids, and antitumor immunity. Cell. Mol. Immunol. 2022, 19, 432–444. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed]




| Datasets | Type | Sample Size | Platforms | |
|---|---|---|---|---|
| Normal | Acute Pancreatitis | |||
| GSE3644 | RNA | GSM84549 GSM84550 GSM84551 GSM84555 GSM84556 GSM84557 | GSM84552 GSM84553 GSM84554 GSM84558 GSM84559 GSM84560 | GPL339 |
| GSE65146 | RNA | GSM1588057 GSM1588058 GSM1588059 GSM1588060 GSM1588086 GSM1588087 GSM1588088 GSM1588089 GSM1588090 | GSM1588081 GSM1588082 GSM1588123 GSM1588124 GSM1588125 | GPL6246 |
| GSE121038 | RNA | GSM3424897 GSM3424898 GSM3424899 GSM3424904 GSM3424905 GSM3424906 GSM3424907 | GSM3424900 GSM3424901 GSM3424902 GSM3424903 GSM3424908 GSM3424909 GSM3424910 GSM3424911 | GPL10787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, Y.; Jin, T.; Zheng, M.; Yap, Y.; Min, X.; Chen, J.; Yuan, L.; He, F.; Zhou, B. Identification of Key Biomarkers Related to Lipid Metabolism in Acute Pancreatitis and Their Regulatory Mechanisms Based on Bioinformatics and Machine Learning. Biomedicines 2025, 13, 2132. https://doi.org/10.3390/biomedicines13092132
Zhang L, Jiang Y, Jin T, Zheng M, Yap Y, Min X, Chen J, Yuan L, He F, Zhou B. Identification of Key Biomarkers Related to Lipid Metabolism in Acute Pancreatitis and Their Regulatory Mechanisms Based on Bioinformatics and Machine Learning. Biomedicines. 2025; 13(9):2132. https://doi.org/10.3390/biomedicines13092132
Chicago/Turabian StyleZhang, Liang, Yujie Jiang, Taojun Jin, Mingxian Zheng, Yixuan Yap, Xuanyang Min, Jiayue Chen, Lin Yuan, Feng He, and Bingduo Zhou. 2025. "Identification of Key Biomarkers Related to Lipid Metabolism in Acute Pancreatitis and Their Regulatory Mechanisms Based on Bioinformatics and Machine Learning" Biomedicines 13, no. 9: 2132. https://doi.org/10.3390/biomedicines13092132
APA StyleZhang, L., Jiang, Y., Jin, T., Zheng, M., Yap, Y., Min, X., Chen, J., Yuan, L., He, F., & Zhou, B. (2025). Identification of Key Biomarkers Related to Lipid Metabolism in Acute Pancreatitis and Their Regulatory Mechanisms Based on Bioinformatics and Machine Learning. Biomedicines, 13(9), 2132. https://doi.org/10.3390/biomedicines13092132








