Cardiac Ischaemia–Reperfusion Injury: Pathophysiology, Therapeutic Targets and Future Interventions
Abstract
1. Introduction
Significance of Ischaemia–Reperfusion Injury
2. Objectives and Scope of the Review
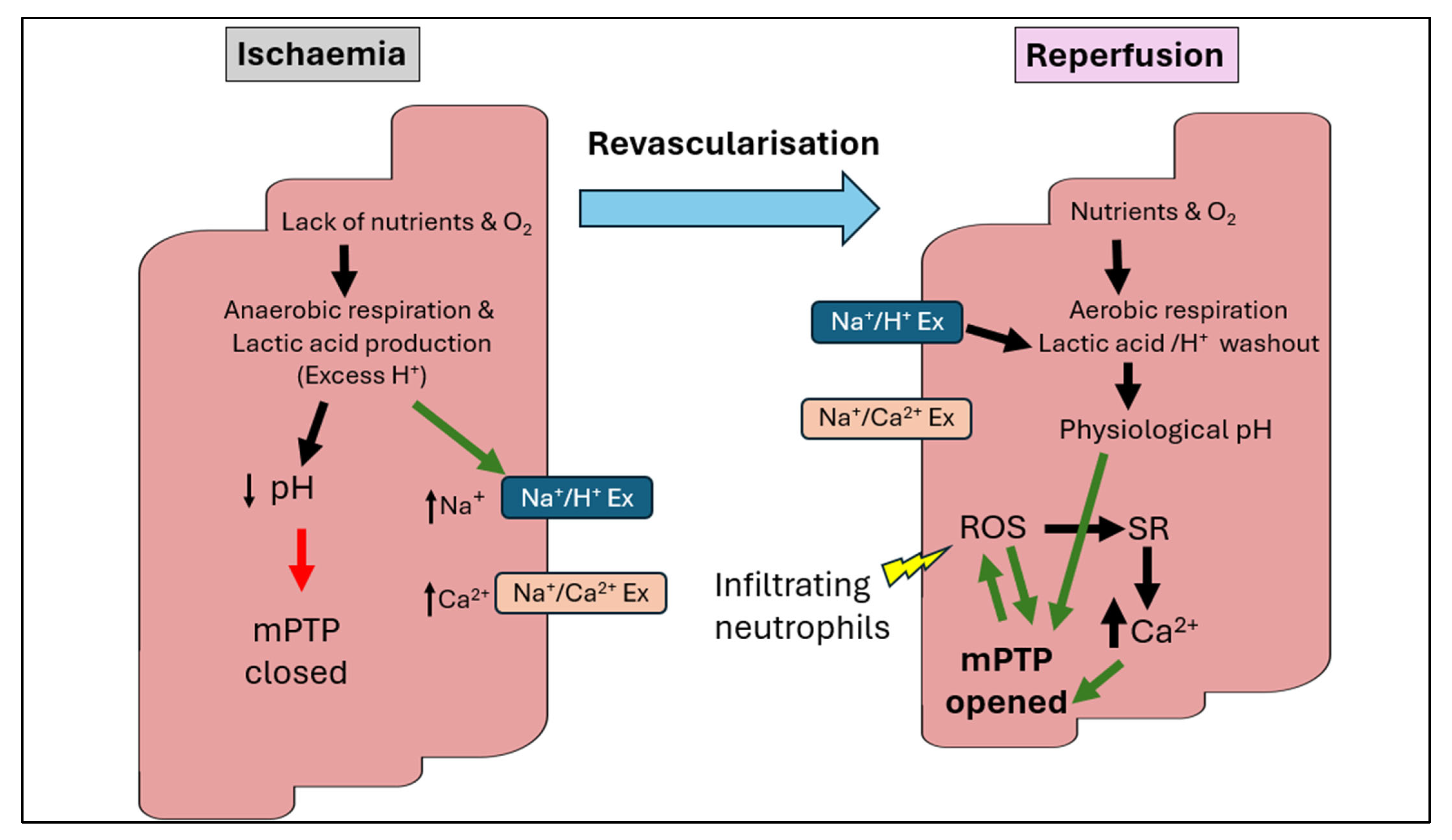
3. Pathophysiology of Ischaemia–Reperfusion Injury
3.1. Cellular and Molecular Changes
3.2. The Paradoxical Damage of Reperfusion
3.3. Inflammatory Response
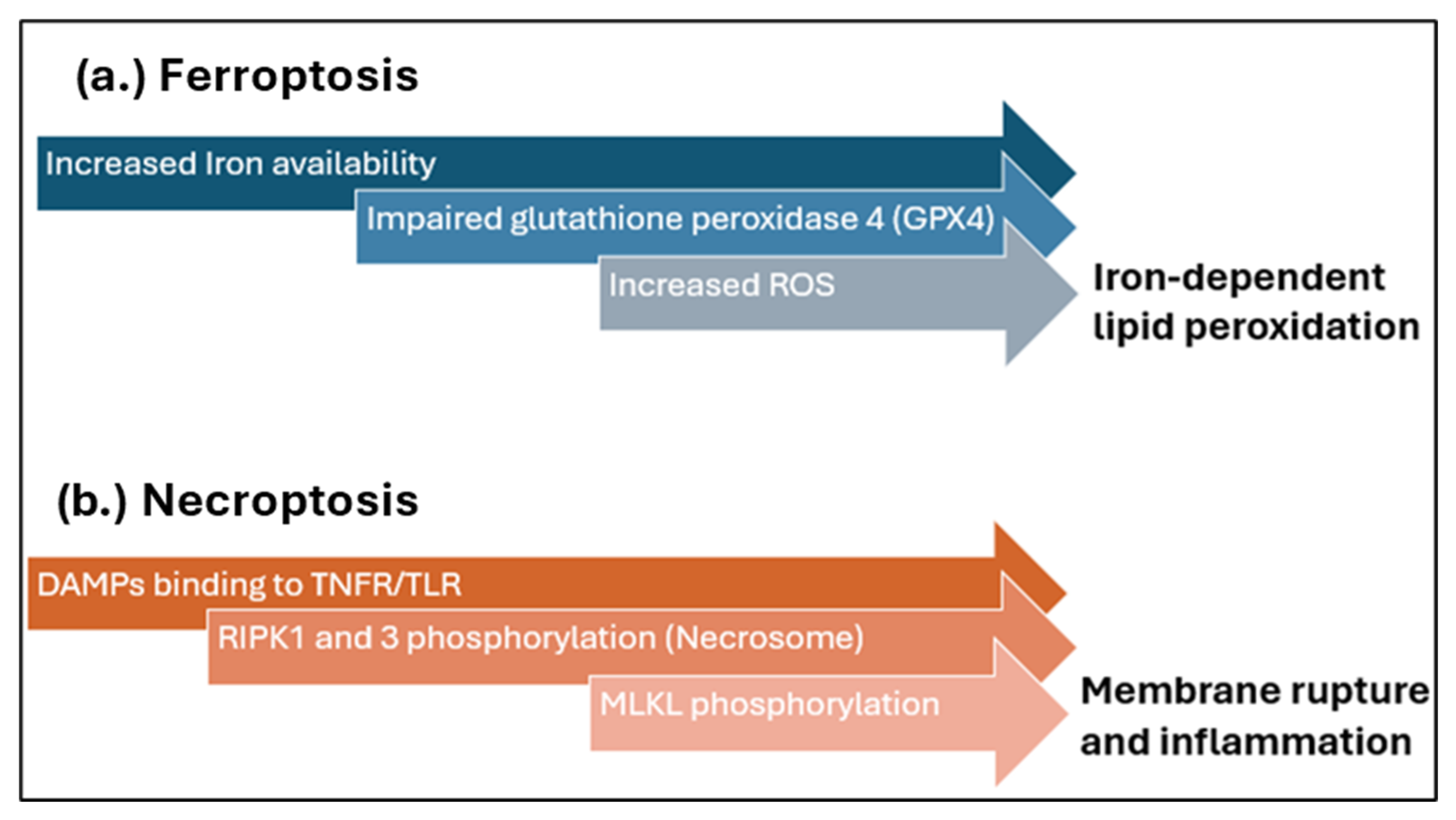
3.4. Apoptosis and Necrosis
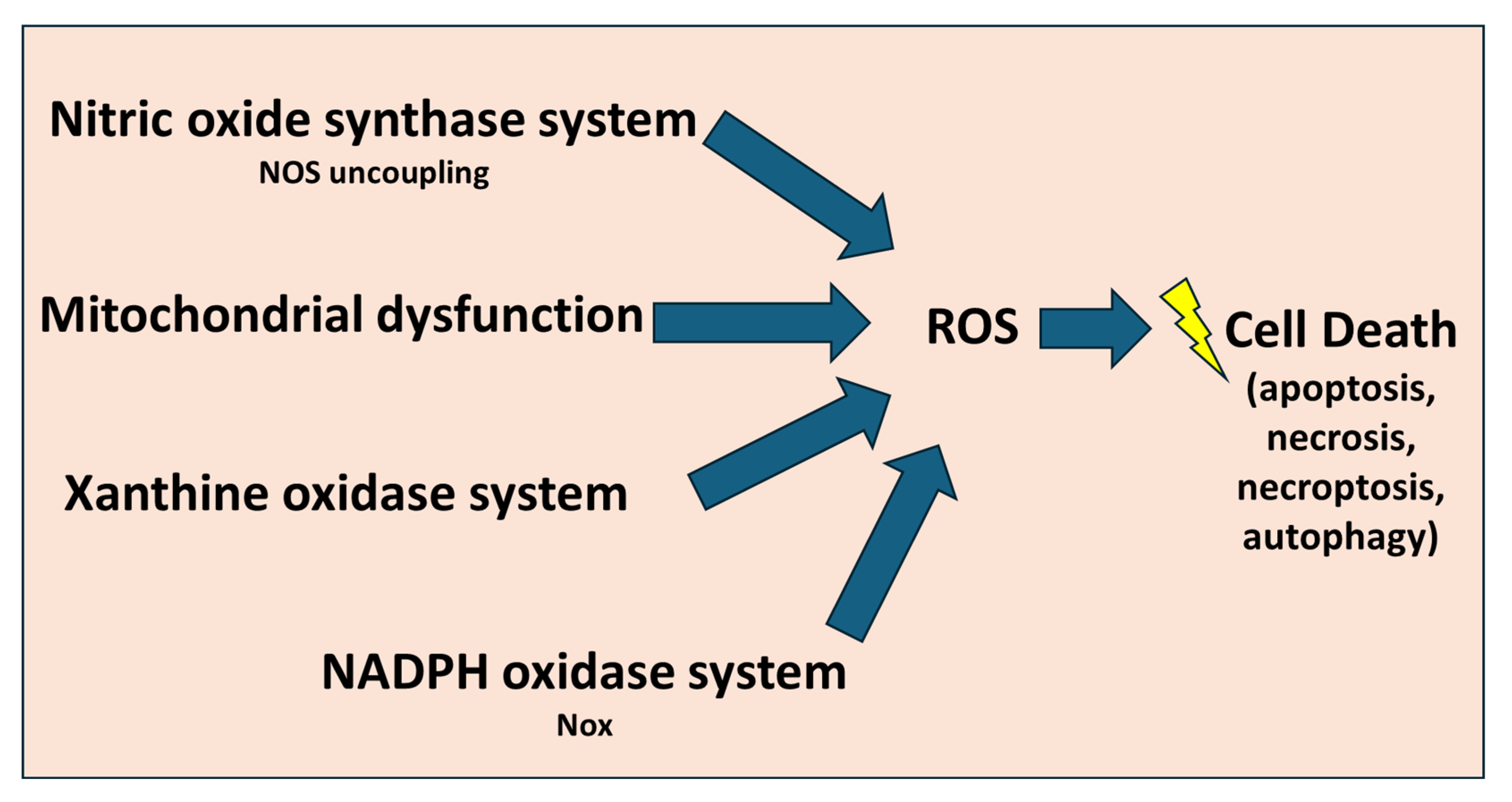
3.5. Oxidative Stress
3.6. Endothelial Dysfunction
3.7. Mitochondrial Dysfunction
4. Experimental Models in I/R Research
4.1. Ex Vivo Models
4.2. In Vivo Models
4.3. Alternative Models and Approaches
5. Current Therapeutic Strategies and Advances in IRI Research
5.1. Pharmacological Interventions
5.2. Molecular Targets and Gene Therapy
5.3. Stem Cell and Regenerative Therapies
5.4. Biomarkers and Precision Medicine
5.5. Future Perspectives: Nanotechnology and Biophysical Approaches and Biomaterial-Based Therapies
5.6. Natural Compounds and Phytochemicals
6. Limitations in Current Research and Future Interventions
6.1. Current Research Limitations
6.2. Future Interventions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| AI | Artificial Intelligence |
| AMPK | AMP-activated Protein Kinase |
| Apoe−/− | Apolipoprotein E-deficient |
| ASICs | Acid-sensing ion channels |
| CABG | Coronary Artery Bypass Grafting |
| DAMPs | Damage-associated molecular patterns |
| Drp1 | Dynamin-related protein 1 |
| EndMT | Endothelial-To-Mesenchymal Transition |
| eNOS | Endothelial Nitric Oxide Synthase |
| ETC | Electron Transport Chain |
| EV | Extracellular Vesicles |
| H/R | Hypoxia-reoxygenation |
| hiPSCs | human-induced Pluripotent Stem Cells |
| I/R | Ischaemia Reperfusion |
| IHD | Ischaemic Heart Disease |
| IL | Interleukin |
| IPC | Ischaemic Preconditioning |
| IPOC | Ischaemic Postconditioning |
| IRI | Ischaemia–Reperfusion Injury |
| LAD | Left Anterior Descending |
| MCS | Mechanical Circulatory Support |
| MDA | Malondialdehyde |
| MI | Myocardial Infarction |
| Mfn1/2 | Mitofusins 1/2 |
| MLKL | Mixed Lineage Kinase Domain-like Protein |
| mPTP | Mitochondrial Permeability Transition Pore |
| MSCs | Mesenchymal Stem Cells |
| NF-κB | Nuclear Factor-kappa B |
| NLRP3 | NOD-like Receptor Protein 3 |
| NO | Nitric Oxide |
| OGD | Oxygen-Glucose Deprivation |
| Opa1 | Optic atrophy 1 |
| PCI | Percutaneous Coronary Intervention |
| PINK1 | PTEN-induced Putative Kinase 1 |
| RIPK | Receptor-Interacting Protein Kinase |
| RISK | Reperfusion Injury Salvage Kinase |
| ROS | Reactive Oxygen Species |
| SAFE | Survivor Activating Factor Enhancement |
| SHR | Spontaneously Hypertensive Rats |
| SIRT1 | Sirtuin 1 |
| TGF-β | Transforming Growth Factor-beta |
| TLR | Toll-like Receptor |
| TNF-α | Tumour Necrosis Factor-alpha |
| TNFR | Tumour Necrosis Factor Receptor |
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Altamirano, F.; Wang, Z.V.; Hill, J.A. Cardioprotection in ischaemia-reperfusion injury: Novel mechanisms and clinical translation. J. Physiol. 2015, 593, 3773–3788. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Taub, P.R.; Epstein, E.; Michos, E.D.; Ferraro, R.A.; Bailey, A.L.; Kelli, H.M.; Ferdinand, K.C.; Echols, M.R.; Weintraub, H.; et al. Ten things to know about ten cardiovascular disease risk factors. Am. J. Prev. Cardiol. 2021, 5, 100149. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases; Key Facts; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, Q.; Wang, T.; Wang, S.; Li, R.; Wang, Y.; Zhang, J.; Gan, J.; Guo, M. Myocardial Ischemia/Reperfusion Injury: Mechanism and Targeted Treatment for Ferroptosis. Anatol. J. Cardiol. 2024, 28, 133–141. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- Nakamura, Y.; Saito, S.; Miyagawa, S.; Yoshikawa, Y.; Hata, H.; Yoshioka, D.; Toda, K.; Sawa, Y. Perioperative ischaemic reperfusion injury and allograft function in the early post-transplantation period. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 230–236. [Google Scholar] [CrossRef]
- Andras, I.; Piana, A.; Verri, P.; Telecan, T.; Gallioli, A.; Prudhomme, T.; Hevia, V.; Baboudjian, M.; Boissier, R.; Crisan, N.; et al. Systematic review of techniques and devices used to avoid warm ischemia time injury during kidney transplantation. World J. Urol. 2023, 41, 993–1003. [Google Scholar] [CrossRef]
- Ibáñez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving Therapies for Myocardial Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef]
- Varró, A.; Tomek, J.; Nagy, N.; Virág, L.; Passini, E.; Rodriguez, B.; Baczkó, I. Cardiac transmembrane ion channels and action potentials: Cellular physiology and arrhythmogenic behavior. Physiol. Rev. 2021, 101, 1083–1176. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Redd, M.A.; Scheuer, S.E.; Saez, N.J.; Yoshikawa, Y.; Chiu, H.S.; Gao, L.; Hicks, M.; Villanueva, J.E.; Joshi, Y.; Chow, C.Y.; et al. Therapeutic Inhibition of Acid-Sensing Ion Channel 1a Recovers Heart Function After Ischemia-Reperfusion Injury. Circulation 2021, 144, 947–960. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro, E.S.O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Park, M.; Kang, C.; Dilmen, S.; Kang, T.H.; Kang, D.G.; Ke, Q.; Lee, S.U.; Lee, D.; Kang, P.M. Hydrogen Peroxide-Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003697. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Zuurbier, C.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef]
- Bulluck, H.; Yellon, D.M.; Hausenloy, D.J. Reducing myocardial infarct size: Challenges and future opportunities. Heart 2016, 102, 341–348. [Google Scholar] [CrossRef]
- López-Ramírez, O.; González-Garrido, A. The role of acid sensing ion channels in the cardiovascular function. Front. Physiol. 2023, 14, 1194948. [Google Scholar] [CrossRef]
- Gunata, M.; Parlakpinar, H. A review of myocardial ischaemia/reperfusion injury: Pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem. Funct. 2021, 39, 190–217. [Google Scholar] [CrossRef]
- Sadat Ebrahimi, S.R.; Amini, H.; Rahbarghazi, R.; Habibollahi, P.; Ghaderi, S.; Rajabi, H.; Rezabakhsh, A. Putative therapeutic impacts of cardiac CTRP9 in ischaemia/reperfusion injury. J. Cell. Mol. Med. 2022, 26, 3120–3132. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Horvath, C.; Abdul-Ghani, S.; Varga, Z.V.; Suleiman, M.S.; Dhalla, N.S. Interplay of Oxidative Stress and Necrosis-like Cell Death in Cardiac Ischemia/Reperfusion Injury: A Focus on Necroptosis. Biomedicines 2022, 10, 127. [Google Scholar] [CrossRef]
- González-Montero, J.; Brito, R.; Gajardo, A.I.; Rodrigo, R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J. Cardiol. 2018, 10, 74–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Wang, T. Reactive oxygen species (ROS)-responsive biomaterials for treating myocardial ischemia-reperfusion injury. Front. Bioeng. Biotechnol. 2024, 12, 1469393. [Google Scholar] [CrossRef]
- Xiang, Q.; Yi, X.; Zhu, X.H.; Wei, X.; Jiang, D.S. Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol. Metab. 2024, 35, 219–234. [Google Scholar] [CrossRef]
- Huang, W.; Yang, J.; He, C.; Yang, J. RP105 plays a cardioprotective role in myocardial ischemia reperfusion injury by regulating the Toll-like receptor 2/4 signaling pathways. Mol. Med. Rep. 2020, 22, 1373–1381. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Francisco, J.; Del Re, D.P. Inflammation in Myocardial Ischemia/Reperfusion Injury: Underlying Mechanisms and Therapeutic Potential. Antioxidants 2023, 12, 1944. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Schofield, Z.V.; Woodruff, T.M.; Halai, R.; Wu, M.C.; Cooper, M.A. Neutrophils--a key component of ischemia-reperfusion injury. Shock 2013, 40, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Montone, R.A. Pathophysiology of coronary microvascular dysfunction. Vasc. Pharmacol. 2023, 153, 107239. [Google Scholar] [CrossRef]
- Pluijmert, N.J.; Atsma, D.E.; Quax, P.H.A. Post-ischemic Myocardial Inflammatory Response: A Complex and Dynamic Process Susceptible to Immunomodulatory Therapies. Front. Cardiovasc. Med. 2021, 8, 647785. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; van Geuns, R.M.; Berry, C.; Riksen, N.P.; Escaned, J.; van Royen, N. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef]
- Zhou, H.; Toan, S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules 2020, 10, 85. [Google Scholar] [CrossRef]
- Wang, J.; Toan, S.; Zhou, H. Mitochondrial quality control in cardiac microvascular ischemia-reperfusion injury: New insights into the mechanisms and therapeutic potentials. Pharmacol. Res. 2020, 156, 104771. [Google Scholar] [CrossRef]
- Wang, D.; Tian, Z.; Zhang, P.; Zhen, L.; Meng, Q.; Sun, B.; Xu, X.; Jia, T.; Li, S. The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease. Biomed. Pharmacother. 2023, 163, 114830. [Google Scholar] [CrossRef]
- You, N.; Liu, G.; Yu, M.; Chen, W.; Fei, X.; Sun, T.; Han, M.; Qin, Z.; Wei, Z.; Wang, D. Reconceptualizing Endothelial-to-mesenchymal transition in atherosclerosis: Signaling pathways and prospective targeting strategies. J. Adv. Res. 2025, 3. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, F.; Guo, Q.; Li, M.; Wang, L.; Zhang, Z.; Jiang, S.; Jin, H.; Chen, A.; Tan, S.; et al. Curcumol induces RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling in hepatic stellate cells. Redox Biol. 2018, 19, 375–387. [Google Scholar] [CrossRef]
- Ying, L.; Benjanuwattra, J.; Chattipakorn, S.C.; Chattipakorn, N. The role of RIPK3-regulated cell death pathways and necroptosis in the pathogenesis of cardiac ischaemia-reperfusion injury. Acta Physiol. 2021, 231, e13541. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, H.; Wang, S.; Tang, G.; Zhai, C.; Shen, L. Ferroptosis: A Novel Therapeutic Target for Ischemia-Reperfusion Injury. Front. Cell Dev. Biol. 2021, 9, 688605. [Google Scholar] [CrossRef]
- Shen, W.C.; Li, H.Y.; Chen, G.C.; Chern, Y.; Tu, P.H. Mutations in the ubiquitin-binding domain of OPTN/optineurin interfere with autophagy-mediated degradation of misfolded proteins by a dominant-negative mechanism. Autophagy 2015, 11, 685–700. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Kumar, R.A.; Thome, T.; Sharaf, O.M.; Ryan, T.E.; Arnaoutakis, G.J.; Jeng, E.I.; Ferreira, L.F. Reversible Thiol Oxidation Increases Mitochondrial Electron Transport Complex Enzyme Activity but Not Respiration in Cardiomyocytes from Patients with End-Stage Heart Failure. Cells 2022, 11, 2292. [Google Scholar] [CrossRef]
- Kura, B.; Slezak, J. The Protective Role of Molecular Hydrogen in Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2024, 25, 7884. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Liu, G.; Yao, K.; Tan, B.; Yin, Y. Mitochondria-Targeted Antioxidants: A Step towards Disease Treatment. Oxidative Med. Cell. Longev. 2020, 2020, 8837893. [Google Scholar] [CrossRef]
- Ma, L.; Zou, R.; Shi, W.; Zhou, N.; Chen, S.; Zhou, H.; Chen, X.; Wu, Y. SGLT2 inhibitor dapagliflozin reduces endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury through normalizing the XO-SERCA2-CaMKII-coffilin pathways. Theranostics 2022, 12, 5034–5050. [Google Scholar] [CrossRef]
- Bekkers Sebastiaan, C.A.M.; Yazdani Saami, K.; Virmani, R.; Waltenberger, J. Microvascular Obstruction. JACC 2010, 55, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.; Dick, A.; Strauss, B.H. Prevention and Treatment of Microvascular Obstruction-Related Myocardial Injury and Coronary No-Reflow Following Percutaneous Coronary Intervention: A Systematic Approach. JACC Cardiovasc. Interv. 2010, 3, 695–704. [Google Scholar] [CrossRef]
- Zhang, C.X.; Cheng, Y.; Liu, D.Z.; Liu, M.; Cui, H.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J. Nanobiotechnol. 2019, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Anzell, A.R.; Maizy, R.; Przyklenk, K.; Sanderson, T.H. Mitochondrial Quality Control and Disease: Insights into Ischemia-Reperfusion Injury. Mol. Neurobiol. 2018, 55, 2547–2564. [Google Scholar] [CrossRef]
- Uchikado, Y.; Ikeda, Y.; Ohishi, M. Current Understanding of the Pivotal Role of Mitochondrial Dynamics in Cardiovascular Diseases and Senescence. Front. Cardiovasc. Med. 2022, 9, 905072. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Shi, H.; Li, F.; Duan, Y.; Guo, Q. New insights into the role of mitochondrial dynamics in oxidative stress-induced diseases. Biomed. Pharmacother. 2024, 178, 117084. [Google Scholar] [CrossRef]
- Ong, S.B.; Kwek, X.Y.; Katwadi, K.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Ismail, N.I.; Lin, Y.H.; Yap, E.P.; Lim, S.Y.; Ja, K.; et al. Targeting Mitochondrial Fission Using Mdivi-1 in A Clinically Relevant Large Animal Model of Acute Myocardial Infarction: A Pilot Study. Int. J. Mol. Sci. 2019, 20, 3972. [Google Scholar] [CrossRef]
- Kulek, A.R.; Anzell, A.; Wider, J.M.; Sanderson, T.H.; Przyklenk, K. Mitochondrial Quality Control: Role in Cardiac Models of Lethal Ischemia-Reperfusion Injury. Cells 2020, 9, 214. [Google Scholar] [CrossRef]
- Peletier, M.; Zhang, X.; Klein, S.; Kroon, J. Multicellular 3D models to study myocardial ischemia–reperfusion injury. Front. Cell Dev. Biol. 2024, 12, 1494911. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, X.; Zhang, Y.; Xia, Z. Abnormalities of glucose and lipid metabolism in myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2023, 163, 114827. [Google Scholar] [CrossRef]
- Watkins, S.J.; Borthwick, G.M.; Arthur, H.M. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 125–131. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Zhang, X.; Liu, G.; Yue, X. Isoflurane reduces oxygen-glucose deprivation-induced oxidative, inflammatory, and apoptotic responses in H9c2 cardiomyocytes. Am. J. Transl. Res. 2016, 8, 2597–2608. [Google Scholar]
- An, Y.; Talwar, C.S.; Park, K.-H.; Ahn, W.-C.; Lee, S.-J.; Go, S.-R.; Cho, J.H.; Kim, D.Y.; Kim, Y.-S.; Cho, S.; et al. Design of hypoxia responsive CRISPR-Cas9 for target gene regulation. Sci. Rep. 2023, 13, 16763. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Li, Y.; Yang, X.; Geng, H.; Lu, X.; Wang, L.; Yang, Z. Protective effects of IL28RA siRNA on cardiomyocytes in hypoxia/reoxygenation injury. Anatol. J. Cardiol. 2017, 18, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Hall, A.R.; Bell, R.M.; Yellon, D.M. Characterization of the Langendorff Perfused Isolated Mouse Heart Model of Global Ischemia–Reperfusion Injury:Impact of Ischemia and Reperfusion Length on Infarct Size and LDH Release. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 286–295. [Google Scholar] [CrossRef]
- Davis, A.; Morris, K.V.; Shevchenko, G. Hypoxia-directed tumor targeting of CRISPR-Cas9 and HSV-TK suicide gene therapy using lipid nanoparticles. Mol. Ther. Methods Clin. Dev. 2022, 25, 158–169. [Google Scholar] [CrossRef]
- Jia, T.; Wang, C.; Han, Z.; Wang, X.; Ding, M.; Wang, Q. Experimental Rodent Models of Cardiovascular Diseases. Front. Cardiovasc. Med. 2020, 7, 588075. [Google Scholar] [CrossRef]
- Sharma, J.; Bhargava, P.; Mishra, P.; Bhatia, J.; Arya, D.S. Molecular mechanisms of flavonoids in myocardial ischemia reperfusion injury: Evidence from in-vitro and in-vivo studies. Vasc. Pharmacol. 2024, 155, 107378. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Brunt, K.R.; Kirk, J.A.; Kleinbongard, P.; Calvert, J.W.; de Castro Brás, L.E.; DeLeon-Pennell, K.Y.; Del Re, D.P.; Frangogiannis, N.G.; Frantz, S.; et al. Guidelines for in vivo mouse models of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H1056–H1073. [Google Scholar] [CrossRef]
- Tao, Z.; Lin, R.; Zhang, R.; He, P.; Lei, C.; Li, Y. Ischemia reperfusion myocardium injuries in type 2 diabetic rats: Effects of ketamine and insulin on LC3-II and mTOR expression. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231196450. [Google Scholar] [CrossRef]
- Snoeckx, L.H.; van der Vusse, G.J.; Coumans, W.A.; Willemsen, P.H.; van der Nagel, T.; Reneman, R.S. Myocardial function in normal and spontaneously hypertensive rats during reperfusion after a period of global ischaemia. Cardiovasc. Res. 1986, 20, 67–75. [Google Scholar] [CrossRef]
- Zaragoza, C.; Gomez-Guerrero, C.; Martin-Ventura, J.L.; Blanco-Colio, L.; Lavin, B.; Mallavia, B.; Tarin, C.; Mas, S.; Ortiz, A.; Egido, J. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011, 2011, 497841. [Google Scholar] [CrossRef]
- Lim, K.; Modi, P.; Nicholson, E.; Lawrance, L.; Halestrap, A.; Angelini, G.; Suleiman, M. A pig model of warm-blood cardioplegic arrest to investigate the cardioprotective effects of propofol. J. Physiol. 2001, 536, 82–83. [Google Scholar]
- Baehr, A.; Klymiuk, N.; Kupatt, C. Evaluating novel targets of ischemia reperfusion injury in pig models. Int. J. Mol. Sci. 2019, 20, 4749. [Google Scholar] [CrossRef] [PubMed]
- Milani-Nejad, N.; Janssen, P.M. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Lerchenmüller, C.; Hastings, M.H.; Rabolli, C.P.; Betge, F.; Roshan, M.; Liu, L.X.; Liu, X.; Heß, C.; Roh, J.D.; Platt, C.; et al. CITED4 gene therapy protects against maladaptive cardiac remodeling after ischemia/reperfusion injury in mice. Mol. Ther. 2024, 32, 3683–3694. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Scalise, M.; Marino, F.; Salerno, L.; Cianflone, E.; Molinaro, C.; Salerno, N.; De Angelis, A.; Viglietto, G.; Urbanek, K.; Torella, D. From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish. Int. J. Mol. Sci. 2021, 22, 13180. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.e22. [Google Scholar] [CrossRef]
- Chen, T.; Vunjak-Novakovic, G. Human Tissue-Engineered Model of Myocardial Ischemia-Reperfusion Injury. Tissue Eng. Part A 2019, 25, 711–724. [Google Scholar] [CrossRef]
- Grass, M.; McDougal, A.D.; Blazeski, A.; Kamm, R.D.; García-Cardeña, G.; Dewey, C.F., Jr. A computational model of cardiomyocyte metabolism predicts unique reperfusion protocols capable of reducing cell damage during ischemia/reperfusion. J. Biol. Chem. 2022, 298, 101693. [Google Scholar] [CrossRef]
- Trayanova, N.A.; Lyon, A.; Shade, J.; Heijman, J. Computational modeling of cardiac electrophysiology and arrhythmogenesis: Toward clinical translation. Physiol. Rev. 2024, 104, 1265–1333. [Google Scholar] [CrossRef]
- Wu, X.Y.; Luo, A.Y.; Zhou, Y.R.; Ren, J.H. N-acetylcysteine reduces oxidative stress, nuclear factor-κB activity and cardiomyocyte apoptosis in heart failure. Mol. Med. Rep. 2014, 10, 615–624. [Google Scholar] [CrossRef]
- Khan, S.A.; Campbell, A.M.; Lu, Y.; An, L.; Alpert, J.S.; Chen, Q.M. N-Acetylcysteine for Cardiac Protection During Coronary Artery Reperfusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 752939. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.; Marcuzzi, A.; Gonelli, A.; Celeghini, C.; Maximova, N.; Rimondi, E. Mitochondria-Targeted Antioxidants, an Innovative Class of Antioxidant Compounds for Neurodegenerative Diseases: Perspectives and Limitations. Int. J. Mol. Sci. 2023, 24, 3739. [Google Scholar] [CrossRef]
- Apostolova, N.; Victor, V.M. Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications. Antioxid. Redox Signal. 2014, 22, 686–729. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.I.; Anto Michel, N.; Katwadi, K.; Mim, M.; Chan, T.-K.; Rahman, A.; Xu, D.; Ong, S.-G.; Hausenloy, D.; Ong, S.-B. Ischemic Preconditioning and Postconditioning Protect the Heart by Preserving the Mitochondrial Network. BioMed Res. Int. 2022, 2022, 6889278. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Vander Heide, R.S.; Steenbergen, C. Cardioprotection and myocardial reperfusion: Pitfalls to clinical application. Circ. Res. 2013, 113, 464–477. [Google Scholar] [CrossRef]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Clemmensen, P.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamaki, K.; et al. Effect of Ischemic Postconditioning During Primary Percutaneous Coronary Intervention for Patients With ST-Segment Elevation Myocardial Infarction: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 490–497. [Google Scholar] [CrossRef]
- Manintveld, O.C.; te Lintel Hekkert, M.; van den Bos, E.J.; Suurenbroek, G.M.; Dekkers, D.H.; Verdouw, P.D.; Lamers, J.M.; Duncker, D.J. Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H1551–H1560. [Google Scholar] [CrossRef]
- Reyes Gaido, O.E.; Anderson, M.E. CRISPR Editing Takes Aim at Ischemia/Reperfusion Injury. JAMA Cardiol. 2023, 8, 522–523. [Google Scholar] [CrossRef]
- Xu, C.; Lu, Z.; Luo, Y.; Liu, Y.; Cao, Z.; Shen, S.; Li, H.; Liu, J.; Chen, K.; Chen, Z.; et al. Targeting of NLRP3 inflammasome with gene editing for the amelioration of inflammatory diseases. Nat. Commun. 2018, 9, 4092. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Burns, N.; Zhao, K.S.; Song, R. SIRT1 is required for mitochondrial biogenesis reprogramming in hypoxic human pulmonary arteriolar smooth muscle cells. Int. J. Mol. Med. 2017, 39, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-R.; Park, B.-W.; Kim, J.; Choo, Y.W.; Kim, H.Y.; Yoon, J.-K.; Kim, H.; Hwang, J.-W.; Kang, M.; Kwon, S.P.; et al. Nanovesicles derived from iron oxide nanoparticles–incorporated mesenchymal stem cells for cardiac repair. Sci. Adv. 2020, 6, eaaz0952. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Y.; Lv, K.; Wang, Y.; Zhong, Z.; Xiao, C.; Zhu, K.; Ni, C.; Wang, K.; Kong, M.; et al. Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci. Transl. Med. 2021, 13, eabb0202. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Madonna, R.; Van Laake, L.W.; Davidson, S.M.; Engel, F.B.; Hausenloy, D.J.; Lecour, S.; Leor, J.; Perrino, C.; Schulz, R.; Ytrehus, K.; et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef]
- Mohammed, O.A.; Alghamdi, M.; Alfaifi, J.; Alamri, M.M.S.; Al-Shahrani, A.M.; Alharthi, M.H.; Alshahrani, A.M.; Alhalafi, A.H.; Adam, M.I.E.; Bahashwan, E.; et al. The emerging role of miRNAs in myocardial infarction: From molecular signatures to therapeutic targets. Pathol. Res. Pract. 2024, 253, 155087. [Google Scholar] [CrossRef]
- Sharma, A.; Lysenko, A.; Jia, S.; Boroevich, K.A.; Tsunoda, T. Advances in AI and machine learning for predictive medicine. J. Hum. Genet. 2024, 69, 487–497. [Google Scholar] [CrossRef]
- Peng, X.; Du, J.; Wang, Y. Metabolic signatures in post-myocardial infarction heart failure, including insights into prediction, intervention, and prognosis. Biomed. Pharmacother. 2024, 170, 116079. [Google Scholar] [CrossRef]
- Binek, A.; Fernández-Jiménez, R.; Jorge, I.; Camafeita, E.; López, J.A.; Bagwan, N.; Galán-Arriola, C.; Pun, A.; Agüero, J.; Fuster, V.; et al. Proteomic footprint of myocardial ischemia/reperfusion injury: Longitudinal study of the at-risk and remote regions in the pig model. Sci. Rep. 2017, 7, 12343. [Google Scholar] [CrossRef]
- Lai, L.; Leone, T.C.; Keller, M.P.; Martin, O.J.; Broman, A.T.; Nigro, J.; Kapoor, K.; Koves, T.R.; Stevens, R.; Ilkayeva, O.R.; et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: A multisystems approach. Circ. Heart Fail. 2014, 7, 1022–1031. [Google Scholar] [CrossRef]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef]
- Skourtis, D.; Stavroulaki, D.; Athanasiou, V.; Fragouli, P.G.; Iatrou, H. Nanostructured Polymeric, Liposomal and Other Materials to Control the Drug Delivery for Cardiovascular Diseases. Pharmaceutics 2020, 12, 1160. [Google Scholar] [CrossRef]
- Song, L.; Jia, K.; Yang, F.; Wang, J. Advanced Nanomedicine Approaches for Myocardial Infarction Treatment. Int. J. Nanomed. 2024, 19, 6399–6425. [Google Scholar] [CrossRef]
- Berg, J.; Jablonowski, R.; Nordlund, D.; Ryd, D.; Heiberg, E.; Carlsson, M.; Arheden, H. Mild hypothermia attenuates ischaemia/reperfusion injury: Insights from serial non-invasive pressure-volume loops. Cardiovasc. Res. 2023, 119, 2230–2243. [Google Scholar] [CrossRef]
- Atti, V.; Narayanan, M.A.; Patel, B.; Balla, S.; Siddique, A.; Lundgren, S.; Velagapudi, P. A Comprehensive Review of Mechanical Circulatory Support Devices. Heart Int. 2022, 16, 37–48. [Google Scholar] [CrossRef]
- Baran, D.A.; Jaiswal, A.; Hennig, F.; Potapov, E. Temporary mechanical circulatory support: Devices, outcomes, and future directions. J. Heart Lung Transplant. 2022, 41, 678–691. [Google Scholar] [CrossRef]
- Vasu, S.; Zhou, J.; Chen, J.; Johnston, P.V.; Kim, D.H. Biomaterials-based Approaches for Cardiac Regeneration. Korean Circ. J. 2021, 51, 943–960. [Google Scholar] [CrossRef]
- Yu, T.; Xu, Q.; Chen, X.; Deng, X.; Chen, N.; Kou, M.T.; Huang, Y.; Guo, J.; Xiao, Z.; Wang, J. Biomimetic nanomaterials in myocardial infarction treatment: Harnessing bionic strategies for advanced therapeutics. Mater. Today Bio 2024, 25, 100957. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Bai, Y.; Wei, Y. Application of biomedical materials in the diagnosis and treatment of myocardial infarction. J. Nanobiotechnol. 2023, 21, 298. [Google Scholar] [CrossRef]
- Zheng, M.; Bai, Y.; Sun, X.; Fu, R.; Liu, L.; Liu, M.; Li, Z.; Huang, X. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α Pathway. Molecules 2022, 27, 5545. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-T.; Qiu, Z.-C.; Xu, Z.-Q.; Tao, E.-D.; Qiu, R.-B.; Peng, H.-Z.; Zhou, L.-F.; Zeng, R.-Y.; Lai, S.-Q.; Wan, L. Curcumin attenuates myocardial ischemia-reperfusion-induced autophagy-dependent ferroptosis via Sirt1/AKT/FoxO3a signaling. Int. J. Mol. Med. 2025, 55, 51. [Google Scholar] [CrossRef] [PubMed]
- Abdulredha, A.; Abosaooda, M.; Al-Amran, F.; Hadi, N.R. Berberine Protests the Heart from Ischemic Reperfusion Injury via Interference with Oxidative and Inflammatory Pathways. Med. Arch. 2021, 75, 174–179. [Google Scholar] [CrossRef]
- Azizidoost, S.; Adelipour, M.; Haybar, H.; Shabaninejad, Z.; Rashidi, M. Implications of Quercetin in Mitigating Myocardial Ischemia-Reperfusion Injury: A Review Study. Adv. Biomed. Res. 2025, 14, 17. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Med 2024, 5, 10–31. [Google Scholar] [CrossRef]
- Dirksen, M.T.; Laarman, G.J.; Simoons, M.L.; Duncker, D.J.G.M. Reperfusion injury in humans: A review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc. Res. 2007, 74, 343–355. [Google Scholar] [CrossRef]
- Sufian, M.A.; Hamzi, W.; Zaman, S.; Alsadder, L.; Hamzi, B.; Varadarajan, J.; Azad, M.A.K. Enhancing Clinical Validation for Early Cardiovascular Disease Prediction through Simulation, AI, and Web Technology. Diagnostics 2024, 14, 1308. [Google Scholar] [CrossRef]
- Casotti, M.C.; Meira, D.D.; Alves, L.N.R.; Bessa, B.G.O.; Campanharo, C.V.; Vicente, C.R.; Aguiar, C.C.; Duque, D.A.; Barbosa, D.G.; Santos, E.; et al. Translational Bioinformatics Applied to the Study of Complex Diseases. Genes 2023, 14, 419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsadder, L.; Hamadah, A. Cardiac Ischaemia–Reperfusion Injury: Pathophysiology, Therapeutic Targets and Future Interventions. Biomedicines 2025, 13, 2084. https://doi.org/10.3390/biomedicines13092084
Alsadder L, Hamadah A. Cardiac Ischaemia–Reperfusion Injury: Pathophysiology, Therapeutic Targets and Future Interventions. Biomedicines. 2025; 13(9):2084. https://doi.org/10.3390/biomedicines13092084
Chicago/Turabian StyleAlsadder, Lujain, and Abdulaziz Hamadah. 2025. "Cardiac Ischaemia–Reperfusion Injury: Pathophysiology, Therapeutic Targets and Future Interventions" Biomedicines 13, no. 9: 2084. https://doi.org/10.3390/biomedicines13092084
APA StyleAlsadder, L., & Hamadah, A. (2025). Cardiac Ischaemia–Reperfusion Injury: Pathophysiology, Therapeutic Targets and Future Interventions. Biomedicines, 13(9), 2084. https://doi.org/10.3390/biomedicines13092084






