Endovascular Treatment Outcomes for TASC C and D Lesions in Chronic Peripheral Arterial Disease: A Retrospective Study and Literature Review
Abstract
1. Introduction
2. Materials and Methods
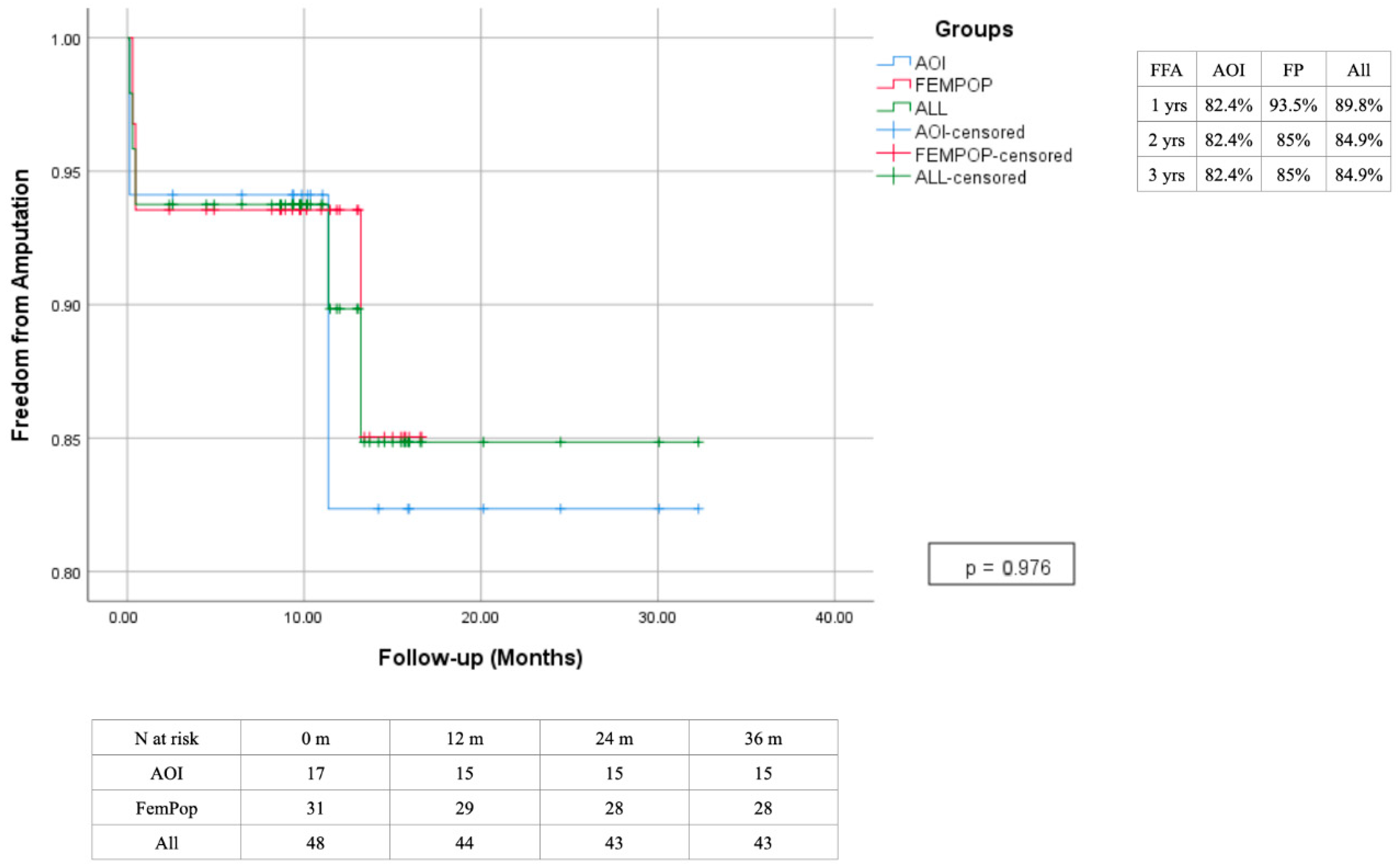
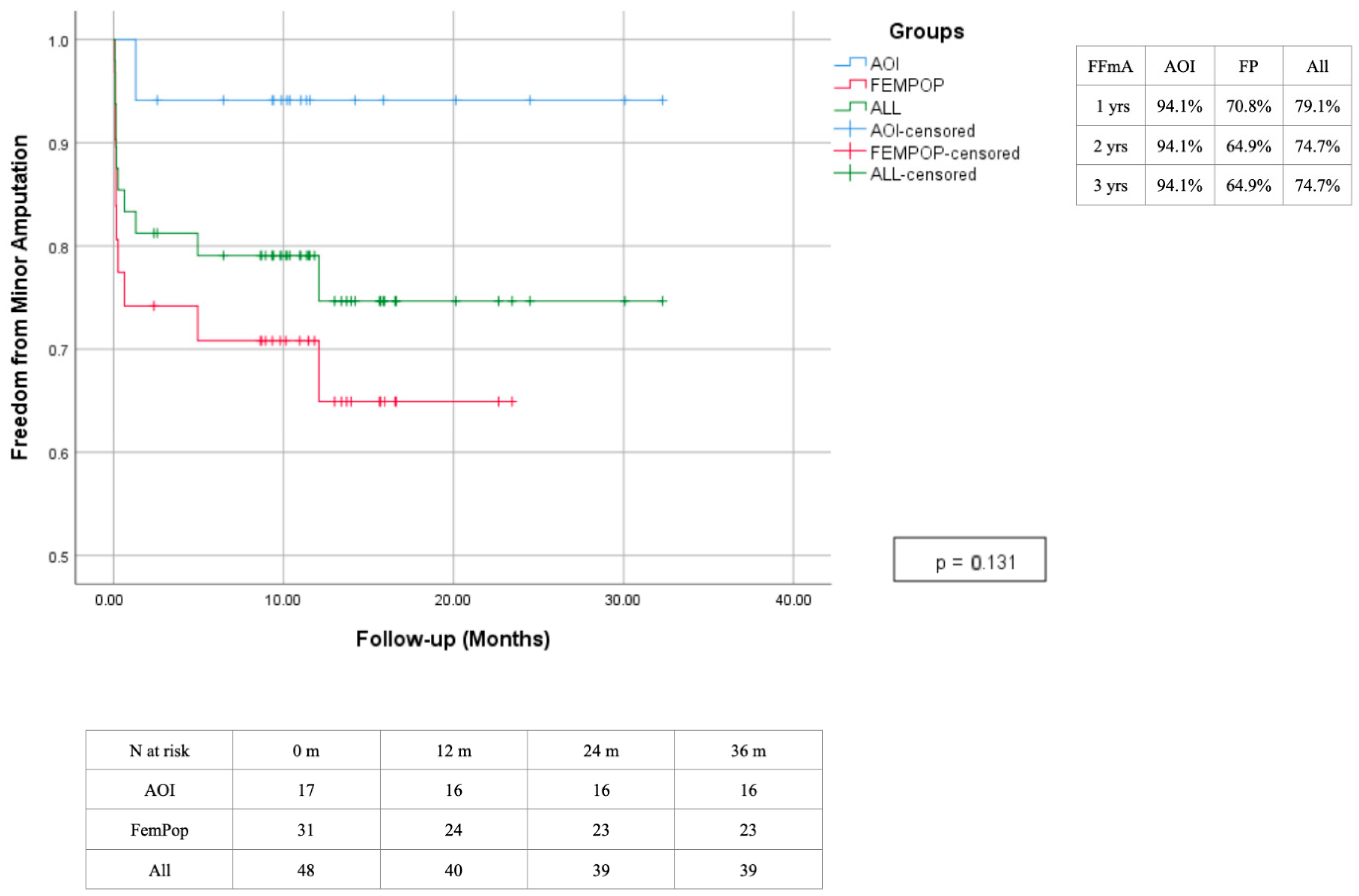
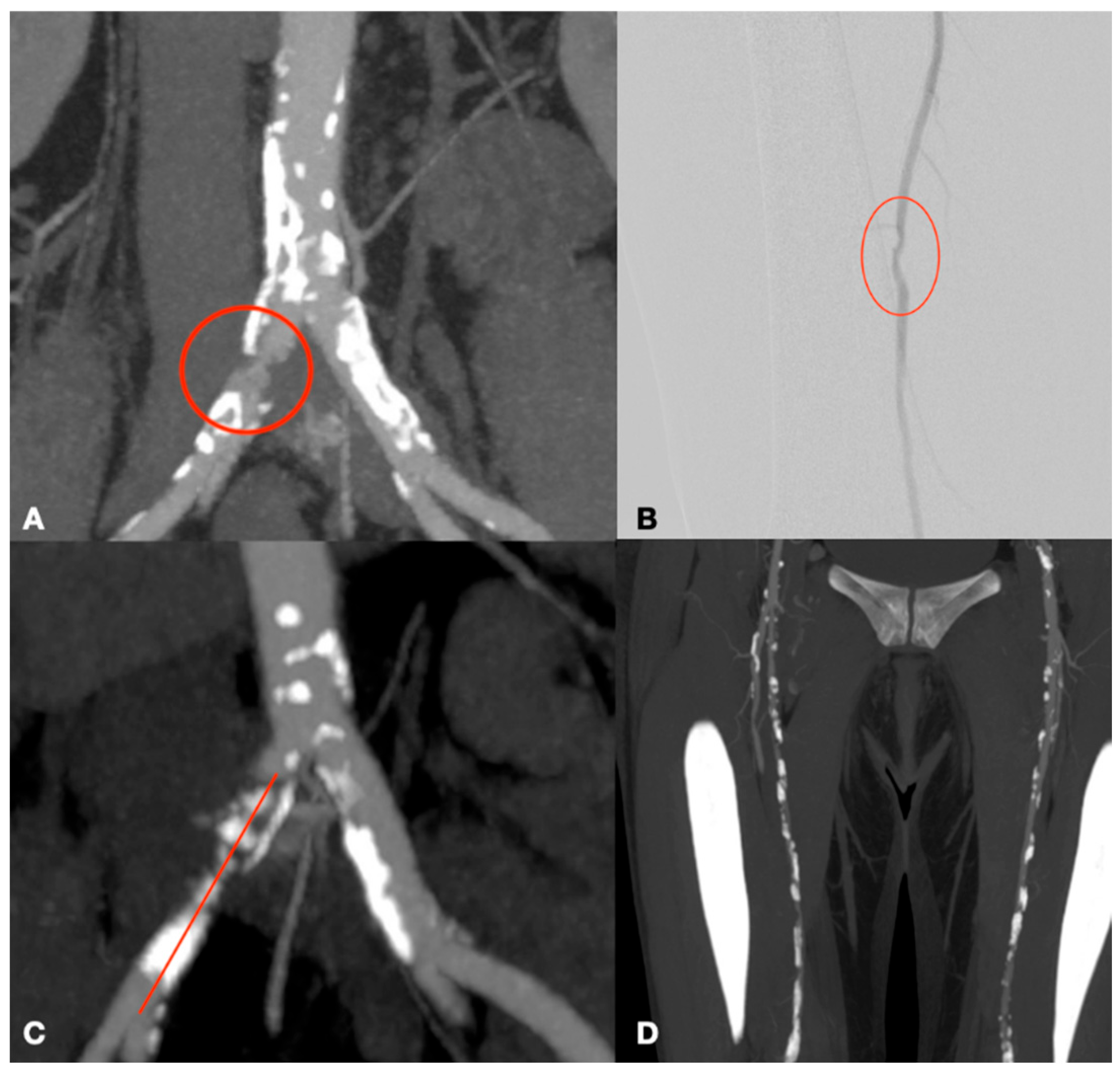
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinoto, E.; Bajardi, G.; La Marca, M.; Ferlito, F.; Mirabella, D.; Pecoraro, F. Composite femoro-tibial bypass as alternative solution in complicated revascolarization: Case report. Int. J. Surg. Case Rep. 2021, 84, 106103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kannel, W.B.; McGee, D.L. Update on some epidemiologic features of intermittent claudication: The framingham study. J. Am. Geriatr. Soc. 1985, 33, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Dinoto, E.; Ferlito, F.; La Marca, M.A.; Tortomasi, G.; Urso, F.; Evola, S.; Guercio, G.; Marcianò, M.; Pakeliani, D.; Bajardi, G.; et al. The Role of Early Revascularization and Biomarkers in the Management of Diabetic Foot Ulcers: A Single Center Experience. Diagnostics 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freyermuth, M.; Roisin, S.; Saidak, Z.; Matray, L.; Sevestre, M.A.; Reix, T.; Soudet, S. Contemporary Minimally Invasive Surgery for TASC-D Aorto-Iliac Lesions: Analysis of Outcomes and Risk Factors for Primary and Secondary Patency. Ann. Vasc. Surg. 2023, 97, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Dinoto, E.; Pecoraro, F.; Ferlito, F.; Tortomasi, G.; Mirabella, D.; Bajardi, G. Multilevel diabetic foot revascularization in COVID 19 patient: Case report. Int. J. Surg. Case Rep. 2021, 84, 106132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pecoraro, F.; Pakeliani, D.; Bruno, S.; Dinoto, E.; Ferlito, F.; Mirabella, D.; Lachat, M.; Cudia, B.; Bajardi, G. Simultaneous Hybrid Treatment of Multilevel Peripheral Arterial Disease in Patients with Chronic Limb-Threatening Ischemia. J. Clin. Med. 2021, 10, 2865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinoto, E.; Pecoraro, F.; Mirabella, D.; Ferlito, F.; Farina, A.; Lo Biundo, N.; Orlando-Conti, P.; Bajardi, G. A Single-Center Experience on Below-The-Knee Endovascular Treatment in Diabetic Patients. Transl. Med. UniSa 2020, 21, 21–23. [Google Scholar] [PubMed] [PubMed Central]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef]
- Doyle, D.J.; Hendrix, J.M.; Garmon, E.H. American Society of Anesthesiologists Classification. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441940/ (accessed on 11 February 2025).
- Humphries, M.D.; Armstrong, E.; Laird, J.; Paz, J.; Pevec, W. Outcomes of covered versus bare-metal balloon-expandable stents for aortoiliac occlusive disease. J. Vasc. Surg. 2014, 60, 337–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehrotra, S.; Paramasivam, G.; Mishra, S. Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. Curr. Cardiol. Rep. 2017, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, S.; Katz, S.; Ochoa, C. Long-Term Patency and Clinical Outcomes of Nitinol Stenting for Femoropopliteal Atherosclerotic Disease. Ann. Vasc. Surg. 2020, 66, 566–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pecoraro, F.; Volpe, P.; Boccalon, L.; Migliara, B.; Rivolta, N.; Silvestro, A.; Trabattoni, P.L.M.; Massara, M.; Diaco, D.A.; Dinoto, E.; et al. Outcome Analysis From a Multicenter Registry on Unibody Stent-Graft System for the Treatment of Spontaneous Infrarenal Acute Aortic Syndrome (MURUSSIAS Registry). J. Endovasc. Ther. 2024, 31, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; McCarthy, R.; Hardman, J.; Chalmers, A.; Horrocks, M. The increasing role of percutaneous transluminal angioplasty in the primary management of critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Dosluoglu, H.H.; Lall, P.; Cherr, G.S.; Harris, L.M.; Dryjski, M.L. Role of simple and complex hybrid revascularization procedures for symptomatic lower extremity occlusive disease. J. Vasc. Surg. 2010, 51, 1425–1435.e1. [Google Scholar] [CrossRef] [PubMed]
- Dosluoglu, H.H.; O’brien-Irr, M.S.; Lukan, J.; Harris, L.M.; Dryjski, M.L.; Cherr, G.S. Does preferential use of endovascular interventions by vascular surgeons improve limb salvage, control of symptoms, and survival of patients with critical limb ischemia? Am. J. Surg. 2006, 192, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.J.; Beard, J.D.; Cleveland, T.; Bell, J.; Bradbury, A.W.; Forbes, J.F.; Fowkes, F.G.R.; Gillepsie, I.; Ruckley, C.V.; Raab, G.; et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet 2005, 366, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Parwani, D.; A Ahmed, M.; Mahawar, A.; Gorantla, V.R. Peripheral Arterial Disease: A Narrative Review. Cureus 2023, 15, e40267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, L.; Paravastu, S.C.V.; Thomas, S.D.; Tan, E.; Farmer, E.; Varcoe, R.L. Cost Analysis of Initial Treatment With Endovascular Revascularization, Open Surgery, or Primary Major Amputation in Patients With Peripheral Artery Disease. J. Endovasc. Ther. 2018, 25, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Park, K.-M.; Kim, Y.K.; Kim, J.I.; Moon, I.S.; Hong, K.C.; Jeon, Y.S.; Kim, J.Y. Outcomes of endovascular treatment for TASC C and D aorto-iliac lesions. Asian J. Surg. 2017, 40, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Fazzini, S.; Pennetta, F.F.; Torsello, G.; Turriziani, V.; Vona, S.; Marchetti, A.A.; Ippoliti, A.; Austermann, M.; Bosiers, M.J. Intravascular Iliac Artery Lithotripsy to Facilitate Aortic Endograft Delivery: Midterm Results of a Dual-Center Experience. J. Endovasc. Ther. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, N.; Tsilimparis, N.; Stavroulakis, K. Intravascular Lithotripsy and Aortic Bare-Metal Stenting: A Low-Profile Solution for the Treatment of Heavily Calcified Aorto-Iliac Disease. J. Endovasc. Ther. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Leville, C.D.; Kashyap, V.S.; Clair, D.G.; Bena, J.F.; Lyden, S.P.; Greenberg, R.K.; O’hAra, P.J.; Sarac, T.P.; Ouriel, K. Endovascular management of iliac artery occlusions: Extending treatment to TransAtlantic Inter-Society Consensus class C and D patients. J. Vasc. Surg. 2006, 43, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Bracale, U.M.; Giribono, A.M.; Spinelli, D.; Del Guercio, L.; Pipitò, N.; Ferrara, D.; Barillà, D.; Barbarisi, D.; Derone, G.; Benedetto, F. Long-term Results of Endovascular Treatment of TASC C and D Aortoiliac Occlusive Disease with Expanded Polytetrafluoroethylene Stent Graft. Ann. Vasc. Surg. 2019, 56, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Faglia, E.; Paola, L.D.; Clerici, G.; Clerissi, J.; Graziani, L.; Fusaro, M.; Gabrielli, L.; Losa, S.; Stella, A.; Gargiulo, M.; et al. Peripheral angioplasty as the first-choice revascularization procedure in diabetic patients with critical limb ischemia: Prospective study of 993 consecutive patients hospitalized and followed between 1999 and 2003. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Faglia, E.; Mantero, M.; Caminiti, M.; CaravaggI, C.; De Giglio, R.; Pritelli, C.; Clerici, G.; Fratino, P.; De Cata, P.; Paola, L.D.; et al. Extensive use of peripheral angioplasty, particularly infrapopliteal, in the treatment of ischaemic diabetic foot ulcers: Clinical results of a multicentric study of 221 consecutive diabetic subjects. J. Intern. Med. 2002, 252, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Graziani, L.; Silvestro, A.; Bertone, V.; Manara, E.; Andreini, R.; Sigala, A.; Mingardi, R.; De Giglio, R. Vascular Involvement in diabetic subjects with ischemic foot ulcer: A new morphologic categorization of disease severity. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Elwan, H.; Eldaly, W.; Khairy, H.; Taha, A.; Gad, A.; Ghoneim, B. Management of critical lower limb ischemia in endovascular era: Experience from 511 Patients. Int. J. Angiol. 2014, 23, 197–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iida, O.; Soga, Y.; Hirano, K.; Kawasaki, D.; Suzuki, K.; Miyashita, Y.; Nanto, S.; Uematsu, M. Midterm outcomes and risk stratification after endovascular therapy for patients with critical limb ischaemia due to isolated below-the-knee lesions. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Iida, O.; Shiraki, T.; Soga, Y.; Hirano, K.; Suzuki, K.; Yamaoka, T.; Miyashita, Y.; Kitayama, M.; Kajinami, K. Clinical characteristics of patients with Rutherford category IV, compared with V and VI. SAGE Open Med. 2015, 3, 2050312115597087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kohler, C.; Gaizauskaite, K.; Kotopoulos, K.; Kotelis, D.; Schmidli, J.; Makaloski, V.; Weiss, S. Amputation-Free Survival, WIfI Stage, and GLASS Classifications in Distal Crural or Pedal Bypass for Chronic Limb-Threatening Ischemia. J. Clin. Med. 2024, 13, 6649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheun, T.J.; Hart, J.P.; Davies, M.G. The Value of Restaging WIfI (Wound, Ischemia, and Foot Infection) After Initial Vascular and Podiatric Intervention. Ann. Vasc. Surg. 2024, 111, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lou, V.; Dossabhoy, S.S.; Tran, K.; Yawary, F.; Ross, E.G.; Stern, J.R.; Dalman, R.L.; Chandra, V. Validity of the Global Vascular Guidelines in Predicting Outcomes Based on First-Time Revascularization Strategy. Ann. Vasc. Surg. 2023, 95, 142–153. [Google Scholar] [CrossRef] [PubMed]
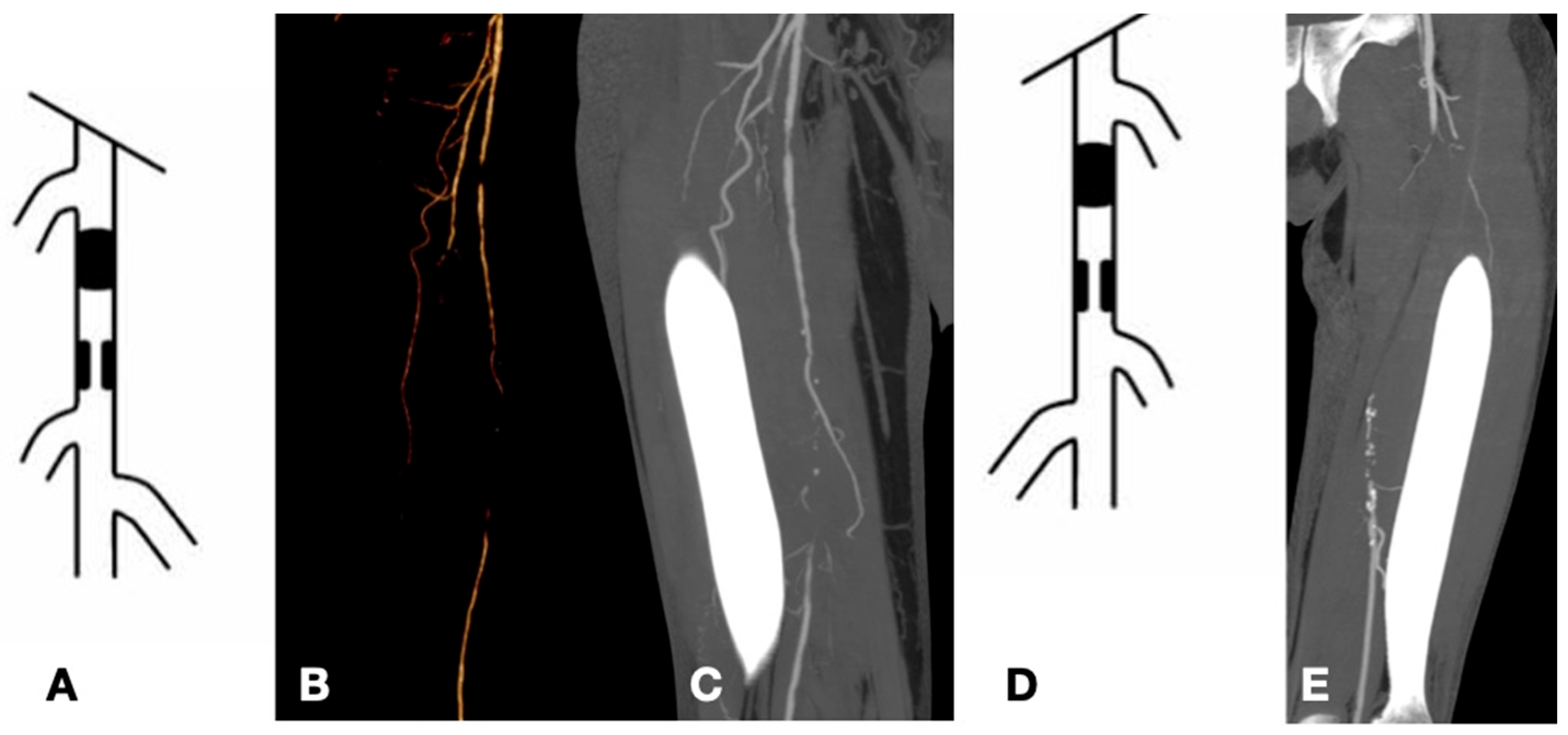
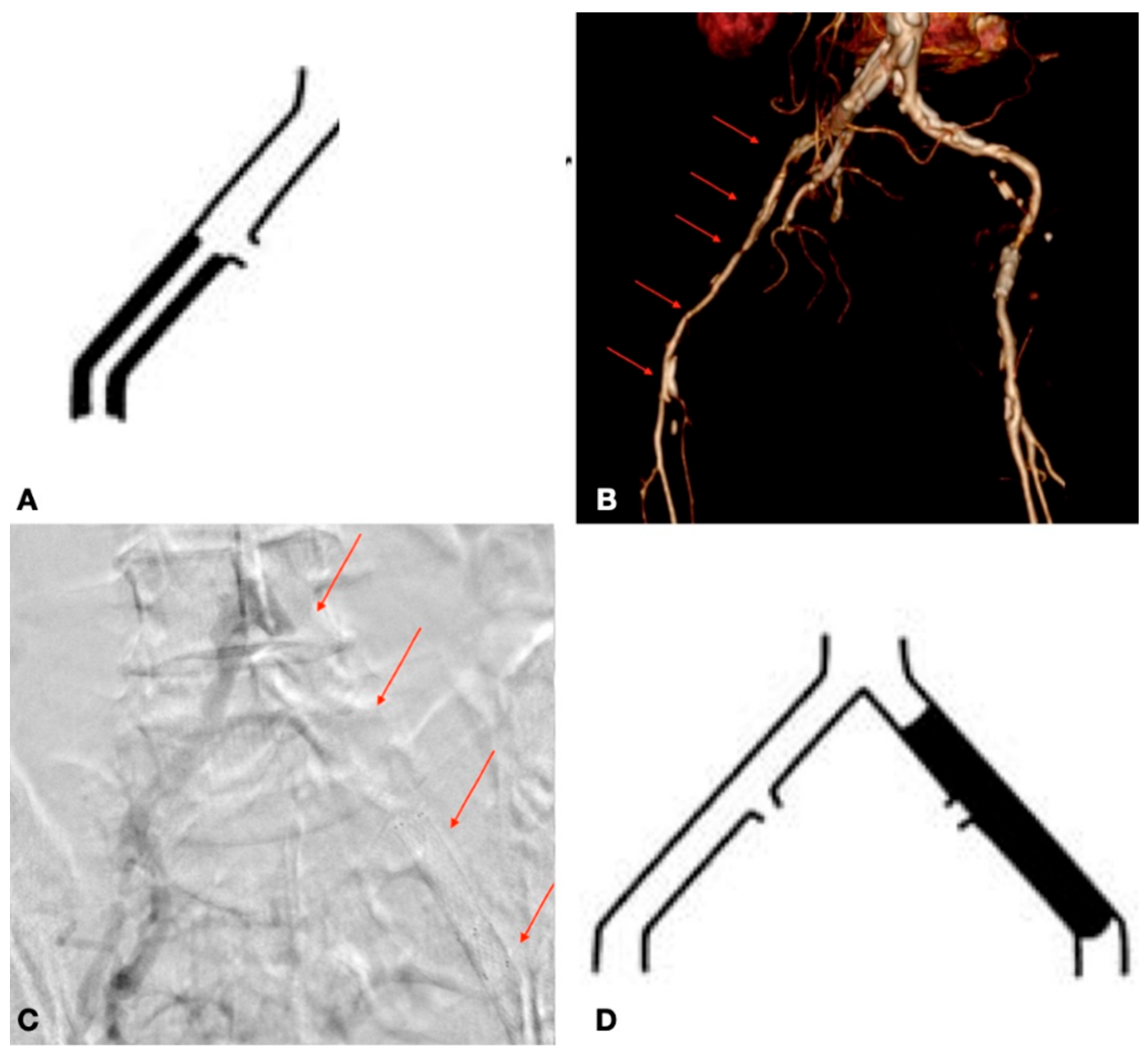
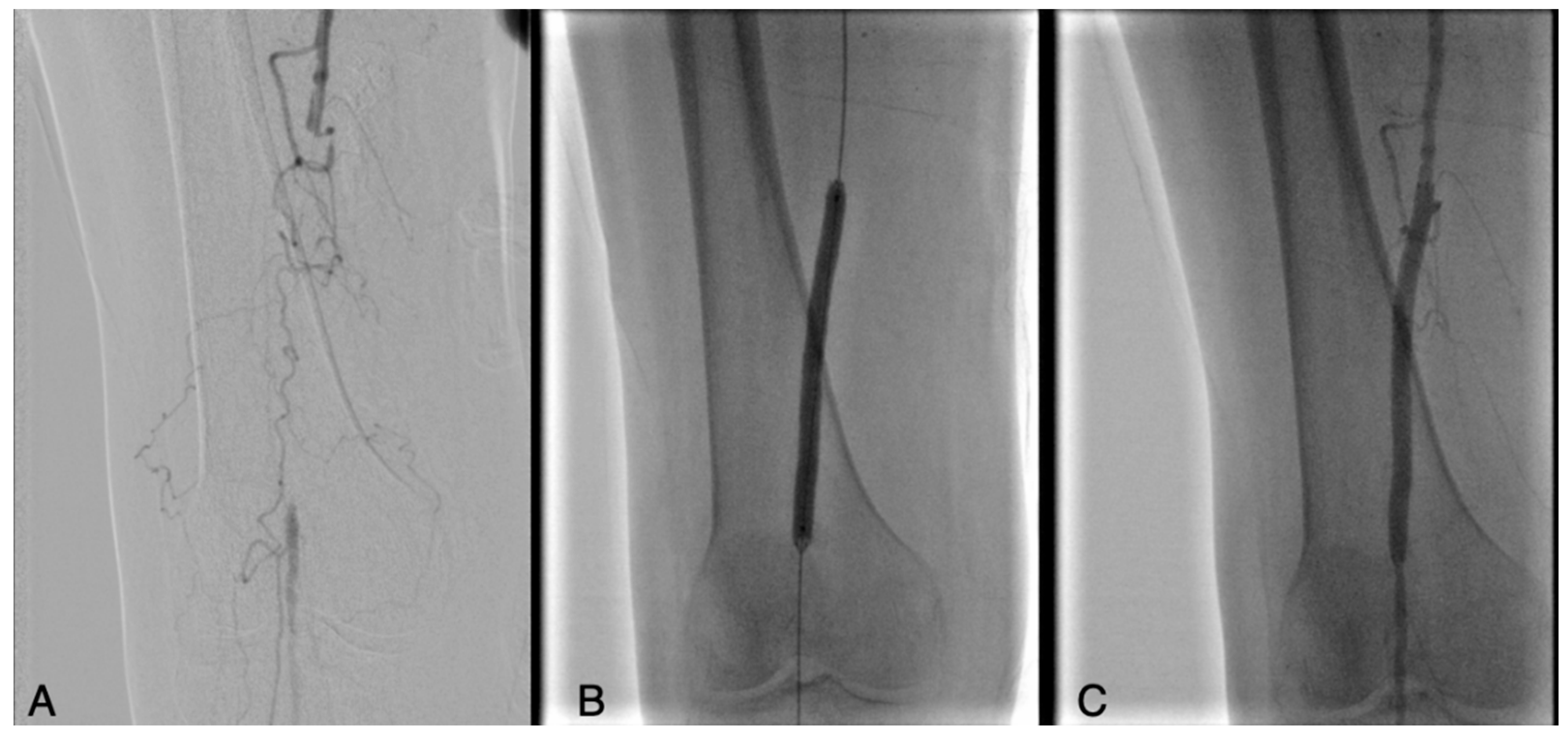
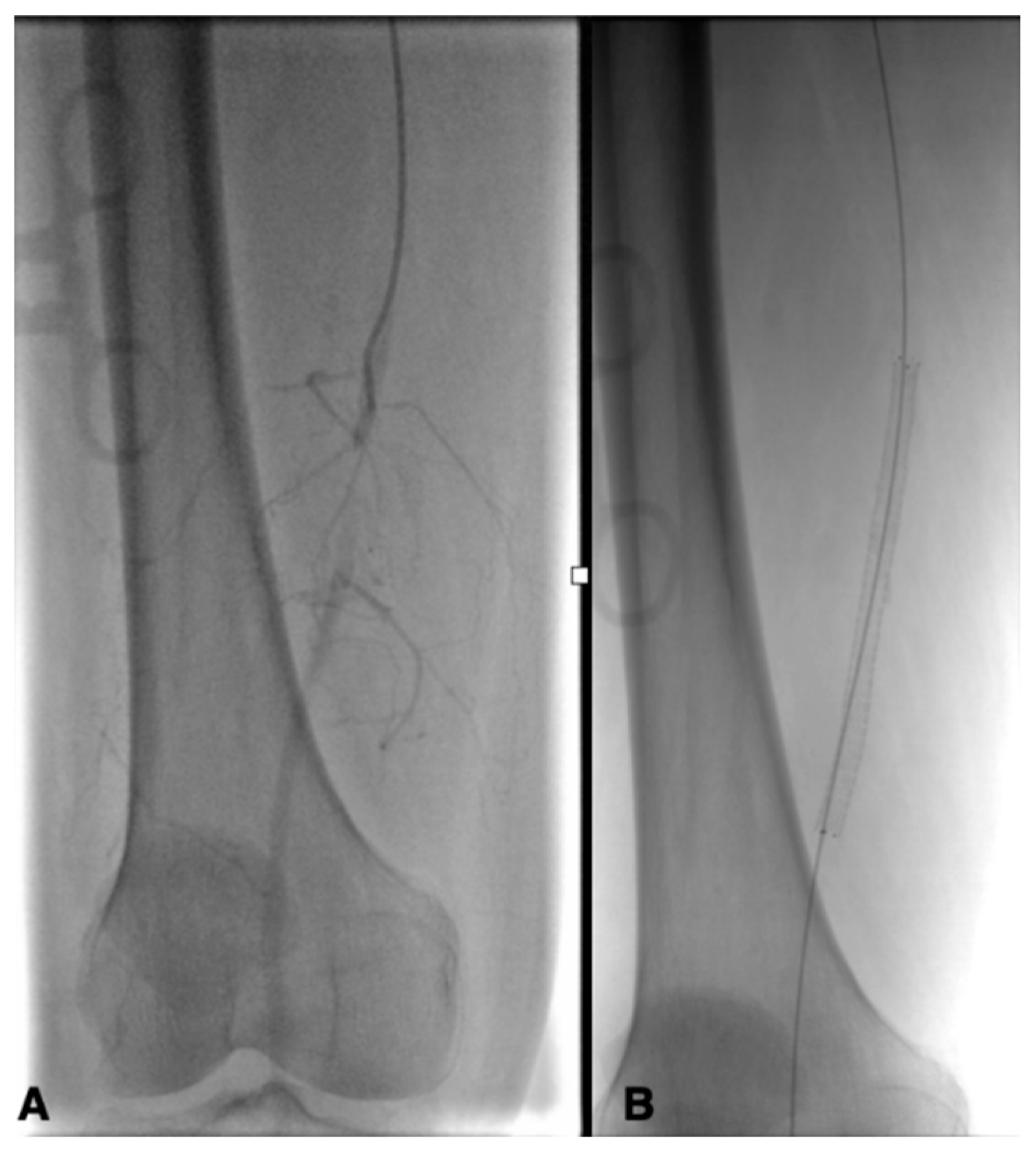
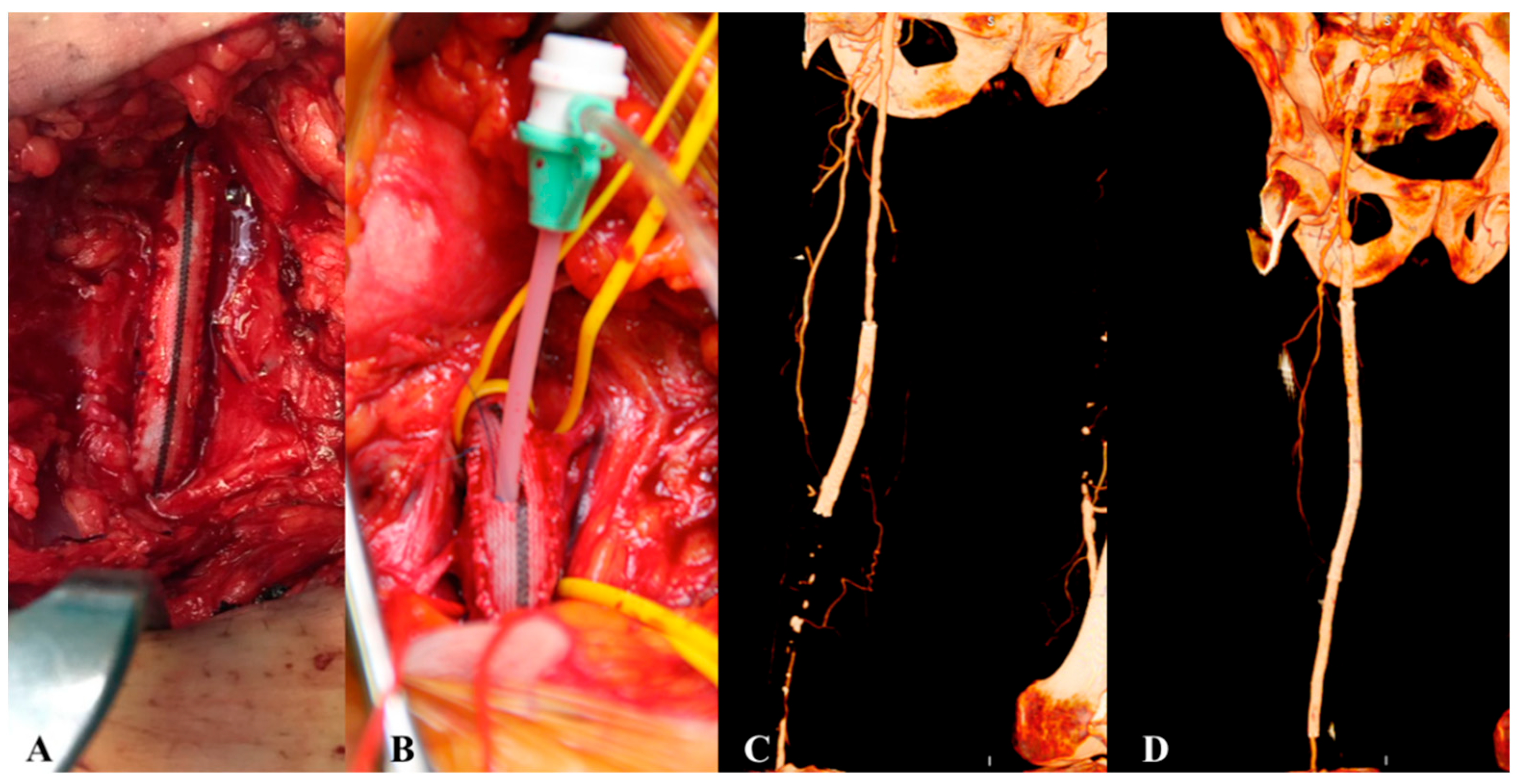
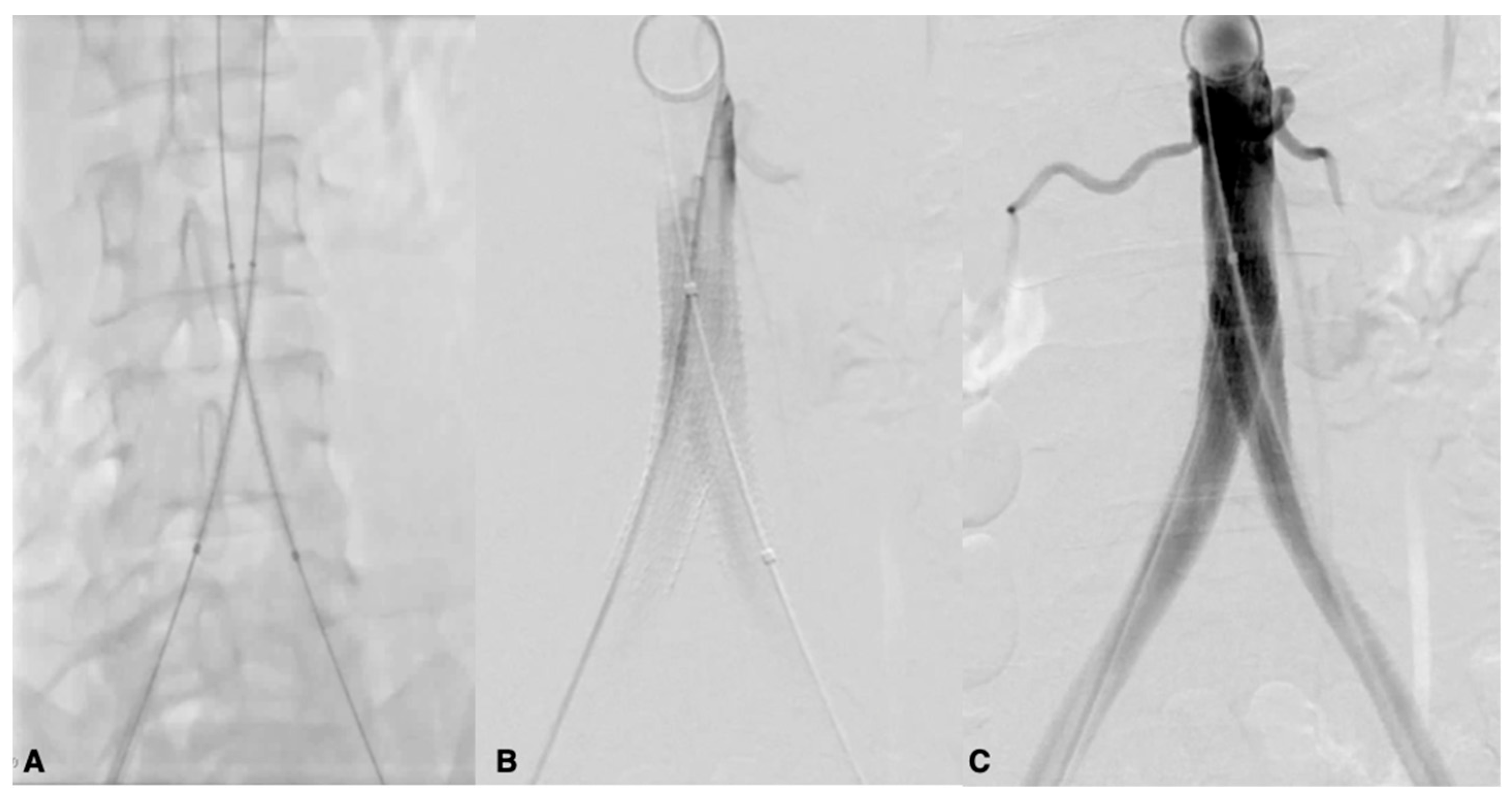
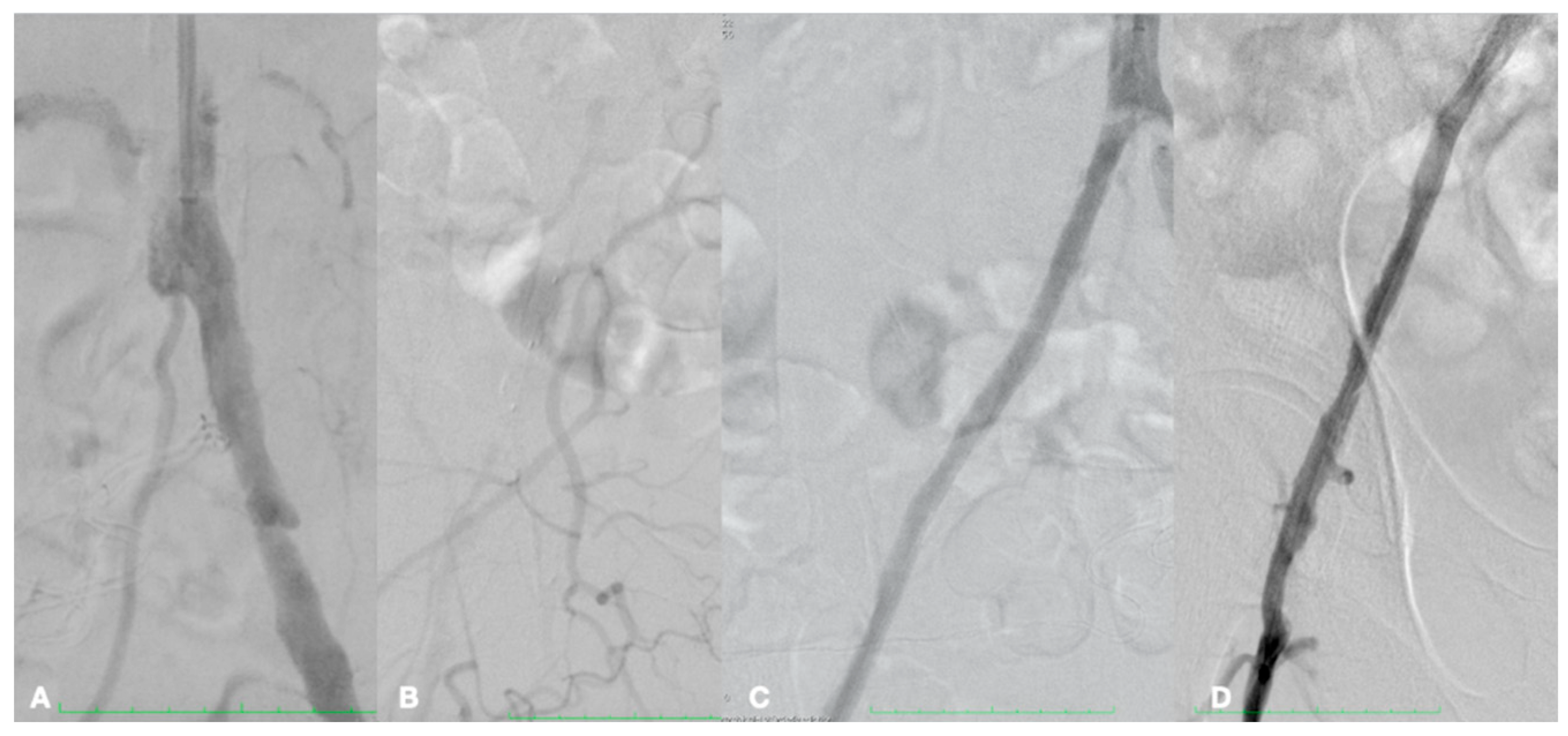
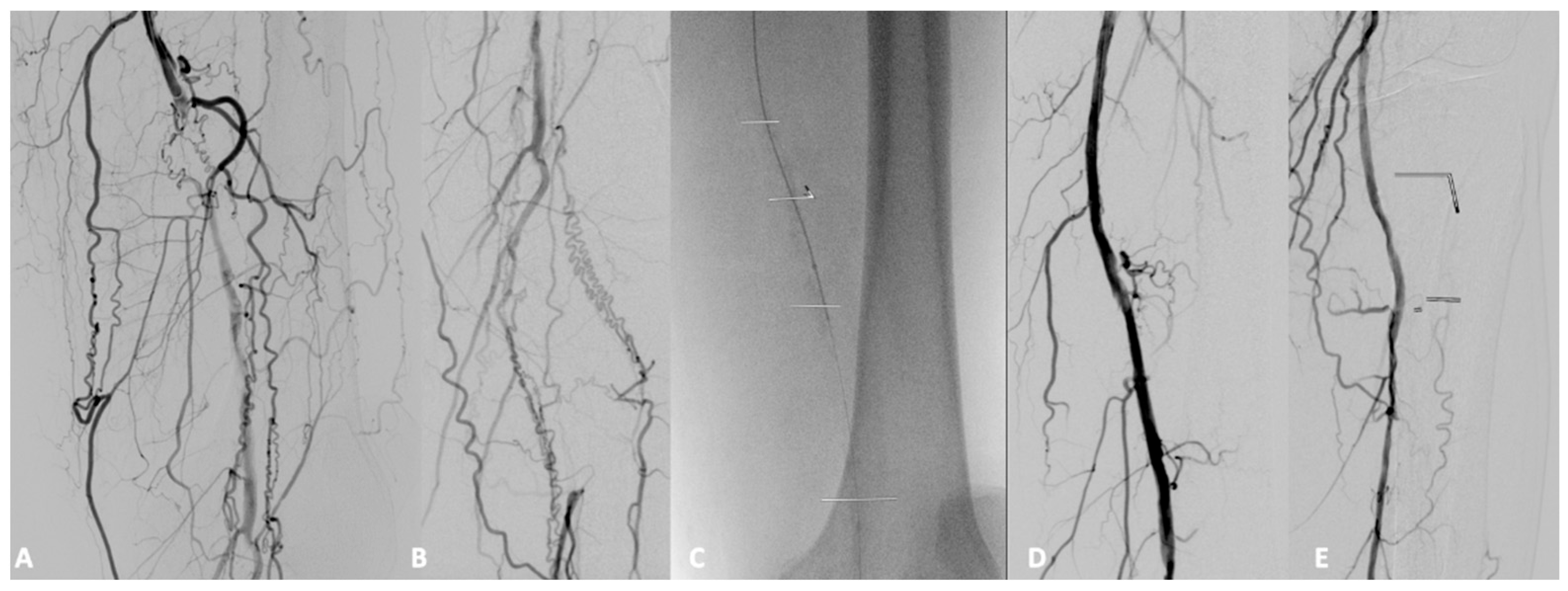
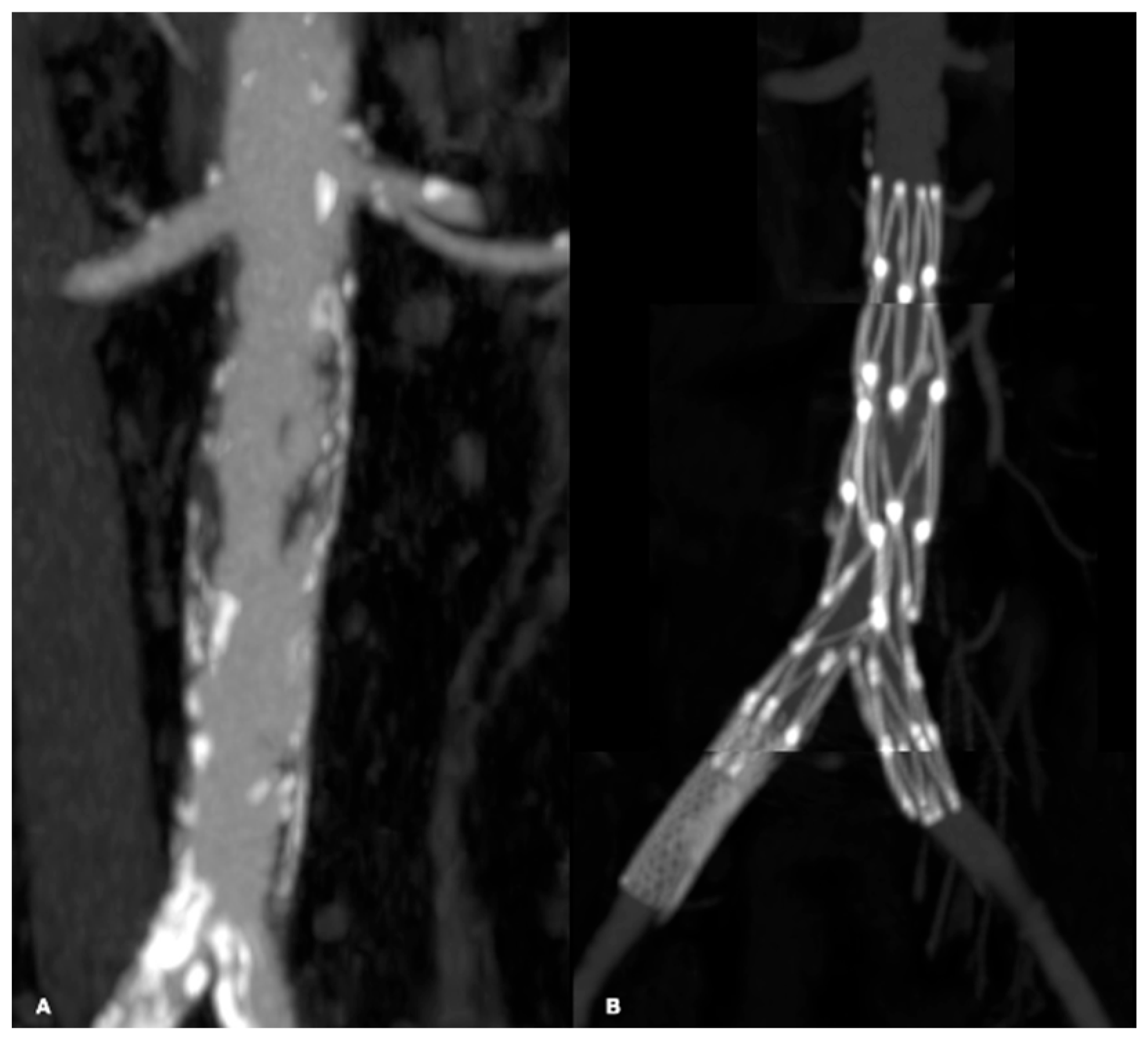
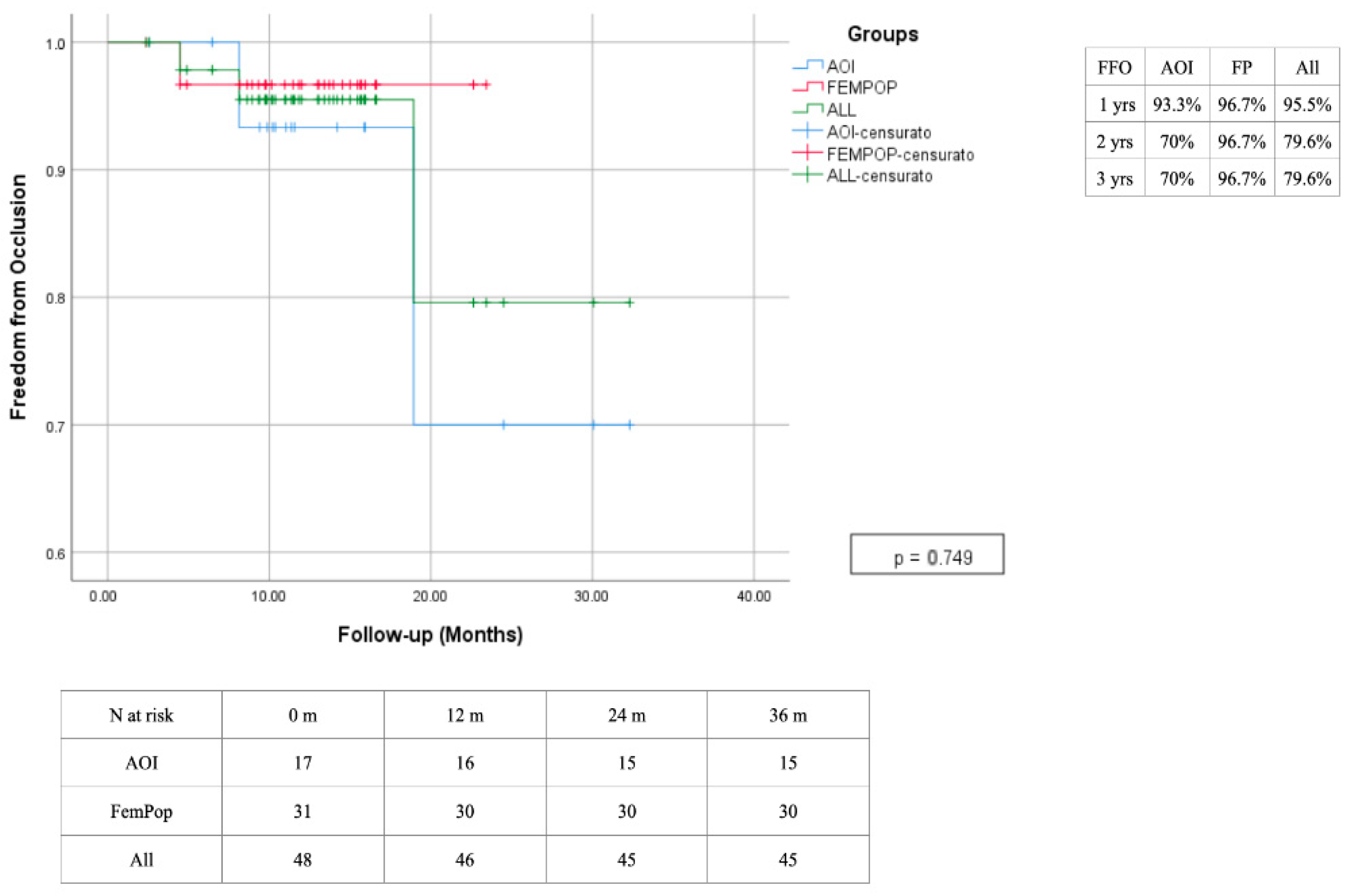
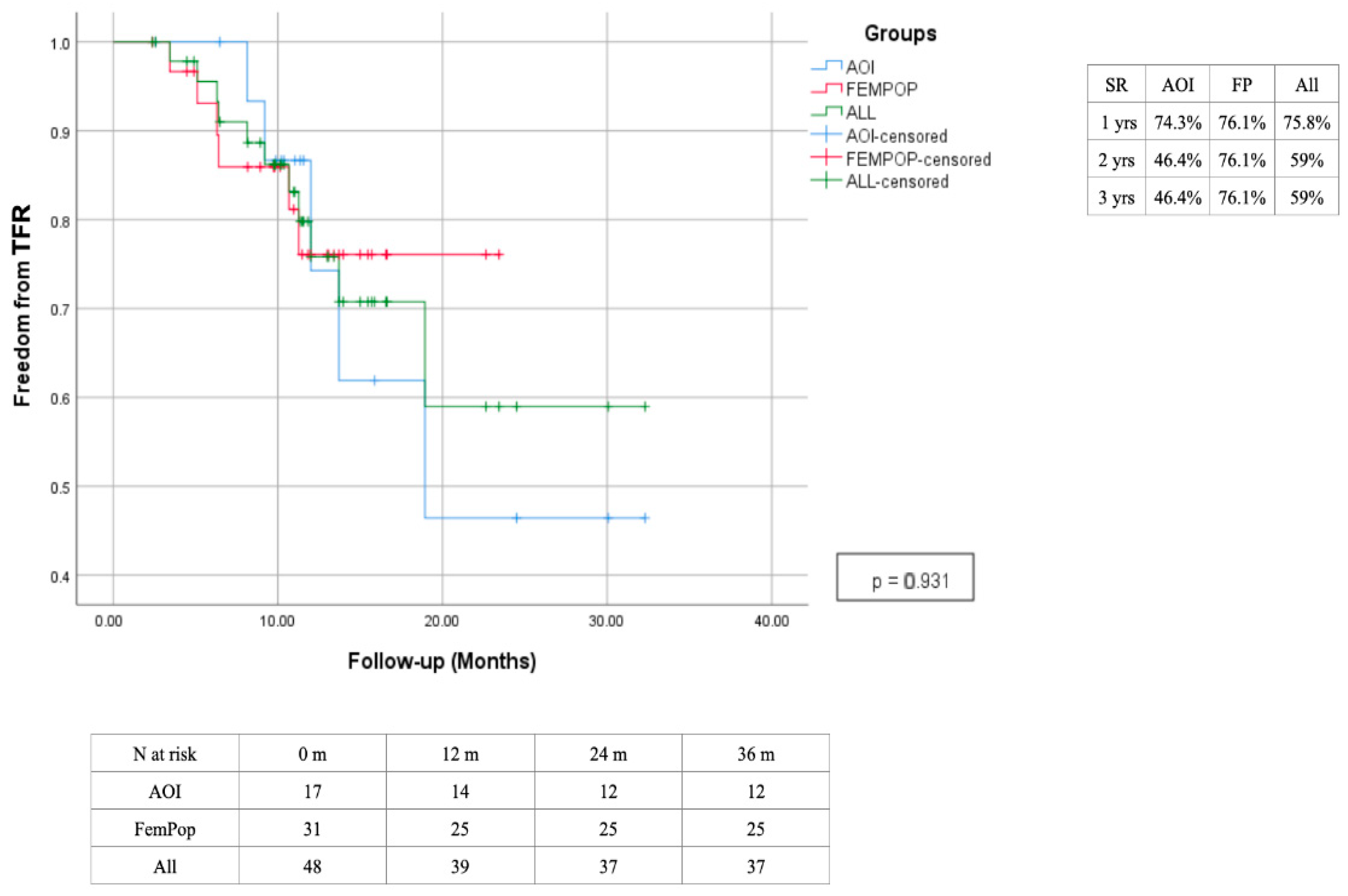
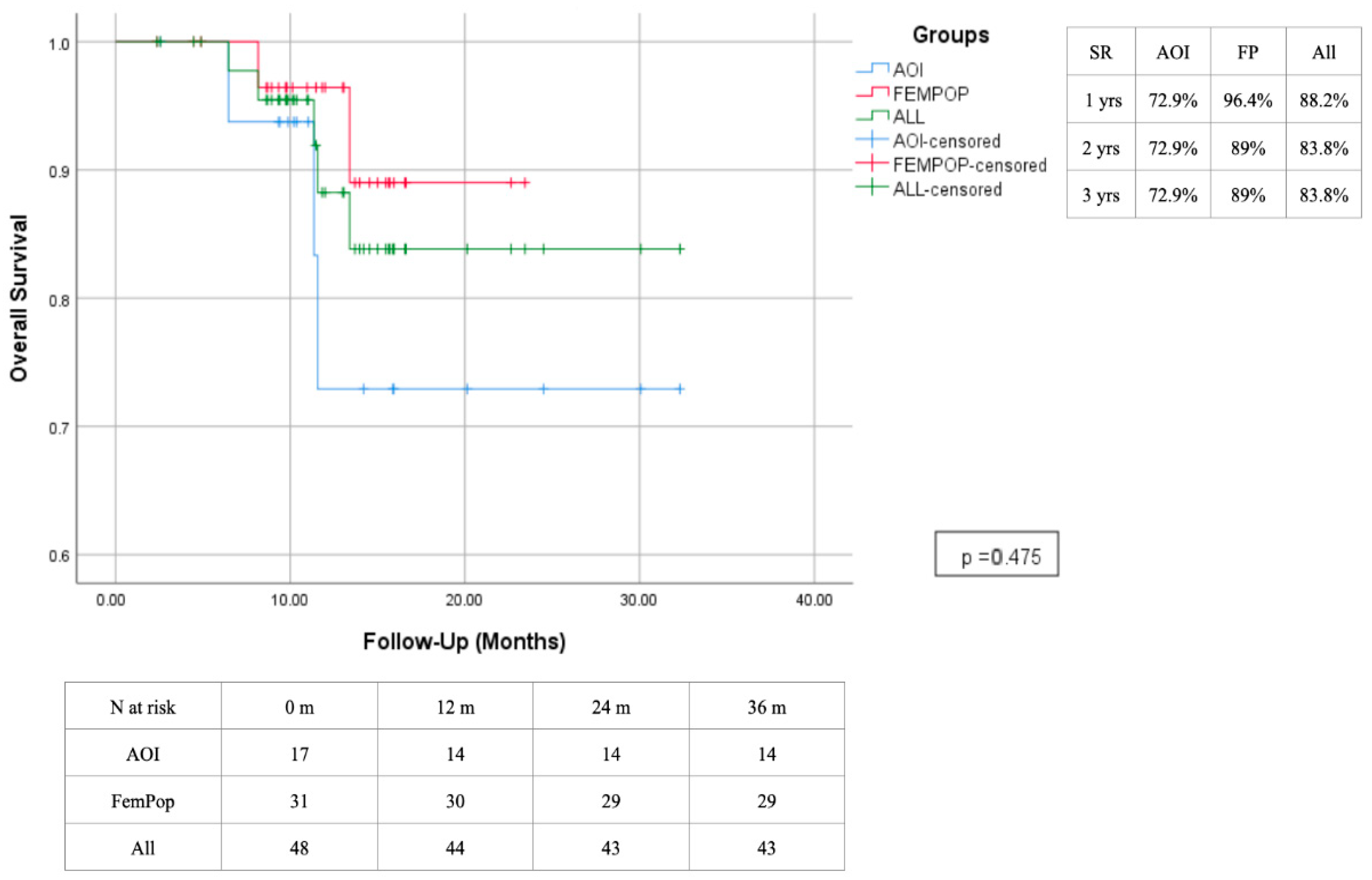
| Risk Factor | Total | Aorto-Iliac | Femoropopliteal | P |
|---|---|---|---|---|
| N | 48 | 17 | 31 | |
| Male | 30 (62.5%) | 11 (64.7%) | 19 (61.3%) | 0.815 |
| Female | 18 (37.5%) | 6 (35.3%) | 12 (38.7%) | |
| Mean Age | 67.5 ± 6.3 | 64.3 ± 5.1 | 69.2 ± 7.4 | 0.018 |
| Cerebrovascular disease | 7 (14.6%) | 0 (0%) | 7 (22.6%) | 0.034 |
| Hypertension | 37 (77.1%) | 12 (70.6%) | 25 (80.6%) | 0.428 |
| Diabetes | 32 (66.7%) | 8 (47.1%) | 24 (77.4%) | 0.033 |
| Ischemic heart disease | 16 (33.3%) | 6 (35.3%) | 10 (32.3%) | 0.831 |
| PCI/CABG | 11 (22.9%) | 4 (23.5%) | 7 (22.6%) | 0.940 |
| Oncologic Disease | 5 (10.4%) | 4 (23.5%) | 1 (3.2%) | 0.028 |
| Dialysis | 1 (2.1%) | 0 (0%) | 1 (3.2%) | 0.454 |
| Tobacco Use | 27 (56.3%) | 9 (52.9%) | 18 (58.1%) | 0.732 |
| Renal Failure | 30 (62.5%) | 12 (70.6%) | 18 (58.1%) | 0.391 |
| Therapy | Total | Aorto-Iliac | Femoropopliteal | P |
|---|---|---|---|---|
| N | 48 | 17 | 31 | |
| Statin | 31 (64.6%) | 11 (64.7%) | 20 (64.5%) | 0.990 |
| ASA | 15 (31.3%) | 3 (17.6%) | 12 (38.7%) | 0.132 |
| Clopidogrel | 8 (16.7%) | 4 (23.5%) | 4 (12.9%) | 0.345 |
| DAPT | 22 (45.8%) | 8 (47.1%) | 14 (45.2%) | 0.900 |
| TAO | 6 (12.5%) | 6 (35.3%) | 0 (0%) | 0.001 |
| NOAC | 7 (14.5%) | 0 (0%) | 7 (22.6%) | 0.034 |
| Total | Aorto-Iliac | Femoropopliteal | P | |
|---|---|---|---|---|
| N | 48 | 17 | 31 | |
| Rutherford | ||||
| Rutherford 3, n (%) | 7 (14.6%) | 5 (29.4%) | 2 (6.5%) | 0.031 |
| Rutherford 4, n (%) | 10 (20.8%) | 6 (35.3%) | 4 (12.9%) | 0.068 |
| Rutherford 5, n (%) | 26 (54.2%) | 5 (29.4%) | 21 (67.7%) | 0.011 |
| Rutherford 6, n (%) | 5 (12.5%) | 1 (5.9%) | 4 (12.9%) | 0.446 |
| TASC | 6 (12.5%) | 6 (35.3%) | 0 (0%) | 0.001 |
| TASC C, n (%) | 23 (47.9%) | 8 (47.1%) | 15 (48.4%) | 0.930 |
| TASC D, n (%) | 25 (52.1%) | 9 (52.9%) | 16 (51.6%) | 0.930 |
| ASA | ||||
| ASA III, n (%) | 47 (97.9%) | 17 (100%) | 30 (96.8%) | 0.454 |
| ASA IV, n (%) | 1 (2.1%) | 0 (0%) | 1 (3.2%) | 0.454 |
| Total | Aorto-Iliac | Femoropopliteal | P | |
|---|---|---|---|---|
| N | 210 | 47 | 163 | |
| Aorta, n % | 6 (2.9%) | 6 (12.8%) | 0 (0%) | |
| Occlusion, n (%) | 4 (1.9%) | 4 (8.5%) | 0 (0%) | 0.005 |
| Stenosis, n (%) | 2 (1%) | 2 (4.3%) | 0 (0%) | 0.051 |
| Common Iliac Artery | 23 (10.9%) | 23 (48.9%) | 0 (0%) | |
| Occlusion, n (%) | 11 (5.2%) | 11 (23.4%) | 0 (0%) | 0.001 |
| Stenosis, n (%) | 12 (5.7%) | 12 (25.5%) | 0 (0%) | 0.001 |
| External Iliaca Artery | 12 (5.7%) | 12 (25.5%) | 0 (0%) | |
| Occlusion, n (%) | 5 (2.4%) | 5 (10.6%) | 0 (0%) | 0.001 |
| Stenosis, n (%) | 7 (3.3%) | 7 (14.9%) | 0 (0%) | 0.001 |
| Internal Iliaca Artery | 6 (2.9%) | 6 (12.8%) | 0 (0%) | |
| Occlusion, n (%) | 3 (1.4%) | 3(6.4%) | 0 (0%) | 0.016 |
| Stenosis, n (%) | 3 (1.4%) | 3(6.4%) | 0 (0%) | 0.051 |
| Common Femoral Artery | 13 (6.2%) | 6 (12.8%) | 7 (4.3%) | |
| Occlusion, n (%) | 3 (1.4%) | 2 (4.3%) | 1 (0.6%) | 0.242 |
| Stenosis, n (%) | 10 (4.8%) | 4 (8.5%) | 6 (3.7%) | 0.733 |
| Superficial Femoral Artery | 29 (13.8%) | 0 (0%) | 29 (17.8%) | |
| Occlusion, n (%) | 17 (8.1%) | 0 (0%) | 17 (10.4%) | 0.001 |
| Stenosis, n (%) | 12 (5.7%) | 0 (0%) | 12 (7.4%) | 0.003 |
| Popliteal Artery | 29 (13.8%) | 0 (0%) | 29 (17.8%) | |
| Occlusion, n (%) | 13 (6.2%) | 0 (0%) | 13 (8%) | 0.002 |
| Stenosis, n (%) | 16 (7.6%) | 0 (0%) | 16 (9.8%) | 0.001 |
| Anterior Tibial Artery | 27 (12.9%) | 0 (0%) | 27 (16.6%) | |
| Occlusion, n (%) | 21 (10%) | 0 (0%) | 21 (12.9%) | 0.001 |
| Stenosis, n (%) | 6 (2.9%) | 0 (0%) | 6 (3.7%) | 0.052 |
| Tibio-peroneal trunk | 21 (10%) | 0 (0%) | 21 (12.9%) | |
| Occlusion, n (%) | 11 (5.2%) | 0 (0%) | 11 (6.8%) | 0.005 |
| Stenosis, n (%) | 10 (4.8%) | 0 (0%) | 10 (6.1%) | 0.008 |
| Peroneal Artery | 16 (7.6%) | 0 (0%) | 16 (9.8%) | |
| Occlusion, n (%) | 8 (3.8%) | 0 (0%) | 8 (4.9%) | 0.022 |
| Stenosis, n (%) | 8 (3.8%) | 0 (0%) | 8 (4.9%) | 0.022 |
| Posterior Tibial Artery | 28 (13.3%) | 0 (0%) | 28 (17.2%) | |
| Occlusion, n (%) | 26 (12.4%) | 0 (0%) | 26 (16%) | 0.001 |
| Stenosis, n (%) | 2 (1%) | 0 (0%) | 2 (1.2%) | 0.285 |
| Total | Aorto-Iliac | Femoropopliteal | P | |
|---|---|---|---|---|
| N | 48 | 17 | 31 | |
| PTA | 32 (66.7%) | 6 (35.3%) | 26 (83.9%) | 0.001 |
| Stenting | 24 (50%) | 16 (94.1%) | 8 (25.8%) | 0.001 |
| Bebulking | 4 (8.3%) | 0 (0%) | 4 (12.9%) | 0.122 |
| Unibody AFX | 2 (4.2%) | 2 (11.8%) | 0 (0%) | 0.051 |
| Intravascular Lithotripsy | 6 (12.5%) | 1 (5.9%) | 5 (16.1%) | 0.052 |
| Hybrid procedure | 30 (62.5%) | 9 (52.9%) | 21 (67.7%) | 0.311 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Marca, M.A.; Bruno, S.; Gagliardo, G.; Dinoto, E.; Federico, R.; Pecoraro, F.; Mirabella, D. Endovascular Treatment Outcomes for TASC C and D Lesions in Chronic Peripheral Arterial Disease: A Retrospective Study and Literature Review. Biomedicines 2025, 13, 2771. https://doi.org/10.3390/biomedicines13112771
La Marca MA, Bruno S, Gagliardo G, Dinoto E, Federico R, Pecoraro F, Mirabella D. Endovascular Treatment Outcomes for TASC C and D Lesions in Chronic Peripheral Arterial Disease: A Retrospective Study and Literature Review. Biomedicines. 2025; 13(11):2771. https://doi.org/10.3390/biomedicines13112771
Chicago/Turabian StyleLa Marca, Manfredi Agostino, Salvatore Bruno, Giovanni Gagliardo, Ettore Dinoto, Rosa Federico, Felice Pecoraro, and Domenico Mirabella. 2025. "Endovascular Treatment Outcomes for TASC C and D Lesions in Chronic Peripheral Arterial Disease: A Retrospective Study and Literature Review" Biomedicines 13, no. 11: 2771. https://doi.org/10.3390/biomedicines13112771
APA StyleLa Marca, M. A., Bruno, S., Gagliardo, G., Dinoto, E., Federico, R., Pecoraro, F., & Mirabella, D. (2025). Endovascular Treatment Outcomes for TASC C and D Lesions in Chronic Peripheral Arterial Disease: A Retrospective Study and Literature Review. Biomedicines, 13(11), 2771. https://doi.org/10.3390/biomedicines13112771







