Associations of First-Trimester Screening Markers and Hematological Indices with Placenta Accreta Spectrum in Pregnancies Complicated by Placenta Previa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Diagnostic Criteria
2.4. Biomarker and Hematological Assessment
2.5. Statistical Analysis
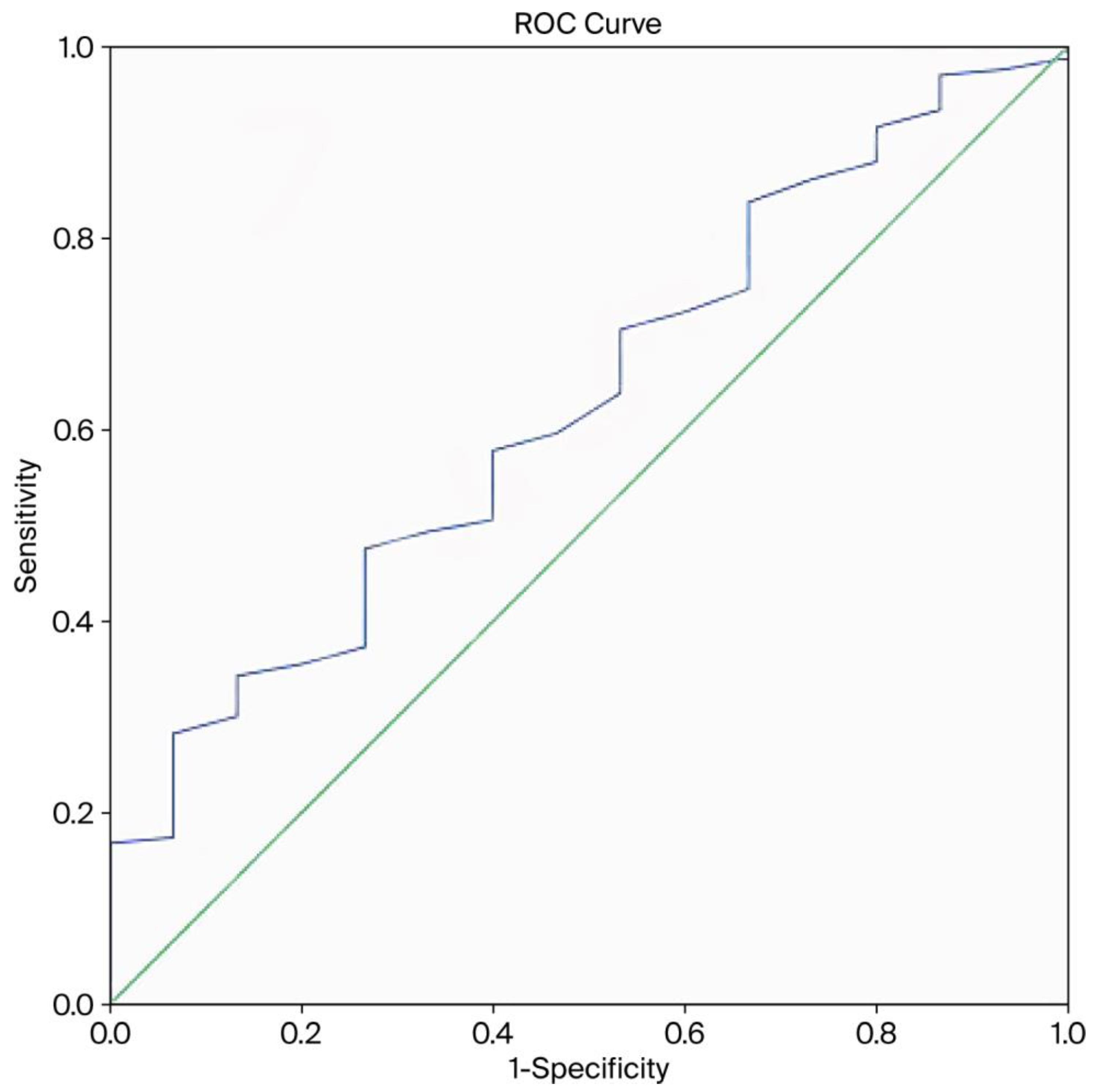
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jauniaux, E.; Bhide, A.; Kennedy, A.; Woodward, P.; Hubinont, C.; Collins, S.; Diagnosis, F.P.A.; Panel, M.E.C. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int. J. Gynecol. Obstet. 2018, 140, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Chantraine, F.; Silver, R.M.; Langhoff-Roos, J.; Diagnosis, F.P.A.; Panel, M.E.C. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int. J. Gynecol. Obstet. 2018, 140, 265–273. [Google Scholar] [CrossRef]
- Matsuo, K.; Einerson, B.D.; Matsuzaki, S.; Pon, F.F.; Jimenez, Z.N.C.; Yao, J.A.; de Meritens, A.B.; Benipal, S.; Givens, M.B.; Mandelbaum, R.S. Nationwide assessment of gestational age distribution at delivery for patients with placenta accreta spectrum disorder. Obstet. Gynecol. 2025, 145, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.M.; Fox, K.A.; Barton, J.R.; Abuhamad, A.Z.; Simhan, H.; Huls, C.K.; Belfort, M.A.; Wright, J.D. Center of excellence for placenta accreta. Am. J. Obstet. Gynecol. 2015, 212, 561–568. [Google Scholar] [CrossRef]
- Einerson, B.D.; Gilner, J.B.; Zuckerwise, L.C. Placenta accreta spectrum. Obstet. Gynecol. 2023, 142, 31–50. [Google Scholar] [CrossRef]
- Anastasia, M.; Athanasios, D.; Michail, P.; Anastasia, D.M.; Vasilios, P.; Panagiotis, A.; George, D.; Marianna, T. First and second trimester aneuploidy screening biomarkers and risk assessment of placenta previa and accreta: A systematic review and meta-analysis. J. Obstet. Gynaecol. Can. 2024, 46, 102663. [Google Scholar] [CrossRef]
- Sugai, S.; Yamawaki, K.; Sekizuka, T.; Haino, K.; Yoshihara, K.; Nishijima, K. Comparison of maternal outcomes and clinical characteristics of prenatally vs nonprenatally diagnosed placenta accreta spectrum: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 101197. [Google Scholar] [CrossRef] [PubMed]
- Bartels, H.C.; Postle, J.D.; Downey, P.; Brennan, D.J. Placenta accreta spectrum: A review of pathology, molecular biology, and biomarkers. Dis. Markers 2018, 2018, 1507674. [Google Scholar] [CrossRef]
- Lizárraga-Verdugo, E.; Beltrán-Ontiveros, S.A.; Gutiérrez-Grijalva, E.P.; Montoya-Moreno, M.; Gutiérrez-Arzapalo, P.Y.; Avendaño-Félix, M.; Gutiérrez-Castro, K.P.; Cuén-Lazcano, D.E.; González-Quintero, P.; Mora-Palazuelos, C.E. The underlying molecular mechanisms of the placenta accreta apectrum: A narrative review. Int. J. Mol. Sci. 2024, 25, 9722. [Google Scholar] [CrossRef]
- Wenqiang, D.; Novin, A.; Liu, Y.; Afzal, J.; Suhail, Y.; Liu, S.; Gavin, N.R.; Jorgensen, J.R.; Morosky, C.M.; Figueroa, R. Scar matrix drives Piezo1 mediated stromal inflammation leading to placenta accreta spectrum. Nat. Commun. 2024, 15, 8379. [Google Scholar] [CrossRef]
- Dugoff, L.; Hobbins, J.C.; Malone, F.D.; Porter, T.F.; Luthy, D.; Comstock, C.H.; Hankins, G.; Berkowitz, R.L.; Merkatz, I.; Craigo, S.D. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (the FASTER Trial). Am. J. Obstet. Gynecol. 2004, 191, 1446–1451. [Google Scholar] [CrossRef]
- Desai, N.; Krantz, D.; Roman, A.; Fleischer, A.; Boulis, S.; Rochelson, B. Elevated first trimester PAPP-A is associated with increased risk of placenta accreta. Prenat. Diagn. 2014, 34, 159–162. [Google Scholar] [CrossRef]
- Çakır, B.T.; Aktemur, G.; Karabay, G.; Şeyhanlı, Z.; Çetin, S.; Filiz, A.A.; Tonyalı, N.V.; Çağlar, A.T. Evaluation of platelet indices and inflammation markers in preeclampsia. J. Clin. Med. 2025, 14, 1406. [Google Scholar] [CrossRef]
- Keles, A.; Dagdeviren, G.; Celik, O.Y.; Sahin, E.K.; Obut, M.; Kahraman, N.C.; Celen, S. Systemic immune-inflammation index to predict placenta accreta spectrum and its histological subtypes. J. Obstet. Gynaecol. Res. 2022, 48, 1675–1682. [Google Scholar] [CrossRef]
- Balkaş, G.; Çelen, Ş. Role of inflammatory and coagulation biomarkers in distinguishing placenta accreta from placenta previa and associated hemorrhage. J. Clin. Med. 2025, 14, 3884. [Google Scholar] [CrossRef]
- Collins, S.L.; Ashcroft, A.; Braun, T.; Calda, P.; Langhoff-Roos, J.; Morel, O.; Stefanovic, V.; Tutschek, B.; Chantraine, F. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet. Gynecol. 2016, 47, 271–275. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Wang, J.; Wang, Y.; Ruan, F.; Shu, H.; Zhu, L.; Man, D. First trimester serum PAPP-A is associated with placenta accreta: A retrospective study. Arch. Gynecol. Obstet. 2021, 303, 645–652. [Google Scholar] [CrossRef]
- Penzhoyan, G.A.; Makukhina, T.B. Significance of the routine first-trimester antenatal screening program for aneuploidy in the assessment of the risk of placenta accreta spectrum disorders. J. Perinat. Med. 2019, 48, 21–26. [Google Scholar] [CrossRef]
- Ersoy, A.O.; Ozler, S.; Oztas, E.; Ersoy, E.; Kirbas, A.; Danisman, N. The association between placenta previa and leukocyte and platelet indices-A case control study. Ginekol. Pol. 2016, 87, 367–371. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Mikhailidis, D.P.; Kitas, G.D. Mean platelet volume: A link between thrombosis and inflammation? Curr. Pharm. Des. 2011, 17, 47–58. [Google Scholar] [CrossRef]
- Lyell, D.; Faucett, A.; Baer, R.; Blumenfeld, Y.; Druzin, M.; El-Sayed, Y.; Shaw, G.; Currier, R.; Jelliffe-Pawlowski, L. Maternal serum markers, characteristics and morbidly adherent placenta in women with previa. J. Perinatol. 2015, 35, 570–574. [Google Scholar] [CrossRef]
- Büke, B.; Akkaya, H.; Demir, S.; Sağol, S.; Şimşek, D.; Başol, G.; Barutçuoğlu, B. Relationship between first trimester aneuploidy screening test serum analytes and placenta accreta. J. Matern. Fetal Neonatal Med. 2018, 31, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Thompson, O.; Otigbah, C.; Nnochiri, A.; Sumithran, E.; Spencer, K. First trimester maternal serum biochemical markers of aneuploidy in pregnancies with abnormally invasive placentation. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1370–1376. [Google Scholar] [CrossRef]
- Al-Nuaimi, A.M.A.; Al-Nuaimi, Z.M.A. Early hematological indices as a predictor of placenta accreta in women with high suspicion of accreta. Medicine 2025, 104, e41084. [Google Scholar] [CrossRef]
- Yayla, C.A.; Ozkaya, E.; Tayyar, A.; Senol, T.; Senturk, M.B.; Karateke, A. Predictive value of complete blood count parameters for placental invasion anomalies. J. Matern. Fetal Neonat. Med. 2017, 30, 2324–2328. [Google Scholar] [CrossRef]
- Karakoç, G.; Yalcin, S.E.; Sarsmaz, K.; Şengül, M.; Yucel, A. Delta neutrophil index as a promising biomarker for placental implantation disorders. Z. Geburtshilfe Neonatol. 2021, 225, 412–417. [Google Scholar] [CrossRef]
- Tikkanen, M.; Paavonen, J.; Loukovaara, M.; Stefanovic, V. Antenatal diagnosis of placenta accreta leads to reduced blood loss. Acta Obstet. Gynecol. Scand. 2011, 90, 1140–1146. [Google Scholar] [CrossRef]
- Oztas, E.; Ozler, S.; Caglar, A.T.; Yucel, A. Analysis of first and second trimester maternal serum analytes for the prediction of morbidly adherent placenta requiring hysterectomy. Kaohsiung J. Med. Sci. 2016, 32, 579–585. [Google Scholar] [CrossRef]
- Munoz, J.L.; Ramsey, P.S.; Greebon, L.J.; Salazar, E.; McCann, G.A.; Byrne, J.J. Risk factors of massive blood transfusion (MTP) in cesarean hysterectomy for placenta accreta spectrum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 293, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, A.; Jauniaux, E.; Hussein, A.; Coutinho, C.; Tinari, S.; Khalil, A.; Shamshirsaz, A.; Palacios-Jaraquemada, J.; D’Antonio, F. Urological complications in women undergoing Cesarean section for placenta accreta spectrum disorders: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2023, 62, 633–643. [Google Scholar] [CrossRef] [PubMed]
| Placenta Accreta Spectrum (n = 15) | Non-Adherent Placenta Previa (n = 166) | p Value | |
|---|---|---|---|
| Age (years), mean ± SD | 25.3 ± 5.1 | 30.0 ± 6.3 | <0.001 |
| BMI (kg/m2), mean ± SD | 23.6 ± 1.7 | 24.0 ± 1.9 | 0.358 |
| Gravida, mean ± SD | 3.0 ± 0.4 | 3.1 ± 0.5 | 0.254 |
| Parity, n (%) | |||
| Nulliparity | 2 (13.3%) | 47 (28.3%) | 0.173 |
| Multiparity | 13 (86.7%) | 119 (72.7%) | |
| Fetal sex, n (%) | |||
| Male | 9 (60%) | 83 (50%) | 0.458 |
| Female | 6 (40%) | 83 (50%) | |
| Prior cesarean section, n (%) | |||
| Yes | 10 (66.7%) | 12 (7.2%) | <0.001 |
| No | 5 (33.3%) | 154 (92.8%) | |
| Gestational age at birth (weeks), mean ± SD | 36.3 ± 1.3 | 35.6 ± 3.0 | 0.475 |
| Birth weight (g), mean ± SD | 2869 ± 640.9 | 2656 ± 701.0 | 0.237 |
| Admission to neonatal intensive care unit, n (%) | |||
| Yes | 1 (6.7%) | 30 (18.1%) | 0.232 |
| No | 14 (93.3%) | 136 (81.9%) | |
| Blood transfusion, n (%) | |||
| Yes | 14 (93.3%) | 49 (29.5%) | <0.001 |
| No | 1 (6.7%) | 117 (70.5%) | |
| Surgical complications, n (%) | |||
| Hysterectomy | 6 (40.0%) | 0 (0%) | <0.001 |
| Hypogastric artery ligation | 3 (20.0%) | 6 (3.6%) | 0.005 |
| Urinary bladder injury | 3 (20.0%) | 0 (0%) | <0.001 |
| Placenta Accreta Spectrum (n = 15) | Non-Adherent Placenta Previa (n = 166) | p Value | |
|---|---|---|---|
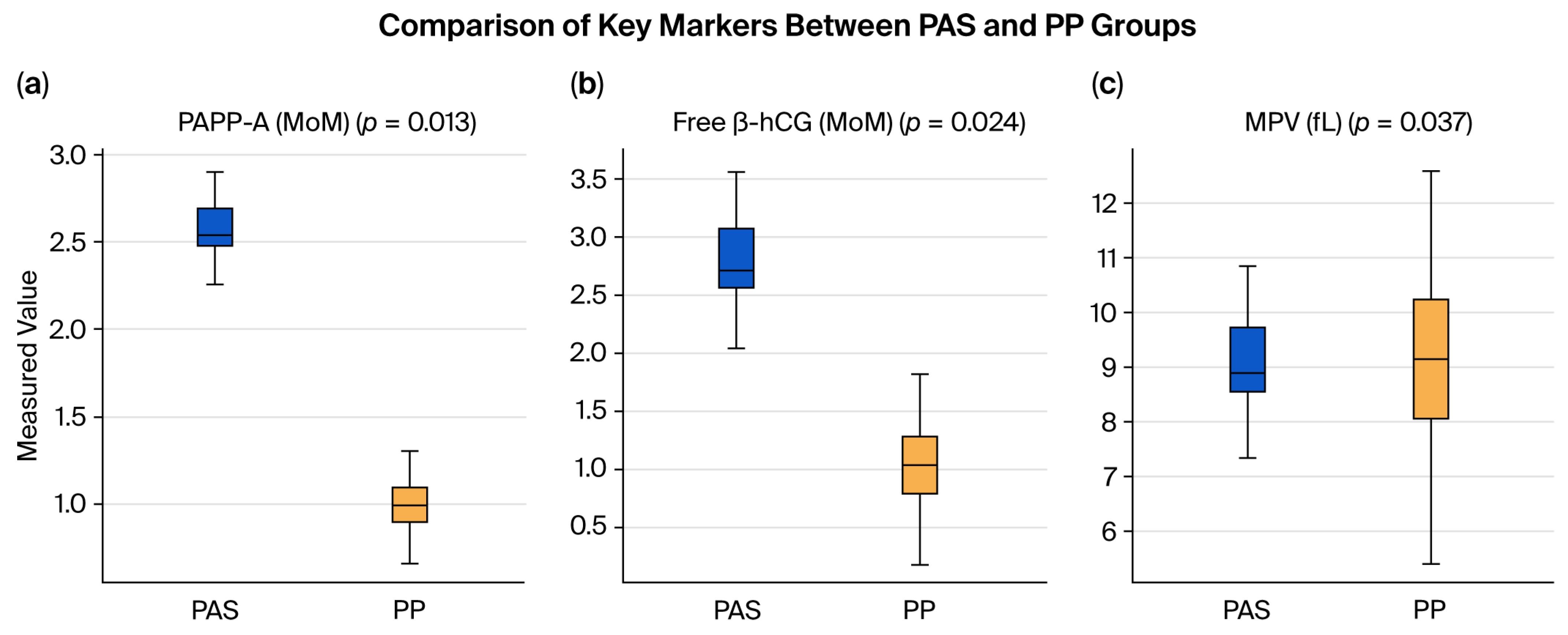
| PAPP-A (MoM), mean ± SD | 2.45 ± 0.2 | 0.99 ± 0.13 | 0.013 |
| Free β-hCG (MoM), mean ± SD | 2.5 ± 0.47 | 1.03 ± 0.33 | 0.024 |
| Placenta Accreta Spectrum (n = 15) | Non-Adherent Placenta Previa (n = 166) | p Value | |
|---|---|---|---|
| WBC count (103/μL), median (range) | 11.6 (7.1–13.5) | 11.2 (6.1–28.5) | 0.945 |
| Neutrophil count (103/μL), median (range) | 8.5 (5.4–10.9) | 8.2 (2.5–25.3) | 0.951 |
| Lymphocyte count (103/μL), median (range) | 2.1 (1.2–2.7) | 2 (0.4–6.6) | 0.633 |
| Hemoglobin (g/dL), mean ± SD | 10.7 ± 1.25 | 11.1 ± 1.23 | 0.284 |
| Hematocrit (%), mean ± SD | 32.9 ± 3.88 | 33.7 ± 3.52 | 0.433 |
| Platelet count (103/μL), median (range) | 228 (127–327) | 220 (81–515) | 0.582 |
| MPV (fL), mean ± SD | 8.4 ± 1.09 | 9.1 ± 1.45 | 0.037 |
| RDW (%), median (range) | 15.9 (12.7–22.8) | 14.2 (12.0–23.7) | 0.099 |
| NLR, median (range) | 3.56 (2.67–9.08) | 4.13 (0.38–24.0) | 0.740 |
| PLR, median (range) | 108.0 (60.4–230 | 111.4 (33.3–420) | 0.975 |
| Placenta Accreta Spectrum (Our Study) | Normal Pregnancy (Literature) * | Reference | |
|---|---|---|---|
| PAPP-A (MoM), mean ± SD | 2.45 ± 0.2 | 0.99 ± 0.13 | Wang et al., 2021 [17] |
| Free β-hCG (MoM), mean ± SD | 2.5 ± 0.47 | 1.08 ± 0.69 (0.5–2.0) | Penzhoyan et al., 2019 [18] |
| MPV (fL), mean ± SD | 8.02 ± 0.94 | 10.54 ± 0.90 (7.4–13.1) | Ersoy et al., 2016 [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatasli, V.; Kanmaz, A.G.; Karabulut, A.; Inan, A.H. Associations of First-Trimester Screening Markers and Hematological Indices with Placenta Accreta Spectrum in Pregnancies Complicated by Placenta Previa. Biomedicines 2025, 13, 2082. https://doi.org/10.3390/biomedicines13092082
Karatasli V, Kanmaz AG, Karabulut A, Inan AH. Associations of First-Trimester Screening Markers and Hematological Indices with Placenta Accreta Spectrum in Pregnancies Complicated by Placenta Previa. Biomedicines. 2025; 13(9):2082. https://doi.org/10.3390/biomedicines13092082
Chicago/Turabian StyleKaratasli, Volkan, Ahkam Goksel Kanmaz, Alaattin Karabulut, and Abdurrahman Hamdi Inan. 2025. "Associations of First-Trimester Screening Markers and Hematological Indices with Placenta Accreta Spectrum in Pregnancies Complicated by Placenta Previa" Biomedicines 13, no. 9: 2082. https://doi.org/10.3390/biomedicines13092082
APA StyleKaratasli, V., Kanmaz, A. G., Karabulut, A., & Inan, A. H. (2025). Associations of First-Trimester Screening Markers and Hematological Indices with Placenta Accreta Spectrum in Pregnancies Complicated by Placenta Previa. Biomedicines, 13(9), 2082. https://doi.org/10.3390/biomedicines13092082






