Epoxide Hydrolase Inhibitors for the Treatment of Alzheimer’s Disease and Other Neurological Disorders: A Comprehensive Review
Abstract
1. Introduction
2. Methodology
3. Epoxide Hydrolase
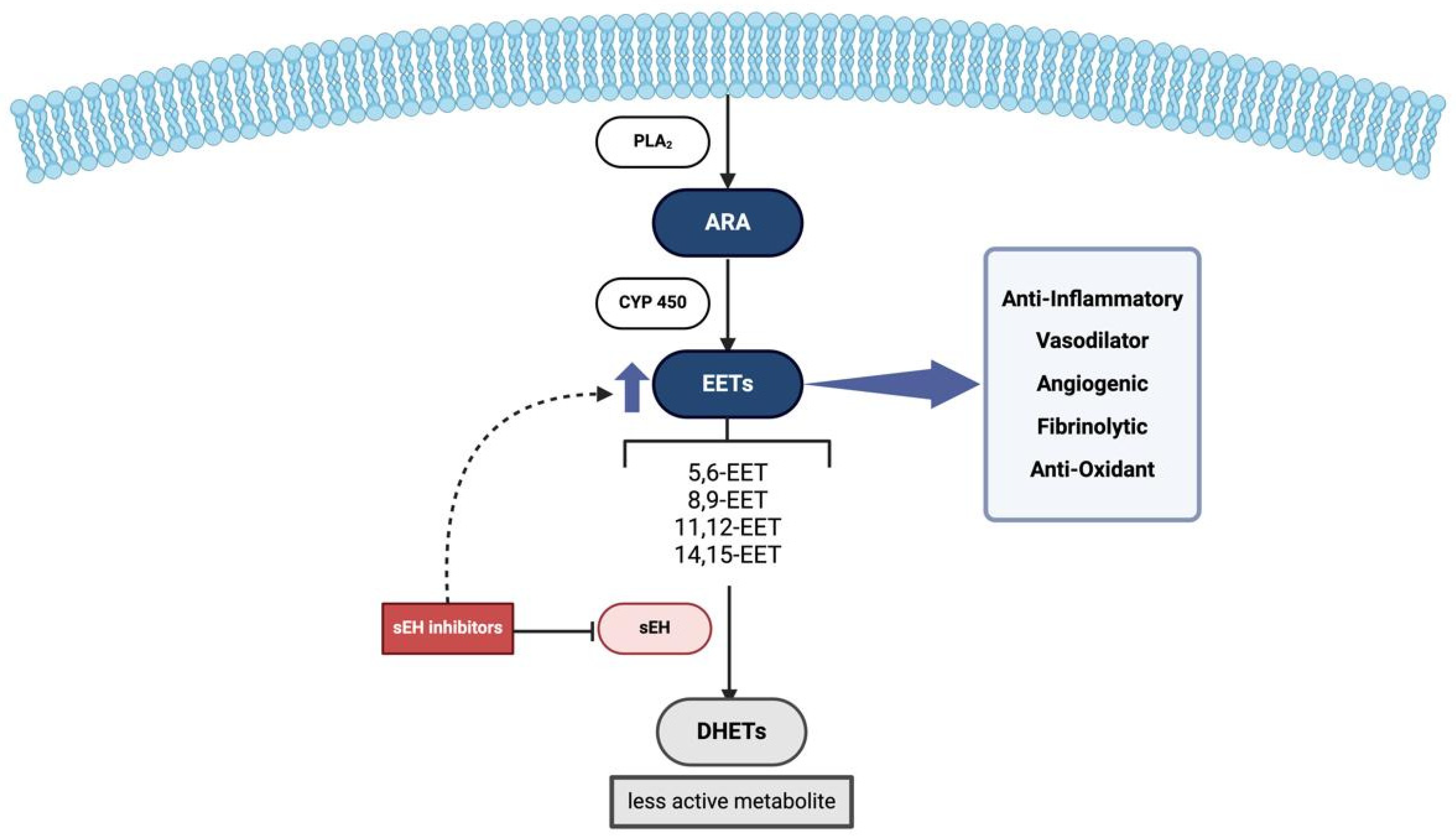
3.1. The Enzyme
3.2. Epoxyeicosatrienoic Acids (EETs) and Epoxide Hydrolase (EH) Inhibitors
4. Alzheimer’s Disease Pathogenesis and Relation to Epoxide Hydrolase
4.1. Use of Soluble Epoxide Hydrolase (sEH) Inhibitors in Alzheimer’s Disease
4.1.1. TPPU
4.1.2. UB-SCG-51
4.1.3. UB-SCG-74
4.1.4. UB-SCG-55
4.1.5. UB-BJ02
4.1.6. TPPU-6-Chlorotacrine/TPPU-Huprine Y
4.1.7. AS-2586114 and UB-EV-52
4.1.8. Other Inhibitors
5. The Role of Epoxide Hydrolase Inhibitors in Cognitive Impairment/Decline in Other CNS Disorders
5.1. Parkinson’s Disease
5.2. Vascular Dementia
5.3. Stroke
5.4. Diabetes-Related Cognitive Impairment
6. Conclusions
7. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Statement on Use of AI
References
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global Cognitive Impairment Prevalence and Incidence in Community Dwelling Older Adults-A Systematic Review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dementia; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Pathak, A.; Samaiya, P.K. Alzheimer’s disease: From early pathogenesis to novel therapeutic approaches. Metab. Brain Dis. 2024, 39, 1231–1254. [Google Scholar] [CrossRef]
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, L. Research Progress on the Pathogenesis, Diagnosis, and Drug Therapy of Alzheimer’s Disease. Brain Sci. 2024, 14, 590. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 2006, Cd005593. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Hampel, H.; Vergallo, A.; Aguilar, L.F.; Benda, N.; Broich, K.; Cuello, A.C.; Cummings, J.; Dubois, B.; Federoff, H.J.; Fiandaca, M.; et al. Precision pharmacology for Alzheimer’s disease. Pharmacol. Res. 2018, 130, 331–365. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.P.; Zhang, X.Y.; Morisseau, C.; Hwang, S.H.; Zhang, Z.J.; Hammock, B.D.; Ma, X.C. Discovery of Soluble Epoxide Hydrolase Inhibitors from Chemical Synthesis and Natural Products. J. Med. Chem. 2021, 64, 184–215. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Codony, S.; Pujol, E.; Yang, J.; Leiva, R.; Escolano, C.; Puigoriol-Illamola, D.; Companys-Alemany, J.; Corpas, R.; Sanfeliu, C.; et al. Pharmacological Inhibition of Soluble Epoxide Hydrolase as a New Therapy for Alzheimer’s Disease. Neurotherapeutics 2020, 17, 1825–1835. [Google Scholar] [CrossRef]
- Choi, W.J.; Choi, C.Y. Production of chiral epoxides: Epoxide hydrolase-catalyzed enantioselective hydrolysis. Biotechnol. Bioprocess Eng. 2005, 10, 167–179. [Google Scholar] [CrossRef]
- Morisseau, C.; Kodani, S.D.; Kamita, S.G.; Yang, J.; Lee, K.S.S.; Hammock, B.D. Relative Importance of Soluble and Microsomal Epoxide Hydrolases for the Hydrolysis of Epoxy-Fatty Acids in Human Tissues. Int. J. Mol. Sci. 2021, 22, 4993. [Google Scholar] [CrossRef] [PubMed]
- Gurung, A.B.; Mayengbam, B.; Bhattacharjee, A. Discovery of novel drug candidates for inhibition of soluble epoxide hydrolase of arachidonic acid cascade pathway implicated in atherosclerosis. Comput. Biol. Chem. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Newman, J.W.; Morisseau, C.; Hammock, B.D. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Prog. Lipid Res. 2005, 44, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Chiamvimonvat, N.; Ho, C.M.; Tsai, H.J.; Hammock, B.D. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J. Cardiovasc. Pharmacol. 2007, 50, 225–237. [Google Scholar] [CrossRef]
- Enayetallah, A.E.; French, R.A.; Barber, M.; Grant, D.F. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J. Histochem. Cytochem. 2006, 54, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sura, P.; Sura, R.; Enayetallah, A.E.; Grant, D.F. Distribution and expression of soluble epoxide hydrolase in human brain. J. Histochem. Cytochem. 2008, 56, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Schmelzer, K.; Lee, T.S.; Fang, X.; Zhu, Y.; Spector, A.A.; Gill, S.; Morisseau, C.; Hammock, B.D.; et al. The antiinflammatory effect of laminar flow: The role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2005, 102, 16747–16752. [Google Scholar] [CrossRef] [PubMed]
- Pinot, F.; Grant, D.F.; Spearow, J.L.; Parker, A.G.; Hammock, B.D. Differential regulation of soluble epoxide hydrolase by clofibrate and sexual hormones in the liver and kidneys of mice. Biochem. Pharmacol. 1995, 50, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Li, N.; Ai, D.; Niu, X.L.; Guan, Y.F.; Zhu, Y. Activation of peroxisome proliferator-activated receptor-γ downregulates soluble epoxide hydrolase in cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2011, 38, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, M.O.D.; Checa, A.; Yang, M.; Dahlén, S.E.; Wheelock, Å.; Eklund, A.; Grunewald, J.; Wheelock, C.E. Soluble epoxide hydrolase derived lipid mediators are elevated in bronchoalveolar lavage fluid from patients with sarcoidosis: A cross-sectional study. Respir. Res. 2018, 19, 236. [Google Scholar] [CrossRef] [PubMed]
- Zarriello, S.; Tuazon, J.P.; Corey, S.; Schimmel, S.; Rajani, M.; Gorsky, A.; Incontri, D.; Hammock, B.D.; Borlongan, C.V. Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders. Prog. Neurobiol. 2019, 172, 23–39. [Google Scholar] [CrossRef]
- Marowsky, A.; Meyer, I.; Erismann-Ebner, K.; Pellegrini, G.; Mule, N.; Arand, M. Beyond detoxification: A role for mouse mEH in the hepatic metabolism of endogenous lipids. Arch. Toxicol. 2017, 91, 3571–3585. [Google Scholar] [CrossRef]
- Barnych, B.; Singh, N.; Negrel, S.; Zhang, Y.; Magis, D.; Roux, C.; Hua, X.; Ding, Z.; Morisseau, C.; Tantillo, D.J.; et al. Development of potent inhibitors of the human microsomal epoxide hydrolase. Eur. J. Med. Chem. 2020, 193, 112206. [Google Scholar] [CrossRef]
- Duan, H.; Yoshimura, K.; Kobayashi, N.; Sugiyama, K.; Sawada, J.; Saito, Y.; Morisseau, C.; Hammock, B.D.; Akatsuka, T. Development of monoclonal antibodies to human microsomal epoxide hydrolase and analysis of “preneoplastic antigen”-like molecules. Toxicol. Appl. Pharmacol. 2012, 260, 17–26. [Google Scholar] [CrossRef]
- Hammock, B.D.; Loury, D.N.; Moody, D.E.; Ruebner, B.; Baselt, R.; Milam, K.M.; Volberding, P.; Ketterman, A.; Talcott, R. A methodology for the analysis of the preneoplastic antigen. Carcinogenesis 1984, 5, 1467–1473. [Google Scholar] [CrossRef]
- Kessler, R.; Hamou, M.F.; Albertoni, M.; de Tribolet, N.; Arand, M.; Van Meir, E.G. Identification of the putative brain tumor antigen BF7/GE2 as the (de)toxifying enzyme microsomal epoxide hydrolase. Cancer Res. 2000, 60, 1403–1409. [Google Scholar] [PubMed]
- Václavíková, R.; Hughes, D.J.; Souček, P. Microsomal epoxide hydrolase 1 (EPHX1): Gene, structure, function, and role in human disease. Gene 2015, 571, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, A.; Shin, E.J.; Liu, X.; Kim, S.G.; Runyons, C.R.; Markesbery, W.; Kim, H.C.; Bing, G. Expression of microsomal epoxide hydrolase is elevated in Alzheimer’s hippocampus and induced by exogenous beta-amyloid and trimethyl-tin. Eur. J. Neurosci. 2006, 23, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Morisseau, C.; Hammock, B.D.; Shivachar, A.C. Differential subcellular distribution and colocalization of the microsomal and soluble epoxide hydrolases in cultured neonatal rat brain cortical astrocytes. J. Neurosci. Res. 2009, 87, 218–227. [Google Scholar] [CrossRef]
- Tacconelli, S.; Patrignani, P. Inside epoxyeicosatrienoic acids and cardiovascular disease. Front. Pharmacol. 2014, 5, 239. [Google Scholar] [CrossRef]
- Edin, M.L.; Hamedani, B.G.; Gruzdev, A.; Graves, J.P.; Lih, F.B.; Arbes, S.J.; Singh, R.; Orjuela Leon, A.C.; Bradbury, J.A.; DeGraff, L.M.; et al. Epoxide hydrolase 1 (EPHX1) hydrolyzes epoxyeicosanoids and impairs cardiac recovery after ischemia. J. Biol. Chem. 2018, 293, 3281–3292. [Google Scholar] [CrossRef]
- Decker, M.; Adamska, M.; Cronin, A.; Di Giallonardo, F.; Burgener, J.; Marowsky, A.; Falck, J.R.; Morisseau, C.; Hammock, B.D.; Gruzdev, A.; et al. EH3 (ABHD9): The first member of a new epoxide hydrolase family with high activity for fatty acid epoxides. J. Lipid Res. 2012, 53, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, S.L.; Gruzdev, A.; Edin, M.L.; Graves, J.P.; Bradbury, J.A.; Flake, G.P.; Lih, F.B.; DeGraff, L.M.; Zeldin, D.C. Generation and characterization of epoxide hydrolase 3 (EPHX3)-deficient mice. PLoS ONE 2017, 12, e0175348. [Google Scholar] [CrossRef]
- Edin, M.L.; Yamanashi, H.; Boeglin, W.E.; Graves, J.P.; DeGraff, L.M.; Lih, F.B.; Zeldin, D.C.; Brash, A.R. Epoxide hydrolase 3 (Ephx3) gene disruption reduces ceramide linoleate epoxide hydrolysis and impairs skin barrier function. J. Biol. Chem. 2021, 296, 100198. [Google Scholar] [CrossRef]
- Dahlhoff, M.; Fröhlich, T.; Arnold, G.J.; Müller, U.; Leonhardt, H.; Zouboulis, C.C.; Schneider, M.R. Characterization of the sebocyte lipid droplet proteome reveals novel potential regulators of sebaceous lipogenesis. Exp. Cell Res. 2015, 332, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.L.; O’Dwyer, S.T.; Stern, P.L.; Renehan, A.G. Global gene expression in pseudomyxoma peritonei, with parallel development of two immortalized cell lines. Oncotarget 2015, 6, 10786–10800. [Google Scholar] [CrossRef] [PubMed]
- Flebbe, H.; Hamdan, F.H.; Kari, V.; Kitz, J.; Gaedcke, J.; Ghadimi, B.M.; Johnsen, S.A.; Grade, M. Epigenome Mapping Identifies Tumor-Specific Gene Expression in Primary Rectal Cancer. Cancers 2019, 11, 1142. [Google Scholar] [CrossRef]
- Shen, N.; Gao, G.; Lu, X.; Jin, J.; Lin, L.; Qian, M.; Qin, Y. Comprehensive analysis of the immune implication of EPHX4 gene in laryngeal squamous cell carcinoma. Braz. J. Otorhinolaryngol. 2024, 90, 101411. [Google Scholar] [CrossRef]
- Kodani, S.D.; Morisseau, C. Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. Biochimie 2019, 159, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Atone, J.; Wagner, K.; Hashimoto, K.; Hammock, B.D. Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106385. [Google Scholar] [CrossRef] [PubMed]
- Konkel, A.; Schunck, W.H. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta 2011, 1814, 210–222. [Google Scholar] [CrossRef]
- Graves, J.P.; Edin, M.L.; Bradbury, J.A.; Gruzdev, A.; Cheng, J.; Lih, F.B.; Masinde, T.A.; Qu, W.; Clayton, N.P.; Morrison, J.P.; et al. Characterization of four new mouse cytochrome P450 enzymes of the CYP2J subfamily. Drug Metab. Dispos. 2013, 41, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.M.; Lee, Y.H. Epoxyeicosatrienoic acids and soluble epoxide hydrolase in physiology and diseases of the central nervous system. Chin. J. Physiol. 2022, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Epoxyeicosanoids in hypertension. Physiol. Res. 2019, 68, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.M.; Gomes, A.; McReynolds, C.B.; Hammock, B.D. Soluble Epoxide Hydrolase Regulation of Lipid Mediators Limits Pain. Neurotherapeutics 2020, 17, 900–916. [Google Scholar] [CrossRef]
- Lazaar, A.L.; Yang, L.; Boardley, R.L.; Goyal, N.S.; Robertson, J.; Baldwin, S.J.; Newby, D.E.; Wilkinson, I.B.; Tal-Singer, R.; Mayer, R.J.; et al. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br. J. Clin. Pharmacol. 2016, 81, 971–979. [Google Scholar] [CrossRef]
- Luther, J.M.; Ray, J.; Wei, D.; Koethe, J.R.; Hannah, L.; DeMatteo, A.; Manning, R.; Terker, A.S.; Peng, D.; Nian, H.; et al. GSK2256294 Decreases sEH (Soluble Epoxide Hydrolase) Activity in Plasma, Muscle, and Adipose and Reduces F2-Isoprostanes but Does Not Alter Insulin Sensitivity in Humans. Hypertension 2021, 78, 1092–1102. [Google Scholar] [CrossRef]
- Martini, R.P.; Siler, D.; Cetas, J.; Alkayed, N.J.; Allen, E.; Treggiari, M.M. A Double-Blind, Randomized, Placebo-Controlled Trial of Soluble Epoxide Hydrolase Inhibition in Patients with Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 2022, 36, 905–915. [Google Scholar] [CrossRef]
- Luo, J.; Hu, S.; Fu, M.; Luo, L.; Li, Y.; Li, W.; Cai, Y.; Dong, R.; Yang, Y.; Tu, L.; et al. Inhibition of soluble epoxide hydrolase alleviates insulin resistance and hypertension via downregulation of SGLT2 in the mouse kidney. J. Biol. Chem. 2021, 296, 100667. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Wanjalla, C.N.; Warren, C.M.; Simmons, J.D.; Ghoshal, K.; Pilkinton, M.; Bailin, S.S.; Gabriel, C.L.; Pozzi, A.; Koethe, J.R.; et al. The soluble epoxide hydrolase inhibitor GSK2256294 decreases the proportion of adipose pro-inflammatory T cells. Prostaglandins Other Lipid Mediat. 2022, 158, 106604. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zhao, C.; Kong, Q.; Li, D.; Cai, Z.; Wang, M. Vascular repair and anti-inflammatory effects of soluble epoxide hydrolase inhibitor. Exp. Ther. Med. 2019, 17, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Hammock, B.D.; McReynolds, C.B.; Wagner, K.; Buckpitt, A.; Cortes-Puch, I.; Croston, G.; Lee, K.S.S.; Yang, J.; Schmidt, W.K.; Hwang, S.H. Movement to the Clinic of Soluble Epoxide Hydrolase Inhibitor EC5026 as an Analgesic for Neuropathic Pain and for Use as a Nonaddictive Opioid Alternative. J. Med. Chem. 2021, 64, 1856–1872. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Shyue, S.K.; Hung, T.H.; Wen, S.; Lin, C.C.; Chang, C.F.; Chen, S.F. Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase reduces brain damage and attenuates neuroinflammation after intracerebral hemorrhage. J. Neuroinflammation 2017, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, X.; Huang, X.; Qin, C.; Fang, Y.; Liu, Y.; Zhang, G.; Pan, D.; Wang, W.; Xie, M. Soluble epoxide hydrolase inhibition provides multi-target therapeutic effects in rats after spinal cord injury. Mol. Neurobiol. 2016, 53, 1565–1578. [Google Scholar] [CrossRef]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019, 10, 1312. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 107. [Google Scholar] [CrossRef]
- Chen, W.; Wang, M.; Zhu, M.; Xiong, W.; Qin, X.; Zhu, X. 14,15-Epoxyeicosatrienoic Acid Alleviates Pathology in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2020, 40, 8188–8203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Q.; Zhang, Y.W.; Xu, H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. 2012, 120 (Suppl. 1), 9–21. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Wingo, A.P.; Liu, Y.; Gerasimov, E.S.; Gockley, J.; Logsdon, B.A.; Duong, D.M.; Dammer, E.B.; Robins, C.; Beach, T.G.; Reiman, E.M.; et al. Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat. Genet. 2021, 53, 143–146. [Google Scholar] [CrossRef]
- Chapuis, J.; Flaig, A.; Grenier-Boley, B.; Eysert, F.; Pottiez, V.; Deloison, G.; Vandeputte, A.; Ayral, A.M.; Mendes, T.; Desai, S.; et al. Genome-wide, high-content siRNA screening identifies the Alzheimer’s genetic risk factor FERMT2 as a major modulator of APP metabolism. Acta Neuropathol. 2017, 133, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Su, W.M.; Gu, X.J.; Dou, M.; Duan, Q.Q.; Jiang, Z.; Yin, K.F.; Cai, W.C.; Cao, B.; Wang, Y.; Chen, Y.P. Systematic druggable genome-wide Mendelian randomisation identifies therapeutic targets for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2023, 94, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Border, J.J.; Zhang, H.; Challagundla, L.; Kaur, J.; Hwang, S.H.; Hammock, B.D.; Fan, F.; Roman, R.J. A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 2433. [Google Scholar] [CrossRef]
- Wang, S.; Qi, C.; Rajpurohit, C.; Ghosh, B.; Xiong, W.; Wang, B.; Qi, Y.; Hwang, S.H.; Hammock, B.D.; Li, H.; et al. Inhibition of Soluble Epoxide Hydrolase Confers Neuroprotection and Restores Microglial Homeostasis in a Tauopathy Mouse Model. Mol. Neurodegener. 2025, 20, 44. [Google Scholar] [CrossRef]
- Padhy, B.; Kapuganti, R.S.; Hayat, B.; Mohanty, P.P.; Alone, D.P. Wide-spread enhancer effect of SNP rs2279590 on regulating epoxide hydrolase-2 and protein tyrosine kinase 2-beta gene expression. Gene 2023, 854, 147096. [Google Scholar] [CrossRef]
- Charisis, S.; Lin, H.; Ray, R.; Joehanes, R.; Beiser, A.S.; Levy, D.; Seshadri, S.; Sargurupremraj, M.; Satizabal, C.L. Obesity impacts the expression of Alzheimer’s disease-related genes: The Framingham Heart Study. Alzheimers Dement. 2023, 19, 3496–3505. [Google Scholar] [CrossRef]
- Kivimäki, M.; Luukkonen, R.; Batty, G.D.; Ferrie, J.E.; Pentti, J.; Nyberg, S.T.; Shipley, M.J.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; et al. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimers Dement. 2018, 14, 601–609. [Google Scholar] [CrossRef]
- Zhang, G.; Kodani, S.; Hammock, B.D. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 2014, 53, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Kytikova, O.Y.; Denisenko, Y.K.; Novgorodtseva, T.P.; Bocharova, N.V.; Kovalenko, I.S. Fatty acid epoxides in the regulation of the inflammation. Biomeditsinskaya Khimiya 2022, 68, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 2009, 8, 794–805. [Google Scholar] [CrossRef]
- Bartra, C.; Vuraić, K.; Yuan, Y.; Codony, S.; Valdés-Quiroz, H.; Casal, C.; Slevin, M.; Máquez-Kisinousky, L.; Planas, A.M.; Griñán-Ferré, C.; et al. Microglial pro-inflammatory mechanisms induced by monomeric C-reactive protein are counteracted by soluble epoxide hydrolase inhibitors. Int. Immunopharmacol. 2025, 155, 114644. [Google Scholar] [CrossRef]
- Sun, C.-P.; Zhang, X.-Y.; Zhou, J.-J.; Huo, X.-K.; Yu, Z.-L.; Morisseau, C.; Hammock, B.D.; Ma, X.-C. Inhibition of sEH via stabilizing the level of EETs alleviated Alzheimer’s disease through GSK3β signaling pathway. Food Chem. Toxicol. 2021, 156, 112516. [Google Scholar] [CrossRef]
- Tang, C.; Border, J.J.; Zhang, H.; Gregory, A.; Bai, S.; Fang, X.; Liu, Y.; Wang, S.; Hwang, S.H.; Gao, W.; et al. Inhibition of soluble epoxide hydrolase ameliorates cerebral blood flow autoregulation and cognition in alzheimer’s disease and diabetes-related dementia rat models. Geroscience 2025, 47, 4429–4449. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Shah, R.; Alford, N.; Mishra, S.P.; Jain, S.; Hansen, B.; Sanberg, P.; Molina, A.J.A.; Yadav, H. The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer’s Disease. J. Gerontol. Ser. A 2023, 78, 2187–2202. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Li, W.; Li, L.; Tian, Q.; Cao, Q.; Meng, Y.; Shen, Y.; Che, F.; Chiu, J.C.; et al. The soluble epoxide hydrolase inhibitor TPPU alleviates Aβ-mediated neuroinflammatory responses in Drosophila melanogaster and cellular models of alzheimer’s disease. J. Inflamm. 2025, 22, 25. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Fan, Z.; Chen, Q.; Chen, J.; Sun, Y.; Jiang, X.; Xiao, Q. Soluble epoxide hydrolase inhibitor protects against blood-brain barrier dysfunction in a mouse model of type 2 diabetes via the AMPK/HO-1 pathway. Biochem. Biophys. Res. Commun. 2020, 524, 354–359. [Google Scholar] [CrossRef]
- Sarkar, P.; Narayanan, J.; Harder, D.R. Differential effect of amyloid β on the cytochrome P450 epoxygenase activity in rat brain. Neuroscience 2011, 194, 241–249. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, J.H.; Dai, Y.F.; Zhu, M.Z.; Wang, M.Y.; Zhang, Y.; Pan, Y.D.; Yuan, X.R.; Guo, Z.X.; Wang, C.X.; et al. Hepatic soluble epoxide hydrolase activity regulates cerebral Aβ metabolism and the pathogenesis of Alzheimer’s disease in mice. Neuron 2023, 111, 2847–2862.e10. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Hammock, B.D.; Hwang, S.H.; Whitelegge, J.; Paul, K.; Kaczor-Urbanowicz, K.E.; Urbanowicz, A.; Kesari, S. Inhibitors of soluble epoxide hydrolase and cGAS/STING repair defects in amyloid-β clearance underlying vascular complications of Alzheimer’s disease. J. Alzheimers Dis. 2025, 104, 150–157. [Google Scholar] [CrossRef]
- Borkowski, K.; Pedersen, T.L.; Seyfried, N.T.; Lah, J.J.; Levey, A.I.; Hales, C.M.; Dammer, E.B.; Blach, C.; Louie, G.; Kaddurah-Daouk, R.; et al. Association of plasma and CSF cytochrome P450, soluble epoxide hydrolase, and ethanolamide metabolism with Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 149. [Google Scholar] [CrossRef]
- Borkowski, K.; Seyfried, N.T.; Arnold, M.; Lah, J.J.; Levey, A.I.; Hales, C.M.; Dammer, E.B.; Blach, C.; Louie, G.; Kaddurah-Daouk, R.; et al. Integration of plasma and CSF metabolomics with CSF proteomic reveals novel associations between lipid mediators and central nervous system vascular and energy metabolism. Sci. Rep. 2023, 13, 13752. [Google Scholar] [CrossRef]
- Borkowski, K.; Yin, C.; Kindt, A.; Liang, N.; de Lange, E.; Blach, C.; Newman, J.W.; Kaddurah-Daouk, R.; Hankemeier, T. Metabolic Alteration in Oxylipins and Endocannabinoids Point to an Important Role for Soluble Epoxide Hydrolase and Inflammation in Alzheimer’s Disease—Finding from Alzheimer’s Disease Neuroimaging Initiative. BioRxiv 2025. [Google Scholar] [CrossRef]
- Yu, L.; Tasaki, S.; Schneider, J.A.; Arfanakis, K.; Duong, D.M.; Wingo, A.P.; Wingo, T.S.; Kearns, N.; Thatcher, G.R.J.; Seyfried, N.T.; et al. Cortical Proteins Associated With Cognitive Resilience in Community-Dwelling Older Persons. JAMA Psychiatry 2020, 77, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Lee, K.I.; Chen, C.H.; Lee, T.S. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflammation 2019, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Lee, Y.H.; Kuo, Y.M.; Hsu, P.C.; Tsay, H.J.; Hsu, Y.T.; Lee, C.C.; Liang, J.J.; Shie, F.S. Soluble epoxide hydrolase modulates immune responses in activated astrocytes involving regulation of STAT3 activity. J. Neuroinflammation 2019, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, B.; Xu, M.; Morisseau, C.; Hwang, S.H.; Hammock, B.D.; Li, Q.X. 1-Trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) Urea, a Selective and Potent Dual Inhibitor of Soluble Epoxide Hydrolase and p38 Kinase Intervenes in Alzheimer’s Signaling in Human Nerve Cells. ACS Chem. Neurosci. 2019, 10, 4018–4030. [Google Scholar] [CrossRef]
- Bartra, C.; Irisarri, A.; Villoslada, A.; Corpas, R.; Aguirre, S.; García-Lara, E.; Suñol, C.; Pallàs, M.; Griñán-Ferré, C.; Sanfeliu, C. Neuroprotective Epigenetic Changes Induced by Maternal Treatment with an Inhibitor of Soluble Epoxide Hydrolase Prevents Early Alzheimer’s Disease Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 15151. [Google Scholar] [CrossRef] [PubMed]
- Jarne-Ferrer, J.; Griñán-Ferré, C.; Bellver-Sanchis, A.; Vázquez, S.; Muñoz-Torrero, D.; Pallàs, M. A Combined Chronic Low-Dose Soluble Epoxide Hydrolase and Acetylcholinesterase Pharmacological Inhibition Promotes Memory Reinstatement in Alzheimer’s Disease Mice Models. Pharmaceuticals 2022, 15, 908. [Google Scholar] [CrossRef]
- Ghosh, A.; Comerota, M.M.; Wan, D.; Chen, F.; Propson, N.E.; Hwang, S.H.; Hammock, B.D.; Zheng, H. An epoxide hydrolase inhibitor reduces neuroinflammation in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2020, 12, eabb1206. [Google Scholar] [CrossRef]
- Yao, E.S.; Tang, Y.; Liu, X.H.; Wang, M.H. TPPU protects tau from H2O2-induced hyperphosphorylation in HEK293/tau cells by regulating PI3K/AKT/GSK-3β pathway. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, R.; Bolshette, N.; Gadhave, K.; Arfeen, M.; Ahmed, S.; Jamwal, R.; Hammock, B.D.; Lahkar, M.; Goswami, S.K. Docosahexaenoic Acid Increases the Potency of Soluble Epoxide Hydrolase Inhibitor in Alleviating Streptozotocin-Induced Alzheimer’s Disease-Like Complications of Diabetes. Front. Pharmacol. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Potjewyd, F.M.; Annor-Gyamfi, J.K.; Aubé, J.; Chu, S.; Conlon, I.L.; Frankowski, K.J.; Guduru, S.K.R.; Hardy, B.P.; Hopkins, M.D.; Kinoshita, C.; et al. Use of AD Informer Set compounds to explore validity of novel targets in Alzheimer’s disease pathology. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2022, 8, e12253. [Google Scholar] [CrossRef] [PubMed]
- Codony, S.; Calvó-Tusell, C.; Valverde, E.; Osuna, S.; Morisseau, C.; Loza, M.I.; Brea, J.; Pérez, C.; Rodríguez-Franco, M.I.; Pizarro-Delgado, J.; et al. From the Design to the In Vivo Evaluation of Benzohomoadamantane-Derived Soluble Epoxide Hydrolase Inhibitors for the Treatment of Acute Pancreatitis. J. Med. Chem. 2021, 64, 5429–5446. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Jarné-Ferrer, J.; Bellver-Sanchís, A.; Codony, S.; Puigoriol-Illamola, D.; Sanfeliu, C.; Oh, Y.; Lee, S.; Vázquez, S.; Pallàs, M. Novel molecular mechanism driving neuroprotection after soluble epoxide hydrolase inhibition: Insights for Alzheimer’s disease therapeutics. CNS Neurosci. Ther. 2024, 30, e14511. [Google Scholar] [CrossRef]
- Jarne-Ferrer, J.; Sánchez, J.; Codony, S.; Schneider, M.; Müller, C.E.; Sanfeliu, C.; Franco, R.; Vazquez, S.; Griñán-Ferré, C.; Pallàs, M. Novel Soluble Epoxide Hydrolase Inhibitor: Toward Regulatory Preclinical Studies. ACS Pharmacol. Transl. Sci. 2025, 8, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Jarne-Ferrer, J.; Griñán-Ferré, C.; Jora, B.; Codony, S.; Miró, L.; Rosell-Cardona, C.; Miñana-Galbis, D.; Pérez-Bosque, A.; Vazquez, S.; Pallàs, M. Soluble Epoxide Hydrolase Inhibition Improves Alzheimer’s Disease Hallmarks: Correlation with Peripheral Inflammation and Gut Microbiota Modulation. Aging Dis. 2025. [Google Scholar] [CrossRef]
- Arora, P.; Swati; Rani, S.; Jha, S.; Gupta, S.; Kumar, S. Innovative approaches in acetylcholinesterase inhibition: A pathway to effective Alzheimer’s disease treatment. Mol. Divers. 2025. [Google Scholar] [CrossRef]
- Codony, S.; Pont, C.; Griñán-Ferré, C.; Di Pede-Mattatelli, A.; Calvó-Tusell, C.; Feixas, F.; Osuna, S.; Jarné-Ferrer, J.; Naldi, M.; Bartolini, M.; et al. Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2022, 65, 4909–4925. [Google Scholar] [CrossRef]
- Almenara-Fuentes, L. Modulation of the Soluble Epoxide Hydrolase Enzyme as a Therapeutic Target Against Alzheimer’s Disease. Master’s Thesis, University of Barcelona, Catalonia, Spain, 2018. [Google Scholar]
- Zhang, J.; Yan, J.; Dong, H.; Zhang, R.; Chang, J.; Feng, Y.; Xu, X.; Li, W.; Qiu, F.; Sun, C. Dimeric sesquiterpenoids with anti-inflammatory activities from Inula britannica. Chin. J. Nat. Med. 2025, 23, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.L.; Xu, X.R.; Feng, Y.L.; Zhu, Q.M.; Morisseau, C.; Qiu, F.; Hammock, B.D.; Sun, C.P. Targeting PBK with small-molecule 1-O-acetyl-4R,6S-britannilactone for the treatment of neuroinflammation. Proc. Natl. Acad. Sci. USA 2025, 122, e2502593122. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tan, Y.; Zheng, Y.; Du, X.; Liu, Q. Ebselen ameliorates β-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer’s disease mice. J. Biol. Inorg. Chem. 2017, 22, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.F.; Callai-Silva, N.; Piovesan, A.R.; Carlini, C.R. Soluble Epoxide Hydrolase and Brain Cholesterol Metabolism. Front. Mol. Neurosci. 2019, 12, 325. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, H.; Yao, E.; Tian, Y.; Zhang, H.; Xu, L.; Yu, Z.; Fang, Y.; Wang, W.; Du, P.; et al. Soluble epoxide hydrolase inhibition Promotes White Matter Integrity and Long-Term Functional Recovery after chronic hypoperfusion in mice. Sci. Rep. 2017, 7, 7758. [Google Scholar] [CrossRef]
- Tu, R.; Armstrong, J.; Lee, K.S.S.; Hammock, B.D.; Sapirstein, A.; Koehler, R.C. Soluble epoxide hydrolase inhibition decreases reperfusion injury after focal cerebral ischemia. Sci. Rep. 2018, 8, 5279. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Chen, Y.; Yao, E.; Liu, X. Soluble epoxide hydrolase inhibition alleviated cognitive impairments via NRG1/ErbB4 signaling after chronic cerebral hypoperfusion induced by bilateral carotid artery stenosis in mice. Brain Res. 2018, 1699, 89–99. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Wu, X.; Muse, F.M.; Chen, J.; Cao, Y.; Yan, J.; Cheng, Z.; Yi, X.; Han, Z. Protective Effects of the Soluble Epoxide Hydrolase Inhibitor 1-Trifluoromethoxyphenyl-3-(1-Propionylpiperidin-4-yl) Urea in a Rat Model of Permanent Middle Cerebral Artery Occlusion. Front. Pharmacol. 2020, 11, 182. [Google Scholar] [CrossRef]
- Matin, N.; Fisher, C.; Lansdell, T.A.; Hammock, B.D.; Yang, J.; Jackson, W.F.; Dorrance, A.M. Soluble epoxide hydrolase inhibition improves cognitive function and parenchymal artery dilation in a hypertensive model of chronic cerebral hypoperfusion. Microcirculation 2021, 28, e12653. [Google Scholar] [CrossRef]
- Yi, X.; Fan, D.; Yi, T.; Chen, H.; Qing, T.; Han, Z.; Bao, S. 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) Urea Exerts Neuro-Protective Effects Against Ischemic Injury via Suppressing JNK/p38 MAPK-Mediated Mitochondrial Apoptosis Pathway. J. Stroke Cerebrovasc. Dis. 2021, 30, 105957. [Google Scholar] [CrossRef]
- Yi, X.; Xu, C.; Huang, P.; Zhang, L.; Qing, T.; Li, J.; Wang, C.; Zeng, T.; Lu, J.; Han, Z. 1-Trifluoromethoxyphenyl-3-(1-Propionylpiperidin-4-yl) Urea Protects the Blood-Brain Barrier Against Ischemic Injury by Upregulating Tight Junction Protein Expression, Mitigating Apoptosis and Inflammation. Front. Pharmacol. 2020, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ma, M.; Yang, J.; Nonaka, R.; Yamaguchi, A.; Ishikawa, K.I.; Kobayashi, K.; Murayama, S.; Hwang, S.H.; Saiki, S.; et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E5815–E5823. [Google Scholar] [CrossRef]
- Oguro, A.; Ishihara, Y.; Siswanto, F.M.; Yamazaki, T.; Ishida, A.; Imaishi, H.; Imaoka, S. Contribution of DHA diols (19,20-DHDP) produced by cytochrome P450s and soluble epoxide hydrolase to the beneficial effects of DHA supplementation in the brains of rotenone-induced rat models of Parkinson’s disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158858. [Google Scholar] [CrossRef]
- Huang, H.J.; Wang, Y.T.; Lin, H.C.; Lee, Y.H.; Lin, A.M. Soluble Epoxide Hydrolase Inhibition Attenuates MPTP-Induced Neurotoxicity in the Nigrostriatal Dopaminergic System: Involvement of α-Synuclein Aggregation and ER Stress. Mol. Neurobiol. 2018, 55, 138–144. [Google Scholar] [CrossRef]
- Luo, A.; Wu, Z.; Li, S.; McReynolds, C.B.; Wang, D.; Liu, H.; Huang, C.; He, T.; Zhang, X.; Wang, Y.; et al. The soluble epoxide hydrolase inhibitor TPPU improves comorbidity of chronic pain and depression via the AHR and TSPO signaling. J. Transl. Med. 2023, 21, 71. [Google Scholar] [CrossRef]
- Wu, Q.; Song, J.; Meng, D.; Chang, Q. TPPU, a sEH Inhibitor, Attenuates Corticosterone-Induced PC12 Cell Injury by Modulation of BDNF-TrkB Pathway. J. Mol. Neurosci. 2019, 67, 364–372. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, H.; Song, J.; Chang, Q. The effects of sEH inhibitor on depression-like behavior and neurogenesis in male mice. J. Neurosci. Res. 2017, 95, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ma, M.; Ishima, T.; Morisseau, C.; Yang, J.; Wagner, K.M.; Zhang, J.C.; Yang, C.; Yao, W.; Dong, C.; et al. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc. Natl. Acad. Sci. USA 2016, 113, E1944–E1952. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Peng, W.; Shen, Y.; Wang, Q.; Ding, J.; Wang, X. TPPU Pre-Treatment Rescues Dendritic Spine Loss and Alleviates Depressive Behaviours during the Latent Period in the Lithium Chloride-Pilocarpine-Induced Status Epilepticus Rat Model. Brain Sci. 2021, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.W.; Hung, S.W.; Wu, Y.C.; Wong, L.K.; Lai, M.T.; Shih, Y.H.; Lee, T.S.; Lin, Y.Y. Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain Behav. Immun. 2015, 43, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Peng, W.; Chen, Q.; Hammock, B.D.; Liu, J.; Li, D.; Yang, J.; Ding, J.; Wang, X. Anti-inflammatory treatment with a soluble epoxide hydrolase inhibitor attenuates seizures and epilepsy-associated depression in the LiCl-pilocarpine post-status epilepticus rat model. Brain Behav. Immun. 2019, 81, 535–544. [Google Scholar] [CrossRef]
- Peng, W.; Hu, Z.; Shen, Y.; Wang, X. Inhibiting the soluble epoxide hydrolase increases the EpFAs and ERK1/2 expression in the hippocampus of LiCl-pilocarpine post-status epilepticus rat model. IBRO Neurosci. Rep. 2024, 17, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Ren, Q.; Yang, J.; Zhang, K.; Xiong, Z.; Ishima, T.; Pu, Y.; Hwang, S.H.; Toyoshima, M.; Iwayama, Y.; et al. Key role of soluble epoxide hydrolase in the neurodevelopmental disorders of offspring after maternal immune activation. Proc. Natl. Acad. Sci. USA 2019, 116, 7083–7088. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Q.; Fujita, Y.; Ishima, T.; Zhang, J.C.; Hashimoto, K. Effects of AS2586114, a soluble epoxide hydrolase inhibitor, on hyperlocomotion and prepulse inhibition deficits in mice after administration of phencyclidine. Pharmacol. Biochem. Behav. 2013, 110, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Companys-Alemany, J.; Cozar, M.; Grinberg, D.; Vilageliu, L.; Codony, S.; Vázquez, S.; Pallàs, M.; Griñán-Ferré, C. Pharmacological inhibition of soluble epoxide hydrolase protects cognitive impairment in a Niemann-Pick mice model. In Proceedings of the 33rd ECNP Congress, Virtual, 12–15 September 2020. [Google Scholar]
- Zhang, W.; Koerner, I.P.; Noppens, R.; Grafe, M.; Tsai, H.J.; Morisseau, C.; Luria, A.; Hammock, B.D.; Falck, J.R.; Alkayed, N.J. Soluble epoxide hydrolase: A novel therapeutic target in stroke. J. Cereb. Blood Flow. Metab. 2007, 27, 1931–1940. [Google Scholar] [CrossRef]
- Yeh, C.F.; Chuang, T.Y.; Hung, Y.W.; Lan, M.Y.; Tsai, C.H.; Huang, H.X.; Lin, Y.Y. Soluble epoxide hydrolase inhibition enhances anti-inflammatory and antioxidative processes, modulates microglia polarization, and promotes recovery after ischemic stroke. Neuropsychiatr. Dis. Treat. 2019, 15, 2927–2941. [Google Scholar] [CrossRef]
- Hung, T.H.; Shyue, S.K.; Wu, C.H.; Chen, C.C.; Lin, C.C.; Chang, C.F.; Chen, S.F. Deletion or inhibition of soluble epoxide hydrolase protects against brain damage and reduces microglia-mediated neuroinflammation in traumatic brain injury. Oncotarget 2017, 8, 103236–103260. [Google Scholar] [CrossRef] [PubMed]
- Jingya, L.; Song, L.; Lu, L.; Zhang, Q.; Zhang, W. Effect of Shenqi Jieyu formula on inflammatory response pathway in hippocampus of postpartum depression rats. Heliyon 2024, 10, e29978. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Song, L.; Qiu, C.; Li, J. Effect of the sEH inhibitor AUDA on arachidonic acid metabolism and NF-κB signaling of rats with postpartum depression-like behavior. J. Neuroimmunol. 2023, 385, 578250. [Google Scholar] [CrossRef]
- Sun, C.P.; Zhou, J.J.; Yu, Z.L.; Huo, X.K.; Zhang, J.; Morisseau, C.; Hammock, B.D.; Ma, X.C. Kurarinone alleviated Parkinson’s disease via stabilization of epoxyeicosatrienoic acids in animal model. Proc. Natl. Acad. Sci. USA 2022, 119, e2118818119. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, Y. Neuroprotective effect of Kurarinone against corticosterone-induced cytotoxicity on rat hippocampal neurons by targeting BACE1 to activate P13K-AKT signaling—A potential treatment in insomnia disorder. Pharmacol. Res. Perspect. 2023, 11, e01132. [Google Scholar] [CrossRef]
- Darwish, S.F.; Elbadry, A.M.M.; Elbokhomy, A.S.; Salama, G.A.; Salama, R.M. The dual face of microglia (M1/M2) as a potential target in the protective effect of nutraceuticals against neurodegenerative diseases. Front. Aging 2023, 4, 1231706. [Google Scholar] [CrossRef]
- Prajapati, R.; Seong, S.H.; Paudel, P.; Park, S.E.; Jung, H.A.; Choi, J.S. In Vitro and In Silico Characterization of Kurarinone as a Dopamine D(1A) Receptor Antagonist and D(2L) and D(4) Receptor Agonist. ACS Omega 2021, 6, 33443–33453. [Google Scholar] [CrossRef]
- Jeong, G.S.; Li, B.; Lee, D.S.; Byun, E.; An, R.B.; Pae, H.O.; Chung, H.T.; Youn, K.H.; Kim, Y.C. Lavandulyl flavanones from Sophora flavescens protect mouse hippocampal cells against glutamate-induced neurotoxicity via the induction of heme oxygenase-1. Biol. Pharm. Bull. 2008, 31, 1964–1967. [Google Scholar] [CrossRef]
- Park, S.J.; Nam, K.W.; Lee, H.J.; Cho, E.Y.; Koo, U.; Mar, W. Neuroprotective effects of an alkaloid-free ethyl acetate extract from the root of Sophora flavescens Ait. against focal cerebral ischemia in rats. Phytomedicine 2009, 16, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.Q.; Zuo, C.; Yue, W.F. Kurarinone alleviates hemin-induced neuroinflammation and microglia-mediated neurotoxicity by shifting microglial M1/M2 polarization via regulating the IGF1/PI3K/Akt signaling. Kaohsiung J. Med. Sci. 2022, 38, 1213–1223. [Google Scholar] [CrossRef]
- Wu, J.; Fan, Z.; Zhao, Y.; Chen, Q.; Xiao, Q. Inhibition of soluble epoxide hydrolase (sEH) protects hippocampal neurons and reduces cognitive decline in type 2 diabetic mice. Eur. J. Neurosci. 2021, 53, 2532–2540. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin sensitizes glioblastoma to t-AUCB by dual inhibition of Hsp27 and COX-2 in vitro and in vivo. J. Exp. Clin. Cancer Res. 2016, 35, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Sang, D.; Lan, Q. Phosphorylation of AKT induced by phosphorylated Hsp27 confers the apoptosis-resistance in t-AUCB-treated glioblastoma cells in vitro. J. Neuro-Oncol. 2015, 121, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Li, R.J.; Wang, H.D. γ-secretase inhibitor DAPT sensitizes t-AUCB-induced apoptosis of human glioblastoma cells in vitro via blocking the p38 MAPK/MAPKAPK2/Hsp27 pathway. Acta Pharmacol. Sin. 2014, 35, 825–831. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Xing, B.; Yu, Y.; Wang, H.; Chen, G.; Gu, B.; Zhang, G.; Wei, D.; Gu, P.; et al. t-AUCB, an improved sEH inhibitor, suppresses human glioblastoma cell growth by activating NF-κB-p65. J. Neuro-Oncol. 2012, 108, 385–393. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Lan, Q. The apoptosis-resistance in t-AUCB-treated glioblastoma cells depends on activation of Hsp27. J. Neuro-Oncol. 2012, 110, 187–194. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin blocks t-AUCB-induced autophagy by Hsp27 and Atg7 inhibition in glioblastoma cells in vitro. J. Neuro-Oncol. 2016, 129, 39–45. [Google Scholar] [CrossRef]
- Poli, G.; Corda, E.; Martino, P.A.; Dall’ara, P.; Bareggi, S.R.; Bondiolotti, G.; Iulini, B.; Mazza, M.; Casalone, C.; Hwang, S.H.; et al. Therapeutic activity of inhibition of the soluble epoxide hydrolase in a mouse model of scrapie. Life Sci. 2013, 92, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; Zhang, W.H.; Allen, E.M.; Bah, T.M.; Shangraw, R.E.; Alkayed, N.J. Soluble Epoxide Hydrolase Blockade after Stroke Onset Protects Normal but Not Diabetic Mice. Int. J. Mol. Sci. 2021, 22, 5419. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, K.L.; Zhang, W.; Roese, N.E.; Alkayed, N.J. Soluble epoxide hydrolase gene deletion improves blood flow and reduces infarct size after cerebral ischemia in reproductively senescent female mice. Front. Pharmacol. 2014, 5, 290. [Google Scholar] [CrossRef]
- Shaik, J.S.; Ahmad, M.; Li, W.; Rose, M.E.; Foley, L.M.; Hitchens, T.K.; Graham, S.H.; Hwang, S.H.; Hammock, B.D.; Poloyac, S.M. Soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is neuroprotective in rat model of ischemic stroke. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1605–H1613. [Google Scholar] [CrossRef]
- Chaudhary, K.R.; Abukhashim, M.; Hwang, S.H.; Hammock, B.D.; Seubert, J.M. Inhibition of soluble epoxide hydrolase by trans-4- [4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is protective against ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. 2010, 55, 67–73. [Google Scholar] [CrossRef]
- Jouihan, S.A.; Zuloaga, K.L.; Zhang, W.; Shangraw, R.E.; Krasnow, S.M.; Marks, D.L.; Alkayed, N.J. Role of soluble epoxide hydrolase in exacerbation of stroke by streptozotocin-induced type 1 diabetes mellitus. J. Cereb. Blood Flow. Metab. 2013, 33, 1650–1656. [Google Scholar] [CrossRef]
- Bah, T.M.; Davis, C.M.; Allen, E.M.; Borkar, R.N.; Perez, R.; Grafe, M.R.; Raber, J.; Pike, M.M.; Alkayed, N.J. Soluble epoxide hydrolase inhibition reverses cognitive dysfunction in a mouse model of metabolic syndrome by modulating inflammation. Prostaglandins Other Lipid Mediat. 2024, 173, 106850. [Google Scholar] [CrossRef]
- Norman, J.E.; Nuthikattu, S.; Milenkovic, D.; Rutledge, J.C.; Villablanca, A.C. Sex-Specific Response of the Brain Free Oxylipin Profile to Soluble Epoxide Hydrolase Inhibition. Nutrients 2023, 15, 1214. [Google Scholar] [CrossRef]
- Nuthikattu, S.; Milenkovic, D.; Norman, J.E.; Rutledge, J.; Villablanca, A. High Glycemia and Soluble Epoxide Hydrolase in Females: Differential Multiomics in Murine Brain Microvasculature. Int. J. Mol. Sci. 2022, 23, 13044. [Google Scholar] [CrossRef]
- Nuthikattu, S.; Milenkovic, D.; Norman, J.E.; Rutledge, J.; Villablanca, A. The Brain’s Microvascular Response to High Glycemia and to the Inhibition of Soluble Epoxide Hydrolase Is Sexually Dimorphic. Nutrients 2022, 14, 3451. [Google Scholar] [CrossRef] [PubMed]
- Nuthikattu, S.; Milenkovic, D.; Norman, J.E.; Rutledge, J.; Villablanca, A. Inhibition of Soluble Epoxide Hydrolase Is Protective against the Multiomic Effects of a High Glycemic Diet on Brain Microvascular Inflammation and Cognitive Dysfunction. Nutrients 2021, 13, 3913. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, K.L.; Krasnow, S.M.; Zhu, X.; Zhang, W.; Jouihan, S.A.; Shangraw, R.E.; Alkayed, N.J.; Marks, D.L. Mechanism of protection by soluble epoxide hydrolase inhibition in type 2 diabetic stroke. PLoS ONE 2014, 9, e97529. [Google Scholar] [CrossRef]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Joshi, N.; Singh, S. Updates on immunity and inflammation in Parkinson disease pathology. J. Neurosci. Res. 2018, 96, 379–390. [Google Scholar] [CrossRef]
- Toulorge, D.; Schapira, A.H.; Hajj, R. Molecular changes in the postmortem parkinsonian brain. J Neurochem. 2016, 139 (Suppl. 1), 27–58. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Erkinjuntti, T.; Reisberg, B.; Roman, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B.; et al. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef]
- Nelson, J.W.; Young, J.M.; Borkar, R.N.; Woltjer, R.L.; Quinn, J.F.; Silbert, L.C.; Grafe, M.R.; Alkayed, N.J. Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat. 2014, 113–115, 30–37. [Google Scholar] [CrossRef]
- Yu, D.; Hennebelle, M.; Sahlas, D.J.; Ramirez, J.; Gao, F.; Masellis, M.; Cogo-Moreira, H.; Swartz, R.H.; Herrmann, N.; Chan, P.C.; et al. Soluble Epoxide Hydrolase-Derived Linoleic Acid Oxylipins in Serum Are Associated with Periventricular White Matter Hyperintensities and Vascular Cognitive Impairment. Transl. Stroke Res. 2019, 10, 522–533. [Google Scholar] [CrossRef]
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- Emsley, H.C.; Smith, C.J.; Gavin, C.M.; Georgiou, R.F.; Vail, A.; Barberan, E.M.; Hallenbeck, J.M.; del Zoppo, G.J.; Rothwell, N.J.; Tyrrell, P.J.; et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: Relationships with infection and atherosclerosis. J. Neuroimmunol. 2003, 139, 93–101. [Google Scholar] [CrossRef]
- Ward, N.C.; Croft, K.D.; Blacker, D.; Hankey, G.J.; Barden, A.; Mori, T.A.; Puddey, I.B.; Beer, C.D. Cytochrome P450 metabolites of arachidonic acid are elevated in stroke patients compared with healthy controls. Clin. Sci. (Lond) 2011, 121, 501–507. [Google Scholar] [CrossRef]
- Iliff, J.J.; Alkayed, N.J. Soluble Epoxide Hydrolase Inhibition: Targeting Multiple Mechanisms of Ischemic Brain Injury with a Single Agent. Future Neurol. 2009, 4, 179–199. [Google Scholar] [CrossRef] [PubMed]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef]
- Dorrance, A.M.; Rupp, N.; Pollock, D.M.; Newman, J.W.; Hammock, B.D.; Imig, J.D. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2005, 46, 842–848. [Google Scholar] [CrossRef]
- Kim, I.H.; Nishi, K.; Tsai, H.J.; Bradford, T.; Koda, Y.; Watanabe, T.; Morisseau, C.; Blanchfield, J.; Toth, I.; Hammock, B.D. Design of bioavailable derivatives of 12-(3-adamantan-1-yl-ureido)dodecanoic acid, a potent inhibitor of the soluble epoxide hydrolase. Bioorg. Med. Chem. 2007, 15, 312–323. [Google Scholar] [CrossRef]
- Hirabayashi, N.; Hata, J.; Ohara, T.; Mukai, N.; Nagata, M.; Shibata, M.; Gotoh, S.; Furuta, Y.; Yamashita, F.; Yoshihara, K.; et al. Association Between Diabetes and Hippocampal Atrophy in Elderly Japanese: The Hisayama Study. Diabetes Care 2016, 39, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Sadeghi, R.; Walker, D.G.; Lue, L.F.; Sabbagh, M.N. Consequences of Aberrant Insulin Regulation in the Brain: Can Treating Diabetes be Effective for Alzheimer’s Disease. Curr. Neuropharmacol. 2011, 9, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Romashko, M.; Schragenheim, J.; Abraham, N.G.; McClung, J.A. Epoxyeicosatrienoic Acid as Therapy for Diabetic and Ischemic Cardiomyopathy. Trends Pharmacol. Sci. 2016, 37, 945–962. [Google Scholar] [CrossRef]
- Minaz, N.; Razdan, R.; Hammock, B.D.; Goswami, S.K. An inhibitor of soluble epoxide hydrolase ameliorates diabetes-induced learning and memory impairment in rats. Prostaglandins Other Lipid Mediat. 2018, 136, 84–89. [Google Scholar] [CrossRef]
- Liu, L.; He, H.; Du, B.; He, Y. Nanoscale drug formulations for the treatment of Alzheimer’s disease progression. RSC Adv. 2025, 15, 4031–4078. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.E.; Greig, N.H.; Giacobini, E. Why do so many drugs for Alzheimer’s disease fail in development? Time for new methods and new practices? J. Alzheimers Dis. 2008, 15, 303–325. [Google Scholar] [CrossRef]
- Chen, D.; Whitcomb, R.; MacIntyre, E.; Tran, V.; Do, Z.N.; Sabry, J.; Patel, D.V.; Anandan, S.K.; Gless, R.; Webb, H.K. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J. Clin. Pharmacol. 2012, 52, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Sellwood, C.D.; Schmidt, W.K. Safety, Tolerability, and Pharmacokinetics of Multiple Doses of Oral EC5026 in Healthy Subjects. 2023. Available online: https://clinicaltrials.gov/study/NCT06089837 (accessed on 21 August 2025).


| No. | Drugs Used in Alzheimer’s Disease Studies | Mechanism of Action | Alzheimer’s Disease Research Findings | Experimental In Vivo Models Used in Alzheimer’s Disease Studies | Other Explored Neurological/Psychiatric Conditions | References |
|---|---|---|---|---|---|---|
| 1. | TPPU | Potent sEH inhibitor with p38 blocking activity | The reduction of Aβ plaques, hyperphosphorylated tau, neuroinflammation, astrogliosis, microglial activation, and oxidative stress. Improved cerebral blood flow, neuroprotection, memory, and cognitive function. | Rodents: Familial Alzheimer’s Disease (5xFAD) mice Senescence-Accelerated Mouse-Prone 8 (SAMP8) C57BL/6 mice CD1 mice Sprague-Dawley rats TgF344-AD rats PS19 Tau P301S transgenic mice Doses: 1–5 mg/kg/day Route of Administration: Oral, intraperitoneal injections Duration: 2–20 weeks Insects: Aβ42 transgenic Drosophila. | Stroke Parkinson’s disease Depression Epilepsy Neurodevelopmental disorders | [13,61,69,70,77,78,79,81,85,92,93,94,95,96,97,98,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] |
| 2. | TPPU-6-chlorotacrine | A hybrid of TPPU and 6-chlorotacrine (huperine Y), an acetylcholine esterase inhibitor | The reduction of Aβ plaques, hyperphosphorylated tau, and neuroinflammation. Improved memory and cognitive function. | Mice (SAMP8) Dose: 2 mg/kg/day Route of Administration: Oral Duration: 4 weeks | - | [104] |
| 3. | UB-SCG-51 | Potent and selective sEH inhibitor derived from Benzohomoadamantane | The reduction of Aβ plaques, hyperphosphorylated tau, neuroinflammation, astrogliosis, microglial activation, and oxidative stress. Improved neuroprotection, memory, and cognitive function. | 5XFAD mice. Dose: 5 mg/kg/day Route of Administration: Oral Duration: 4 weeks | - | [100] |
| 4. | UB-SCG-55 | Safe and potent sEH inhibitor derived from Benzohomoadamantane | The reduction of neuroinflammation and microglial inflammation. | Experiments were performed in vitro: (Primary microglial and BV2 cells (murine)). | - | [77] |
| 5. | UB-SCG-74 | Selective, potent, and safe sEH inhibitor derived from Benzohomoadamantane. Has good oral absorption and BBB permeability. | Improved memory, cognitive, and synaptic function. | 5XFAD mice. Doses: 0.5, 1.5, and 3 mg/kg/day Route of Administration: Oral Duration: 4 weeks | - | [101] |
| 6. | UB-BJ02 | Novel soluble epoxide hydrolase inhibitor | The reduction of Aβ plaques, neuroinflammation, astrogliosis, microglial activation. Improved memory and cognitive function. Improved mitochondrial function and gut microbiota diversity | 5XFAD mice Dose: 5 mg/kg/day Route of Administration: Oral Duration: 4 weeks | - | [102] |
| 7. | AS-2586114 and UB-EV-52 | Newer inhibitors with BBB crossing ability | The reduction of Aβ plaques, hyperphosphorylated tau, neuroinflammation, and oxidative stress. Improved memory and cognitive function. | SAMP8 and 5XFAD mice Doses: AS-2586114: 7.5 mg/kg/day UB-EV-52: 5 mg/kg/day Route of Administration: Oral Duration: 4 weeks | Antipsychotic activity (AS-2586114) Cognitive decline in Niemann-pick disease (UB-EV-52) | [13,130,131] |
| 8. | UB23 and UB28 | Novel sEH inhibitors | The reduction of neuroinflammation. Improved neuroprotection. | Experiments were performed in vitro: SH-SY5Y cells Murine microglial cells (BV2). | - | [105] |
| Other Drugs | ||||||
| 9. | AUDA | sEH inhibitor with anti-inflammatory effects | Protective against stroke through inhibition of EETs synthesis, microglial M2 polarization. Has anti-inflammatory, antioxidant, and neuroprotective properties. | C57Bl/6 mice. Wistar Kyoto rats. Dose: 10–20 mg/kg/day Route of Administration: Intraperitoneal Duration:1–2 days | Intracerebral hemorrhage Brain and spinal cord injury Postpartum depression Epilepsy | [57,58,126,132,133,134,135,136] |
| 10. | Kurarinone | A natural flavonoid derived from Sophora flavescens, with a non-competitive sEH inhibitory function. | Showed behavioural alleviation in Parkinson’s disease-, reduced neurotoxicity and neuroinflammation. | C57BL/6 mice Dose: 5–10–20 mg/kg/day Route of Administration: Oral Duration: 12–19 days | Insomnia Neurodegeneration Cerebral ischemia. Cerebral hemorrhage | [137,138,139,140,141,142,143] |
| 11. | t-AUCB | Potent sEH inhibitor | Protective against DM-related cognitive dysfunction, through antioxidation and neuroprotection. | db/db mice Dose: 2 mg/L in drinking water (0.6–0.8 μg/kg/day) Route of Administration: Oral Duration: 4 weeks | Glioblastoma Scrapie Stroke Metabolic neurological complications | [82,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.; Ibrahim, M.; Alkorbi, N.; Alkuwari, S.; Pedersen, S.; Rathore, H.A. Epoxide Hydrolase Inhibitors for the Treatment of Alzheimer’s Disease and Other Neurological Disorders: A Comprehensive Review. Biomedicines 2025, 13, 2073. https://doi.org/10.3390/biomedicines13092073
Abdalla M, Ibrahim M, Alkorbi N, Alkuwari S, Pedersen S, Rathore HA. Epoxide Hydrolase Inhibitors for the Treatment of Alzheimer’s Disease and Other Neurological Disorders: A Comprehensive Review. Biomedicines. 2025; 13(9):2073. https://doi.org/10.3390/biomedicines13092073
Chicago/Turabian StyleAbdalla, Manal, Mohamed Ibrahim, Noora Alkorbi, Shaika Alkuwari, Shona Pedersen, and Hassaan Anwer Rathore. 2025. "Epoxide Hydrolase Inhibitors for the Treatment of Alzheimer’s Disease and Other Neurological Disorders: A Comprehensive Review" Biomedicines 13, no. 9: 2073. https://doi.org/10.3390/biomedicines13092073
APA StyleAbdalla, M., Ibrahim, M., Alkorbi, N., Alkuwari, S., Pedersen, S., & Rathore, H. A. (2025). Epoxide Hydrolase Inhibitors for the Treatment of Alzheimer’s Disease and Other Neurological Disorders: A Comprehensive Review. Biomedicines, 13(9), 2073. https://doi.org/10.3390/biomedicines13092073








