Increased IGF2 and Immunosuppressive Cell Populations in Ascites of Patients with Recurrent High-Grade Serous Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Eligibility and Consent
2.2. RNA Isolation
2.3. RNA Sequencing
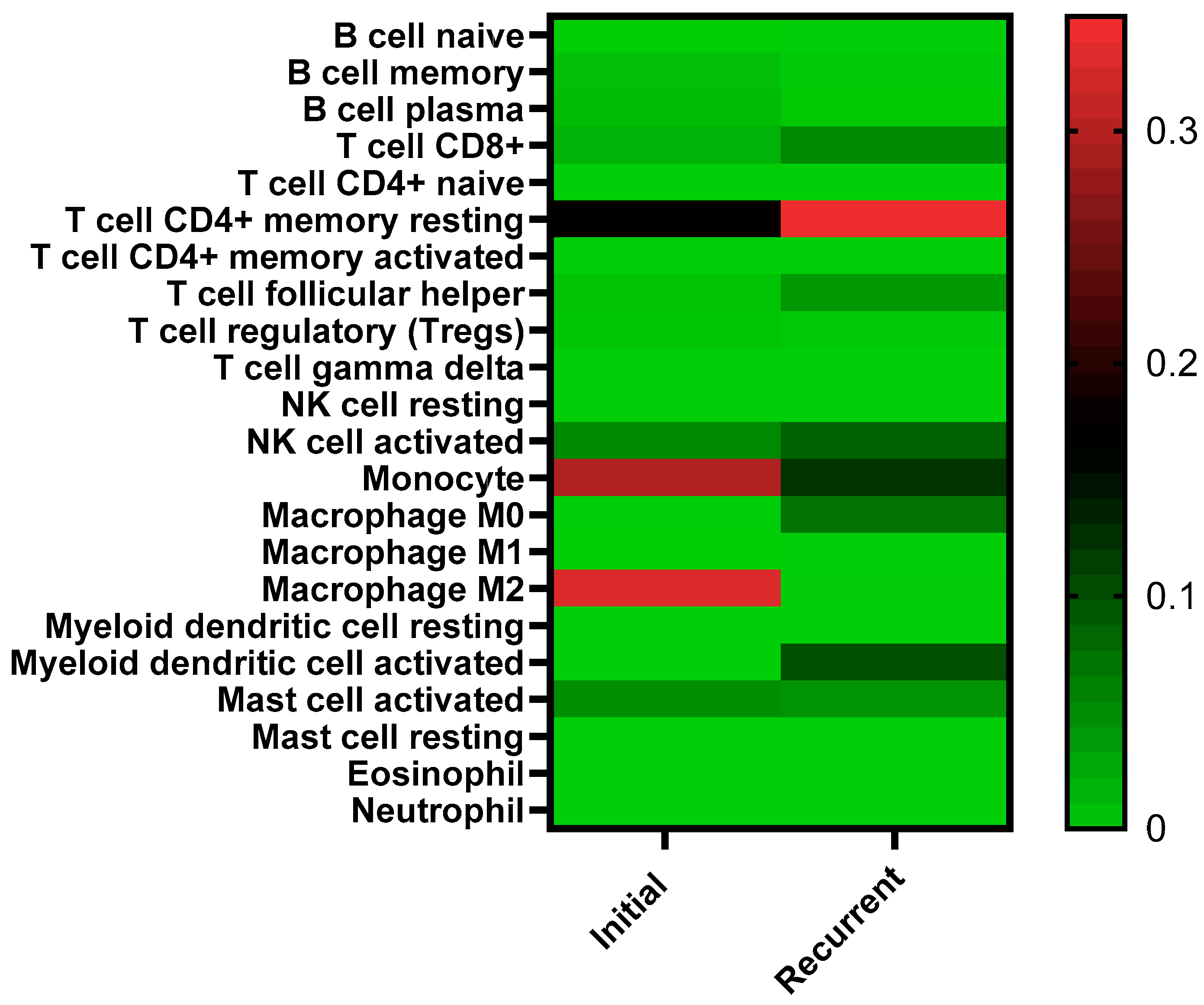
2.4. Deconvolution Analysis
2.5. Pro-Detect Antibody Isotyping
2.6. IgG Analysis
2.7. Protein Isolation
2.8. Insulin-like Growth Factor 2 (IGF2) Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Survival Analysis
3. Results
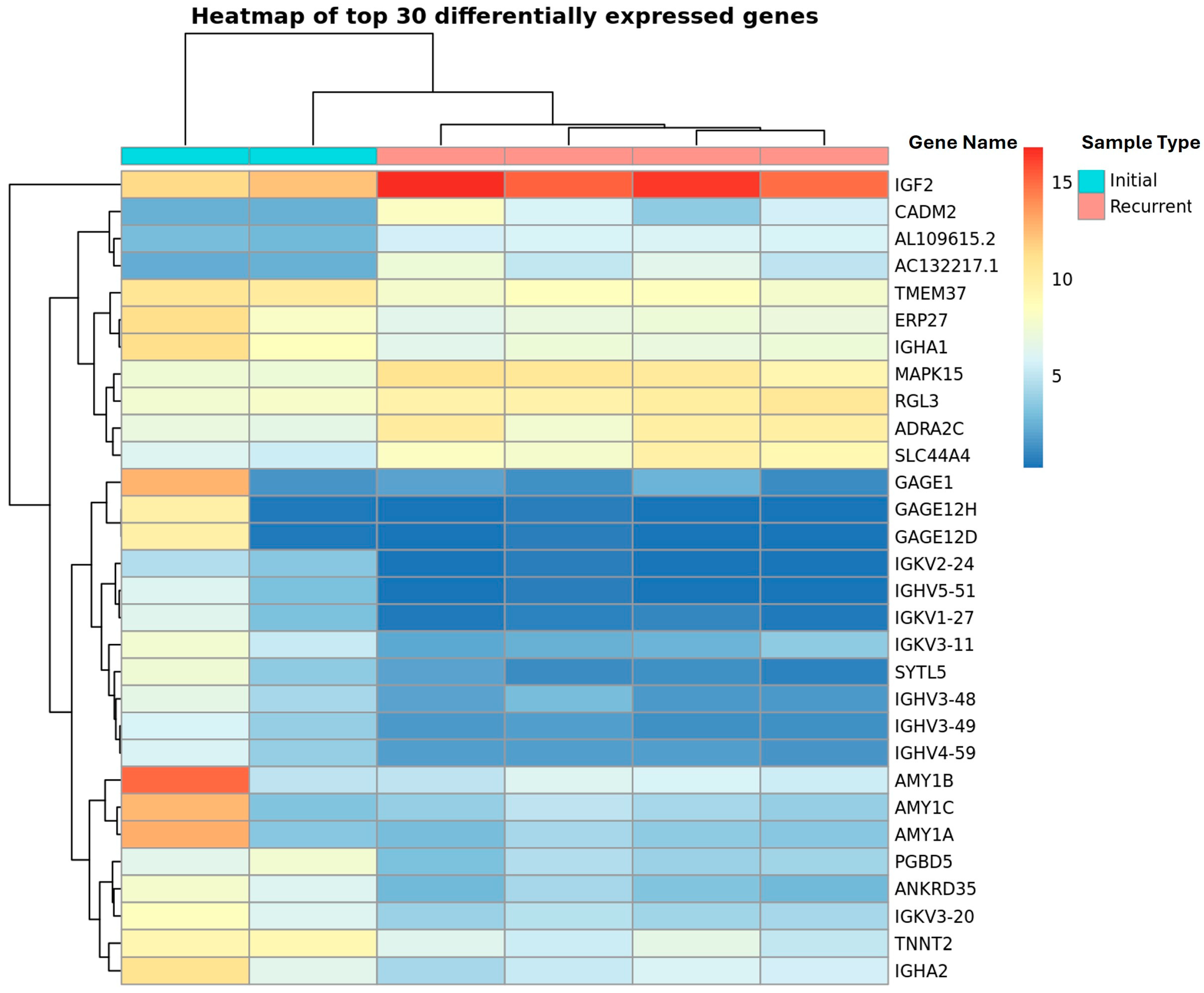
3.1. Identification of IGF2 and Decreased B-Cells in HGSOC Recurrence
3.2. Immunoglobulins Expressed in Initial Compared to Recurrent Ascites
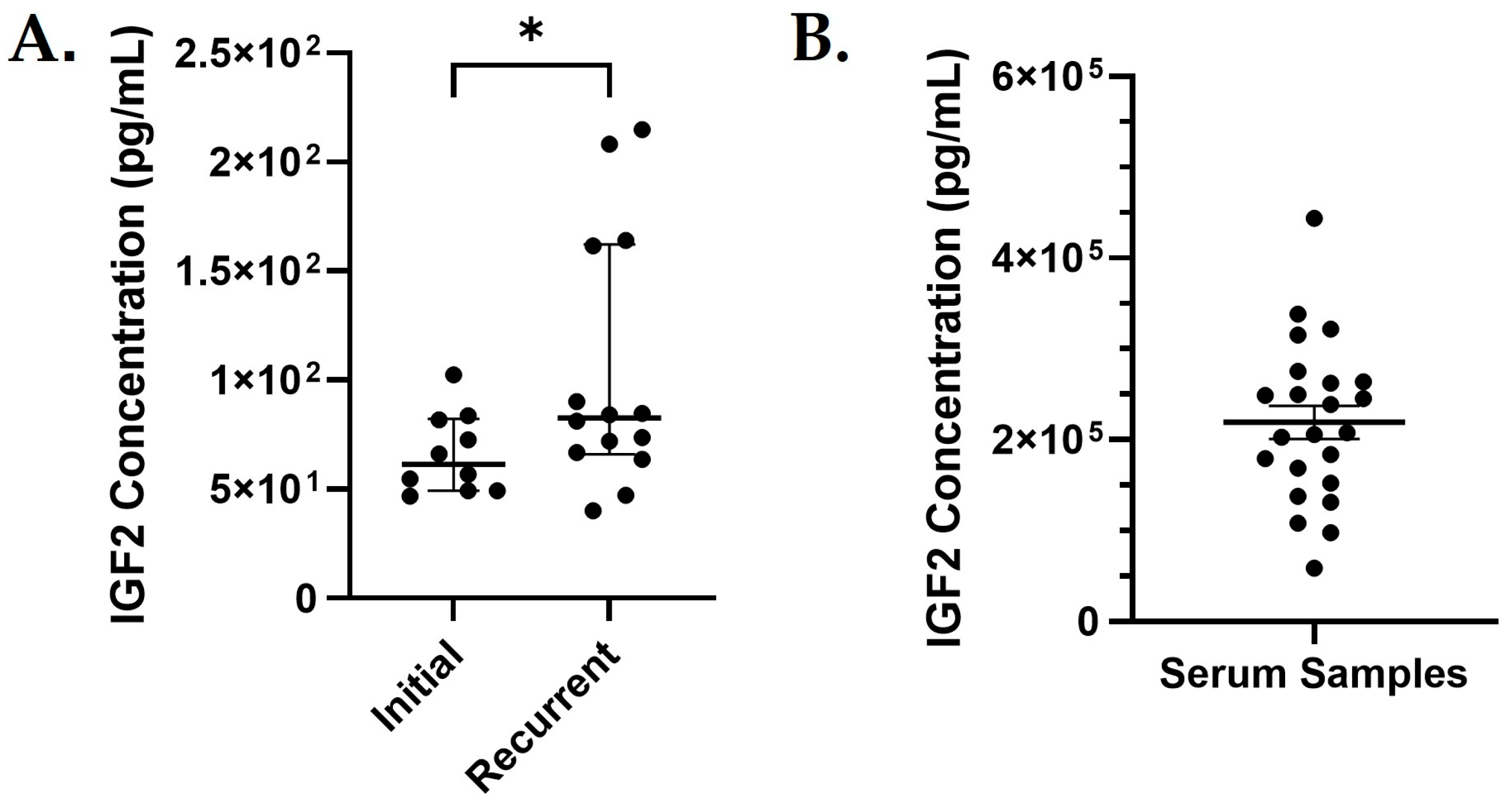
3.3. IGF2 Expression Analysis
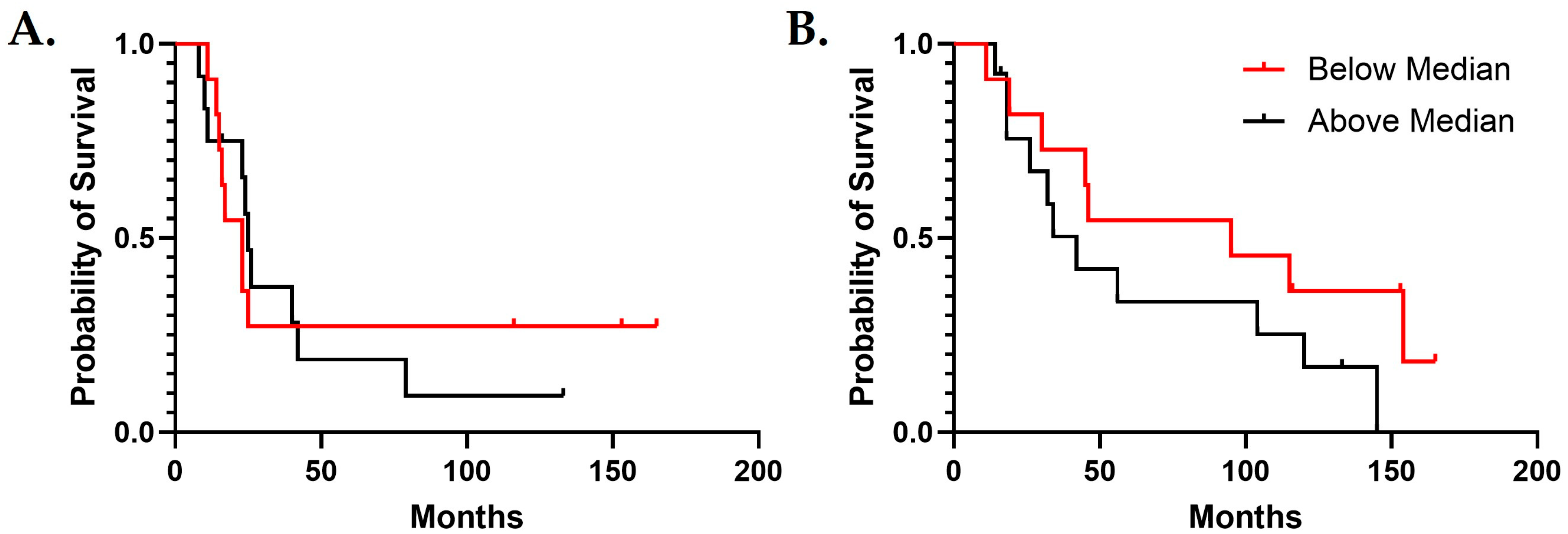
3.4. IGF2 Is Useful for Predicting Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI/AN | American Indian/Alaskan Native |
| BCA | Bicinchoninic Acid |
| BMI | Body Mass Index |
| CD4+ | Cluster of Differentiation 4 Positive |
| CD8+ | Cluster of Differentiation 8 Positive |
| DESeq2 | Differential Expression Sequencing 2 |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FCER | Fc Epsilon Receptor |
| FCGR | Fc Gamma Receptor |
| GRCh38 | Genome Reference Consortium Human Build 38 |
| HGSOC | High-Grade Serous Ovarian Cancer |
| IDT | Integrated DNA Technologies |
| IgA | Immunoglobulin A |
| IGF1-R | Insulin-like growth factor 1 receptor |
| IGF2 | Insulin-like growth factor 2 |
| IgG1 | Immunoglobulin G Subclass 1 |
| IgG2 | Immunoglobulin G Subclass 2 |
| IgG3 | Immunoglobulin G Subclass 3 |
| IgG4 | Immunoglobulin G Subclass 4 |
| IGHA1/2 | Immunoglobulin Heavy Constant Alpha 1/2 |
| IGHG2/4 | Immunoglobulin Heavy Constant Gamma 2/4 |
| IGHV | Immunoglobulin Heavy Variable |
| IGKV | Immunoglobulin Kappa Variable |
| IGLV | Immunoglobulin Lambda Variable |
| IgM | Immunoglobulin M |
| IPA | Ingenuity Pathway Analysis |
| IRB | Institutional Review Board |
| KM | Kaplan–Meier |
| MAPK15 | Mitogen-Activated Protein Kinase 15 |
| MCPCounter | Microenvironment Cell Populations Counter |
| NEB | New England Biolabs |
| OD | Optical Density |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| PCA | Principal Component Analysis |
| RNA | Ribonucleic Acid |
| RIN | RNA Integrity Number |
| STIC | Serous Tubal Intraepithelial Carcinoma |
| T-PER | Tissue Protein Extraction Reagent |
| TLR9 | Toll-Like Receptor 9 |
| TP53 | Tumor Protein 53 Gene |
| USA | United States of America |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Kim, J.; Park, E.; Kim, O.; Schilder, J.; Coffey, D.; Cho, C.-H.; Bast, R. Cell Origins of High-Grade Serous Ovarian Cancer. Cancers 2018, 10, 433. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Stat Facts: Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 6 August 2025).
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib Tablets as Maintenance Therapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a/Mutation (SOLO2/ENGOT-Ov21): A Final Analysis of a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Obstet. Gynecol. Surv. 2021, 76, 535–536. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 7, CD005343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yu, P.; Qu, X.; Liu, Y.; Zhang, J. Phase III Trials of Standard Chemotherapy with or without Bevacizumab for Ovarian Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e81858. [Google Scholar] [CrossRef]
- Giannini, A.; Di Dio, C.; Di Donato, V.; D’Oria, O.; Salerno, M.G.; Capalbo, G.; Cuccu, I.; Perniola, G.; Muzii, L.; Bogani, G. PARP Inhibitors in Newly Diagnosed and Recurrent Ovarian Cancer. Am. J. Clin. Oncol. 2023, 46, 414–419. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Tian, M. Efficacy and safety of antiangiogenic therapy (bevacizumab or apatinib) plus chemotherapy in patients with platinum-resistant recurrent ovarian cancer: A retrospective study. Oncol. Lett. 2024, 29, 44. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Nunes, D.; Ricardo, S. Ovarian Cancer Ascites as a Liquid Tumor Microenvironment. In Ovarian Cancer; Lele, S., Ed.; Exon Publications: Brisbane, QLD, Australia, 2022; pp. 43–55. [Google Scholar] [CrossRef]
- Tan, D.S.; Agarwal, R.; Kaye, S.B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006, 7, 925–934. [Google Scholar] [CrossRef]
- Kurman, R.J.; Visvanathan, K.; Roden, R.; Wu, T.C.; Shih, I.-M. Early detection and treatment of ovarian cancer: Shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am. J. Obstet. Gynecol. 2008, 198, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Ishikawa, N.; Nakamura, K.; Nakayama, K. The fallopian tube as origin of ovarian cancer: Change of diagnostic and preventive strategies. Cancer Med. 2020, 9, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.M.; Wang, Y.; Wang, T.L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am. J. Pathol. 2021, 191, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Lo Riso, P.; Villa, C.E.; Gasparoni, G.; Vingiani, A.; Luongo, R.; Manfredi, A.; Jungmann, A.; Bertolotti, A.; Borgo, F.; Garbi, A.; et al. A cell-of-origin epigenetic tracer reveals clinically distinct subtypes of high-grade serous ovarian cancer. Genome Med. 2020, 12, 94. [Google Scholar] [CrossRef]
- Kim, J.; Coffey, D.M.; Ma, L.; Matzuk, M.M. The ovary is an alternative site of origin for high-grade serous ovarian cancer in mice. Endocrinology 2015, 156, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Doig, T.; Monaghan, H. Sampling the omentum in ovarian neoplasia: When one block is enough. Int. J. Gynecol. Cancer 2006, 16, 36–40. [Google Scholar] [CrossRef]
- Latifi, A.; Luwor, R.B.; Bilandzic, M.; Nazaretian, S.; Stenvers, K.; Pyman, J.; Zhu, H.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Isolation and Characterization of Tumor Cells from the Ascites of Ovarian Cancer Patients: Molecular Phenotype of Chemoresistant Ovarian Tumors. PLoS ONE 2012, 7, e46858. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.J.; Lan, C.Y.; Huang, Q.D.; Feng, Y.L.; Huang, Y.W.; Liu, J.H. Clinical significance of ascites in epithelial ovarian cancer. Neoplasma 2013, 60, 546–552. [Google Scholar] [CrossRef]
- Szender, J.B.; Emmons, T.; Belliotti, S.; Dickson, D.; Khan, A.; Morrell, K.; Khan, A.N.M.N.; Singel, K.L.; Mayor, P.C.; Moysich, K.B.; et al. Impact of ascites volume on clinical outcomes in ovarian cancer: A cohort study. Gynecol. Oncol. 2017, 146, 491–497. [Google Scholar] [CrossRef]
- Krugmann, J.; Schwarz, C.L.; Melcher, B.; Sterlacci, W.; Ozalinskaite, A.; Lermann, J.; Agaimy, A.; Vieth, M. Malignant ascites occurs most often in patients with high-grade serous papillary ovarian cancer at initial diagnosis: A retrospective analysis of 191 women treated at Bayreuth Hospital, 2006-2015. Arch. Gynecol. Obstet. 2019, 299, 515–523. [Google Scholar] [CrossRef]
- Yoshihara, M.; Emoto, R.; Kitami, K.; Iyoshi, S.; Uno, K.; Mogi, K.; Tano, S.; Yoshikawa, N.; Matsui, S.; Kajiyama, H. A large-scale multi-institutional study evaluating prognostic aspects of positive ascites cytology and effects of therapeutic interventions in epithelial ovarian cancer. Sci. Rep. 2021, 11, 15154. [Google Scholar] [CrossRef]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef]
- Geng, Z.; Pan, X.; Xu, J.; Jia, X. Friend and foe: The regulation network of ascites components in ovarian cancer progression. J. Cell Commun. Signal. 2023, 17, 391–407. [Google Scholar] [CrossRef]
- Vanja Tadic, V.; Zhang, W.; Brozovic, A. The high-grade serous ovarian cancer metastasis and chemoresistance in 3D models. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 1879, 189052. [Google Scholar]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; Finotello, F.; Petitprez, F.; Zhang, J.D.; Baumbach, J.; Fridman, W.H.; List, M.; Aneichyk, T. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics 2019, 35, i436–i445. [Google Scholar] [CrossRef]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautès-Fridman, C.; Fridman, W.H.; et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016, 17, 218. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Liefers-Visser, J.; Meijering, R.; Reyners, A.; Van Der Zee, A.; De Jong, S. IGF system targeted therapy: Therapeutic opportunities for ovarian cancer. Cancer Treat. Rev. 2017, 60, 90–99. [Google Scholar] [CrossRef]
- Belfiore, A.; Rapicavoli, R.V.; Le Moli, R.; Lappano, R.; Morrione, A.; De Francesco, E.M.; Vella, V. IGF2: A Role in Metastasis and Tumor Evasion from Immune Surveillance? Biomedicines 2023, 11, 229. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Han, F.; Chen, H.; Zhao, X.; Qin, Q.; Shi, R.; Liu, J. High IGF2 expression is associated with poor clinical outcome in human ovarian cancer. Oncol. Rep. 2015, 34, 936–942. [Google Scholar] [CrossRef]
- Sayer, R.A.; Lancaster, J.M.; Pittman, J.; Gray, J.; Whitaker, R.; Marks, J.R.; Berchuck, A. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol. Oncol. 2005, 96, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Brouwer-Visser, J.; Ramirez, M.J.; Kim, C.H.; Hebert, T.M.; Lin, J.; Arias-Pulido, H.; Qualls, C.R.; Prossnitz, E.R.; Goldberg, G.L.; et al. Insulin-like Growth Factor 2 Expression Modulates Taxol Resistance and Is a Candidate Biomarker for Reduced Disease-Free Survival in Ovarian Cancer. Clin. Cancer Res. 2010, 16, 2999–3010. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Wang, J.; Li, Y.; Chen, Y. SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis. J. Gynecol. Oncol. 2022, 33, e75. [Google Scholar] [CrossRef]
- Brouwer-Visser, J.; Lee, J.; McCullagh, K.; Cossio, M.J.; Wang, Y.; Huang, G.S. Insulin-like growth factor 2 silencing restores taxol sensitivity in drug resistant ovarian cancer. PLoS ONE 2014, 9, e100165. [Google Scholar] [CrossRef]
- Bergqvist, M.; Bondarenko, I.; Thuresson, M.; Klockare, M.; Harmenberg, J. Randomized, controlled, multicenter, multinational phase 2 study of docetaxel (DCT) or AXL1717 treatment in patients with squamous cell carcinoma (SCC) or adenocarcinoma (AC) of non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2014, 32, 8091. [Google Scholar] [CrossRef]
- Schmid, P.; Sablin, M.-P.; Bergh, J.; Im, S.-A.; Lu, Y.-S.; Martínez, N.; Neven, P.; Lee, K.S.; Morales, S.; Pérez-Fidalgo, J.A.; et al. A phase Ib/II study of xentuzumab, an IGF-neutralising antibody, combined with exemestane and everolimus in hormone receptor-positive, HER2-negative locally advanced/metastatic breast cancer. Breast Cancer Res. 2021, 23, 8. [Google Scholar] [CrossRef]
- Hjortebjerg, R.; Høgdall, C.; Hansen, K.H.; Høgdall, E.; Frystyk, J. The IGF–PAPP-A–Stanniocalcin Axis in Serum and Ascites Associates with Prognosis in Patients with Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 2014. [Google Scholar] [CrossRef]
- Ji, J.; Bi, F.; Zhang, X.; Zhang, Z.; Xie, Y.; Yang, Q. Single-cell transcriptome analysis revealed heterogeneity in glycolysis and identified IGF2 as a therapeutic target for ovarian cancer subtypes. BMC Cancer 2024, 24, 926. [Google Scholar] [CrossRef]

| Pathway | z-Score | Gene Names | p Value |
|---|---|---|---|
| Communication between Innate and Adaptive Immunity | −4.264 | IGHV3-74, IGHV4-4, IGHV4-39, IGHV4-59, IGHV4-61, IGHV5-51, IGKV1-9, IGKV1-27, IGKV2-24, IGKV3-11, IGKV3-20, IGLV2-8, TLR9 | 1.53 × 10−12 |
| Binding and Uptake of ligands by Scavenger Receptors | −3 | IGHA1, IGHA2, IGHV1-69, IGHV3-48, IGHV4-39, IGHV4-59, IGKV3-11, IGKV3-20, IGLV2-8 | 2.88 × 10−10 |
| Fc gamma receptor (FCGR) dependent phagocytosis | −3 | IGHG2, IGHG4, IGHV1-69, IGHV3-48, IGHV4-39, IGHV4-59, IGKV3-11, IGKV3-20, IGLV2-8 | 5.81 × 10−9 |
| Cell Surface interactions at the vascular wall | −3 | IGHA1, IGHA2, IGHV1-69, IGHV3-48, IGHV4-39, IGHV4-59, IGKV3-11, IGKV3-20, IGLV2-8 | 7.54 × 10−8 |
| Signaling by the B Cell Receptor | −2.646 | IGHV1-69, IGHV3-48, IGHV4-39, IGHV4-59, IGKV3-11, IGKV3-20, IGLV2-8 | 2.88 × 10−6 |
| Immunoregulatory interactions between Lymphoid and non-Lymphoid cells | −2.646 | IGHV1-69, IGHV3-48, IGHV4-39, IGHV4-59, IGKV3-11, IGKV3-20, IGLV2-8 | 8.95 × 10−6 |
| Fc epsilon receptor (FCER) signaling Pathway | −2.464 | IGHV1-69, IGHV3-48, IGHV4-39, IGHV4-59, IGKV3-11, IGKV3-20, IGLV2-8 | 1.02 × 10−5 |
| Neutrophil Extracellular Trap Signaling Pathway | −1.342 | IGHV1-69, IGHV3-48, IGHV4-39, IGHV4-59, IGKV3-11, IGKV3-20, IGLV2-8 | 3.34 × 103 |
| CLEAR Signaling Pathway | −1 | ATP6V0D2, BMPR1B, MAPK15, TLR9 | 2.01 × 10−2 |
| Neuroinflammation Signaling Pathway | 1 | IL34, MAPK15, PLA2G2D, TLR9 | 2.83 × 10−2 |
| Pathogen-Induced Cytokine Storm Signaling Pathway | 1 | CXCL17, MAPK15, SLC2A1, TLR9 | 4.61 × 10−2 |
| Initial HGSOC Ascites Samples | Recurrent Platinum-Resistant HGSOC Ascites Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Isotype | |||||||||||
| IgG4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IgG3 | 0 | 0 | 1 | 1 | 2 | 1 | 2 | 0 | 2 | 0 | |
| IgG2 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | |
| IgG1 | 3 | 3 | 3 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | |
| IgA | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | |
| IgM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Initial HGSOC Ascites (N = 10) | Recurrent HGSOC Ascites (N = 14) | * p-Value | |
|---|---|---|---|
| Age, N (%) | <65 years-old: 6 (60) | <65 years-old: 3 (21) | 0.09 |
| ≥65 years-old: 4 (40) | ≥65 years-old: 11 (79) | ||
| Initial diagnosis, N (%) | 10 (100) | N/A | |
| Recurrent diagnosis | 14 (100) | ||
| Platinum sensitive | 3 patients | ||
| Platinum resistant | 11 patients | ||
| Stage at Initial diagnosis, N (%) | I: 0 | I: 0 | n.s. |
| II: 0 | II: 0 | ||
| III: 7 (70) | III: 9 (64) | ||
| IV: 3 (30) | IV: 5 (36) | ||
| BMI (kg/m2), N (%) | <18.5: 0 | <18.5: 1 (7) | n.s. |
| 18.5–24.9: 3 (30) | 18.5–24.9: 8 (53) | ||
| 25.0–29.9: 2 (20) | 25.0–29.9: 3 (21) | ||
| >30.0: 5 (50) | >30.0: 2 (14) | ||
| Race, N (%) | AI/AN: 1 (10) | AI/AN: 1 (7) | n.s. |
| White: 9 (90) | White: 13 (93) |
| IGF2 Serum Concentration High (N = 12) | IGF2 Serum Concentration Low (N = 11) | * p-Value | |
|---|---|---|---|
| Age, N (%) | <65 years-old: 6 (50) | <65 years-old: 7 (64) | n.s. |
| ≥65 years-old: 5 (42) | ≥65 years-old: 4 (36) | ||
| Unknown: 1 (8) | |||
| Stage at Initial diagnosis, N (%) | I: 0 | I: 0 | n.s. |
| II: 0 | II: 1 (9) | ||
| III: 6 (50) | III: 4 (36) | ||
| IV: 1 (8) | IV: 3 (27) | ||
| Unknown: 5 (42) | Unknown: 3 (27) | ||
| BMI (kg/m2), N (%) | <18.5: 1 (8) | <18.5: 0 | n.s. |
| 18.5–24.9: 1 (8) | 18.5–24.9: 3 (27) | ||
| 25.0–29.9: 3 (25) | 25.0–29.9: 3 (27) | ||
| >30.0: 1 (8) | >30.0: 1 (9) | ||
| Unknown: 6 (50) | Unknown: 4 (36) | ||
| Race, N (%) | AI/AN: 1 (8) | AI/AN: 0 | n.s. |
| Black: 2 (16) | Black: 1 (9) | ||
| White: 9 (75) | White: 10 (91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortan, L.F.; Bohn, J.A.; Benbrook, D.M. Increased IGF2 and Immunosuppressive Cell Populations in Ascites of Patients with Recurrent High-Grade Serous Ovarian Cancer. Biomedicines 2025, 13, 2074. https://doi.org/10.3390/biomedicines13092074
Mortan LF, Bohn JA, Benbrook DM. Increased IGF2 and Immunosuppressive Cell Populations in Ascites of Patients with Recurrent High-Grade Serous Ovarian Cancer. Biomedicines. 2025; 13(9):2074. https://doi.org/10.3390/biomedicines13092074
Chicago/Turabian StyleMortan, Laura F., Jacqueline A. Bohn, and Doris Mangiaracina Benbrook. 2025. "Increased IGF2 and Immunosuppressive Cell Populations in Ascites of Patients with Recurrent High-Grade Serous Ovarian Cancer" Biomedicines 13, no. 9: 2074. https://doi.org/10.3390/biomedicines13092074
APA StyleMortan, L. F., Bohn, J. A., & Benbrook, D. M. (2025). Increased IGF2 and Immunosuppressive Cell Populations in Ascites of Patients with Recurrent High-Grade Serous Ovarian Cancer. Biomedicines, 13(9), 2074. https://doi.org/10.3390/biomedicines13092074







