Advances in Techniques for the Structure and Functional Optimization of Therapeutic Monoclonal Antibodies
Abstract
1. Introduction
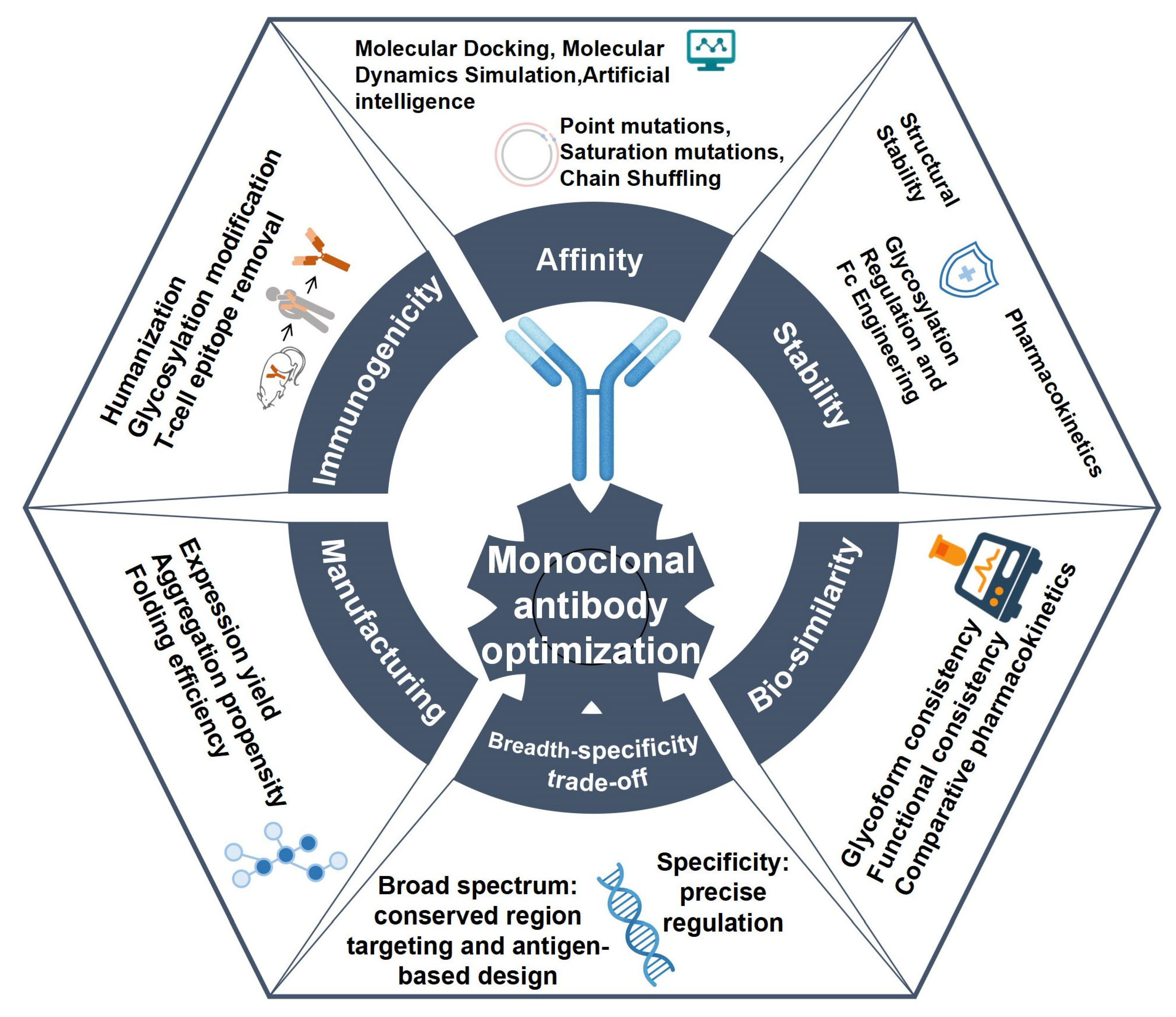
2. Fundamental Principles of Monoclonal Antibody Optimization
3. Strategies for Monoclonal Antibody Optimization
3.1. Affinity Optimization
3.1.1. Structure-Based Affinity Optimization Approaches
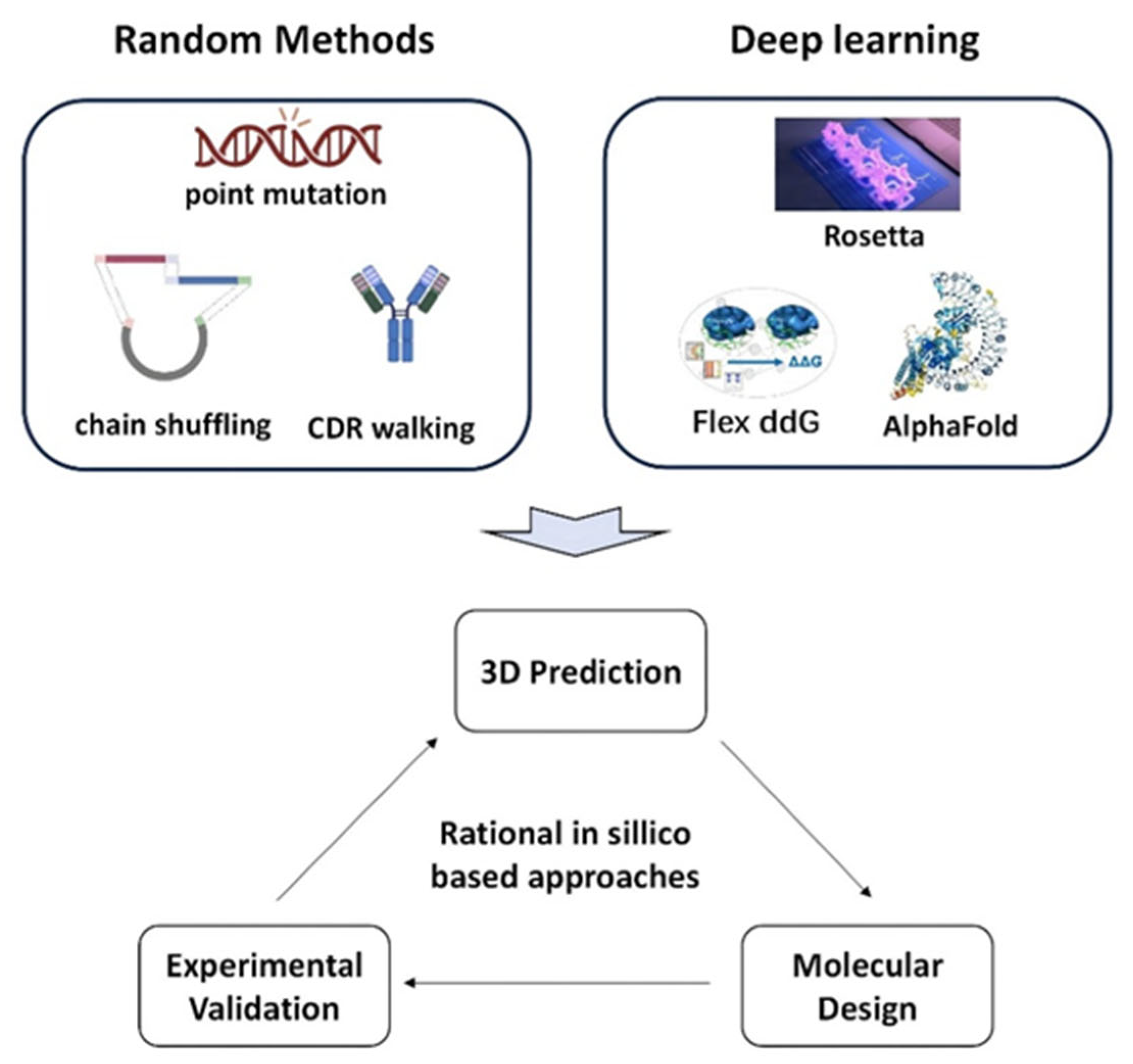
3.1.2. Optimization Approaches Based on Computational Design
3.2. Breadth-Specificity Trade-Off
3.2.1. Broad-Spectrum Optimization Strategies
3.2.2. Specificity Optimization Strategies
3.3. Reducing Immunogenicity
3.3.1. Antibody Humanization
3.3.2. Glycosyl Modification
3.3.3. Elimination of T-Cell Epitopes
3.3.4. Immune Response Risks in Long-Term Use of Therapeutic Antibodies
3.4. Stability Optimization
3.4.1. Structural Optimization
3.4.2. Modulation of Glycosylation and Fc Engineering
3.4.3. Pharmacokinetic Optimization
3.5. Computationally Driven Antibody Optimization Technologies
4. Challenges and Future Directions in Monoclonal Antibody Optimization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, P.N.; Reynolds, G.M.; Waldron, E.E.; Ward, E.; Giannopoulos, K.; Murray, P.G. Demystified …: Monoclonal Antibodies. Mol. Pathol. 2000, 53, 111–117. [Google Scholar] [CrossRef]
- Vitek, G.E.; Decourt, B.; Sabbagh, M.N. Lecanemab (BAN2401): An Anti–Beta-Amyloid Monoclonal Antibody for the Treatment of Alzheimer Disease. Expert. Opin. Investig. Drugs 2023, 32, 89–94. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Donanemab: First Approval. Drugs 2024, 84, 1313–1318. [Google Scholar] [CrossRef]
- Gupta, S.; Jiskoot, W.; Schöneich, C.; Rathore, A.S. Oxidation and Deamidation of Monoclonal Antibody Products: Potential Impact on Stability, Biological Activity, and Efficacy. J. Pharm. Sci. 2022, 111, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Shi, H.; Liu, C.; Liu, J.; Liu, X.; Sun, Y. Construction and Evaluation of a Novel Humanized HER2-Specific Chimeric Receptor. Breast Cancer Res. 2014, 16, R61. [Google Scholar] [CrossRef]
- Liu, L.; Lu, J.; Allan, B.W.; Tang, Y.; Tetreault, J.; Chow, C.; Barmettler, B.; Nelson, J.; Bina, H.; Huang, L.; et al. Generation and Characterization of Ixekizumab, a Humanized Monoclonal Antibody That Neutralizes Interleukin-17A. J. Inflamm. Res. 2016, 9, 39–50. [Google Scholar] [CrossRef]
- Grom, M.; Kozorog, M.; Caserman, S.; Pohar, A.; Likozar, B. Protein A Affinity Chromatography of Chinese Hamster Ovary (CHO) Cell Culture Broths Containing Biopharmaceutical Monoclonal Antibody (mAb): Experiments and Mechanistic Transport, Binding and Equilibrium Modeling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1083, 44–56. [Google Scholar] [CrossRef]
- Zhang, W.; DiNenna, V.; Przybycien, T. An Affinity Complex Titration Isotherm for Mechanistic Modeling in Protein A Chromatography. Biotechnol. Bioeng. 2025, 122, 2433–2455. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Mojca Cajnko, M.; Vicente, F.A.; Novak, U.; Likozar, B. Natural Deep Eutectic Solvents (NaDES): Translating Cell Biology to Processing. Green. Chem. 2023, 25, 9045–9062. [Google Scholar] [CrossRef]
- Tiller, K.E.; Tessier, P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015, 17, 191–216. [Google Scholar] [CrossRef] [PubMed]
- Zinn, S.; Vazquez-Lombardi, R.; Zimmermann, C.; Sapra, P.; Jermutus, L.; Christ, D. Advances in Antibody-Based Therapy in Oncology. Nat. Cancer 2023, 4, 165–180. [Google Scholar] [CrossRef]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P.W.I. Avidity in Antibody Effector Functions and Biotherapeutic Drug Design. Nat. Reviews Drug Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef]
- Castelli, M.S.; McGonigle, P.; Hornby, P.J. The Pharmacology and Therapeutic Applications of Monoclonal Antibodies. Pharmacol. Res. Perspe 2019, 7, e00535. [Google Scholar] [CrossRef]
- Wen, K.; Cai, J.-P.; Fan, X.; Zhang, X.; Luo, C.; Tang, K.-M.; Shuai, H.; Chen, L.-L.; Zhang, R.R.; Situ, J.; et al. Broad-Spectrum Humanized Monoclonal Neutralizing Antibody against SARS-CoV-2 Variants, Including the Omicron Variant. Front. Cell Infect. Mi 2023, 13, 1213806. [Google Scholar] [CrossRef]
- Wang, P.; Casner, R.G.; Nair, M.S.; Yu, J.; Guo, Y.; Wang, M.; Chan, J.F.-W.; Cerutti, G.; Iketani, S.; Liu, L.; et al. A Monoclonal Antibody That Neutralizes SARS-CoV-2 Variants, SARS-CoV, and Other Sarbecoviruses. Emerg. Microbes Infect. 2022, 11, 147–157. [Google Scholar] [CrossRef]
- Wei, R.; Liao, X.; Li, J.; Mu, X.; Ming, Y.; Peng, Y. Novel Humanized Monoclonal Antibodies against ROR1 for Cancer Therapy. Mol. Cancer 2024, 23, 165. [Google Scholar] [CrossRef]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-Drug Conjugates Come of Age in Oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef]
- Carter, P.J.; Quarmby, V. Immunogenicity Risk Assessment and Mitigation for Engineered Antibody and Protein Therapeutics. Nat. Rev. Drug Discov. 2024, 23, 898–913. [Google Scholar] [CrossRef]
- Basle, Y.L.; Chennell, P.; Tokhadze, N.; Astier, A.; Sautou, V. Physicochemical Stability of Monoclonal Antibodies: A Review. J. Pharm. Sci. 2020, 109, 169–190. [Google Scholar] [CrossRef]
- Manning, M.C.; Holcomb, R.E.; Payne, R.W.; Stillahn, J.M.; Connolly, B.D.; Katayama, D.S.; Liu, H.; Matsuura, J.E.; Murphy, B.M.; Henry, C.S.; et al. Stability of Protein Pharmaceuticals: Recent Advances. Pharm. Res. 2024, 41, 1301–1367. [Google Scholar] [CrossRef]
- Ma, H.; Ó’Fágáin, C.; O’Kennedy, R. Antibody Stability: A Key to Performance—Analysis, Influences and Improvement. Biochimie 2020, 177, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Duensing, T.D.; Watson, S.R. Antibody Screening Using High-Throughput Flow Cytometry. Cold Spring Harb. Protoc. 2018, 2018, pdb-top093773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, N.; Zhou, Y.; Shi, N.; Shen, B.; Luo, L.; Feng, J. Structure-Guided Affinity Maturation of a Novel Human Antibody Targeting the SARS-CoV-2 Nucleocapsid Protein. Sci. Rep. 2022, 12, 8469. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Jin, J.; Kim, S.I.; Kang, M.J.; Yi, E.C.; Kim, J.E.; Park, J.B.; Kim, H.; Chung, J. A Point Mutation in the Heavy Chain Complementarity-Determining Region 3 (HCDR3) Significantly Enhances the Specificity of an Anti-ROS1 Antibody. Biochem. Biophys. Res. Commun. 2017, 493, 325–331. [Google Scholar] [CrossRef]
- Dong, S.; Gao, M.; Bo, Z.; Guan, L.; Hu, X.; Zhang, H.; Liu, B.; Li, P.; He, K.; Liu, X.; et al. Production and Characterization of a Single-Chain Variable Fragment Antibody from a Site-Saturation Mutagenesis Library Derived from the Anti-Cry1A Monoclonal Antibody. Int. J. Biol. Macromol. 2020, 149, 60–69. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Luo, Y.; Wang, B.; Wang, J.; Li, J.; Li, J.; Ye, B.; Wang, Y.; Xi, J.J. High-Throughput Saturation Mutagenesis Generates a High-Affinity Antibody against SARS-CoV-2 Variants Using Protein Surface Display Assay on a Human Cell. PLoS Pathog. 2023, 19, e1011119. [Google Scholar] [CrossRef]
- Tulika, T.; Ruso-Julve, F.; Ahmadi, S.; Ljungars, A.; Rivera-de-Torre, E.; Wade, J.; Fernández-Quintero, M.L.; Jenkins, T.P.; Belfakir, S.B.; Ross, G.M.S.; et al. Engineering of pH-Dependent Antigen Binding Properties for Toxin-Targeting IgG1 Antibodies Using Light-Chain Shuffling. Structure 2024, 32, 1404–1418.e7. [Google Scholar] [CrossRef]
- Cummins, M.; Dogovski, C.; Robert, R.; Alderton, M.; Chong, D.; Proll, D.; Pontes-Braz, L.; Raicevic, A.; Hattarki, M.; Nuttall, S.; et al. Kinetic Characterization of a Panel of High-Affinity Monoclonal Antibodies Targeting Ricin and Recombinant Re-Formatting for Biosensor Applications. Antibodies 2014, 3, 215–231. [Google Scholar] [CrossRef]
- Hummer, A.M.; Abanades, B.; Deane, C.M. Advances in Computational Structure-Based Antibody Design. Curr. Opin. Struc Biol. 2022, 74, 102379. [Google Scholar] [CrossRef]
- Ghani, S.; Bandehpour, M.; Yarian, F.; Baghaei, K.; Kazemi, B. Production of a Ribosome-Displayed Mouse scFv Antibody Against CD133, Analysis of Its Molecular Docking, and Molecular Dynamic Simulations of Their Interactions. Appl. Biochem. Biotechnol. 2024, 196, 1399–1418. [Google Scholar] [CrossRef]
- Kunamneni, A.; Montera, M.A.; Durvasula, R.; Alles, S.R.A.; Goyal, S.; Westlund, K.N. Rapid Generation and Molecular Docking Analysis of Single-Chain Fragment Variable (scFv) Antibody Selected by Ribosome Display Targeting Cholecystokinin B Receptor (CCK-BR) for Reduction of Chronic Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 11035. [Google Scholar] [CrossRef]
- Childers, M.C.; Daggett, V. Molecular Dynamics Methods for Antibody Design. Methods Mol. Biol. 2023, 2552, 109–124. [Google Scholar] [CrossRef]
- Yang, B.; Lin, S.-J.; Ren, J.-Y.; Liu, T.; Wang, Y.-M.; Li, C.-M.; Xu, W.-W.; He, Y.-W.; Zheng, W.-H.; Zhao, J.; et al. Molecular Docking and Molecular Dynamics (MD) Simulation of Human Anti-Complement Factor H (CFH) Antibody Ab42 and CFH Polypeptide. Int. J. Mol. Sci. 2019, 20, 2568. [Google Scholar] [CrossRef]
- Sivasubramanian, A.; Chao, G.; Pressler, H.M.; Wittrup, K.D.; Gray, J.J. Structural Model of the mAb 806-EGFR Complex Using Computational Docking Followed by Computational and Experimental Mutagenesis. Structure 2006, 14, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Desautels, T.A.; Arrildt, K.T.; Zemla, A.T.; Lau, E.Y.; Zhu, F.; Ricci, D.; Cronin, S.; Zost, S.J.; Binshtein, E.; Scheaffer, S.M.; et al. Computationally Restoring the Potency of a Clinical Antibody against Omicron. Nature 2024, 629, 878–885. [Google Scholar] [CrossRef]
- Jonniya, N.A.; Poddar, S.; Mahapatra, S.; Kar, P. Computer-Aided Affinity Enhancement of a Cross-Reactive Antibody against Dengue Virus Envelope Domain III. Cell Biochem. Biophys. 2023, 81, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; McFee, M.; Fang, Q.; Abdin, O.; Kim, P.M. Computational and Artificial Intelligence-Based Methods for Antibody Development. Trends Pharmacol. Sci. 2023, 44, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gupta, E.; Spaeth, J.; Shing, L.; Jaimes, R.; Engelhart, E.; Lopez, R.; Caceres, R.S.; Bepler, T.; Walsh, M.E. Machine Learning Optimization of Candidate Antibody Yields Highly Diverse Sub-Nanomolar Affinity Antibody Libraries. Nat. Commun. 2023, 14, 3454. [Google Scholar] [CrossRef]
- Zambaldi, V.; La, D.; Chu, A.E.; Patani, H.; Danson, A.E.; Kwan, T.O.C.; Frerix, T.; Schneider, R.G.; Saxton, D.; Thillaisundaram, A.; et al. De Novo Design of High-Affinity Protein Binders with AlphaProteo. arXiv 2024, arXiv:2409.08022. [Google Scholar] [CrossRef]
- Prihoda, D.; Maamary, J.; Waight, A.; Juan, V.; Fayadat-Dilman, L.; Svozil, D.; Bitton, D.A. BioPhi: A Platform for Antibody Design, Humanization, and Humanness Evaluation Based on Natural Antibody Repertoires and Deep Learning. mAbs 2022, 14, 2020203. [Google Scholar] [CrossRef]
- He, H.; He, B.; Guan, L.; Zhao, Y.; Jiang, F.; Chen, G.; Zhu, Q.; Chen, C.Y.-C.; Li, T.; Yao, J. De Novo Generation of SARS-CoV-2 Antibody CDRH3 with a Pre-Trained Generative Large Language Model. Nat. Commun. 2024, 15, 6867. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, H.; Mueller, J.; Carter, B.; Wang, Z.; Schilz, J.; Horny, G.; Birnbaum, M.E.; Ewert, S.; Gifford, D.K. Antibody Complementarity Determining Region Design Using High-Capacity Machine Learning. Bioinformatics 2020, 36, 2126–2133. [Google Scholar] [CrossRef]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.-C.D.; Lin, T.-H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly Neutralizing Antibodies Target the Coronavirus Fusion Peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef]
- Henderson, R.; Anasti, K.; Manne, K.; Stalls, V.; Saunders, C.; Bililign, Y.; Williams, A.; Bubphamala, P.; Montani, M.; Kachhap, S.; et al. Engineering Immunogens That Select for Specific Mutations in HIV Broadly Neutralizing Antibodies. Nat. Commun. 2024, 15, 9503. [Google Scholar] [CrossRef]
- Jardine, J.G.; Ota, T.; Sok, D.; Pauthner, M.; Kulp, D.W.; Kalyuzhniy, O.; Skog, P.D.; Thinnes, T.C.; Bhullar, D.; Briney, B.; et al. Priming a Broadly Neutralizing Antibody Response to HIV-1 Using a Germline-Targeting Immunogen. Science 2015, 349, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Escolano, A.; Steichen, J.M.; Dosenovic, P.; Kulp, D.W.; Golijanin, J.; Sok, D.; Freund, N.T.; Gitlin, A.D.; Oliveira, T.; Araki, T.; et al. Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knock-in Mice. Cell 2016, 166, 1445–1458.e12. [Google Scholar] [CrossRef] [PubMed]
- Steichen, J.M.; Lin, Y.-C.; Havenar-Daughton, C.; Pecetta, S.; Ozorowski, G.; Willis, J.R.; Toy, L.; Sok, D.; Liguori, A.; Kratochvil, S.; et al. A Generalized HIV Vaccine Design Strategy for Priming of Broadly Neutralizing Antibody Responses. Science 2019, 366, eaax4380. [Google Scholar] [CrossRef]
- Akbar, R.; Robert, P.A.; Pavlović, M.; Jeliazkov, J.R.; Snapkov, I.; Slabodkin, A.; Weber, C.R.; Scheffer, L.; Miho, E.; Haff, I.H.; et al. A Compact Vocabulary of Paratope-Epitope Interactions Enables Predictability of Antibody-Antigen Binding. Cell Rep. 2021, 34, 108856. [Google Scholar] [CrossRef]
- Weitzner, B.D.; Jeliazkov, J.R.; Lyskov, S.; Marze, N.; Kuroda, D.; Frick, R.; Adolf-Bryfogle, J.; Biswas, N.; Dunbrack, R.L.; Gray, J.J. Modeling and Docking of Antibody Structures with Rosetta. Nat. Protoc. 2017, 12, 401–416. [Google Scholar] [CrossRef]
- Krawczyk, K.; Kelm, S.; Kovaltsuk, A.; Galson, J.D.; Kelly, D.; Trück, J.; Regep, C.; Leem, J.; Wong, W.K.; Nowak, J.; et al. Structurally Mapping Antibody Repertoires. Front. Immunol. 2018, 9, 1698. [Google Scholar] [CrossRef]
- Xu, H.; Palpant, T.; Wang, Q.; Shaw, D.E. Design of Immunogens to Present a Tumor-Specific Cryptic Epitope. Sci. Rep. 2025, 15, 11322. [Google Scholar] [CrossRef]
- Morrison, S.L.; Johnson, M.J.; Herzenberg, L.A.; Oi, V.T. Chimeric Human Antibody Molecules: Mouse Antigen-Binding Domains with Human Constant Region Domains. Proc. Natl. Acad. Sci. USA 1984, 81, 6851–6855. [Google Scholar] [CrossRef]
- Jones, P.T.; Dear, P.H.; Foote, J.; Neuberger, M.S.; Winter, G. Replacing the Complementarity-Determining Regions in a Human Antibody with Those from a Mouse. Nature 1986, 321, 522–525. [Google Scholar] [CrossRef]
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to Watch in 2023. mAbs 2023, 15, 2153410. [Google Scholar] [CrossRef]
- Crescioli, S.; Kaplon, H.; Chenoweth, A.; Wang, L.; Visweswaraiah, J.; Reichert, J.M. Antibodies to Watch in 2024. mAbs 2024, 16, 2297450. [Google Scholar] [CrossRef]
- Crescioli, S.; Kaplon, H.; Wang, L.; Visweswaraiah, J.; Kapoor, V.; Reichert, J.M. Antibodies to Watch in 2025. mAbs 2025, 17, 2443538. [Google Scholar] [CrossRef]
- Hitt, E.M. Rozanolixizumab: A New Therapy in the Treatment of Myasthenia Gravis. Ann. Pharmacother. 2024, 58, 1140–1148. [Google Scholar] [CrossRef]
- Hoy, S.M. Rozanolixizumab: First Approval. Drugs 2023, 83, 1341–1347. [Google Scholar] [CrossRef]
- Smith, M.R. Rituximab (Monoclonal Anti-CD20 Antibody): Mechanisms of Action and Resistance. Oncogene 2003, 22, 7359–7368. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, C.; Wuest, B.; Schreiner, J.; Steiner, U.C.; Vultaggio, A.; Matucci, A.; Crowley, C.; Boyman, O. Systematic Review of Safety and Efficacy of Rituximab in Treating Immune-Mediated Disorders. Front. Immunol. 2019, 10, 1990. [Google Scholar] [CrossRef]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R. The Immunogenicity of Humanized and Fully Human Antibodies: Residual Immunogenicity Resides in the CDR Regions. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef]
- See, K.; Kadonosono, T.; Ota, Y.; Miyamoto, K.; Yimchuen, W.; Kizaka-Kondoh, S. Reconstitution of an Anti-HER2 Antibody Paratope by Grafting Dual CDR-Derived Peptides onto a Small Protein Scaffold. Biotechnol. J. 2020, 15, e2000078. [Google Scholar] [CrossRef]
- Kurella, V.B.; Gali, R. Structure Guided Homology Model Based Design and Engineering of Mouse Antibodies for Humanization. Bioinformation 2014, 10, 180–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, Y.; Schultz, P.G.; Wang, F. Rational Design of Humanized Dual-Agonist Antibodies. J. Am. Chem. Soc. 2015, 137, 38–41. [Google Scholar] [CrossRef]
- Desmet, J.; Vanhoorelbeke, K.; Deckmyn, H. Humanization by Resurfacing. In Antibody Engineering; Kontermann, R., Dübel, S., Eds.; Springer: Berlin, Heidelberg, 2010; pp. 341–353. [Google Scholar] [CrossRef]
- Ceesay, M.M.; Matutes, E.; Taylor, G.P.; Fields, P.; Cavenagh, J.; Simpson, S.; Ho, A.; Devereux, S.; Mufti, G.J.; Pagliuca, A. Phase II Study on Combination Therapy with CHOP-Zenapax for HTLV-I Associated Adult T-Cell Leukaemia/Lymphoma (ATLL). Leuk. Res. 2012, 36, 857–861. [Google Scholar] [CrossRef]
- Tsurushita, N.; Hinton, P.R.; Kumar, S. Design of Humanized Antibodies: From Anti-Tac to Zenapax. Methods 2005, 36, 69–83. [Google Scholar] [CrossRef]
- Kashmiri, S.V.S.; De Pascalis, R.; Gonzales, N.R.; Schlom, J. SDR Grafting—A New Approach to Antibody Humanization. Methods 2005, 36, 25–34. [Google Scholar] [CrossRef]
- Choi, Y.; Hua, C.; Sentman, C.L.; Ackerman, M.E.; Bailey-Kellogg, C. Antibody Humanization by Structure-Based Computational Protein Design. mAbs 2015, 7, 1045–1057. [Google Scholar] [CrossRef]
- Kim, J.H.; Gripon, P.; Bouezzedine, F.; Jeong, M.S.; Chi, S.-W.; Ryu, S.-E.; Hong, H.J. Enhanced Humanization and Affinity Maturation of Neutralizing Anti-Hepatitis B Virus preS1 Antibody Based on Antigen-Antibody Complex Structure. FEBS Lett. 2015, 589, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Marks, C.; Hummer, A.M.; Chin, M.; Deane, C.M. Humanization of Antibodies Using a Machine Learning Approach on Large-Scale Repertoire Data. Bioinformatics 2021, 37, 4041–4047. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, D.; Fan, L. YabXnization Platform: A Monoclonal Antibody Heterologization Server Based on Rational Design and Artificial Intelligence-Assisted Computation. Comput. Struct. Biotec 2024, 23, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Gogesch, P.; Dudek, S.; van Zandbergen, G.; Waibler, Z.; Anzaghe, M. The Role of Fc Receptors on the Effectiveness of Therapeutic Monoclonal Antibodies. Int. J. Mol. Sci. 2021, 22, 8947. [Google Scholar] [CrossRef]
- Golay, J.; Andrea, A.E.; Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front. Immunol. 2022, 13, 929895. [Google Scholar] [CrossRef]
- Kanda, Y.; Yamada, T.; Mori, K.; Okazaki, A.; Inoue, M.; Kitajima-Miyama, K.; Kuni-Kamochi, R.; Nakano, R.; Yano, K.; Kakita, S.; et al. Comparison of Biological Activity among Nonfucosylated Therapeutic IgG1 Antibodies with Three Different N-Linked Fc Oligosaccharides: The High-Mannose, Hybrid, and Complex Types. Glycobiology 2007, 17, 104–118. [Google Scholar] [CrossRef]
- Higel, F.; Sandl, T.; Kao, C.-Y.; Pechinger, N.; Sörgel, F.; Friess, W.; Wolschin, F.; Seidl, A. N-Glycans of Complex Glycosylated Biopharmaceuticals and Their Impact on Protein Clearance. Eur. J. Pharm. Biopharm. 2019, 139, 123–131. [Google Scholar] [CrossRef]
- Chang, M.M.; Gaidukov, L.; Jung, G.; Tseng, W.A.; Scarcelli, J.J.; Cornell, R.; Marshall, J.K.; Lyles, J.L.; Sakorafas, P.; Chu, A.-H.A.; et al. Small-Molecule Control of Antibody N-Glycosylation in Engineered Mammalian Cells. Nat. Chem. Biol. 2019, 15, 730–736. [Google Scholar] [CrossRef]
- Zhou, Q.; Qiu, H. The Mechanistic Impact of N-Glycosylation on Stability, Pharmacokinetics, and Immunogenicity of Therapeutic Proteins. J. Pharm. Sci. 2019, 108, 1366–1377. [Google Scholar] [CrossRef]
- Peters, B.; Nielsen, M.; Sette, A. T Cell Epitope Predictions. Annu. Rev. Immunol. 2020, 38, 123–145. [Google Scholar] [CrossRef]
- King, C.; Garza, E.N.; Mazor, R.; Linehan, J.L.; Pastan, I.; Pepper, M.; Baker, D. Removing T-Cell Epitopes with Computational Protein Design. Proc. Natl. Acad. Sci. USA 2014, 111, 8577–8582. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-Drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef]
- Baker, M.P.; Reynolds, H.M.; Lumicisi, B.; Bryson, C.J. Immunogenicity of Protein Therapeutics. Self Nonself 2010, 1, 314–322. [Google Scholar] [CrossRef]
- Endo, K.; Kakuta, Y.; Moroi, R.; Yamamoto, K.; Shiga, H.; Kuroha, M.; Naito, T.; Kinouchi, Y.; Masamune, A. TL1A (TNFSF15) Genotype Affects the Long-term Therapeutic Outcomes of anti-TNFα Antibodies for Crohn’s Disease Patients. JGH Open 2020, 4, 1108–1113. [Google Scholar] [CrossRef]
- Lin, K.H.; Stone, N.J. Therapeutic Persistence in the Management of Familial Hypercholesterolemia. JACC Case Rep. 2024, 29, 102712. [Google Scholar] [CrossRef]
- Shankar, G.; Arkin, S.; Cocea, L.; Devanarayan, V.; Kirshner, S.; Kromminga, A.; Quarmby, V.; Richards, S.; Schneider, C.K.; Subramanyam, M.; et al. Assessment and Reporting of the Clinical Immunogenicity of Therapeutic Proteins and Peptides—Harmonized Terminology and Tactical Recommendations. AAPS J. 2014, 16, 658–673. [Google Scholar] [CrossRef]
- Sun, R.; Qian, M.G.; Zhang, X. T and B Cell Epitope Analysis for the Immunogenicity Evaluation and Mitigation of Antibody-Based Therapeutics. mAbs 2024, 16, 2324836. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Tan, Z.; Ehamparanathan, V.; Lewandowski, A.; Ghose, S.; Li, Z.J. Antibody Disulfide Bond Reduction and Recovery during Biopharmaceutical Process Development—A Review. Biotechnol. Bioeng. 2021, 118, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Fitriana, W.; Sakai, T.; Duan, L.; Hengphasatporn, K.; Shigeta, Y.; Mashima, T.; Uda, T.; Hifumi, E.; Hirota, S. Experimental and Computational Studies on Domain-Swapped Structure Stabilization of an Antibody Light Chain by Disulfide Bond Introduction. J. Med. Chem. 2024, 67, 22313–22321. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Tan, Z.; Ehamparanathan, V.; Ren, T.; Hoffman, L.; Du, C.; Song, Y.; Tao, L.; Lewandowski, A.; Ghose, S.; et al. Optimization and Kinetic Modeling of Interchain Disulfide Bond Reoxidation of Monoclonal Antibodies in Bioprocesses. mAbs 2020, 12, 1829336. [Google Scholar] [CrossRef]
- Bozhanova, N.G.; Flyak, A.I.; Brown, B.P.; Ruiz, S.E.; Salas, J.; Rho, S.; Bombardi, R.G.; Myers, L.; Soto, C.; Bailey, J.R.; et al. Computational Identification of HCV Neutralizing Antibodies with a Common HCDR3 Disulfide Bond Motif in the Antibody Repertoires of Infected Individuals. Nat. Commun. 2022, 13, 3178. [Google Scholar] [CrossRef]
- Ramdani, Y.; Lamamy, J.; Watier, H.; Gouilleux-Gruart, V. Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 9604. [Google Scholar] [CrossRef]
- Kaur, H. Characterization of Glycosylation in Monoclonal Antibodies and Its Importance in Therapeutic Antibody Development. Crit. Rev. Biotechnol. 2021, 41, 300–315. [Google Scholar] [CrossRef]
- Datta-Mannan, A.; Witcher, D.R.; Tang, Y.; Watkins, J.; Wroblewski, V.J. Monoclonal Antibody Clearance. Impact of Modulating the Interaction of IgG with the Neonatal Fc Receptor. J. Biol. Chem. 2007, 282, 1709–1717. [Google Scholar] [CrossRef]
- Zhu, Q.; McLellan, J.S.; Kallewaard, N.L.; Ulbrandt, N.D.; Palaszynski, S.; Zhang, J.; Moldt, B.; Khan, A.; Svabek, C.; McAuliffe, J.M.; et al. A Highly Potent Extended Half-Life Antibody as a Potential RSV Vaccine Surrogate for All Infants. Sci. Transl. Med. 2017, 9, eaaj1928. [Google Scholar] [CrossRef]
- Reddy, J.V.; Leibiger, T.; Singh, S.K.; Lee, K.H.; Papoutsakis, E.; Ierapetritou, M. A Novel, Site-Specific N-Linked Glycosylation Model Provides Mechanistic Insights Into the Process-Condition Dependent Distinct Fab and Fc Glycosylation of an IgG1 Monoclonal Antibody Produced by CHO VRC01 Cells. Biotechnol. Bioeng. 2025, 122, 761–778. [Google Scholar] [CrossRef]
- Karst, D.J.; Scibona, E.; Serra, E.; Bielser, J.-M.; Souquet, J.; Stettler, M.; Broly, H.; Soos, M.; Morbidelli, M.; Villiger, T.K. Modulation and Modeling of Monoclonal Antibody N-Linked Glycosylation in Mammalian Cell Perfusion Reactors. Biotechnol. Bioeng. 2017, 114, 1978–1990. [Google Scholar] [CrossRef]
- Okamura, K.; Badr, S.; Ichida, Y.; Yamada, A.; Sugiyama, H. Modeling of Cell Cultivation for Monoclonal Antibody Production Processes Considering Lactate Metabolic Shifts. Biotechnol. Progr 2024, 40, e3486. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics of Therapeutic Monoclonal Antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarenko, P.V.; Flynn, G.C. High-Mannose Glycans on the Fc Region of Therapeutic IgG Antibodies Increase Serum Clearance in Humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef]
- Yu, M.; Brown, D.; Reed, C.; Chung, S.; Lutman, J.; Stefanich, E.; Wong, A.; Stephan, J.-P.; Bayer, R. Production, Characterization and Pharmacokinetic Properties of Antibodies with N-Linked Mannose-5 Glycans. mAbs 2012, 4, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Warszawski, S.; Borenstein Katz, A.; Lipsh, R.; Khmelnitsky, L.; Ben Nissan, G.; Javitt, G.; Dym, O.; Unger, T.; Knop, O.; Albeck, S.; et al. Optimizing Antibody Affinity and Stability by the Automated Design of the Variable Light-Heavy Chain Interfaces. PLoS Comput. Biol. 2019, 15, e1007207. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Li, G.; Zheng, X.; Li, P.; Yuan, J.; Yan, W. Characterization of a Novel Monoclonal Antibody with High Affinity and Specificity against Aflatoxins: A Discovery from Rosetta Antibody-Ligand Computational Simulation. J. Chem. Inf. Model. 2024, 64, 6814–6826. [Google Scholar] [CrossRef]
- Barlow, K.A.; Ó Conchúir, S.; Thompson, S.; Suresh, P.; Lucas, J.E.; Heinonen, M.; Kortemme, T. Flex ddG: Rosetta Ensemble-Based Estimation of Changes in Protein-Protein Binding Affinity upon Mutation. J. Phys. Chem. B 2018, 122, 5389–5399. [Google Scholar] [CrossRef]
- Raybould, M.I.J.; Marks, C.; Krawczyk, K.; Taddese, B.; Nowak, J.; Lewis, A.P.; Bujotzek, A.; Shi, J.; Deane, C.M. Five Computational Developability Guidelines for Therapeutic Antibody Profiling. Proc. Natl. Acad. Sci. USA 2019, 116, 4025–4030. [Google Scholar] [CrossRef]
- Sharma, V.K.; Patapoff, T.W.; Kabakoff, B.; Pai, S.; Hilario, E.; Zhang, B.; Li, C.; Borisov, O.; Kelley, R.F.; Chorny, I.; et al. In Silico Selection of Therapeutic Antibodies for Development: Viscosity, Clearance, and Chemical Stability. Proc. Natl. Acad. Sci. USA 2014, 111, 18601–18606. [Google Scholar] [CrossRef]
- Jain, T.; Sun, T.; Durand, S.; Hall, A.; Houston, N.R.; Nett, J.H.; Sharkey, B.; Bobrowicz, B.; Caffry, I.; Yu, Y.; et al. Biophysical Properties of the Clinical-Stage Antibody Landscape. Proc. Natl. Acad. Sci. USA 2017, 114, 944–949. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Shan, L.-L.; Liang, F.; Du, C.-Y.; Li, J.-J. Strategies and Considerations for Improving Recombinant Antibody Production and Quality in Chinese Hamster Ovary Cells. Front. Bioeng. Biotech. 2022, 10, 856049. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Wang, X.; Yuan, K.; Wang, G.; Hu, L.; Zhang, G.; Pei, W.; Wang, L.; Sun, C.; et al. Bispecific Antibodies in Cancer Therapy: Target Selection and Regulatory Requirements. Acta Pharm. Sin. B 2023, 13, 3583–3597. [Google Scholar] [CrossRef]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next Generation of Antibody-Drug Conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody-Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Z.; Zhou, Y. AbAgIntPre: A Deep Learning Method for Predicting Antibody-Antigen Interactions Based on Sequence Information. Front. Immunol. 2022, 13, 1053617. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Nasif, K.F.A.; Xie, Y.; Deng, B.; Niu, S.; Pouriyeh, S.; Dai, Z.; Chen, J.; Xie, C.Y. AI-Driven Deep Learning Techniques in Protein Structure Prediction. Int. J. Mol. Sci. 2024, 25, 8426. [Google Scholar] [CrossRef]
- Arras, P.; Yoo, H.B.; Pekar, L.; Clarke, T.; Friedrich, L.; Schröter, C.; Schanz, J.; Tonillo, J.; Siegmund, V.; Doerner, A.; et al. AI/ML Combined with next-Generation Sequencing of VHH Immune Repertoires Enables the Rapid Identification of de Novo Humanized and Sequence-Optimized Single Domain Antibodies: A Prospective Case Study. Front. Mol. Biosci. 2023, 10, 1249247. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamamoto, N.; Kurosawa, G.; Tajima, K.; Kondo, M.; Hiramatsu, N.; Kato, Y.; Tanaka, M.; Yamaguchi, H.; Kurosawa, Y.; et al. A Novel High-Throughput Screening Method for a Human Multicentric Osteosarcoma-Specific Antibody and Biomarker Using a Phage Display-Derived Monoclonal Antibody. Cancers 2022, 14, 5829. [Google Scholar] [CrossRef] [PubMed]
- Hanning, K.R.; Minot, M.; Warrender, A.K.; Kelton, W.; Reddy, S.T. Deep Mutational Scanning for Therapeutic Antibody Engineering. Trends Pharmacol. Sci. 2022, 43, 123–135. [Google Scholar] [CrossRef]
- Feldhaus, M.J.; Siegel, R.W.; Opresko, L.K.; Coleman, J.R.; Feldhaus, J.M.W.; Yeung, Y.A.; Cochran, J.R.; Heinzelman, P.; Colby, D.; Swers, J.; et al. Flow-Cytometric Isolation of Human Antibodies from a Nonimmune Saccharomyces Cerevisiae Surface Display Library. Nat. Biotechnol. 2003, 21, 163–170. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kankala, R.K.; Yang, Z.; Li, W.; Xie, S.; Li, H.; Chen, A.-Z.; Zou, L. Antibody-Based Drug Delivery Systems for Cancer Therapy: Mechanisms, Challenges, and Prospects. Theranostics 2022, 12, 3719–3746. [Google Scholar] [CrossRef]
- Huynh, U.; Wu, P.; Qiu, J.; Prachyathipsakul, T.; Singh, K.; Jerry, D.J.; Gao, J.; Thayumanavan, S. Targeted Drug Delivery Using a Plug-to-Direct Antibody-Nanogel Conjugate. Biomacromolecules 2023, 24, 849–857. [Google Scholar] [CrossRef]
- Mieczkowski, C.; Zhang, X.; Lee, D.; Nguyen, K.; Lv, W.; Wang, Y.; Zhang, Y.; Way, J.; Gries, J.-M. Blueprint for Antibody Biologics Developability. mAbs 2023, 15, 2185924. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Han, M.; Deng, H.; Wu, F.; Liu, G.; Chen, G.-Q. Lysine β-Hydroxybutyrylation Improves Stability of COVID-19 Antibody. Biomacromolecules 2021, 23, 454–463. [Google Scholar] [CrossRef]
- Khera, E.; Dong, S.; Huang, H.; de Bever, L.; van Delft, F.L.; Thurber, G.M. Cellular-Resolution Imaging of Bystander Payload Tissue Penetration from Antibody-Drug Conjugates. Mol. Cancer Ther. 2022, 21, 310–321. [Google Scholar] [CrossRef] [PubMed]
| Humanization Strategy | Fundamental Principle | Advantages | Limitations | Representative Example(s) |
|---|---|---|---|---|
| Chimeric antibody [53] | Fusion of murine variable regions (VH/VL) with human constant regions (CH/CL) | Simple to construct; high expression efficiency | Retains a large portion of murine sequence; high immunogenicity; potential to induce antidrug antibodies (ADAs) | Rituximab (anti-CD20) [63,64] |
| CDR grafting [57] | Transplantation of murine CDRs onto a human antibody framework | Significantly reduces immunogenicity [65] | Poor framework–CDR compatibility may cause affinity loss and functional impairment | Trastuzumab (anti-HER2) [66] |
| Structural Modification | Identification of key Vernier residues through structural modeling [67] | Restores/enhances binding affinity | Technically complex and highly structure-dependent | Engineered version of Trastuzumab [68] |
| Resurfacing [69] | Replacement of surface-exposed non-human residues to reduce immune recognition | Reduces immunogenicity by masking foreign epitopes | Functional regions may be affected, risking loss of activity | Zenapax [70,71] |
| Specificity-determining residue grafting [72] | Transplantation of only key residues directly involved in antigen binding, rather than full CDRs | Maximally preserves human sequence; further reduces immunogenicity | Unstable binding affinity; requires high-throughput screening; limited current application [73] | HzKR127 [74] |
| AI-assisted humanization | Deep learning algorithms predict optimal humanization mutations and immunogenic hotspots | High-throughput, automated, and design-accurate | Requires experimental validation to confirm functionality of predicted sites | Hu-mAb [75], YabXnization platform [76] |
| Category | Tool | Mechanism of Action | Application Example | |
|---|---|---|---|---|
| Affinity | Molecular docking | ClusPro 2.0 | Performed molecular docking between the antibody and antigen, analyzed key binding residues, automatically masked non-CDR regions under default parameters, and selected the lowest-energy conformation | CD133 scFv2 successfully bound to D-EC3, and the docked complex reached a stable state after 25 ns [34] |
| Rosetta [46] | Modeled the monoclonal antibody–antigen complex and performed structural docking | Obtained the 4E11–DENV1–4 EDIII complex model and validated that single and double mutants exhibited enhanced binding affinity [36] | ||
| Ens-Grad | Identifies key complementarity-determining regions (CDRs) and predicts antibody affinity, optimizes amino acid combinations to enhance the binding ability | Synthesized and tested 12 seed sequences along with 7 Ens-Grad-designed sequences, identifying key Sufficient Input Subset (SIS) residues. The top-performing antibody achieved an EC50 of 0.29 nM, showing significantly enhanced affinity compared to ranibizumab [46] | ||
| Molecular dynamics simulations | Rosetta | Performed in silico point mutation simulations on docking models and filtered out conformations inconsistent with experimental results based on predicted changes in binding affinity | Residues E293 and D297 were predicted as key binding sites for mAb806. Simulations indicated that mutations at these positions would abolish binding, and the docking model was subsequently optimized based on this insight [38] | |
| PALM-H3 | De novo generation of artificial antibody heavy-chain CDRs with desired antigen-binding specificity | The generated candidate antibodies exhibited superior binding energies at the interface compared to natural antibodies and demonstrated outstanding neutralization potency against the SARS-CoV-2 Omicron variant XBB, with an IC50 of 3.01 μg/L [45] | ||
| AlphaSeq | Constructed an scFv library containing random mutations and measured their binding affinities to the target antigen for model training | The top-performing computationally generated scFv exhibited a 28.7-fold improvement of binding compared to the best scFv obtained through directed evolution [42] | ||
| YabXnization | Traditional CDR grafting and backmutation-driven rational design approaches, alongside AI-assisted integrative computational design strategies | SPR-based binding affinity measurement of the humanized 4-1BB agonist antibody revealed a binding affinity of 4.46 × 10−9 M [76] | ||
| AlphaFold 3 | Incorporates a substantially updated diffusion-based architecture capable of accurately predicting the joint structure of antibody–antigen complexes | A high-performance structure prediction tool that significantly improves the accuracy of antibody–antigen complex modeling [105] | ||
| AbLIFT | Allows online input of any VH/VL sequence and automatically generates multipoint interface mutation suggestions to enhance affinity | The affinity of D44.1 was enhanced 10-fold, while the dissociation constant of G6des13 was improved approximately 4.5-fold [106] | ||
| RosettaAntibody and RosettaLigand | Generates a large number of docking models to efficiently screen for high-quality binding sites | Determined that the KD value of the mAb for AFB1 is in the nanomolar range [107] | ||
| Stability | Computational design | Rosetta | Automated design of the heavy–light chain interface to optimize stability | The melting temperature (Tm) of the G6 antibody was increased from 72 to 76 °C [106] |
| Flex ddG | Evaluates the impact of mutations on the binding free energy of the protein complex | Binding stability prediction error reduced by 30% [108] | ||
| Structure prediction | Rosetta | Predicts the impact of mutations on the RBD binding free energy to screen variants that balance binding performance and structural stability | The 2130 1 0114 112 antibody showed no significant difference of thermal stability compared to the original clinical antibody COV2 2130, indicating that the optimization process did not compromise thermal stability [39] | |
| Broad-spectrum activity | Structure-based screening | Rosetta | Guided screening strategy to identify broadly neutralizing antibodies with diverse sequences but conserved structural conformations | Broad-spectrum functional immunological assays confirmed that the selected antibodies possess neutralizing activity against multiple HCV variants [94] |
| Immunogenicity | Humanization | Hu mAb | A random forest classifier built on billions of antibody sequences | The humanness score output by the Hu mAb model is significantly negatively correlated with the experimentally observed immunogenicity of clinical antibodies—i.e., a higher score indicates a lower likelihood of an immune response [75] |
| YabXnization | AI-based antibody variable region identification and humanization recommendation platform, integrating structure–sequence databases and immunogenicity assessment tools for multi-parameter optimization | Successfully humanized a murine-derived antibody while preserving its affinity and function and significantly reduced the potential T-cell epitope score [76] | ||
| Structure prediction | BioPhi | Provides a visualized humanization interface with dynamic display of residue substitution suggestions and corresponding changes in humanness similarity scores | Optimization recommendations show high consistency with experimental structures, with 97% of suggested mutations matching actual mutation sites in published humanized antibodies within a benchmark dataset [44] | |
| T-cell epitope prediction | Rosetta | Designing minimal mutations using a greedy algorithm to disrupt T-cell epitopes while preserving structural stability and functionality | Proposed multiple mutation combinations for sfGFP and exotoxin PE38, effectively eliminating major epitopes while maintaining controllable Rosetta energy changes [84] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Huang, W.; Wu, X.; Xia, H. Advances in Techniques for the Structure and Functional Optimization of Therapeutic Monoclonal Antibodies. Biomedicines 2025, 13, 2055. https://doi.org/10.3390/biomedicines13092055
He C, Huang W, Wu X, Xia H. Advances in Techniques for the Structure and Functional Optimization of Therapeutic Monoclonal Antibodies. Biomedicines. 2025; 13(9):2055. https://doi.org/10.3390/biomedicines13092055
Chicago/Turabian StyleHe, Chenchen, Weijin Huang, Xi Wu, and Huanzhang Xia. 2025. "Advances in Techniques for the Structure and Functional Optimization of Therapeutic Monoclonal Antibodies" Biomedicines 13, no. 9: 2055. https://doi.org/10.3390/biomedicines13092055
APA StyleHe, C., Huang, W., Wu, X., & Xia, H. (2025). Advances in Techniques for the Structure and Functional Optimization of Therapeutic Monoclonal Antibodies. Biomedicines, 13(9), 2055. https://doi.org/10.3390/biomedicines13092055





