Popliteus Tendon Morphology: Anatomical Classification and Clinical Implications—A Narrative Review
Abstract
1. Introduction
2. Anatomy and Morphological Variability of the Popliteus Muscle
2.1. Normal Anatomy of the Popliteus Muscle
2.2. Anatomical Variations
2.2.1. Historical Perspectives on the Anatomy of the Popliteus Muscle
2.2.2. Recent Studies on the Morphological Variability of the Popliteus Muscle
2.2.3. Clinical Implications of the Popliteus Tendon Classification
3. Biomechanics of the Popliteus Tendon
3.1. Primary Functions of the Popliteus Tendon
- To internally rotate the tibia (or externally rotate the femur in weight bearing),
- To unlock the knee from full extension by initiating flexion,
- To stabilize the lateral meniscus during knee motion,
3.2. Cadaveric and In Vitro Evidence
3.3. Role in Cruciate Ligament Deficient Knees
3.4. Dynamic Function in Gait and Athletic Movements
3.5. Implications for Biomechanical Modeling and Surgery
4. Clinical Significance of the Popliteus Tendon
5. Diagnosis and Imaging of the Popliteus Tendon
5.1. Clinical Evaluation
5.2. Magnetic Resonance Imaging (MRI)
5.3. High-Resolution Ultrasound (US)
5.4. Diagnostic Arthroscopy
5.5. Preoperative Imaging and Surgical Planning
6. Surgical Implications of Popliteus Tendon Morphological Variability
6.1. Topographical Considerations and Surgical Exposure Planning
6.2. Posterolateral Corner Reconstructions
6.3. ACL Reconstruction and Rotational Instability
6.4. Arthroscopy and Risk of Iatrogenic Injury
Clinical Pitfalls in Arthroscopic Evaluation of the Popliteus Tendon
- Accessory bands (Types IIc, IVc, IVe) may mimic lateral meniscal tears or fibrotic adhesions.
- Dual tendons (Types III, IV) can be misinterpreted as torn or duplicated structures.
- Lack of awareness of PT variants may lead to unnecessary debridement or incomplete reconstruction.
- Misidentification during revision procedures can result in persistent posterolateral instability.
6.5. Open Surgery and Identification of Variant Attachments
6.6. Surgical Recommendations
- Use of intraoperative ultrasound or dynamic palpation may assist in confirming the presence of accessory bands, especially for open reconstructions where visualization is limited [18].
- Intraoperative mapping of the PT and its insertions guided by classifications such as that proposed by Olewnik et al. [11] can prevent inadvertent damage to stabilizing fascicles.
- Preservation of native PT tissue should be prioritized unless the pathology dictates otherwise; reconstruction of its stabilizing bands should be considered in high-grade PLC injuries [3].
7. The Role of the Popliteus Tendon in Sports Orthopedics
7.1. Mechanisms of Injury in Athletes
- Acutely, due to direct trauma or forced hyperextension with external tibial rotation (e.g., skiing, football).
- Chronically, via repetitive microtrauma from excessive torsional stress, especially in runners, martial artists, and athletes involved in cutting or pivoting sports [3].
7.2. Popliteus Tendon Syndrome and Overuse Conditions
7.3. Popliteomeniscal Fascicle Injury in Pivoting Sports
7.4. Implications for Surgical Decision-Making in Athletes
7.5. Rehabilitation in the Athletic Population
- Restoration of full range of motion,
- Early proprioceptive training,
- Emphasis on controlling rotational movements during weight-bearing activities,
8. Rehabilitation Following Popliteus Tendon Injury or Reconstruction
8.1. Phase-Based Rehabilitation Strategy
- Initial Phase (Weeks 0–2): Focus on minimizing inflammation and pain, while preserving range of motion. Passive mobility exercises are initiated, while weight-bearing may be limited or assisted depending on the surgical method (e.g., PT repair or PLC reconstruction). Early quadriceps and gluteus activation are essential [23,36].
8.2. Tendinopathy Management
8.3. Special Considerations
9. Summary
10. Biomarkers and Laboratory Correlates in Popliteus Tendon-Associated Pathology
11. Conclusions
- The PT exhibits substantial morphological variability, including differences in the number of fascicles, insertion sites, and the presence of accessory bands inserting into the lateral meniscus, the FCL, or the posterior capsule.
- The classification proposed by Olewnik et al. (2021) [11] currently represents the most comprehensive anatomical system for describing these variations, and provides a structured approach for both clinical and anatomical assessment.
- Anatomical variability has direct surgical implications, influencing the planning and success of PLC reconstructions, ACL revisions, and contributing to persistent rotational instability even after anatomically performed procedures.
- The PT is at risk of misinterpretation during arthroscopy, where it may be confused with fibrotic tissue or pathological adhesions, increasing the risk of inadvertent resection.
- Advanced imaging (MRI and high-resolution ultrasound) should include targeted assessment of the PT, particularly for patients with lateral or rotational knee instability or for preoperative planning for complex reconstructions.
- The integration of anatomical classification, high-resolution imaging, and proprioceptive function of the popliteus should become a standard part of interdisciplinary management, involving orthopedic surgeons, radiologists, and physical therapists.
- The classification of the popliteus tendon should also be integrated into arthroscopic training and preoperative planning tools, including surgical navigation and robotic-assisted systems, to enhance anatomical precision and intraoperative decision-making.
12. Future Research Directions
- Multicenter anatomical and cadaveric studies using high-resolution 3D reconstruction, to validate and to expand the existing classification systems of the PT.
- Correlative imaging dissection studies (MRI vs. cadaver) to improve the recognition of accessory fascicles and bifid tendon types in clinical diagnostics.
- Development of arthroscopic and reconstructive surgical protocols that incorporate PT classification, including decision-making algorithms for rotational instability despite intact cruciate ligaments.
- Integration of PT anatomical data into navigation-assisted and robotic surgical systems, especially in the context of personalized tunnel placement and ligament balancing procedures.
- Design of dynamic functional tests for popliteus activity, enabling an evaluation of tendon function without relying solely on imaging—potentially applicable in orthopedic or rehabilitation clinics. Such tests may focus on the control of tibial external rotation under load, posterior translation during knee flexion, and response to multiplanar instability challenges during stance and directional changes.
- Inclusion of the PT anatomy as an evaluated variable in clinical studies assessing the outcomes of PLC reconstruction and ACL revision surgery—possibly as a prognostic factor.
- Investigation of popliteus-specific rehabilitation strategies and their effect on return-to-sport metrics, particularly in pivoting and high-demand athletic populations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PT | Popliteus Tendon |
| PLC | Posterolateral Corner |
| ACL | Anterior Cruciate Ligament |
| PCL | Posterior Cruciate Ligament |
| PPM | Popliteus Muscle |
| FCL | Fibular Collateral Ligament |
| PFL | Popliteofibular Ligament |
References
- LaPrade, R.F.; Wozniczka, J.K.; Stellmaker, M.P.; Wijdicks, C.A. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: The “fifth ligament” of the knee. Am. J. Sports Med. 2010, 38, 543–549. [Google Scholar] [CrossRef]
- Terry, G.C.; LaPrade, R.F. The posterolateral aspect of the knee. Anatomy and surgical approach. Am. J. Sports Med. 1996, 24, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Laprade, R.F.; Griffith, C.J.; Coobs, B.R.; Geeslin, A.G.; Johansen, S.; Engebretsen, L. Improving outcomes for posterolateral knee injuries. J. Orthop. Res. 2014, 32, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Stäubli, H.U.; Birrer, S. The popliteus tendon and its fascicles at the popliteal hiatus: Gross anatomy and functional arthroscopic evaluation with and without anterior cruciate ligament deficiency. Arthroscopy 1990, 6, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Winge, S.; Phadke, P. Isolated popliteus muscle rupture in polo players. Knee Surg. Sports Traumatol. Arthrosc. 1996, 4, 89–91. [Google Scholar] [CrossRef]
- Geeslin, A.G.; LaPrade, R.F. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: A prospective case series and surgical technique. J. Bone Jt. Surg. Am. 2011, 93, 1672–1683. [Google Scholar] [CrossRef]
- Pasque, C.; Noyes, F.R.; Gibbons, M.; Levy, M.; Grood, E. The role of the popliteofibular ligament and the tendon of popliteus in providing stability in the human knee. J. Bone Jt. Surg. Br. 2003, 85, 292–298. [Google Scholar] [CrossRef]
- Kou, J.; Wang, Y.; Chen, Z.; Shi, Y.; Guo, Q. Gait Planning and Multimodal Human-Exoskeleton Cooperative Control Based on Central Pattern Generator. IEEE/ASME Trans. Mechatron. 2024, 30, 2598–2608. [Google Scholar] [CrossRef]
- Musahl, V.; Plakseychuk, A.; VanScyoc, A.; Sasaki, T.; Debski, R.E.; McMahon, P.J.; Fu, F.H. Varying femoral tunnels between the anatomical footprint and isometric positions: Effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am. J. Sports Med. 2005, 33, 712–718. [Google Scholar] [CrossRef]
- Tang, C.; Wang, Z.; Xie, Y.; Fei, Y.; Luo, J.; Wang, C.; Ying, Y.; He, P.; Yan, R.; Chen, Y.; et al. Classification of distinct tendinopathy subtypes for precision therapeutics. Nat. Commun. 2024, 15, 9460. [Google Scholar] [CrossRef]
- Olewnik, Ł.; LaPrade, R.F.; Paulsen, F.; Gonera, B.; Kurtys, K.; Podgórski, M.; Aragonés, P.; Sanudo, J.R.; Polguj, M. A proposal for a new morphological classification of the popliteus muscle tendon with potential clinical and biomechanical significance. Sci. Rep. 2021, 11, 14434. [Google Scholar] [CrossRef]
- Vadgaonkar, R.; Tonse, M.; Blossom, V.; Kalluraya, P.G.G.; Murlimanju, B.V. Morphological study of the popliteus muscle-tendon complex in formalin embalmed adult cadavers. F1000Research 2023, 12, 1329. [Google Scholar] [CrossRef]
- Doral, M.N.; Atay, A.O.; Bozkurt, M.; Ayvaz, M.; Tetik, O.; Leblebicioglu, G. Three-bundle popliteus tendon: A nonsymptomatic anatomical variation. Knee 2006, 13, 342–343. [Google Scholar] [CrossRef]
- Paraskevas, G.; Papaziogas, B.; Kitsoulis, P.; Spanidou, S. A study on the morphology of the popliteus muscle and arcuate popliteal ligament. Folia Morphol. 2006, 65, 381–384. [Google Scholar]
- Feipel, V.; Simonnet, M.L.; Rooze, M. The proximal attachments of the popliteus muscle: A quantitative study and clinical significance. Surg. Radiol. Anat. 2003, 25, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.H.; Kim, J.D.; Kim, H. Location and classification of popliteus tendon’s origin: Cadaveric study. Arch. Orthop. Trauma Surg. 2010, 130, 1027–1032. [Google Scholar] [CrossRef]
- Markolf, K.L.; Graves, B.R.; Sigward, S.M.; Jackson, S.R.; McAllister, D.R. How well do anatomical reconstructions of the posterolateral corner restore varus stability. Am. J. Sports Med. 2007, 35, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Schinhan, M.; Bijak, M.; Unger, E.; Nau, T. Electromyographic study of the popliteus muscle in the dynamic stabilization of the posterolateral corner structures of the knee. Am. J. Sports Med. 2011, 39, 173–179. [Google Scholar] [CrossRef]
- Watanabe, Y.; Moriya, H.; Takahashi, K.; Yamagata, M.; Sonoda, M.; Shimada, Y.; Tamaki, T. Functional anatomy of the posterolateral structures of the knee. Arthroscopy 1993, 9, 57–62. [Google Scholar] [CrossRef]
- Anderson, D.D.; Chubinskaya, S.; Guilak, F.; Martin, J.A.; Oegema, T.R.; Olson, S.A.; Buckwalter, J.A. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J. Orthop. Res. 2011, 29, 802–809. [Google Scholar] [CrossRef]
- Macalister, A. Additional observations on muscular anomalies in human anatomy (third series), with a catalogue of the principal muscular variations hitherto published. Trans. R. Irish Acad. Sci. 1875, 25, 1–130. [Google Scholar]
- Testut, L. Les Anomalies Musculaires Chez L’homme Expliquées par L’anatomie Comparée et leur Importance en Anthropologie; G. Masson: Paris, France, 1884. [Google Scholar]
- Nyland, J.; Lachman, N.; Kocabey, Y.; Brosky, J.; Altun, R.; Caborn, D. Anatomy, function, and rehabilitation of the popliteus musculotendinous complex. J. Orthop. Sports Phys. Ther. 2005, 35, 165–179. [Google Scholar] [CrossRef]
- Gruber, W. Ueber Accessorische Muskeln der Regio Glutaea; Memoires de l’Academie Imperiale des Sciences de St Petersbourg: St. Pétersbourg, Russia, 1868; Volume 7, pp. 161–172. [Google Scholar]
- LeDouble, A. Traité des Variations du Système Musculaire de l’Homme et de leur Signification au Point de Vue de l’Anthropologie Zoologique; Schleicher Frères: Paris, France, 1897. [Google Scholar]
- Stäubli, H. Arthroscopically Assisted ACL Reconstruction Using Autologous Quadriceps Tendon. In Knee Cruciate Ligaments; Springer: Berlin/Heidelberg, Germany, 1992; pp. 443–451. [Google Scholar]
- Gollehon, D.L.; Torzilli, P.A.; Warren, R.F. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J. Bone Jt. Surg. Am. 1987, 69, 233–242. [Google Scholar] [CrossRef]
- Bernhardson, A.S.; Aman, Z.S.; DePhillipo, N.N.; Dornan, G.J.; Storaci, H.W.; Brady, A.W.; Nakama, G.; LaPrade, R.F. Tibial Slope and Its Effect on Graft Force in Posterior Cruciate Ligament Reconstructions. Am. J. Sports Med. 2019, 47, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Bernhardson, A.S.; DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Dornan, G.J.; LaPrade, R.F. Decreased Posterior Tibial Slope Does Not Affect Postoperative Posterior Knee Laxity After Double-Bundle Posterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2019, 47, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Hsiao, M.Y.; Hung, C.Y.; Özçakar, L. An Uncommon Cause of Posterior Knee Pain: Diagnosis and Injection for Popliteus Strain Using Ultrasonography. Pain Med. 2016, 17, 795–796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, J.; Finnoff, J.T.; Santaella-Sante, B.; Henning, T.; Levy, B.A.; Lai, J.K. Sonographically guided popliteus tendon sheath injection: Techniques and accuracy. J. Ultrasound Med. 2010, 29, 775–782. [Google Scholar] [CrossRef]
- Geeslin, A.G.; Chahla, J.; Moatshe, G.; Muckenhirn, K.J.; Kruckeberg, B.M.; Brady, A.W.; Coggins, A.; Dornan, G.J.; Getgood, A.M.; Godin, J.A.; et al. Anterolateral Knee Extra-articular Stabilizers: A Robotic Sectioning Study of the Anterolateral Ligament and Distal Iliotibial Band Kaplan Fibers. Am. J. Sports Med. 2018, 46, 1352–1361. [Google Scholar] [CrossRef]
- Olewnik, Ł.; Gonera, B.; Kurtys, K.; Zielinska, N.; Ruzik, K.; Aragonés, P.; Sanudo, J.R.; Danowska-Klonowska, D.; LaPrade, R.F. Classification of the popliteofibular ligament. Clin. Anat. 2022, 35, 375–382. [Google Scholar] [CrossRef]
- Morrissey, C.D.; Knapik, D.M. Prevalence, Mechanisms, and Return to Sport After Isolated Popliteus Injuries in Athletes: A Systematic Review. Orthop. J. Sports Med. 2022, 10, 23259671211073617. [Google Scholar] [CrossRef]
- Koong, D.P.; An, V.V.G.; Lorentzos, P.; Moussa, P.; Sivakumar, B.S. Non-Operative Rehabilitation of Isolated Popliteus Tendon Rupture in a Rugby Player. Knee Surg. Relat. Res. 2018, 30, 269–272. [Google Scholar] [CrossRef]
- Petsche, T.S.; Selesnick, F.H. Popliteus tendinitis: Tips for diagnosis and management. Phys. Sportsmed. 2002, 30, 27–31. [Google Scholar] [CrossRef]
- Cuellar, J.M.; Scuderi, G.J.; Cuellar, V.G.; Golish, S.R.; Yeomans, D.C. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J. Bone Jt. Surg. Am. 2009, 91, 2313–2320. [Google Scholar] [CrossRef]
- Rai, M.F.; Cai, L.; Zhang, Q.; Townsend, R.R.; Brophy, R.H. Synovial Fluid Proteomics From Serial Aspirations of ACL-Injured Knees Identifies Candidate Biomarkers. Am. J. Sports Med. 2023, 51, 1733–1742. [Google Scholar] [CrossRef]
- Roberts, H.M.; Law, R.J.; Thom, J.M. The time course and mechanisms of change in biomarkers of joint metabolism in response to acute exercise and chronic training in physiologic and pathological conditions. Eur. J. Appl. Physiol. 2019, 119, 2401–2420. [Google Scholar] [CrossRef]
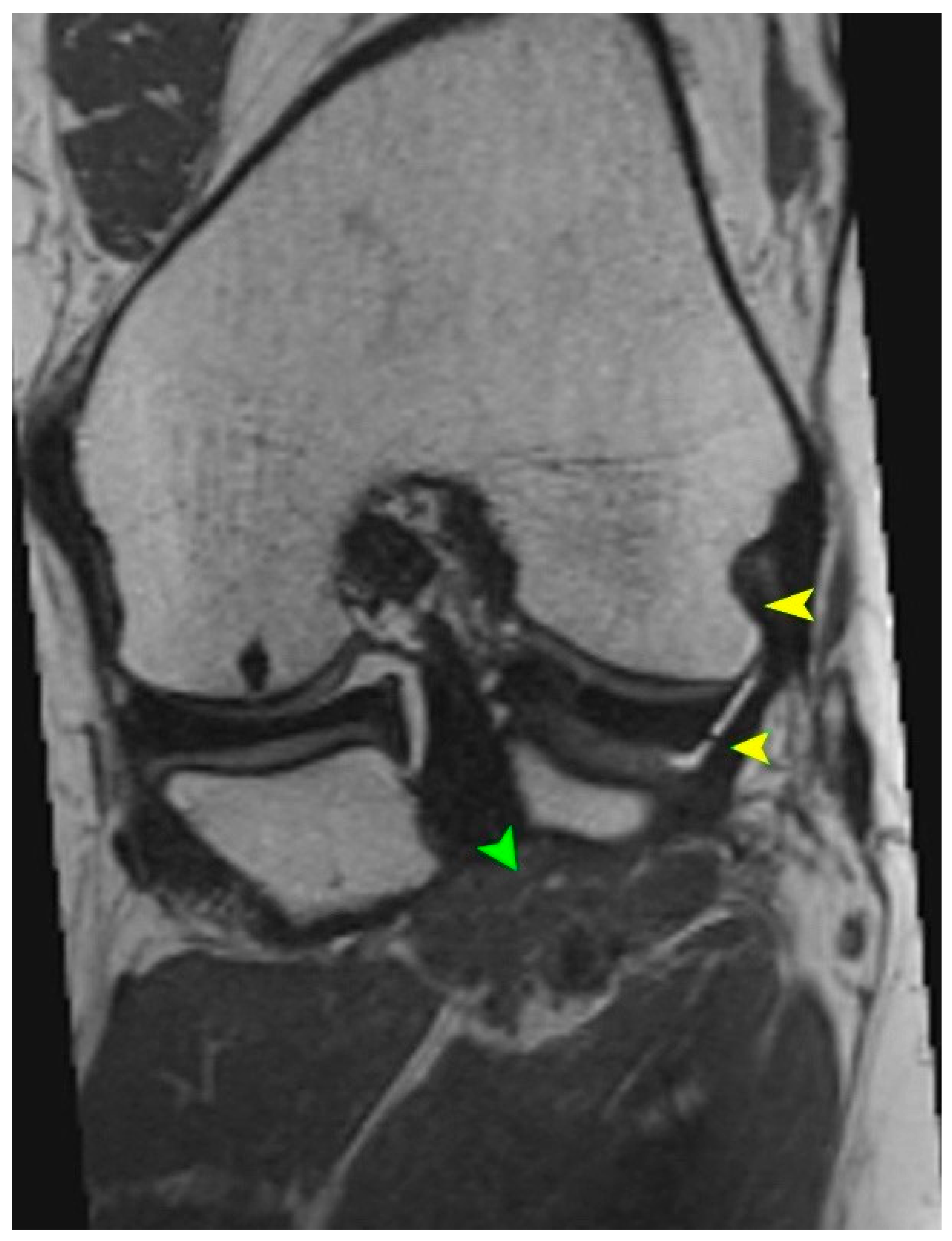
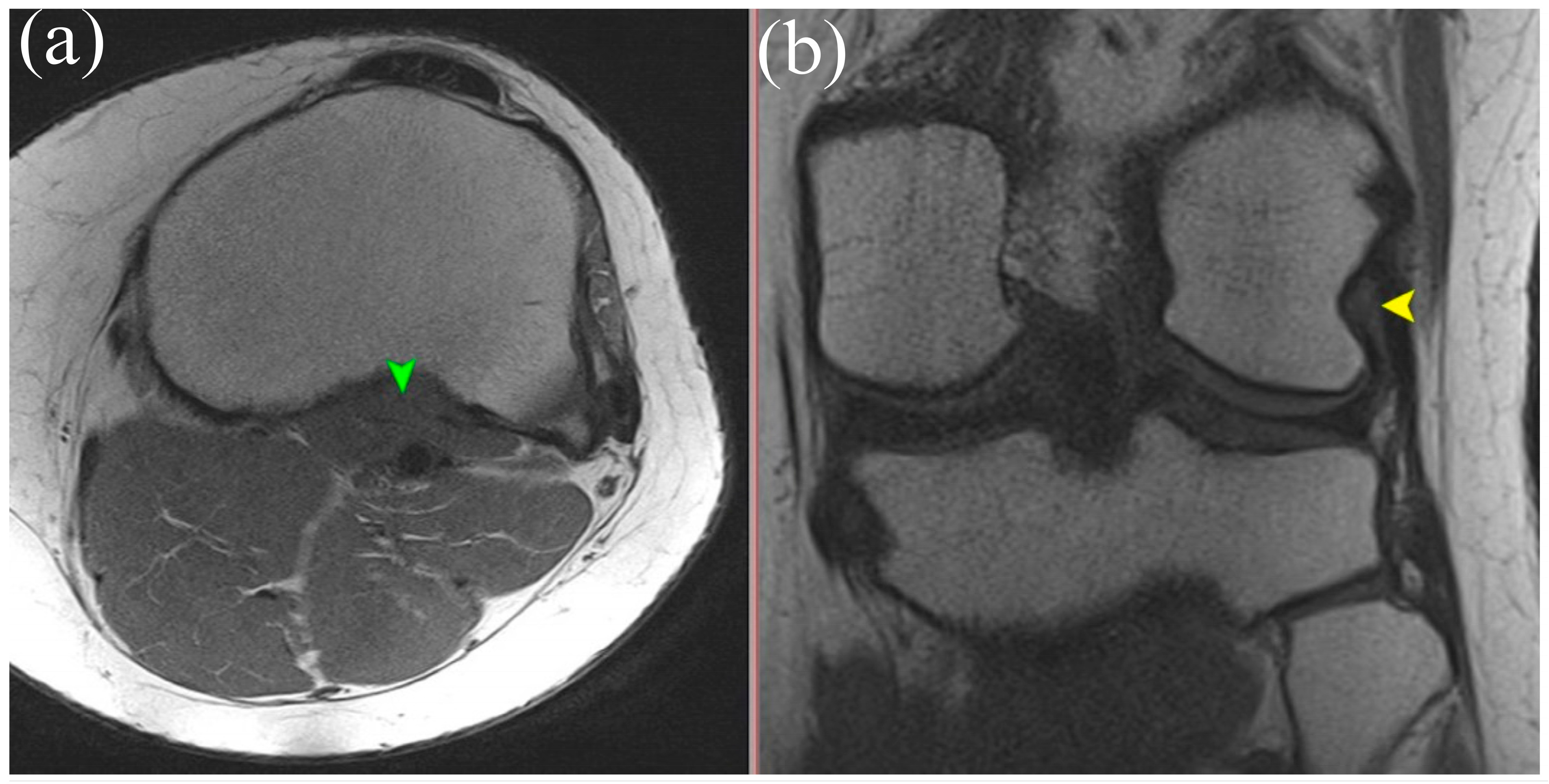

| Type | Description | Prevalence | Clinical and Surgical Implications |
|---|---|---|---|
| Type I | Single tendon attached to the proximal half of the popliteal sulcus | 46 limbs (34.3%) | Most common type; typically visualized clearly on imaging and during surgery; considered the anatomical “baseline”. |
| Type II | Single tendon with accessory bands; main tendon as in Type I | 41 limbs (30.6%) | May be mistaken for fibrous adhesions or scarring; accessory bands can be confused with pathological structures in arthroscopy. |
| IIa | One accessory band attaching to the oblique popliteal ligament | 17 limbs | Potential misdiagnosis as capsular thickening on MRI. |
| IIb | One accessory band attaching to the fibular collateral ligament | 12 limbs | Can complicate interpretation of lateral stabilizer continuity. |
| IIc | One accessory band attaching to lateral part of the lateral meniscus | 6 limbs | May mimic meniscocapsular lesions; should not be resected. |
| IId | Two bands: one to the posterior capsule, one to the oblique popliteal ligament | 4 limbs | Increases surgical complexity; may require modification of tunnel orientation in PLC reconstructions. |
| IIe | Three bands: to the FCL and posterior meniscofemoral ligament (2) | 2 limbs | Rare; risk of neurovascular entrapment if unrecognized. |
| Type III | Two separate tendons inserting into the popliteal sulcus | 21 limbs (15.7%) | Can be confused with a split tear or a bifid tendon; critical to differentiate in preoperative imaging. |
| Type IV | Two tendons as in Type III, with additional accessory bands | 26 limbs (19.4%) | Complex variant; increases the chance of diagnostic and surgical misinterpretation. |
| IVa | One accessory band to the oblique popliteal ligament | 5 limbs | May interfere with posterior capsular release. |
| IVb | Two bands: to the FCL and posterior capsule | 4 limbs | Mimics scarring or chronic trauma-related fibrosis. |
| IVc | Bands to the FCL and lateral meniscus | 7 limbs | Misinterpretable as meniscotibial injury; affects lateral meniscus mobility. |
| IVd | Bands to the lateral meniscus and the posterior capsule | 6 limbs | Requires care during meniscal repair; risk of unintended band resection. |
| IVe | Bands to the medial and lateral portions of the lateral meniscus | 4 limbs | Unusual configuration; risk of overconstraint if reconstructed improperly. |
| Modality | Sensitivity for PT | Advantages | Limitations |
|---|---|---|---|
| MRI | 90–95% | High resolution, detailed soft tissue view | Expensive, limited access in some settings, less useful for chronic injuries |
| Ultrasound | 70–85% | Dynamic assessment, portable, cost effective | Operator dependent, reduced depth penetration |
| Arthroscopy | 100% (direct view) | Gold standard, diagnostic + therapeutic | Invasive, requires anesthesia |
| Phase | Goals | Interventions | Criteria for Progression |
|---|---|---|---|
| Phase I: Acute (0–2 weeks) | Reduce pain and inflammation; protect healing structures; restore passive ROM | RICE, protected weight-bearing, gentle passive ROM (0–90°), isometrics of quadriceps and hamstrings | Pain ≤ 3/10, minimal swelling, passive ROM ≥ 90°, able to contract quadriceps without compensation |
| Phase II: Subacute (2–6 weeks) | Restore full ROM; reintroduce neuromuscular control; begin light strengthening | Active ROM, closed-chain exercises, balance training, resistance band work for hip and core | Full ROM, no gait deviation, single-leg stance ≥ 15 s, pain-free level ground ambulation |
| Phase III: Functional (6–12 weeks) | Enhance strength, proprioception, and dynamic control; initiate sport-specific drills | Dynamic balance, eccentric loading, lateral movements, plyometrics initiation | Symmetric strength ≥ 80%, good control with dynamic valgus tests, tolerance of plyometrics |
| Phase IV: Return to Sport (12+ weeks) | Restore high-level function; prevent reinjury; optimize neuromuscular patterns | Agility drills, high-velocity multiplanar movements, sport-specific reintegration, functional testing | Completion of return-to-sport testing (e.g., hop tests ≥ 90% symmetry), clearance by physician and therapist; typical return between 12–20 weeks depending on sport level |
| Clinical Scenario | Recommended Clinical Action |
|---|---|
| Isolated lateral knee pain of unclear origin | Evaluate the popliteus tendon using MRI or high-resolution ultrasound |
| Positive dial test (>10°) during ACL evaluation | Assess for PLC involvement, including PT status |
| Persistent pivot–shift after ACL reconstruction | Consider missed posterolateral injury involving the PT |
| Lateral meniscus pathology seen on imaging or arthroscopy | Differentiate between true meniscal injury and PT accessory bands |
| PLC reconstruction planning | Map popliteus tendon insertions; anticipate morphological variations (e.g., bifid tendon) |
| Rehabilitation of rotational instability | Include proprioceptive and rotational control exercises targeting popliteus function |
| Chronic tendinopathy in athletes | Use eccentric strengthening and image-guided therapy targeting the PT |
| Posterolateral instability in revision ACL cases | Reconstruct or reinforce PT in combination with PLC repair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olewnik, Ł.; Landfald, I.C.; Gonera, B.; Triantafyllou, G.; Domosławska, D.; Piagkou, M.; LaPrade, R.F. Popliteus Tendon Morphology: Anatomical Classification and Clinical Implications—A Narrative Review. Biomedicines 2025, 13, 2053. https://doi.org/10.3390/biomedicines13092053
Olewnik Ł, Landfald IC, Gonera B, Triantafyllou G, Domosławska D, Piagkou M, LaPrade RF. Popliteus Tendon Morphology: Anatomical Classification and Clinical Implications—A Narrative Review. Biomedicines. 2025; 13(9):2053. https://doi.org/10.3390/biomedicines13092053
Chicago/Turabian StyleOlewnik, Łukasz, Ingrid C. Landfald, Bartosz Gonera, George Triantafyllou, Daria Domosławska, Maria Piagkou, and Robert F. LaPrade. 2025. "Popliteus Tendon Morphology: Anatomical Classification and Clinical Implications—A Narrative Review" Biomedicines 13, no. 9: 2053. https://doi.org/10.3390/biomedicines13092053
APA StyleOlewnik, Ł., Landfald, I. C., Gonera, B., Triantafyllou, G., Domosławska, D., Piagkou, M., & LaPrade, R. F. (2025). Popliteus Tendon Morphology: Anatomical Classification and Clinical Implications—A Narrative Review. Biomedicines, 13(9), 2053. https://doi.org/10.3390/biomedicines13092053










