In Vivo Characterization of ONL1204, a Small Peptide Inhibitor of the Fas Receptor, as a Potential Neuroprotective Therapy for Geographic Atrophy and Dry Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Care
2.2. ONL1204
2.3. Pharmacokinetic Study
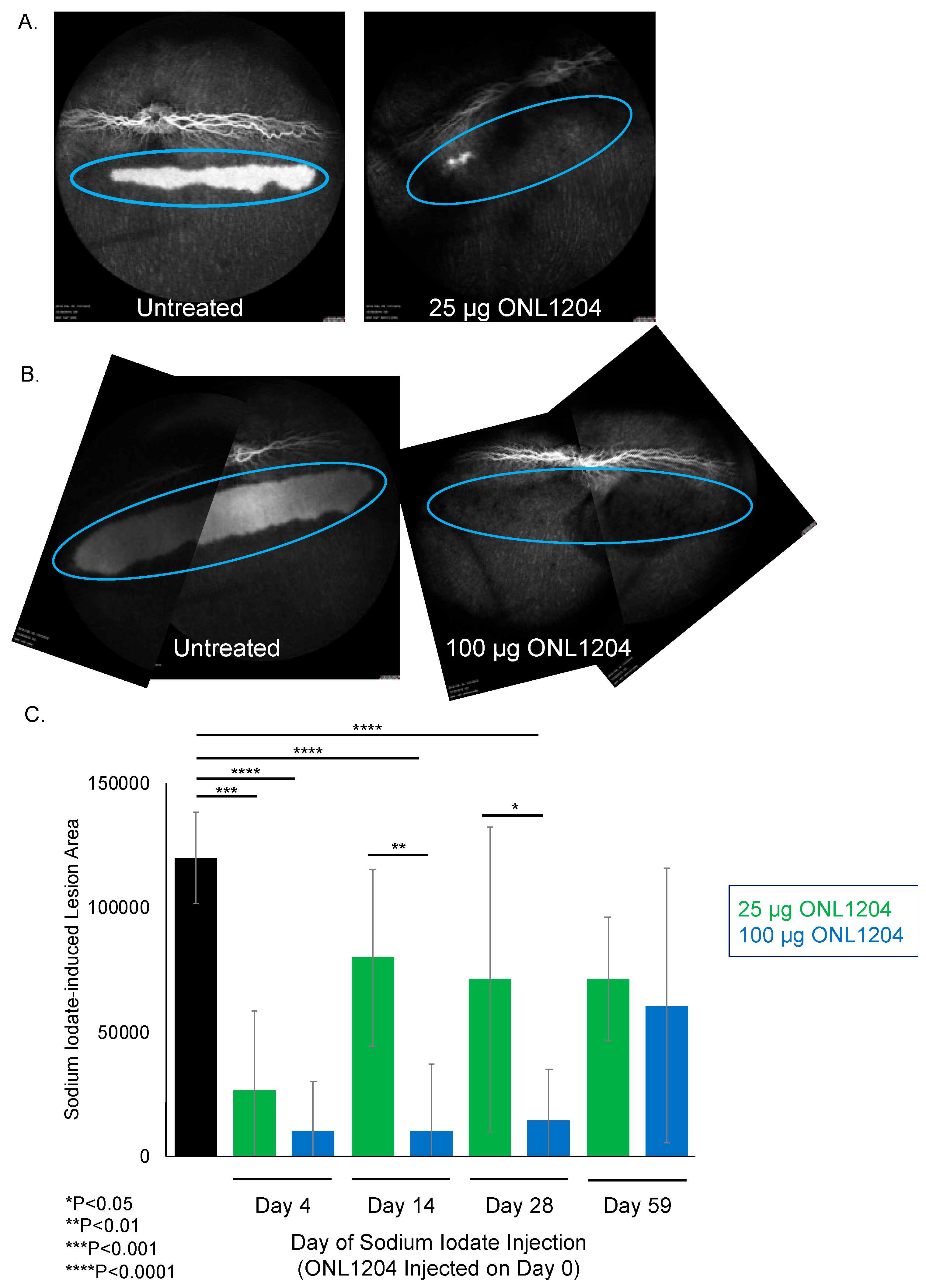
2.4. Sodium Iodate Challenge
2.5. Fluorescein Angiography
2.6. Smoke Exposure
2.7. Immunohistochemistry and Microscopy
2.8. Caspase-8 Activity Assay
3. Results
3.1. ONL1204 Pharmacokinetics
3.2. ONL1204 Pharmacodynamics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| VEGF | Vascular endothelial growth factor |
| GA | Geographic atrophy |
| PK | Pharmacokinetics |
| IVT | Intravitreal |
| RPE | Retinal pigment epithelium |
| FDA | United States Food and Drug Administration |
| TNF | Tumor necrosis factor |
| DISC | Death-inducing signaling complex |
| NaIO3 | Sodium Iodate |
| RIPK | Receptor-interacting protein kinase |
| CSE | Cigarette smoke extract |
| USDA | United States Department of Agriculture |
| IACUC | Institutional Animal Care and Use Committee |
| apoB100 | apolipoproteinB100 |
| HFD | High-fat diet |
| LC-MS/MS | Liquid chromatography-mass spectrometry/mass spectrometry |
| TBS | Tris-buffered saline |
| BSA | Bovine serum albumin |
| AIF-1/IBA1 | Allograft inflammatory factor 1/ionized calcium-binding adapter molecule 1 |
| ZO-1 | Zonula occludens-1 |
| RIPA | Radioimmunoprecipitation assay buffer |
| LLOQ | Lower limit of quantification |
| CS | Cigarette smoke |
| WT | Wild type |
| RD | Retinal detachment |
| IRD | Inherited retinal degeneration |
| RGC | Retinal ganglion cell |
| ILM | Inner limiting membrane |
| EMT | Epithelial–mesenchymal transition |
References
- Friedman, D.; O’Colmain, B.; Munoz, B.; Tomany, S.; McCarty, C.; de Jong, P.; Nemesure, B.; Mitchell, P.; Kempen, J.; Congdon, N. Prevalence of Age-Related Macular Degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Patel, S.S.; Staurenghi, G.; Tadayoni, R.; Danzig, C.J.; Eichenbaum, D.A.; Hsu, J.; Wykoff, C.C.; Heier, J.S.; Lally, D.R.; et al. Efficacy and Safety of Avacincaptad Pegol in Patients with Geographic Atrophy (GATHER2): 12-Month Results from a Randomised, Double-Masked, Phase 3 Trial. Lancet 2023, 402, 1449–1458. [Google Scholar] [CrossRef]
- Heier, J.S.; Lad, E.M.; Holz, F.G.; Rosenfeld, P.J.; Guymer, R.H.; Boyer, D.; Grossi, F.; Baumal, C.R.; Korobelnik, J.-F.; Slakter, J.S.; et al. Pegcetacoplan for the Treatment of Geographic Atrophy Secondary to Age-Related Macular Degeneration (OAKS and DERBY): Two Multicentre, Randomised, Double-Masked, Sham-Controlled, Phase 3 Trials. Lancet 2023, 402, 1434–1448. [Google Scholar] [CrossRef]
- Zacks, D.N.; Kocab, A.J.; Choi, J.J.; Gregory-Ksander, M.S.; Cano, M.; Handa, J.T. Cell Death in AMD: The Rationale for Targeting Fas. J. Clin. Med. 2022, 11, 592. [Google Scholar] [CrossRef]
- van der Schaft, T.L.; Mooy, C.M.; de Bruijn, W.C.; Oron, F.G.; Mulder, P.H.; de Jong, P.T.V.M. Histologic Features of the Early Stages of Age. Related Macular Degeneration A Statistical Analysis. Ophthalmology 1992, 99, 278–286. [Google Scholar] [CrossRef]
- Green, W.R.; Mcdonnell, P.J.; Yeo, J.H. Pathologic Features of Senile Macular Degeneration. Ophthalmology 1985, 92, 615–627. [Google Scholar] [CrossRef]
- Sarks, S.H. Ageing and Degeneration in the Macular Region: A Clinico-Pathological Study. Br. J. Ophthalmol. 1976, 60, 324–341. [Google Scholar] [CrossRef]
- Handa, J.T.; Bowes Rickman, C.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A Systems Biology Approach towards Understanding and Treating Non-Neovascular Age-Related Macular Degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef]
- Del Priore, L.V.; Kuo, Y.-H.; Tezel, T.H. Age-Related Changes in Human RPE Cell Density and Apoptosis Proportion In Situ. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3312–3318. [Google Scholar]
- Dunaief, J.L.; Dentchev, T.; Ying, G.-S.; Milam, A.H. The Role of Apoptosis in Age-Related Macular Degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, M.-W.H.; Sternberg, P.; Jones, D.P. Fas Mediates Apoptosis and Oxidant-Induced Cell Death in Cultured HRPE Cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 645–655. [Google Scholar]
- Ferrington, D.A.; Tran, T.N.; Lew, K.L.; Van Remmen, H.; Gregerson, D.S. Different Death Stimuli Evoke Apoptosis via Multiple Pathways in Retinal Pigment Epithelial Cells. Exp. Eye Res. 2006, 83, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, D.; Wang, V.M.; Yu, C.R.; Wang, R.X.; Tuo, J.; Chan, C.C. Enhanced Apoptosis in Retinal Pigment Epithelium under Inflammatory Stimuli and Oxidative Stress. Apoptosis 2012, 17, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.-T.; Panka, D.J.; Cui, H.; Sherr, D.H.; Stanger, B.Z.; Marshak-Rothstein, A. Fas(CD95)/FasL Interactions Required for Programmed Cell Death after T-Cell Activation. Nature 1995, 373, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.; Mogil, R.J.; LaFace, D.; Yoo, N.J.; Mahboubi, A.; Echeverri, F.; Martin, S.J.; Force, W.R.; Lynch, D.H.; Ware, C.F.; et al. Cell-Autonomous Fas (CD95)/Fas-Ligand Interaction Mediates Activation-Induced Apoptosis in T-Cell Hybridomas. Nature 1995, 373, 441–444. [Google Scholar] [CrossRef]
- Dhein, J.; Walczak, H.; Bäumlert, C.; Debatint, K.-M.; Krammer, P.H. Autocrine T-Cell Suicide Mediated by APO-l/(Fas/CD95). Nature 1995, 373, 438–441. [Google Scholar] [CrossRef]
- Xiao, J.; Yao, J.; Jia, L.; Lin, C.; Zacks, D.N. Protective Effect of Met12, a Small Peptide Inhibitor of Fas, on the Retinal Pigment Epithelium and Photoreceptor after Sodium Iodate Injury. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1801–1810. [Google Scholar] [CrossRef]
- Hohlbaum, A.M.; Gregory, M.S.; Ju, S.-T.; Marshak-Rothstein, A. Fas Ligand Engagement of Resident Peritoneal Macrophages In Vivo Induces Apoptosis and the Production of Neutrophil Chemotactic Factors 1. J. Immunol. 2001, 167, 6217–6224. [Google Scholar] [CrossRef]
- Zou, C.; Ma, J.; Wang, X.; Guo, L.; Zhu, Z.; Stoops, J.; Eaker, A.E.; Johnson, C.J.; Strom, S.; Michalopoulos, G.K.; et al. Lack of Fas Antagonism by Met in Human Fatty Liver Disease. Nat. Med. 2007, 13, 1078–1085. [Google Scholar] [CrossRef]
- Yang, M.; Yao, J.; Jia, L.; Kocab, A.J.; Zacks, D.N. Preservation of Retinal Structure and Function in Two Mouse Models of Inherited Retinal Degeneration by ONL1204, an Inhibitor of the Fas Receptor. Cell Death Dis. 2024, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Kocab, A.J.; Zacks, D.N.; Marshak-Rothstein, A.; Gregory-Ksander, M. A Small Peptide Antagonist of the Fas Receptor Inhibits Neuroinflammation and Prevents Axon Degeneration and Retinal Ganglion Cell Death in an Inducible Mouse Model of Glaucoma. J. Neuroinflamm. 2019, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Besirli, C.G.; Chinskey, N.D.; Zheng, Q.D.; Zacks, D.N. Inhibition of Retinal Detachment-Induced Apoptosis in Photoreceptors by a Small Peptide Inhibitor of the Fas Receptor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Cano, M.; Handa, J.T. Mice That Produce ApoB100 Lipoproteins in the RPE Do Not Develop Drusen yet Are Still a Valuable Experimental System. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7285–7295. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis HHS Public Access. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Fujihara, M.; Nagai, N.; Sussan, T.E.; Biswal, S.; Handa, J.T. Chronic Cigarette Smoke Causes Oxidative Damage and Apoptosis to Retinal Pigmented Epithelial Cells in Mice. PLoS ONE 2008, 3, e3119. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Satyanarayana, G.; Liu, T.; Wang, L.; Wang, J.; Cheng, J.; Itoh, K.; Sharma, A.; Bhutto, I.; et al. Mitophagy Initiates Retrograde Mitochondrial-Nuclear Signaling to Guide Retinal Pigment Cell Heterogeneity. Autophagy 2023, 19, 966–983. [Google Scholar] [CrossRef]
- Shrader, S.M.; Greentree, W.F. Göttingen Minipigs in Ocular Research. Toxicol. Pathol. 2018, 46, 403–407. [Google Scholar] [CrossRef]
- Krishnan, A.; Fei, F.; Jones, A.; Busto, P.; Marshak-Rothstein, A.; Ksander, B.R.; Gregory-Ksander, M. Overexpression of Soluble Fas Ligand Following Adeno-Associated Virus Gene Therapy Prevents Retinal Ganglion Cell Death in Chronic and Acute Murine Models of Glaucoma. J. Immunol. 2016, 197, 4626–4638. [Google Scholar] [CrossRef]
- Nordgaard, C.L.; Berg, K.M.; Kapphahn, R.J.; Reilly, C.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Proteomics of the Retinal Pigment Epithelium Reveals Altered Protein Expression at Progressive Stages of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2006, 47, 815–822. [Google Scholar] [CrossRef]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA Damage as a Potential Mechanism for Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Nordgaard, C.L.; Karunadharma, P.P.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Mitochondrial Proteomics of the Retinal Pigment Epithelium at Progressive Stages of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, K.; De Smedt, S.; Remaut, K.; Peynshaert, K. Toward Successful Retinal Drug Delivery after Intravitreal Injection: Current Strategies to Overcome the Inner Limiting Membrane. J. Control. Release 2025, 384, 113849. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner Limiting Membrane Barriers to Aav-Mediated Retinal Transduction from the Vitreous. Mol. Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Johnson, T.V. The Internal Limiting Membrane: Roles in Retinal Development and Implications for Emerging Ocular Therapies. Exp. Eye Res. 2021, 206, 108545. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal Pigment Epithelial Cell Necroptosis in Response to Sodium Iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsumoto, H.; Roh, M.; Giani, A.; Kataoka, K.; Morizane, Y.; Kayama, M.; Thanos, A.; Nakatake, S.; Notomi, S.; et al. Programmed Necrosis, Not Apoptosis, Is a Key Mediator of Cell Loss and DAMP-Mediated Inflammation in DsRNA-Induced Retinal Degeneration. Cell Death Differ. 2014, 21, 270–277. [Google Scholar] [CrossRef]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.-L.; Schneider, P.; Seed, B.; Tschopp, J. Fas Triggers an Alternative, Caspase-8– Independent Cell Death Pathway using the Kinase RIP as Effector Molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef]
- Liu, C.; Gao, F.; Zhu, L.; Sun, W.; Zhu, T.; Shi, S.; Wang, J.; Ou, Q.; Xu, J.Y.; Li, J.; et al. Extracellular GMFB Activates the Non-Canonical FAS-FAF1 Pathway to Induce Lysosomal Dysfunction in Early Diabetic Retinopathy. Int. J. Biol. Macromol. 2025, 318, 145082. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated Autophagy in the RPE Is Associated with Increased Susceptibility to Oxidative Stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Cao, D.; Leong, B.; Messinger, J.D.; Kar, D.; Ach, T.; Yannuzzi, L.A.; Bailey Freund, K.; Curcio, C.A. Hyperreflective Foci, Optical Coherence Tomography Progression Indicators in Age-Related Macular Degeneration, Include Transdifferentiated Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2021, 62, 34. [Google Scholar] [CrossRef]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated Retinal Pigment Epithelium, an Optical Coherence Tomography Biomarker for Progression in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO211–BIO226. [Google Scholar]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and ENDMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef]
- Gurubaran, I.S.; Koskela, A.; Heloterä, H.; Kaarniranta, K. Secretory Autophagy and Epithelial-to-Mesenchymal Transition in Cadaveric AMD Samples: Novel Pathways in Disease Progression. Acta Ophthalmol. 2025. [Google Scholar] [CrossRef]
- Chowdhury, O.; Bammidi, S.; Gautam, P.; Babu, V.S.; Liu, H.; Shang, P.; Xin, Y.; Mahally, E.; Nemani, M.; Koontz, V.; et al. Activated MTOR Signaling in the RPE Drives EMT, Autophagy, and Metabolic Disruption, Resulting in AMD-Like Pathology in Mice. Aging Cell 2025, 24, e70018. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Zhang, L.; Yang, W.; Fu, S. Molecular Mechanisms of Epithelial–Mesenchymal Transition in Retinal Pigment Epithelial Cells: Implications for Age-Related Macular Degeneration (AMD) Progression. Biomolecules 2025, 15, 771. [Google Scholar] [CrossRef]

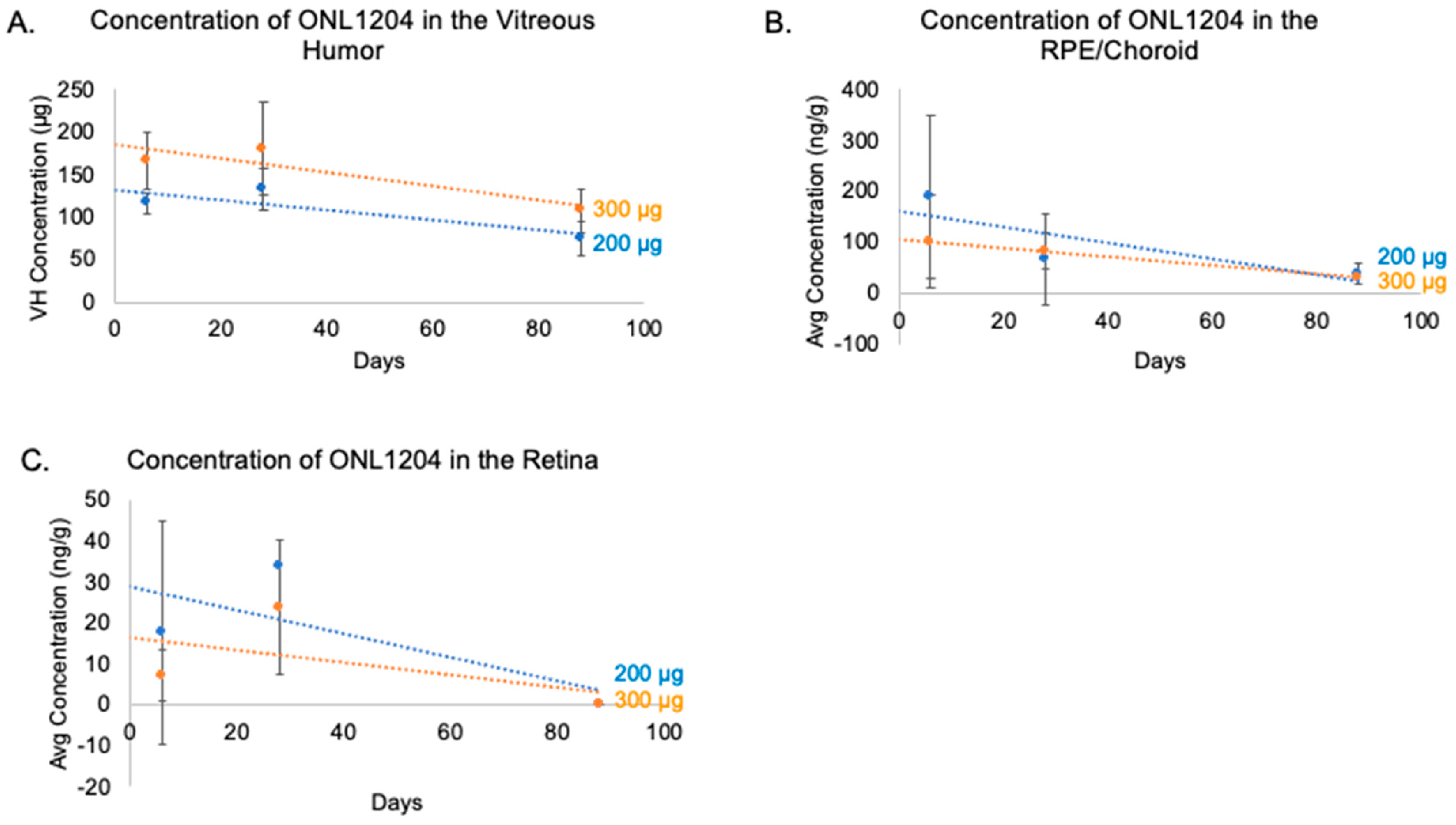

| Tissue | Day | 10 µg/Eye ONL1204 | 50 µg/Eye ONL1204 | 100 µg/Eye ONL1204 |
|---|---|---|---|---|
| Vitreous Humor (µg) 1 | 1 | 9.3 ± 1.2 | 40.6 ± 5.2 | 77.1 ± 2.7 |
| 28 | 7.7 ± 1.0 | 32.5 ± 1.7 | 62.0 ± 3.2 | |
| 56 | 6.9 ± 0.8 | 27.1 ± 5.9 | 50.0 ± 6.7 | |
| 83 | 6.2 ± 0.8 | 24.5 ± 2.4 | 48.7 ± 3.0 | |
| Retina (ng/g) 2 | 1 | 11.2 ± 8.4 | 75.6 ± 144.0 | 183.0 ± 346.0 |
| 28 | 51.0 ± 97.9 | 27.0 ± 36.6 | 42.3 ± 72.1 | |
| 56 | 14.3 ± 15.1 | 37.8 ± 48.4 | 22.7 ± 27.9 | |
| 83 | 125.0 ± 162.0 | 201.0 ± 268.0 | 86.1 ± 103.0 | |
| RPE/Choroid (ng/g) 3 | 1 | 5.45 4 | 58.0 ± 79.8 | 174.0 ± 149 |
| 28 | 10.5 4 | 92.4 4 | 40.6 4 | |
| 56 | 144.0 4 | 61.8 4 | 36.2 ± 41.0 | |
| 83 | 213.0 ± 207.0 | 209.0 ± 251.0 | 182.0 ± 312.0 |
| Tissue | Day | 200 µg/Eye ONL1204 | 300 µg/Eye ONL1204 |
|---|---|---|---|
| Vitreous Humor (µg) 1 | 6 | 116.0 ± 12.7 | 166.0 ± 34.0 |
| 28 | 132.0 ± 24.5 | 180.0 ± 54.1 | |
| 88 | 74.9 ± 20.6 | 108.0 ± 25.5 | |
| Retina (ng/g) 2 | 6 | 17.8 ± 27.3 | 7.20 ± 6.37 |
| 28 | 2.05 4 | 23.8 ± 16.4 | |
| 88 | <LLOQ | <LLOQ | |
| RPE/Choroid (ng/g) 3 | 6 | 189.0 ± 160.0 | 101.0 ± 91.4 |
| 28 | 66.5 ± 87.8 | 82.4 ± 34.5 | |
| 88 | 38.2 ± 21.7 | 29.8 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocab, A.J.; Cano, M.; Bacellar-Galdino, M.; Jamison, J.A.; Brock, W.J.; Zacks, D.N.; Handa, J.T. In Vivo Characterization of ONL1204, a Small Peptide Inhibitor of the Fas Receptor, as a Potential Neuroprotective Therapy for Geographic Atrophy and Dry Age-Related Macular Degeneration. Biomedicines 2025, 13, 2052. https://doi.org/10.3390/biomedicines13092052
Kocab AJ, Cano M, Bacellar-Galdino M, Jamison JA, Brock WJ, Zacks DN, Handa JT. In Vivo Characterization of ONL1204, a Small Peptide Inhibitor of the Fas Receptor, as a Potential Neuroprotective Therapy for Geographic Atrophy and Dry Age-Related Macular Degeneration. Biomedicines. 2025; 13(9):2052. https://doi.org/10.3390/biomedicines13092052
Chicago/Turabian StyleKocab, Andrew J., Marisol Cano, Marianna Bacellar-Galdino, Jeffrey A. Jamison, William J. Brock, David N. Zacks, and James T. Handa. 2025. "In Vivo Characterization of ONL1204, a Small Peptide Inhibitor of the Fas Receptor, as a Potential Neuroprotective Therapy for Geographic Atrophy and Dry Age-Related Macular Degeneration" Biomedicines 13, no. 9: 2052. https://doi.org/10.3390/biomedicines13092052
APA StyleKocab, A. J., Cano, M., Bacellar-Galdino, M., Jamison, J. A., Brock, W. J., Zacks, D. N., & Handa, J. T. (2025). In Vivo Characterization of ONL1204, a Small Peptide Inhibitor of the Fas Receptor, as a Potential Neuroprotective Therapy for Geographic Atrophy and Dry Age-Related Macular Degeneration. Biomedicines, 13(9), 2052. https://doi.org/10.3390/biomedicines13092052






