Anti-Inflammatory and Immunomodulatory Effects of 2-(3-Acetyl-5-(4-Chlorophenyl)-2-Methyl-1H-Pyrrol-1-yl)-3-Phenylpropanoic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Animals
2.3. Evaluation of Anti-Inflammatory Activity
2.4. Cytokine Profiling in a LPS-Induced Inflammation Model
ELISA-Based Cytokine Quantification
- TGF-β1: Sensitivity: 8 pg/mL; Intra-/inter-assay CV: <3.7%/<8.6%
- IL-10: Sensitivity: 1.5 pg/mL; Intra-/inter-assay CV: <5%/<10%
- TNF-α: Sensitivity: 11 pg/mL; Intra-/inter-assay CV: <5%/<10%
2.5. Statistical Analysis
3. Results
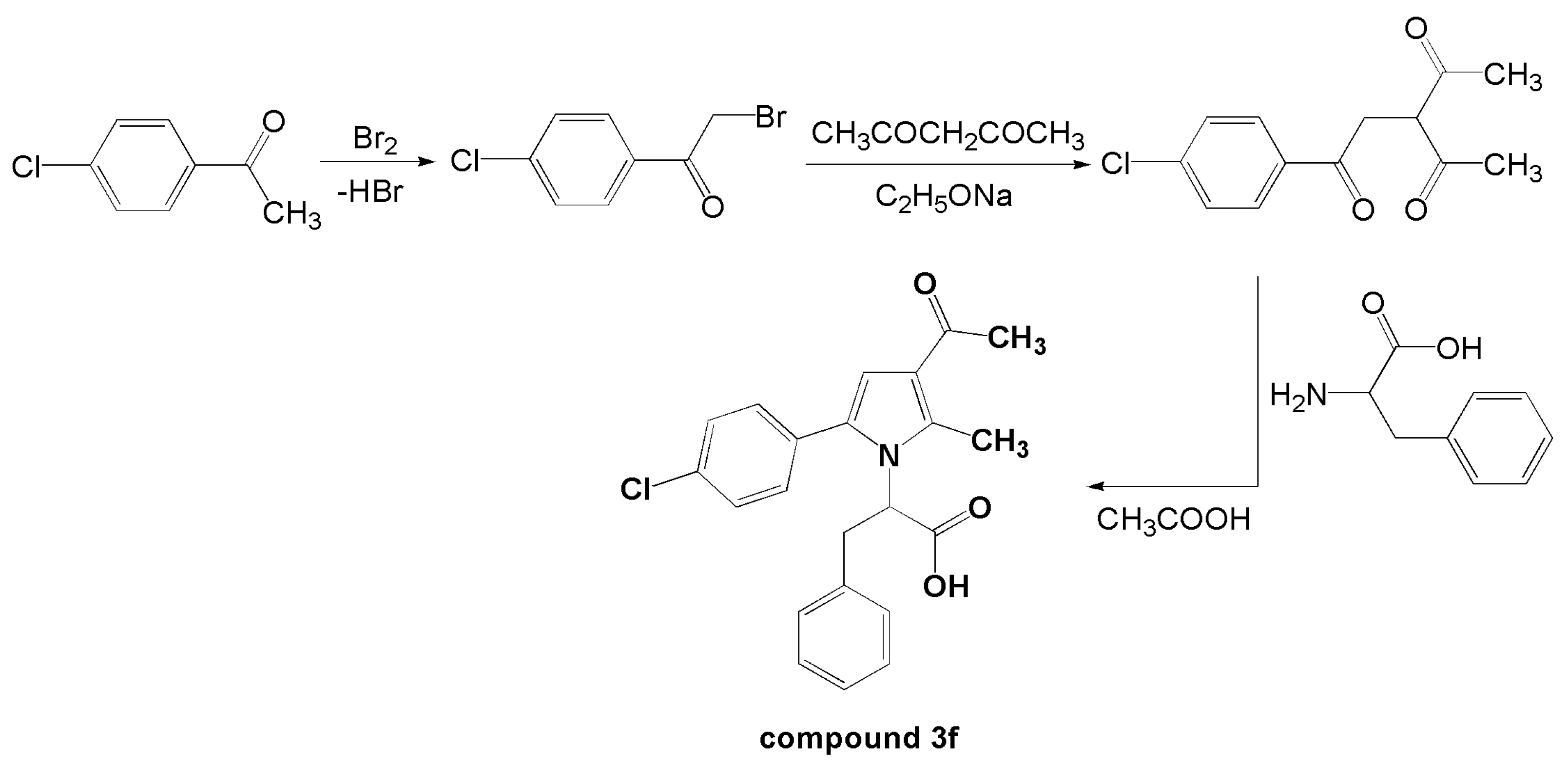
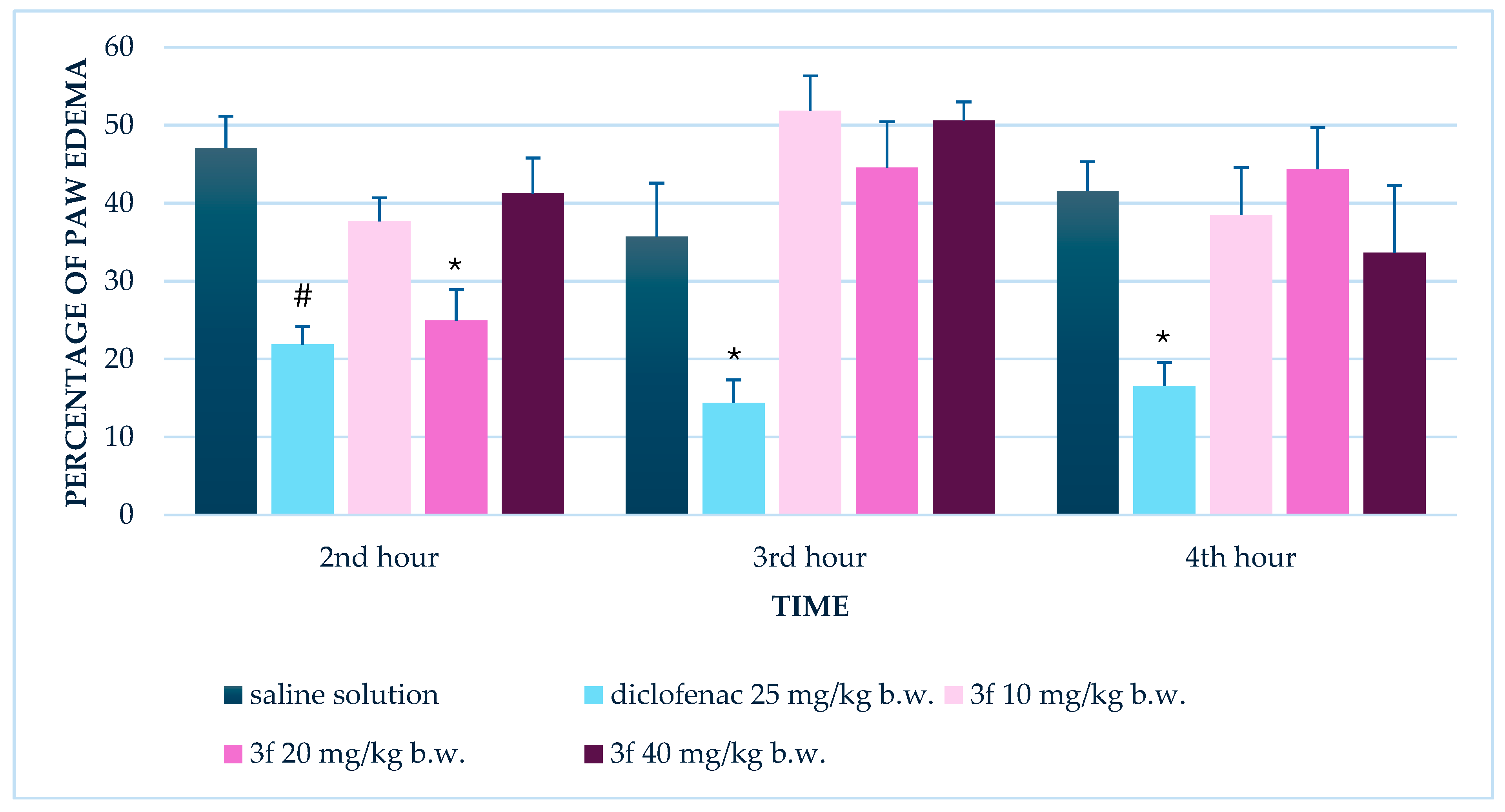
3.1. Anti-Inflammatory Effect of Compound 3f
3.1.1. Single Administration
3.1.2. Repeated Administration (14 Days)
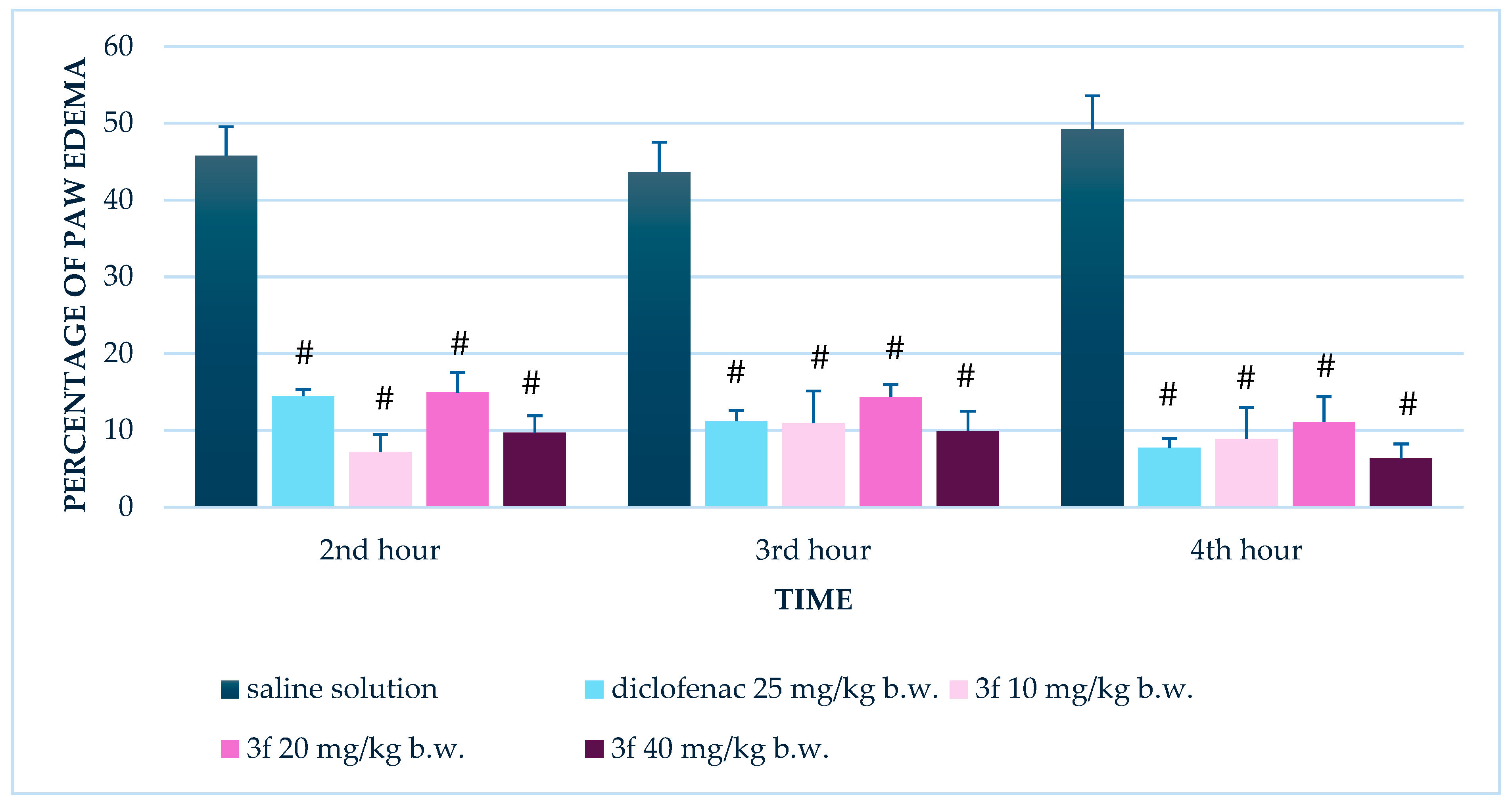
3.2. Effect of Compound 3f on Serum Levels of TNF-α, IL-10, and TGF-β1
3.2.1. Single Administration
3.2.2. Repeated Administration (14 Days)
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| COX | Cyclooxygenase |
| LPS | Lipopolysaccharide |
| TNF-α | Tumor necrosis factor alpha |
| IL-10 | Interleukin 10 |
| TGF-β1 | Transforming growth factor beta 1 |
| NF-κB | Nuclear factor kappa B |
| ELISA | Enzyme-linked immunosorbent assay |
| HPLC | High-performance liquid chromatography |
References
- Bian, M.; Ma, Q.Q.; Wu, Y.; Du, H.H.; Guo-Hua, G. Small molecule compounds with good anti-inflammatory activity reported in the literature from 01/2009 to 05/2021: A review. J. Enzyme Inhib. Med. Chem. 2021, 36, 2139–2159. [Google Scholar] [CrossRef]
- Chahal, S.; Rani, P.; Kiran; Sindhu, J.; Joshi, G.; Ganesan, A.; Kalyaanamoorthy, S.; Mayank; Kumar, P.; Singh, R.; et al. Design and development of COX-II inhibitors: Current scenario and future perspective. ACS Omega 2023, 8, 17446–17498. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Wakode, S. Contemporary advances of cyclic molecules proposed for inflammation. Eur. J. Med. Chem. 2021, 221, 113493. [Google Scholar] [CrossRef] [PubMed]
- Jeelan Basha, N.; Basavarajaiah, S.M.; Shyamsunder, K. Therapeutic potential of pyrrole and pyrrolidine analogs: An update. Mol. Divers. 2022, 26, 2915–2937. [Google Scholar] [CrossRef]
- Javid, H.; Saeedian Moghadam, E.; Farahmandfar, M.; Manouchehrabadi, M.; Amini, M.; Salimi, M.; Torkaman-Boutorabi, A. Biological activity of novel pyrrole derivatives as antioxidant agents against 6-OHDA induced neurotoxicity in PC12 cells. Iran J. Pharm. Res. 2023, 22, e140450. [Google Scholar] [CrossRef]
- Reale, A.; Brogi, S.; Chelini, A.; Paolino, M.; Di Capua, A.; Giuliani, G.; Cappelli, A.; Giorgi, G.; Chemi, G.; Grillo, A.; et al. Synthesis, biological evaluation and molecular modeling of novel selective COX-2 inhibitors: Sulfide, sulfoxide, and sulfone derivatives of 1,5-diarylpyrrol-3-substituted scaffold. Bioorg Med. Chem. 2019, 27, 115045. [Google Scholar] [CrossRef]
- Saletti, M.; Maramai, S.; Reale, A.; Paolino, M.; Brogi, S.; Di Capua, A.; Cappelli, A.; Giorgi, G.; D’AVino, D.; Rossi, A.; et al. Novel analgesic/anti-inflammatory agents: 1,5-Diarylpyrrole nitrooxyethyl sulfides and related compounds as cyclooxygenase-2 inhibitors containing a nitric oxide donor moiety endowed with vasorelaxant properties. Eur. J. Med. Chem. 2022, 241, 114615. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Abdelall, E.K.A.; Labib, M.B.; Fadaly, W.A.A.; Zidan, T.H. Design, synthesis of celecoxib-tolmetin drug hybrids as selective and potent COX-2 inhibitors. Bioorg. Chem. 2019, 90, 103029. [Google Scholar] [CrossRef]
- Almeer, R.S.; Hammad, S.F.; Leheta, O.F.; Abdel Moneim, A.E.; Amin, H.K. Anti-inflammatory and anti-hyperuricemic functions of two synthetic hybrid drugs with dual biological active sites. Int. J. Mol. Sci. 2019, 20, 5635. [Google Scholar] [CrossRef]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Souza, R.F.; Caetano, M.A.F.; Magalhães, H.I.R.; Castelucci, P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.M.; Velegraki, M.; Bolyard, C.; Rosenblum, M.D.; Li, Z. Transforming growth factor-β1 in regulatory T cell biology. Sci. Immunol. 2022, 7, eabi4613. [Google Scholar] [CrossRef]
- Mishra, B.; Bachu, M.; Yuan, R.; Wingert, C.; Chaudhary, V.; Brauner, C.; Bell, R.; Ivashkiv, L.B. IL-10 targets IRF transcription factors to suppress IFN and inflammatory response genes by epigenetic mechanisms. Nat. Immunol. 2025, 26, 748–759. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.-Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J.; et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Moon, S.J. Inflammatory cytokines in psoriatic arthritis: Understanding pathogenesis and implications for treatment. Int. J. Mol. Sci. 2023, 24, 11662. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, C.; Zhou, H.; Yu, L.; Bao, Y.; Mao, Q.; Yang, J.; Wan, H. Salvianolic acid B inhibits atherosclerosis and TNF-α-induced inflammation by regulating NF-κB/NLRP3 signaling pathway. Phytomedicine 2023, 119, 155002. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Feng, J.-Y.; Zhao, L.-N.; Zhao, M.; Wei, X.-F.; Geng, Y.; Yuan, H.-F.; Hou, C.-Y.; Zhang, H.-H.; Wang, G.-W.; et al. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-κB p65-activated SLC7A11 transcription. Acta Pharmacol. Sin. 2023, 44, 1712–1724. [Google Scholar] [CrossRef]
- Sokołowska, P.; Bleibel, L.; Owczarek, J.; Wiktorowska-Owczarek, A. PPARγ, NF-κB and the UPR pathway as new molecular targets in the anti-inflammatory actions of NSAIDs: Novel applications in cancers and central nervous system diseases? Adv. Clin. Exp. Med. 2024, 33, 1007–1022. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Sui, J.; Tu, Y.; Guo, X.; Li, F. Celecoxib prevents tumor necrosis factor-α (TNF-α)-induced cellular senescence in human chondrocytes. Bioengineered 2021, 12, 12812–12820. [Google Scholar] [CrossRef]
- Vladimirova, S.; Bijev, A. An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization. Heterocycl. Commun. 2014, 20, 111–115. [Google Scholar] [CrossRef]
- Hassan, A.S.; Soliman, G.M. Rutin nanocrystals with enhanced anti-inflammatory activity: Preparation and ex vivo/in vivo evaluation in an inflammatory rat model. Pharmaceutics 2022, 14, 2727. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Mancuso, R.I.; Via, F.I.D.; Torello, C.O.; Saad, S.T.O. Protective effect of green tea and epigallocatechin-3-gallate in a LPS-induced systemic inflammation model. J. Nutr. Biochem. 2022, 101, 108920. [Google Scholar] [CrossRef]
- Chaouch, A.C.; Benkaci-Ali, F.; Oukil, S.; Umar, H.I.; Ayoade, I.; Tata, S.; Belkadi, A. Chemical composition and kinetic study of Pinus resin volatile, investigation in its antibacterial, anti-inflammatory, anti-Alzheimer and insecticidal activities. Food Biosci. 2025, 68, 106653. [Google Scholar] [CrossRef]
- Adel, I.; Jarrar, Q.; Ayoub, R.; Jilani, J.; Moshawih, S.; Al-Qadi, E.; Zihlif, M. The toxicity and therapeutic efficacy of mefenamic acid and its hydroxyethyl ester in mice: In vivo comparative study: A promising drug derivative. Jordan J. Pharm. Sci. 2022, 15, 507–522. [Google Scholar] [CrossRef]
- Morais, M.G.; Saldanha, A.A.; Mendes, I.C.; Rodrigues, J.P.C.; Azevedo, L.S.; Ferreira, L.M.; Amado, P.A.; Zanuncio, V.S.; Farias, K.S.; Silva, D.B.; et al. Antinociceptive and anti-inflammatory potential, and chemical characterization of the dichloromethane fraction of Solanum lycocarpum (Solanaceae) ripe fruits by LC-DAD-MS. J. Ethnopharmacol. 2024, 322, 117640. [Google Scholar] [CrossRef] [PubMed]
- Burayk, S.; Oh-Hashi, K.; Kandeel, M. Drug discovery of new anti-inflammatory compounds by targeting cyclooxygenases. Pharmaceuticals 2022, 15, 282. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Maddila, S.; Gorle, S.; Sampath, C.; Lavanya, P. Synthesis and anti-inflammatory activity of some new 1,3,4-thiadiazoles containing pyrazole and pyrrole nucleus. J. Saudi Chem. Soc. 2012, 20, S306–S312. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Kamel, R.; Fatahala, S.S. Synthesis and biological evaluation of some thio containing pyrrolo [2,3-d]pyrimidine derivatives for their anti-inflammatory and anti-microbial activities. Eur. J. Med. Chem. 2010, 45, 2994–3004. [Google Scholar] [CrossRef]
- Di Capua, A.; Sticozzi, C.; Brogi, S.; Brindisi, M.; Cappelli, A.; Sautebin, L.; Rossi, A.; Pace, S.; Ghelardini, C.; Di Cesare Mannelli, L.; et al. Synthesis and biological evaluation of fluorinated 1,5-diarylpyrrole-3-alkoxyethyl ether derivatives as selective COX-2 inhibitors endowed with anti-inflammatory activity. Eur. J. Med. Chem. 2016, 109, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lavrentaki, V.; Kousaxidis, A.; Theodosis-Nobelos, P.; Papagiouvannis, G.; Koutsopoulos, K.; Nicolaou, I. Design, synthesis, and pharmacological evaluation of indazole carboxamides of N-substituted pyrrole derivatives as soybean lipoxygenase inhibitors. Mol. Divers. 2024, 28, 3757–3782. [Google Scholar] [CrossRef]
- Pasha, A.; Mondal, S.; Panigrahi, N. Synthesis of novel aryl (4-aryl-1H-pyrrol-3-yl) (thiophen-2-yl) methanone derivatives: Molecular modelling, in silico ADMET, anti-inflammatory and anti-ulcer activities. Antiinflamm. Antiallergy Agents Med. Chem. 2021, 20, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qin, T.; Feng, X.; Xu, M.; Ling, Z.; Xie, X.; Zhang, M.; Huang, Y.; Luo, J.; Cao, S.; et al. Structural optimizations on the scaffold of 7H-pyrrolo [2,3-d]pyrimidine to develop potent iNOS inhibitors with improved antiarthritis activity in vivo. Arch. Pharm. 2025, 358, e70038. [Google Scholar] [CrossRef]
- Schleiff, M.A.; Payakachat, S.; Schleiff, B.M.; Swamidass, S.J.; Boysen, G.; Miller, G.P. Impacts of diphenylamine NSAID halogenation on bioactivation risks. Toxicology 2021, 458, 152832. [Google Scholar] [CrossRef]
- Cuesta, S.A.; Meneses, L. The role of organic small molecules in pain management. Molecules 2021, 26, 4029. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, P. Inflammation: Biochemistry, cellular targets, anti-inflammatory agents and challenges with special emphasis on cyclooxygenase-2. Bioorg. Chem. 2022, 121, 105663. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Daniels, J.S. Carboxylic acids and their bioisosteres. In Metabolism, Pharmacokinetics and Toxicity of Functional Groups: Impact of Chemical Building Blocks on ADMET; Smith, D.A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2010; Volume 1, pp. 116–118. [Google Scholar]
- Lee, Y.; Kim, J.; Kim, W.; Yoon, I.S.; Jung, Y. Preparation and evaluation of amino acid conjugates of celecoxib as prodrugs to improve the pharmacokinetic and therapeutic properties of celecoxib. Pharmaceutics 2020, 12, 1043. [Google Scholar] [CrossRef]
- Rodríguez-Castillo, A.J.; González-Chávez, S.A.; Portillo-Pantoja, I.; Cruz-Hermosillo, E.; Pacheco-Tena, C.; Chávez-Flores, D.; Delgado-Gardea, M.C.E.; Infante-Ramírez, R.; Ordaz-Ortiz, J.J.; Sánchez-Ramírez, B. Aqueous extracts of Rhus trilobata inhibit the lipopolysaccharide-induced inflammatory response in vitro and in vivo. Plants 2024, 13, 2840. [Google Scholar] [CrossRef]
- Lim, J.S.; Bae, J.; Lee, S.; Lee, D.Y.; Yao, L.; Cho, N.; Bach, T.T.; Yun, N.; Park, S.-J.; Cho, Y.-C. In vitro anti-inflammatory effects of Symplocos sumuntia Buch.-Ham. Ex D. Don extract via blockage of the NF-κB/JNK signaling pathways in LPS-activated microglial cells. Plants 2022, 11, 3095. [Google Scholar] [CrossRef]
- Jung, H.J.; Cho, D.-Y.; Han, J.-H.; Park, K.D.; Choi, D.-K.; Kim, E.; Yoon, S.-H.; Park, J.-Y. Synthesis of 1-(4-(dimethylamino)phenyl)-3,4-diphenyl-1H-pyrrole-2,5-dione analogues and their anti-inflammatory activities in lipopolysaccharide-induced BV2 cells. Bioorg. Med. Chem. Lett. 2023, 92, 129408. [Google Scholar] [CrossRef]
- Pandey, A.R.; Singh, S.P.; Joshi, P.; Srivastav, K.S.; Srivastava, S.; Yadav, K.; Chandra, R.; Bisen, A.C.; Agrawal, S.; Sanap, S.N.; et al. Design, synthesis and evaluation of novel pyrrole-hydroxybutenolide hybrids as promising antiplasmodial and anti-inflammatory agents. Eur. J. Med. Chem. 2023, 254, 115340. [Google Scholar] [CrossRef]
- Paprocka, R.; Pazderski, L.; Mazur, L.; Wiese-Szadkowska, M.; Kutkowska, J.; Nowak, M.; Helmin-Basa, A. Synthesis and structural study of amidrazone derived pyrrole-2,5-dione derivatives: Potential anti-inflammatory agents. Molecules 2022, 27, 2891. [Google Scholar] [CrossRef]
- Xu, Q.; Qiao, Y.; Dai, C.; Pang, L.; Li, L.; Lu, Y. Discovery of anti-inflammatory pyrrole alkaloids from Curvularia lunata CL1. Fitoterapia 2025, 185, 106718. [Google Scholar] [CrossRef]
- de Brito, T.M.; Amendoeira, F.C.; de Oliveira, T.B.; Doro, L.H.; Garcia, E.B.; da Silva, N.M.F.; Chaves, A.d.S.; Muylaert, F.F.; Pádua, T.A.; Rosas, E.C.; et al. Anti-inflammatory activity and chemical analysis of different fractions from Solidago chilensis inflorescence. Oxid. Med. Cell Longev. 2021, 2021, 7612380. [Google Scholar] [CrossRef]
- Liu, T.W.; Chen, C.M.; Chang, K.H. Biomarker of neuroinflammation in Parkinson’s disease. Int. J. Mol. Sci. 2022, 23, 4148. [Google Scholar] [CrossRef] [PubMed]
- Vingren, J.L.; Boyett, J.C.; Lee, E.C.; Levitt, D.E.; Luk, H.Y.; McDermott, B.P.; Munoz, C.X.; Ganio, M.S.; Armstrong, L.E.; Hill, D.W. A single dose of ibuprofen impacts IL-10 response to 164-km road cycling in the heat. Res. Q. Exerc. Sport. 2023, 94, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, B.O.; Adeboboye, C.F.; Adiji, P.I.; Adebolu, T.T. Cytokine-mediated immunoregulatory activity of Lactobacillus species in a carrageenan-induced acute inflammatory model. BioTechnologia 2023, 104, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Abudukeyoumu, A.; Li, M.Q.; Xie, F. Transforming growth factor-β1 in intrauterine adhesion. Am. J. Reprod. Immunol. 2020, 84, e13262. [Google Scholar] [CrossRef]
- de Streel, G.; Lucas, S. Targeting immunosuppression by TGF-β1 for cancer immunotherapy. Biochem. Pharmacol. 2021, 192, 114697. [Google Scholar] [CrossRef]
- Houshmand, G.; Naghizadeh, B.; Ghorbanzadeh, B.; Ghafouri, Z.; Goudarzi, M.; Mansouri, M.T. Celecoxib inhibits acute edema and inflammatory biomarkers through peroxisome proliferator-activated receptor-γ in rats. Iran J. Basic Med. Sci. 2020, 23, 1544–1550. [Google Scholar] [CrossRef]
- Beekhuizen, M.; Tsuchida, A.I.; Saris, D.B.; Dhert, W.J.; Creemers, L.B.; van Osch, G.J. Celecoxib decreases the production of cytokines by osteoarthritic cartilage and synovium without affecting cartilage matrix degradation. Osteoarthr. Cartil. 2012, 20, S239. [Google Scholar] [CrossRef]
- Beyer, I.; Njemini, R.; Bautmans, I.; Demanet, C.; Mets, T. Immunomodulatory effect of NSAID in geriatric patients with acute infection: Effects of piroxicam on chemokine/cytokine secretion patterns and levels of heat shock proteins. Cell Stress Chaperones. 2012, 17, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.K.; Iyer, K.; Senthil, S.A.; Ramalingam, A.P.; Venkatachalam, S. Evaluating the anti-inflammatory efficacy of steroids, COX-2 selective, and nonselective NSAIDs in contusion spinal cord injury: An experimental analysis. J. Pharm. Bioallied Sci. 2025, 17, S1877–S1881. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Lu, Z.; Zhang, H.; Fan, X.; Zhang, X.; Jiang, B.; Li, J.; Xue, M. Aspirin and celecoxib regulate Notch1/Hes1 pathway to prevent pressure overload-induced myocardial hypertrophy. Int. Heart J. 2024, 65, 475–486. [Google Scholar] [CrossRef]
- Shendi, S.S.; Selim, S.M.; Sharaf, S.A.; Gouda, M.A.; Sallam, H.M.; Sweed, D.M.; A Shafey, D. Anti-toxoplasmic effects of celecoxib alone and combined with spiramycin in experimental mice. Acta Trop. 2024, 260, 107448. [Google Scholar] [CrossRef]
- Tajdari, M.; Peyrovinasab, A.; Bayanati, M.; Ismail Mahboubi Rabbani, M.; Abdolghaffari, A.H.; Zarghi, A. Dual COX-2/TNF-α inhibitors as promising anti-inflammatory and cancer chemopreventive agents: A review. Iran J. Pharm. Res. 2024, 23, e151312. [Google Scholar] [CrossRef]
| Group | Treatment | Paw Edema Model | LPS-Induced Inflammation Model | n |
|---|---|---|---|---|
| 1 | Saline (carrageenan control) | Yes—single and repeated administration | No | 8 |
| 2 | Saline (LPS single-dose control) | No | Yes—LPS administered on day of experiment | 8 |
| 3 | Saline (LPS repeated-dose control) | No | Yes—LPS administered on day of experiment after 14 days of saline treatment | 8 |
| 4 | Diclofenac 25 mg/kg | Yes—single and repeated administration | No | 8 |
| 5 | Compound 3f 10 mg/kg | Yes—single and repeated administration | No | 8 |
| 6 | Compound 3f 20 mg/kg | Yes—single and repeated administration | No | 8 |
| 7 | Compound 3f 40 mg/kg | Yes—single and repeated administration | No | 8 |
| 8 | Compound 3f 40 mg/kg (single administration) + LPS | No | Yes—LPS administered on day of experiment | 8 |
| 9 | Compound 3f 40 mg/kg (repeated administration) + LPS | No | Yes—LPS administered on day of experiment after 14 days of treatment | 8 |
| Parameter | Dose (mg/kg) | Time (h)/ Measurement Point | Control (Mean ± SEM) | Treated (Mean ± SEM) | Significance (p) | Administration |
|---|---|---|---|---|---|---|
| Paw Edema (%) | 20 | 2 | 47.1 ± 4.1 | 25.0 ± 3.9 | 0.001 | Single dose |
| 10 | 2 | 45.8 ± 2.9 | 7.2 ± 2.3 | <0.001 | Repeated (14 days) | |
| 10 | 3 | 40.5 ± 3.9 | 11.0 ± 4.2 | <0.001 | Repeated (14 days) | |
| 10 | 4 | 41.0 ± 4.0 | 8.9 ± 4.1 | <0.001 | Repeated (14 days) | |
| 20 | 2 | 45.8 ± 2.9 | 14.9 ± 2.6 | <0.001 | Repeated (14 days) | |
| 20 | 3 | 40.5 ± 3.9 | 14.4 ± 1.6 | <0.001 | Repeated (14 days) | |
| 20 | 4 | 41.0 ± 4.0 | 11.1 ± 3.3 | <0.001 | Repeated (14 days) | |
| 40 | 2 | 45.8 ± 2.9 | 9.7 ± 2.2 | <0.001 | Repeated (14 days) | |
| 40 | 3 | 40.5 ± 3.9 | 9.9 ± 2.6 | <0.001 | Repeated (14 days) | |
| 40 | 4 | 41.0 ± 4.0 | 6.4 ± 1.9 | <0.001 | Repeated (14 days) | |
| TNF-α (pg/mL) | 40 | 4 h post-LPS | 155 ± 38 | 64 ± 14 | 0.064 (trend) | Single dose |
| 40 | 4 h post-LPS | 103 ± 22 | 40 ± 7 | 0.032 | Repeated (14 days) | |
| TGF-β1 (pg/mL) | 40 | 4 h post-LPS | 83 ± 13 | 155 ± 10 | 0.002 | Single dose |
| 40 | 4 h post-LPS | 78 ± 16 | 119 ± 5 | 0.045 | Repeated (14 days) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlatanova-Tenisheva, H.; Vladimirova, S. Anti-Inflammatory and Immunomodulatory Effects of 2-(3-Acetyl-5-(4-Chlorophenyl)-2-Methyl-1H-Pyrrol-1-yl)-3-Phenylpropanoic Acid. Biomedicines 2025, 13, 2003. https://doi.org/10.3390/biomedicines13082003
Zlatanova-Tenisheva H, Vladimirova S. Anti-Inflammatory and Immunomodulatory Effects of 2-(3-Acetyl-5-(4-Chlorophenyl)-2-Methyl-1H-Pyrrol-1-yl)-3-Phenylpropanoic Acid. Biomedicines. 2025; 13(8):2003. https://doi.org/10.3390/biomedicines13082003
Chicago/Turabian StyleZlatanova-Tenisheva, Hristina, and Stanislava Vladimirova. 2025. "Anti-Inflammatory and Immunomodulatory Effects of 2-(3-Acetyl-5-(4-Chlorophenyl)-2-Methyl-1H-Pyrrol-1-yl)-3-Phenylpropanoic Acid" Biomedicines 13, no. 8: 2003. https://doi.org/10.3390/biomedicines13082003
APA StyleZlatanova-Tenisheva, H., & Vladimirova, S. (2025). Anti-Inflammatory and Immunomodulatory Effects of 2-(3-Acetyl-5-(4-Chlorophenyl)-2-Methyl-1H-Pyrrol-1-yl)-3-Phenylpropanoic Acid. Biomedicines, 13(8), 2003. https://doi.org/10.3390/biomedicines13082003






