Biological Actions of Alamandine: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Stage 1: Identification of the Research Question
- In what clinical conditions and outcomes has ALA been described?
- What are the effects of ALA?
2.2. Stage 2: Identification of Relevant Studies (Search Strategy)
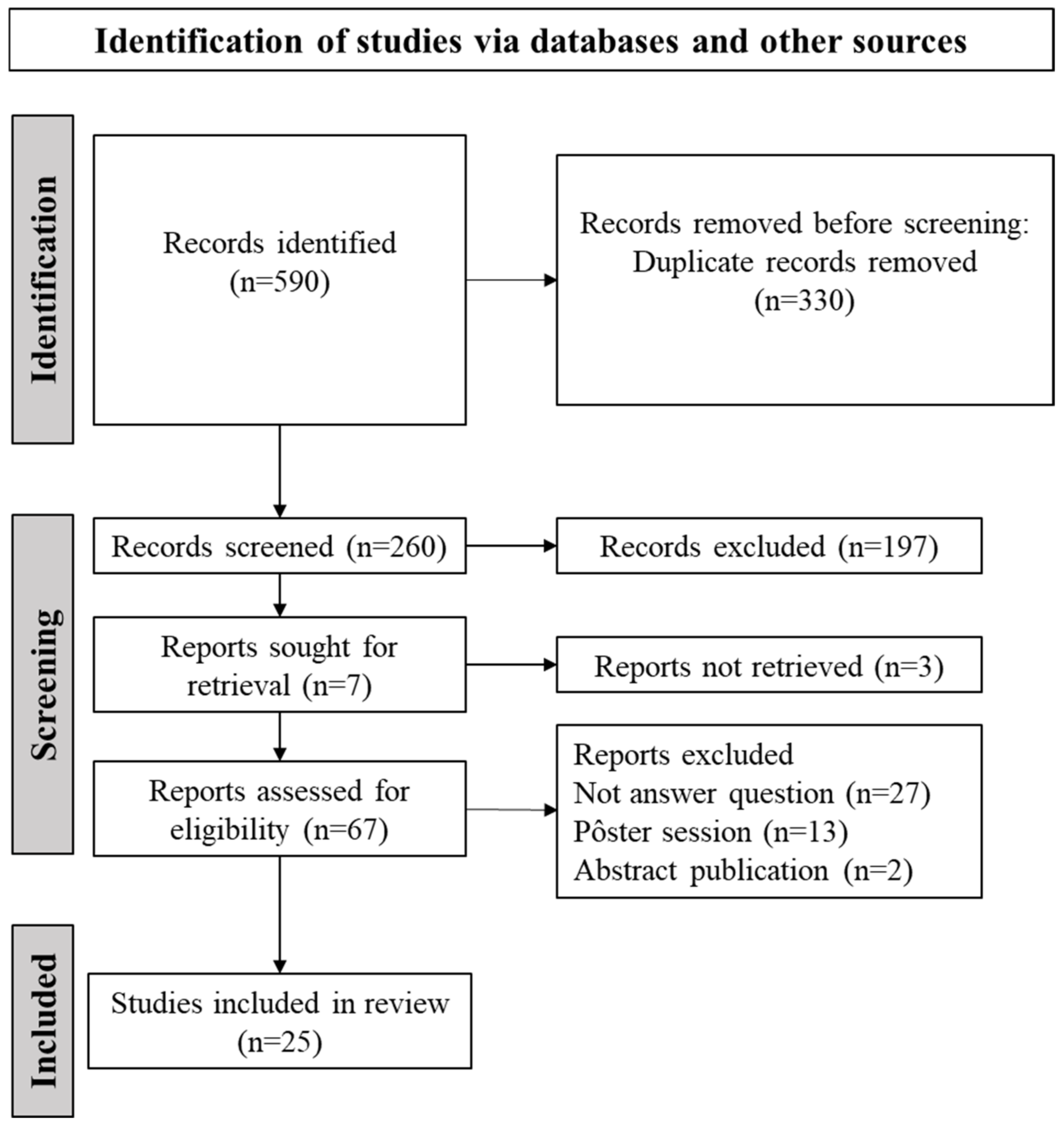
2.3. Stage 3: Selection of Studies for the Review
2.4. Stage 4: Data Extraction
2.5. Stage 5: Summary of Data and Synthesis of Results
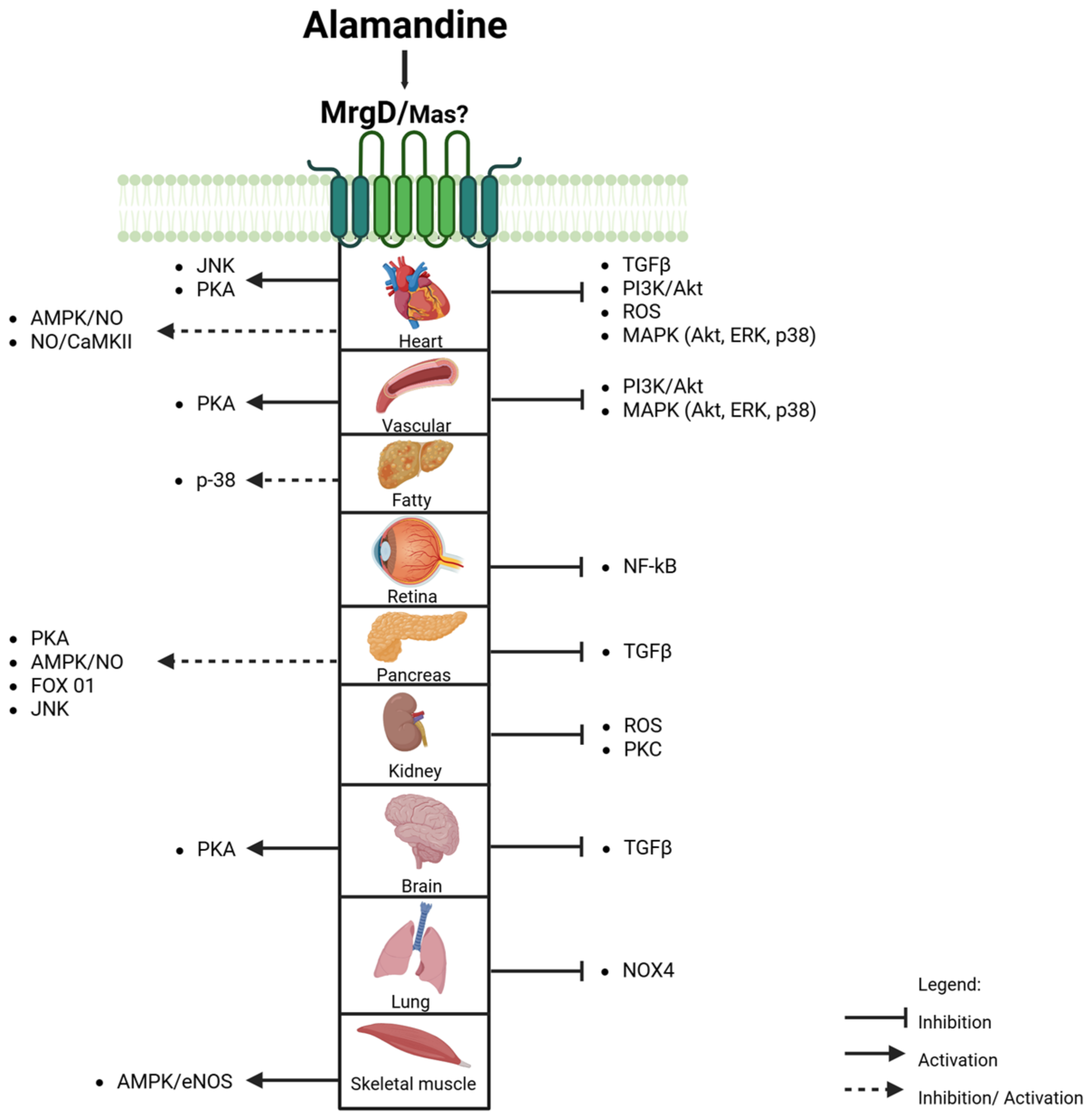
3. Results
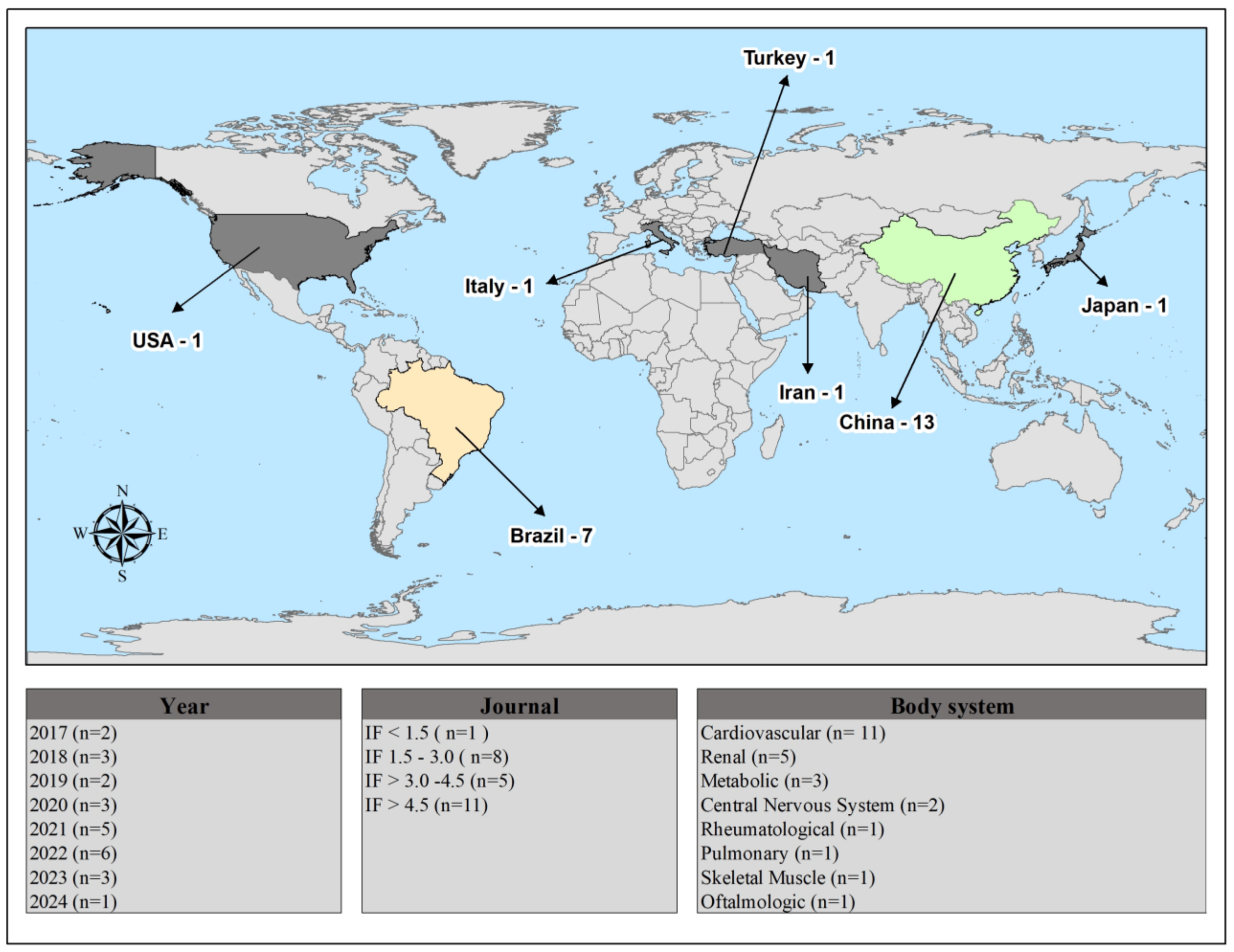
Selection of Studies and General Characteristics of Selected Studies
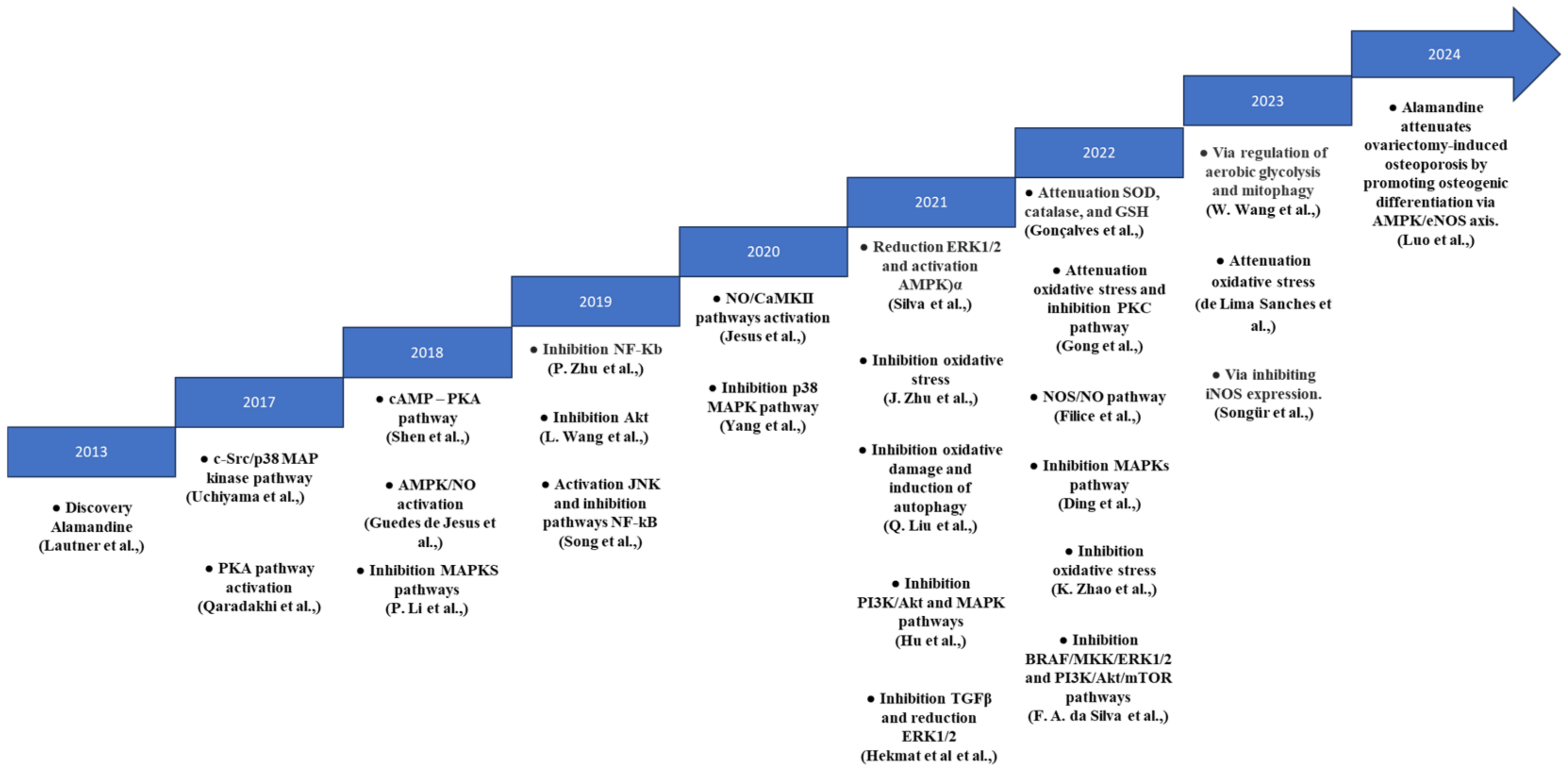
4. Discussion
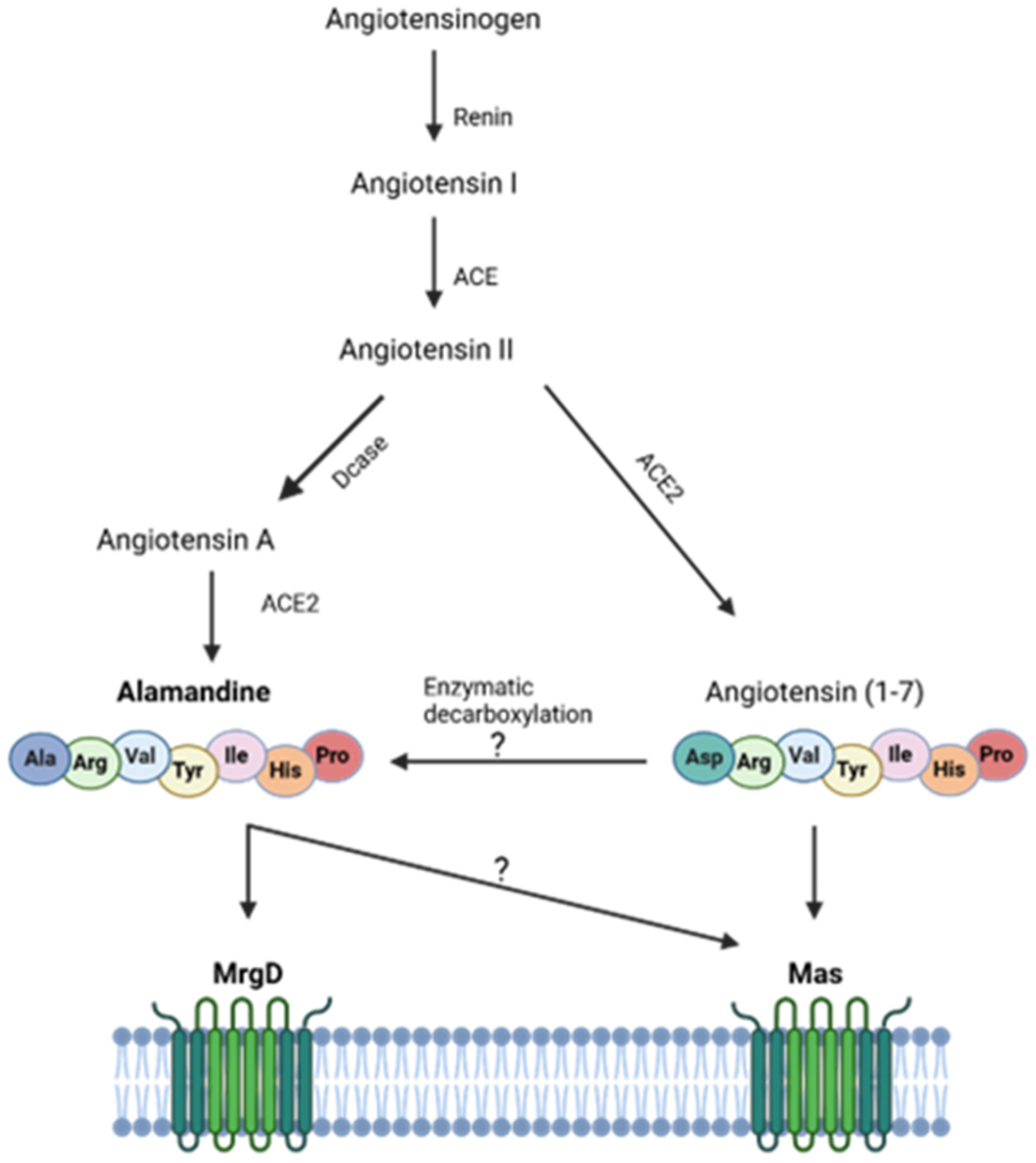
4.1. Mas and MrgD Receptors: Alamandine’s (Un)Specificity
4.2. MAPK and AMPK Signaling Pathways
4.3. Nitric Oxide Production: The Therapeutic Role of ALA in Cardiovascular Health
4.4. Modulation of PI3K Enzyme Activity by ALA
4.5. Alamandine and Oxidative Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ACE2 | Angiotensin-converting enzyme 2 |
| Akt | Protein Kinase B |
| ALA | Alamandine |
| ANG-(1-7) | Angiotensin-(1-7) |
| Ang II | Angiotensin II |
| AMPK | Adenosine monophosphate-activated protein kinase |
| CAMKII | Calmodulin-dependent kinase II |
| Dox | Doxorubicin |
| eNOS | Endothelial Nitric Oxide Synthase |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| HK2 | Hexokinase 2 |
| iNOS | Inducible Nitric Oxide Synthase |
| JNK | c-Jun N-terminal kinase |
| Mas | G protein-coupled receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA | Malondialdehyde |
| MrgD | Mas-related G protein-coupled receptor member D |
| NF-kB | Nuclear Factor Kappa B |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| O2•− | Superoxide anion |
| PCC | Population Concept and Context |
| PI3K | Phosphoinositide 3-kinase |
| PKA | Protein kinase A |
| PKFB3 | Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 |
| RAS | Renin–angiotensin system |
| SHR | Spontaneously hypertensive rat |
| SOD | Superoxide Dismutase |
| TGFβ | Transforming Growth Factor-β |
References
- Hall, J.E. Historical Perspective of the Renin-Angiotensin System. Mol. Biotechnol. 2003, 24, 27–39. [Google Scholar] [CrossRef]
- Suter, C.; Coote, J.H. Intrathecally administered angiotensin II increases sympathetic activity in the rat. J. Auton. Nerv. Syst. 1987, 19, 31–37. [Google Scholar] [CrossRef]
- Lautner, R.Q.; Villela, D.C.; Fraga-Silva, R.A.; Silva, N.; Verano-Braga, T.; Costa-Fraga, F.; Jankowski, J.; Jankowski, V.; Sousa, F.; Alzamora, A.; et al. Discovery and characterization of alamandine: A novel component of the renin-angiotensin system. Circ. Res. 2013, 112, 1104–1111. [Google Scholar] [CrossRef]
- Santos, R.A.; Pesquero, J.; Chernicky, C.L.; Greene, L.J.; Ferrario, C.M. Converting Enzyme Activity and Angiotensin Metabolism in the Dog Brainstem. Hypertension 1988, 11, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Schleifenbaum, J. Alamandine and its receptor mrgd pair up to join the protective arm of the renin-angiotensin system. Front. Med. 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, X.-R.; Xu, F.; Liu, C.; Li, C.; Liu, H.; Wang, H.; Sun, W.; Sheng, Y.-H.; Kong, X.-Q. Alamandine attenuates sepsis-associated cardiac dysfunction via inhibiting MAPKs signaling pathways. Life Sci. 2018, 206, 106–116. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Netto, M.R.T.; Carvalho, F.B.; Rigatto, K. Alamandine: A promising treatment for fibrosis. Peptides 2022, 157, 170848. [Google Scholar] [CrossRef]
- De Jesus, I.C.G.; Scalzo, S.; Alves, F.; Marques, K.; Rocha-Resende, C.; Bader, M.; Santos, R.A.S.; Guatimosim, S. Alamandine acts via MrgD to induce AMPK/NO activation against ANG II hypertrophy in cardiomyocytes. Am. J. Physiol. Cell Physiol. 2018, 314, C702–C711. [Google Scholar] [CrossRef]
- Zhao, K.; Xu, T.; Mao, Y.; Wu, X.; Hua, D.; Sheng, Y.; Li, P. Alamandine alleviated heart failure and fibrosis in myocardial infarction mice. Biol. Direct 2022, 17, 25. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Lou, A.; Wang, G.Z.; Hu, Y.; Zhang, Y.; Huang, W.; Wang, J.; Li, Y.; Zhu, X.; et al. Alamandine attenuates hepatic fibrosis by regulating autophagy induced by NOX4-dependent ROS. Clin. Sci. 2020, 134, 853–869. [Google Scholar] [CrossRef]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. JBI Manual for Evidence Synthesis. 2024. Available online: https://synthesismanual.jbi.global (accessed on 30 April 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Da Silva, F.A.; Rodrigues-Ribeiro, L.; Melo-Braga, M.N.; Passos-Silva, D.G.; Sampaio, W.O.; Gorshkov, V.; Kjeldsen, F.; Verano-Braga, T.; Santos, R.A.S. Phosphoproteomic studies of alamandine signaling in CHO-Mrgd and human pancreatic carcinoma cells: An antiproliferative effect is unveiled. Proteomics 2022, 22, e2100255. [Google Scholar] [CrossRef]
- De Lima Sanches, B.; Souza-Neto, F.; de Alcântara-Leonídeo, T.C.; Silva, M.M.; Guatimosim, S.; Vieira, M.A.R.; Santos, R.A.S.; da Silva, R.F. Alamandine attenuates oxidative stress in the right carotid following transverse aortic constriction in mice. Peptides 2023, 171, 171094. [Google Scholar] [CrossRef]
- Ding, W.; Miao, Z.; Feng, X.; Luo, A.; Tan, W.; Li, P.; Wang, F. Alamandine, a new member of the renin-angiotensin system (RAS), attenuates collagen-induced arthritis in mice via inhibiting cytokine secretion in synovial fibroblasts. Peptides 2022, 154, 170816. [Google Scholar] [CrossRef]
- Filice, M.; Mazza, R.; Imbrogno, S.; Mileti, O.; Baldino, N.; Barca, A.; Del Vecchio, G.; Verri, T.; Gattuso, A.; Cerra, M.C. An ACE2-Alamandine Axis Modulates the Cardiac Performance of the Goldfish Carassius auratus via the NOS/NO System. Antioxidants 2022, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.C.; Bassi, B.L.; Kangussu, L.M.; Alves, D.T.; Ramos, L.K.; Fernandes, L.F.; Alves, M.T.; Sinisterra, R.; Bruch, G.E.; Santos, R.A.; et al. Alamandine Induces Neuroprotection in Ischemic Stroke Models. Curr. Med. Chem. 2022, 29, 3483–3498. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Luo, M.; Yong, Y.; Zhong, S.; Li, P. Alamandine alleviates hypertension and renal damage via oxidative-stress attenuation in Dahl rats. Cell Death Discov. 2022, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, A.S.; Navabi, Z.; Alipanah, H.; Javanmardi, K. Alamandine significantly reduces doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 2021, 40, 1781–1795. [Google Scholar] [CrossRef]
- Hu, W.; Gao, W.; Miao, J.; Xu, Z.; Sun, L. Alamandine, a derivative of angiotensin-(1-7), alleviates sepsis-associated renal inflammation and apoptosis by inhibiting the PI3K/Ak and MAPK pathways. Peptides 2021, 146, 170627. [Google Scholar] [CrossRef]
- Jesus, I.C.G.; Mesquita, T.R.R.; Monteiro, A.L.L.; Parreira, A.B.; Santos, A.K.; Coelho, E.L.X.; Silva, M.M.; Souza, L.A.C.; Campagnole-Santos, M.J.; Santos, R.S.; et al. Alamandine enhances cardiomyocyte contractility in hypertensive rats through a nitric oxide-dependent activation of CaMKII. Am. J. Physiol. Physiol. 2020, 318, C740–C750. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, B.; Zhang, Y.; Huang, W.; Hong, Q.; Meng, Y. Alamandine via MrgD receptor attenuates pulmonary fibrosis via nox4 and autophagy pathway. Can. J. Physiol. Pharmacol. 2021, 99, 885–893. [Google Scholar] [CrossRef]
- Luo, W.; Yao, C.; Sun, J.; Zhang, B.; Chen, H.; Miao, J.; Zhang, Y. Alamandine attenuates ovariectomy-induced osteoporosis by promoting osteogenic differentiation via AMPK/eNOS axis. BMC Musculoskelet. Disord. 2024, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Matsoukas, M.T.; Hayes, A.; Rybalka, E.; Caprnda, M.; Rimarova, K.; Sepsi, M.; Büsselberg, D.; Kruzliak, P.; Matsoukas, J.; et al. Alamandine reverses hyperhomocysteinemia-induced vascular dysfunction via PKA-dependent mechanisms. Cardiovasc. Ther. 2017, 35, e12306. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Chen, X.R.; Yang, C.X.; Liu, B.X.; Li, P. Alamandine injected into the paraventricular nucleus increases blood pressure and sympathetic activation in spontaneously hypertensive rats. Peptides 2018, 103, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; de Souza-Neto, F.P.; de Jesus, I.C.G.; Gonçalves, G.K.; Santuchi, M.d.C.; Sanches, B.d.L.; de Alcântara-Leonídio, T.C.; Melo, M.B.; Vieira, M.A.R.; Guatimosim, S.; et al. Alamandine improves cardiac remodeling induced by transverse aortic constriction in mice. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H352–H363. [Google Scholar] [CrossRef]
- Song, X.D.; Feng, J.P.; Yang, R.X. Alamandine protects rat from myocardial ischemia-reperfusion injury by activating JNK and inhibiting NF-κB. Eur. Rev. Med. Pharmacol. Sci. 2019, 12, 6718–6726. [Google Scholar] [CrossRef]
- Songür, H.S.; Kaya, S.A.; Altınışık, Y.C.; Abanoz, R.; Özçelebi, E.; Özmen, F.; Kösemehmetoğlu, K.; Soydan, G. Alamandine treatment prevents LPS-induced acute renal and systemic dysfunction with multi-organ injury in rats via inhibiting iNOS expression. Eur. J. Pharmacol. 2023, 960, 176160. [Google Scholar] [CrossRef]
- Uchiyama, T.; Okajima, F.; Mogi, C.; Tobo, A.; Tomono, S.; Sato, K. Alamandine reduces leptin expression through the c-Src/p38 MAP kinase pathway in adipose tissue. PLoS ONE 2017, 12, e0178769. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Chen, X.; Li, P. Alamandine attenuates long-term hypertension-induced cardiac fibrosis independent of blood pressure. Mol. Med. Rep. 2019, 19, 4553–4560. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Huang, W.; Yuan, Y.; Hong, Q.; Xie, Z.; Li, L.; Chen, Y.; Li, X.; Meng, Y. Alamandine/MrgD axis prevents TGF-β1-mediated fibroblast activation via regulation of aerobic glycolysis and mitophagy. J. Transl. Med. 2023, 21, 24. [Google Scholar] [CrossRef]
- Yang, C.; Wu, X.; Shen, Y.; Liu, C.; Li, P.; Kong, X. Alamandine attenuates angiotensin II-induced vascular fibrosis via inhibiting p38 MAPK pathway. Eur. J. Pharmacol. 2020, 883, 173384. [Google Scholar] [CrossRef]
- Zhu, P.; Verma, A.; Prasad, T.; Li, Q. Expression and Function of Mas-Related G Protein-Coupled Receptor D and Its Ligand Alamandine in Retina. Mol. Neurobiol. 2020, 57, 513–527. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, J.G.; Xu, W.T.; Ma, H.X.; Jiang, K. Alamandine protects against renal ischaemia–reperfusion injury in rats via inhibiting oxidative stress. J. Pharm. Pharmacol. 2021, 73, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Villela, D.C.; Passos-Silva, D.G.; Santos, R.A.S. Alamandine: A new member of the angiotensin family. Curr. Opin. Nephrol. Hypertens. 2014, 23, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, V.; Vanholder, R.; van der Giet, M.; Toölle, M.; Karadogan, S.; Gobom, J.; Furkert, J.; Oksche, A.; Krause, E.; Tran, T.N.A.; et al. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arter. Thromb. Vasc. Biol. 2007, 27, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Tetzner, A.; Naughton, M.; Gebolys, K.; Eichhorst, J.; Sala, E.; Villacañas, Ó.; Walther, T. Decarboxylation of Ang-(1–7) to Ala1-Ang-(1–7) leads to significant changes in pharmacodynamics. Eur. J. Pharmacol. 2018, 833, 116–123. [Google Scholar] [CrossRef]
- Shinohara, T.; Harada, M.; Ogi, K.; Maruyama, M.; Fujii, R.; Tanaka, H.; Fukusumi, S.; Komatsu, H.; Hosoya, M.; Noguchi, Y.; et al. Identification of a G protein-coupled receptor specifically responsive to β-alanine. J. Biol. Chem. 2004, 279, 23559–23564. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Melo, M.B.; Motta-Santos, D.; Peluso, A.A.; Souza-Neto, F.; da Silva, R.F.; Almeida, J.F.Q.; Canta, G.; Reis, A.M.; Goncalves, G.; et al. Genetic deletion of the alamandine receptor mrgd leads to dilated cardiomyopathy in mice. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H123–H133. [Google Scholar] [CrossRef]
- Hami, J.; von Bohlen und Halbach, V.; Tetzner, A.; Walther, T.; von Bohlen und Halbach, O. Localization and expression of the Mas-related G-protein coupled receptor member D (MrgD) in the mouse brain. Heliyon 2021, 7, e08440. [Google Scholar] [CrossRef]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the brain: The renin angiotensin system. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef]
- Marins, F.R.; Oliveira, A.C.; Qadri, F.; Motta-Santos, D.; Alenina, N.; Bader, M.; Fontes, M.A.P.; Santos, R.A.S. Alamandine but not angiotensin-(1–7) produces cardiovascular effects at the rostral insular cortex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R513–R521. [Google Scholar] [CrossRef] [PubMed]
- Cerri, G.C.; Santos, S.H.S.; Bader, M.; Santos, R.A.S. Brown adipose tissue transcriptome unveils an important role of the Beta-alanine/alamandine receptor, MrgD, in metabolism. J. Nutr. Biochem. 2023, 114, 109268. [Google Scholar] [CrossRef] [PubMed]
- Habiyakare, B.; Alsaadon, H.; Mathai, M.L.; Hayes, A.; Zulli, A. Reduction of angiotensin A and alamandine vasoactivity in the rabbit model of atherogenesis: Differential effects of alamandine and Ang(1-7). Int. J. Exp. Pathol. 2014, 95, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Rukavina Mikusic, N.L.; Silva, M.G.; Erra Díaz, F.A.; Pineda, A.M.; Ferragut, F.; Gómez, K.A.; Mazzitelli, L.; Gonzalez Maglio, D.H.; Nuñez, M.; Santos, R.A.S.; et al. Alamandine, a protective component of the renin-angiotensin system, reduces cellular proliferation and interleukin-6 secretion in human macrophages through MasR–MrgDR heteromerization. Biochem. Pharmacol. 2024, 229, 116480. [Google Scholar] [CrossRef]
- Assis, A.D.; do Prado Mascarenhas, F.N.; de Assis Araújo, F.; Santos, R.A.; Zanon, R.G. Angiotensin-(1-7) receptor Mas antagonist (A779) influenced gliosis and reduced synaptic density in the spinal cord after peripheral axotomy. Peptides 2020, 129, 170329. [Google Scholar] [CrossRef]
- Takano, A.P.C.; Diniz, G.P.; Barreto-Chaves, M.L.M. AMPK signaling pathway is rapidly activated by T3 and regulates the cardiomyocyte growth. Mol. Cell Endocrinol. 2013, 376, 43–50. [Google Scholar] [CrossRef]
- Beauloye, C.; Bertrand, L.; Horman, S.; Hue, L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc. Res. 2011, 90, 224–233. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, S.; Meng, R.; Peng, C.; Xiong, Z.; Chen, B.; Chen, G.; Yao, F.; Chen, Y.; Ma, Y.; et al. Metformin attenuates ventricular hypertrophy by activating the AMP-activated protein kinase-endothelial nitric oxide synthase pathway in rats. Clin. Exp. Pharmacol. Physiol. 2011, 38, 55–62. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK—Sensing Energy while Talking to Other Signaling Pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef]
- Zaha, V.G.; Young, L.H. AMP-Activated Protein Kinase Regulation and Biological Actions in the Heart. Circ. Res. 2012, 111, 800–814. [Google Scholar] [CrossRef]
- Rababa’h, A.; Singh, S.; Suryavanshi, S.; Altarabsheh, S.; Deo, S.; McConnell, B. Compartmentalization Role of A-Kinase Anchoring Proteins (AKAPs) in Mediating Protein Kinase A (PKA) Signaling and Cardiomyocyte Hypertrophy. Int. J. Mol. Sci. 2014, 16, 218–229. [Google Scholar] [CrossRef]
- Peng, T.; Lu, X.; Lei, M.; Feng, Q. Endothelial nitric-oxide synthase enhances lipopolysaccharide-stimulated tumor necrosis factor-α expression via cAMP-mediated p38 MAPK pathway in cardiomyocytes. J. Biol. Chem. 2003, 278, 8099–8105. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, W.; He, J.; Xu, B.; Lei, B.; Wang, Z.; Cates, C.; Rousselle, T.; Li, J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 2018, 83, 256–270. [Google Scholar] [CrossRef]
- Shen, J.; O’Brien, D.; Xu, Y. Matrix metalloproteinase-2 contributes to tumor necrosis factor alpha induced apoptosis in cultured rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2006, 347, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Lu, H.; Bai, Y.; Tian, A.; Yang, Q.; Wu, J.; Yang, C.; Fan, T.P.; Zhang, Y.; Zheng, X.; et al. A metabolite of Danshen formulae attenuates cardiac fibrosis induced by isoprenaline, via a NOX2/ROS/p38 pathway. Br. J. Pharmacol. 2015, 172, 5573–5585. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Díez, R.; Rodrigues-Díez, R.; Lavoz, C.; Rayego-Mateos, S.; Civantos, E.; Rodríguez-Vita, J.; Mezzano, S.; Ortiz, A.; Egido, J.; Ruiz-Ortega, M. Statins inhibit angiotensin II/smad pathway and related vascular fibrosis, by a TGF-β-independent process. PLoS ONE 2010, 5, e14145. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Nadal-Ginard, B.; Kajstura, J.; Anversa, P.; Leri, A. A matter of life and death: Cardiac myocyte apoptosis and regeneration. J. Clin. Investig. 2003, 111, 1457–1459. [Google Scholar] [CrossRef]
- Potthoff, S.; Stamer, S.; Grave, K.; Königshausen, E.; Sivritas, S.; Thieme, M.; Mori, Y.; Woznowski, M.; Rump, L.; Stegbauer, J. Chronic p38 mitogen-activated protein kinase inhibition improves vascular function and remodeling in angiotensin II-dependent hypertension. J. Renin-Angiotensin-Aldosterone Syst. 2016, 17, 147032031665328. [Google Scholar] [CrossRef]
- Kostenko, S.; Shiryaev, A.; Dumitriu, G.; Gerits, N.; Moens, U. Cross-talk between protein kinase A and the MAPK-activated protein kinases RSK1 and MK5. J. Recept. Signal Transduct. 2011, 31, 1–9. [Google Scholar] [CrossRef]
- Imbrogno, S.; Filice, M.; Cerra, M.C.; Gattuso, A. NO, CO and H2S: What about gasotransmitters in fish and amphibian heart? Acta Physiol. 2018, 223, e13035. [Google Scholar] [CrossRef]
- Jeon, M.J.; Leem, J.; Ko, M.S.; Jang, J.E.; Park, H.-S.; Kim, H.S.; Kim, M.; Yoo, H.J.; Lee, C.-H.; Park, I.-S.; et al. Mitochondrial dysfunction and activation of iNOS are responsible for the palmitate-induced decrease in adiponectin synthesis in 3T3L1 adipocytes. Exp. Mol. Med. 2012, 44, 562–570. [Google Scholar] [CrossRef]
- Beckendorf, J.; van den Hoogenhof, M.M.G.; Backs, J. Physiological and unappreciated roles of CaMKII in the heart. Basic Res. Cardiol. 2018, 113, 29. [Google Scholar] [CrossRef]
- Prasad, S.V.N.; Perrino, C.; Rockman, H.A. Role of phosphoinositide 3-kinase in cardiac function and heart failure. Trends Cardiovasc. Med. 2003, 13, 206–212. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hsu, Y.-J.; Hsu, S.-C.; Chen, Y.; Lee, H.-S.; Lin, S.-H.; Huang, S.-M.; Tsai, C.-S.; Shih, C.-C. CB1 cannabinoid receptor antagonist attenuates left ventricular hypertrophy and Akt-mediated cardiac fibrosis in experimental uremia. J. Mol. Cell Cardiol. 2015, 85, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.D.; Viswanadhapalli, S.; Williams, P.; Shi, Q.; Tan, C.; Yi, X.; Bhandari, B.; Abboud, H.E. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways. Circulation 2015, 131, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Srivastava, R.K.; Shankar, S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J. Mol. Signal 2010, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, H.; Liu, X.; Tan, L.; Qiao, X.; Ni, J.; Sun, Y.; Liang, J.; Hou, Y.; Dou, H. Pyruvate kinase isoform M2 impairs cognition in systemic lupus erythematosus by promoting microglial synaptic pruning via the β-catenin signaling pathway. J. Neuroinflamm. 2021, 18, 229. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Chen, B.; Lin, S.; Gu, J.; Tan, L.; Lin, M. Telocytes protect against lung tissue fibrosis through hexokinase 2-dependent pathway by secreting hepatocyte growth factor. Clin. Exp. Pharmacol. Physiol. 2023, 50, 964–972. [Google Scholar] [CrossRef]
- Yin, X.; Choudhury, M.; Kang, J.-H.; Schaefbauer, K.J.; Jung, M.-Y.; Andrianifahanana, M.; Hernandez, D.M.; Leof, E.B. Hexokinase 2 couples glycolysis with the profibrotic actions of TGF-β. Sci. Signal. 2019, 12, eaax4067. [Google Scholar] [CrossRef]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023, 35, 1406–1423.e8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chen, T.; Lin, Y.; Gong, J.; Xu, Q.; Wang, J.; Li, J.; Meng, Y.; Li, Y.; et al. Caveolin-1 depletion attenuates hepatic fibrosis via promoting SQSTM1-mediated PFKL degradation in HSCs. Free Radic. Biol. Med. 2023, 204, 95–107. [Google Scholar] [CrossRef]
- Wu, R.; Smeele, K.M.; Wyatt, E.; Ichikawa, Y.; Eerbeek, O.; Sun, L.; Chawla, K.; Hollmann, M.W.; Nagpal, V.; Heikkinen, S.; et al. Reduction in hexokinase II levels results in decreased cardiac function and altered remodeling after ischemia/reperfusion injury. Circ. Res. 2011, 108, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Jiang, S.; Fu, D.; Lu, X.; Lu, M.; Li, Y.; Luo, D.; Wu, K.; Xu, Y.; et al. The glycolytic enzyme PFKFB3 drives kidney fibrosis through promoting histone lactylation-mediated NF-κB family activation. Kidney Int. 2024, 106, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huo, E.; Cai, Y.; Zhang, Z.; Dong, C.; Asara, J.M.; Wei, Q. PFKFB3-Mediated Glycolysis Boosts Fibroblast Activation and Subsequent Kidney Fibrosis. Cells 2023, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.B.; Hay, N.; Robey, R.B.; Hay, N. Mitochondrial Hexokinases: Guardians of the Mitochondria. Cell Cycle 2005, 4101, 654–658. [Google Scholar] [CrossRef]
- Zeng, H.; Pan, T.; Zhan, M.; Hailiwu, R.; Liu, B.; Yang, H.; Li, P. Suppression of PFKFB3-driven glycolysis restrains endothelial-to-mesenchymal transition and fibrotic response. Signal Transduct. Target. Ther. 2022, 7, 303. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Pandey, M.; Loskutoff, D.J.; Samad, F. Molecular mechanisms of tumor necrosis factor-α-mediated plasminogen activator inhibitor-1 expression in adipocytes. FASEB J. 2005, 19, 1317–1319. [Google Scholar] [CrossRef]
- Kumar, D.; Jugdutt, B.I. Apoptosis and oxidants in the heart. J. Lab. Clin. Med. 2003, 142, 288–297. [Google Scholar] [CrossRef]
- Li, Z.; Bing, O.H.L.; Long, X.; Robinson, K.G.; Lakatta, E.G. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am. J. Physiol. Heart Circ. Physiol. 1997, 272, 41–45. [Google Scholar] [CrossRef]
- Dash, S.K.; Chattopadhyay, S.; Ghosh, T.; Dash, S.S.; Tripathy, S.; Das, B.; Bag, B.G.; Das, D.; Roy, S. Self-assembled betulinic acid protects doxorubicin induced apoptosis followed by reduction of ROS-TNF-α-caspase-3 activity. Biomed. Pharmacother. 2015, 72, 144–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flor, J.; Silveira, A.T.; Martins, I.A.; Otero, L.B.; Silva, F.M.; Vizuete, A.F.K.; Wink, M.R.; Rigatto, K. Biological Actions of Alamandine: A Scoping Review. Biomedicines 2025, 13, 1957. https://doi.org/10.3390/biomedicines13081957
Flor J, Silveira AT, Martins IA, Otero LB, Silva FM, Vizuete AFK, Wink MR, Rigatto K. Biological Actions of Alamandine: A Scoping Review. Biomedicines. 2025; 13(8):1957. https://doi.org/10.3390/biomedicines13081957
Chicago/Turabian StyleFlor, Juliane, Andresa Thomé Silveira, Isabel Amaral Martins, Laura Bastos Otero, Flávia Moraes Silva, Adriana Fernanda K. Vizuete, Márcia Rosângela Wink, and Katya Rigatto. 2025. "Biological Actions of Alamandine: A Scoping Review" Biomedicines 13, no. 8: 1957. https://doi.org/10.3390/biomedicines13081957
APA StyleFlor, J., Silveira, A. T., Martins, I. A., Otero, L. B., Silva, F. M., Vizuete, A. F. K., Wink, M. R., & Rigatto, K. (2025). Biological Actions of Alamandine: A Scoping Review. Biomedicines, 13(8), 1957. https://doi.org/10.3390/biomedicines13081957






