Abstract
Background/Objectives: Chronic kidney disease (CKD) and osteoporosis are critical public health concerns, particularly among older adults, due to their prevalence and associated complications. While CKD-related disruptions in bone mineral metabolism are believed to increase osteoporosis risk, this relationship remains unclear in diverse populations such as Korea. Methods: This longitudinal cohort study utilized data from the Korean National Health Insurance Service Health Screening Cohort (2002–2019), including 13,622 patients with newly diagnosed CKD and 54,488 matched controls. CKD was defined as having at least two outpatient or inpatient records with ICD-10 codes N18 or N19 and/or evidence of dialysis treatment claims, following a 1-year washout period to exclude prevalent cases. Individuals with a prior history of osteoporosis or incomplete baseline data were excluded. Propensity score overlap weighting was applied, and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using Cox proportional hazards models, with subgroup analyses based on demographic and clinical factors. Results: CKD was not associated with an increased risk of osteoporosis. On the contrary, CKD patients exhibited an 18% lower risk of developing osteoporosis compared to controls (HR = 0.82, 95% CI: 0.77–0.87, p < 0.001). This inverse association was more pronounced among women, non-smokers, individuals with low alcohol consumption, and those with a higher comorbidity burden. Conclusions: These findings suggest that certain subgroups of CKD patients may have a reduced risk of osteoporosis, highlighting the importance of individualized risk assessment and tailored preventive strategies.
1. Introduction
Chronic kidney disease (CKD) and osteoporosis are major global health concerns, particularly among older adults, as both conditions are associated with severe complications and significant public health burdens [1,2,3]. Osteoporosis, characterized by reduced bone density and increased fracture risk, affects over 200 million people worldwide, resulting in substantial healthcare costs [4]. In Korea, 22.4% of individuals aged 50 years and older are diagnosed with osteoporosis, with prevalence rates significantly higher in women (35.5%) compared to men (7.5%) [5,6]. CKD is a progressive condition affecting over 10% of the global population, totaling more than 800 million people [2]. In Korea, the prevalence is similar, with 10–13% of the population impacted [1]. This underscores CKD’s significant public health burden worldwide and within Korea. These conditions often overlap [7,8], with CKD-related complications such as disrupted bone mineral metabolism contributing to an increased risk of osteoporosis [9,10].
Both CKD and osteoporosis share common risk factors, including aging, lifestyle behaviors, and socioeconomic status [11,12]. While previous studies have suggested that CKD—particularly in its advanced stages—may contribute to bone mineral density loss through mechanisms such as calcium–phosphorus imbalance, systemic inflammation, and secondary hyperparathyroidism [8,9], the overall relationship between CKD and osteoporosis remains poorly understood. Despite biologically plausible connections, epidemiological evidence is inconsistent. Some studies report an increased risk of osteoporosis and fractures among CKD patients, especially in advanced stages [9,10], whereas others find no significant association or even paradoxical protective trends in certain subgroups [11,13]. These discrepancies may arise from variations in study design, population characteristics, CKD definitions, or confounder adjustment [12,14,15]. Additionally, most available evidence originates from Western populations [8,16], limiting its applicability to Asian populations.
Korea’s rapidly aging population, coupled with the rising prevalence of chronic diseases [17], underscores the need to better understand the intersection of CKD and osteoporosis within this unique demographic. Although healthcare access and health behaviors have improved in recent decades, large-scale, population-based studies exploring this association in Korea are lacking. This gap is particularly concerning given the high prevalence of both CKD and osteoporosis in the Korean population [1,6]. Therefore, research using intensive methodology and nationally representative data is essential to clarify this relationship and inform effective prevention strategies for high-risk groups.
To address this gap, this study evaluates the association between CKD and osteoporosis risk in the Korean population, which may vary based on individual patient characteristics, such as age, sex, socioeconomic status, and comorbid conditions. We examined whether certain lifestyle or demographic factors (e.g., age, sex, smoking, rural residence) might modify this risk within the CKD population. To fulfill this, we conducted a long-term observational study using data from the Korean national public healthcare system, analyzing the association between CKD and osteoporosis risk while accounting for potential confounding factors. By leveraging nationwide, longitudinal data and robust analytical techniques, this research could help to inform more targeted prevention strategies and improve clinical outcomes for CKD patients, addressing an important public health concern.
2. Materials and Methods
2.1. Research Design, Data Resource, and Cohort Selection
This study analyzed data from the Health Screening Cohort of the Korean National Health Insurance Service, a comprehensive dataset that provides sociodemographic and clinical information on a representative sample of Korean adults, encompassing the period from 2002 to 2019. Participants aged 40–79 years who underwent health screenings during 2002–2003 and were followed until 2019 [18]. The dataset comprises 514,866 participants, selected through 10% simple random sampling from all eligible individuals during the enrollment period [19]. The database is fully anonymized by the government and employs International Classification of Diseases, 10th Revision (ICD-10) codes for healthcare information and standardized disease diagnosis. This study was approved by the Ethics Committee of Hallym University (IRB No. 2019-10-023), with a waiver of written informed consent due to the use of anonymized secondary data. All analyses were conducted in compliance with the guidelines and regulations set forth by the Ethics Committee of Hallym University.
This retrospective cohort study evaluated the impact of chronic kidney disease (CKD) on osteoporosis incidence using a large-scale dataset from the Korean National Health Insurance Service, which included 514,866 individuals aged ≥40 years and 895,300,177 medical claims recorded between 2002 and 2019. CKD was defined as having at least two outpatient or inpatient records with ICD-10 codes N18 (chronic kidney disease) or N19 (unspecified kidney failure), and/or receipt of dialysis based on Korean insurance claim codes (O7010, O7020, or O7070) [20]. To improve diagnostic accuracy and exclude prevalent cases, a washout period was applied in 2002, and only newly diagnosed cases from 2003 onward were included. Participants with a single CKD diagnosis, incomplete baseline data (e.g., missing BMI, blood pressure, or fasting glucose), or a prior history of osteoporosis were excluded to minimize misclassification and reverse causation.
For the control group, 497,388 eligible individuals without CKD-related diagnoses were initially identified. We first performed 1:4 exact matching based on age, sex, income level, and region of residence. The index date for each CKD patient—defined as the date of diagnosis—was assigned to a randomly selected matched control. Controls who had died or developed osteoporosis before the assigned index date were excluded, resulting in the removal of 442,340 individuals. After this matching step, we estimated propensity scores using the full set of baseline covariates and applied overlap weighting to further adjust for residual confounding and achieve covariate balance. The final study population included 13,622 CKD patients and 54,488 matched controls.
Osteoporosis was defined using ICD-10 codes M80 (with pathological fracture), M81 (without pathological fracture), and M82 (associated with other diseases) [21,22]. To ensure diagnostic accuracy, only individuals with at least two clinical visits and corresponding bone density assessments by X-ray or computed tomography (claim codes HC341–HC345, E7001–E7004) were considered as osteoporosis cases [21,22]. All participants were followed for osteoporosis incidence from the index date until 31 December 2019 (Figure 1).
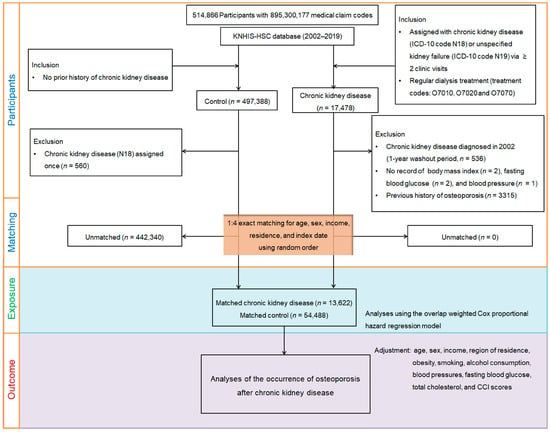
Figure 1.
Flowchart of participant selection, 1:4 exact matching, and overlap weighting. Among 514,866 individuals aged ≥40 years, 13,622 patients with newly diagnosed CKD were identified based on ≥2 claims with ICD-10 codes N18/N19 and/or dialysis. After excluding those with prior osteoporosis, incomplete baseline data, or a single CKD record, 1:4 exact matching was performed by age, sex, income, and region. Each CKD patient’s index date (diagnosis date) was assigned to a randomly selected matched control. Controls with prior osteoporosis or death before the index date were excluded. Propensity score overlap weighting was applied to balance covariates between 13,622 CKD patients and 54,488 matched controls. Participants were followed for incident osteoporosis through 31 December 2019.
2.2. Covariables
Participants were categorized by age (10 groups) and income level (5 tiers), with residential areas classified as urban or rural. Behavioral factors, including BMI, alcohol consumption, and smoking, were analyzed alongside physiological measures such as fasting glycemic values, blood pressure, and total cholesterol. The Charlson Comorbidity Index (CCI) was used to quantify overall health burden based on ICD-10 codes from each participant’s medical history, assigning scores ranging from 0 to 29 depending on the presence and severity of 17 comorbid conditions [23]. This index provides a standardized assessment of cumulative health impact [23]. The conditions included in the CCI are myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disorders, peptic ulcer disease, liver disease, diabetes (with and without complications), paraplegia, renal disease, cancer, metastatic cancer, severe liver disease, and HIV/AIDS [23].
2.3. Statistical Analyses
To address confounding factors and ensure a balanced comparison between CKD patients and controls, this study utilized propensity score overlap weighting. This method was chosen for its ability to focus on the region of common support between groups, minimizing bias by excluding extreme outliers, maximizing the effective sample size by retaining all participants, and achieving balanced baseline characteristics across groups [24,25]. Unlike traditional propensity score matching, which discards unmatched participants and may introduce selection bias, overlap weighting creates a pseudo-population in which the baseline features of CKD and control groups are nearly identical, mimicking the properties of a randomized controlled trial [24,25].
Specifically, propensity scores were estimated through a logistic regression model that included multiple variables [26]. Overlap weights were then derived, assigning higher weights to participants with similar probabilities of being in either group, ensuring better comparability and accounting for complex relationships between covariates and group membership [24,25]. This ensures a more comprehensive adjustment for confounding factors. This approach allowed for a robust comparison of osteoporosis risk between CKD and control groups [25]. A standardized difference of less than 0.20 was considered indicative of a well-balanced dataset between the groups and minimized baseline differences [27].
Crude incidence rates and incidence rate differences were calculated as the number of events per 1000 person-years by dividing event counts by total person-years. Kaplan–Meier estimators were employed to evaluate osteoporosis incidence over time, while Cox proportional hazards regression models were used to estimate HRs and 95% CIs, with proportional hazards assumptions confirmed. Subgroup analyses were performed to examine variations by demographic and clinical factors. Statistical analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA), with significance set at p-values < 0.05 for two-tailed tests.
3. Results
3.1. Baseline Characteristics of the Participants
The initial traits of participants prior to and following propensity score overlap weighting are summarized (Table 1). Prior to adjustment, imbalances were detected between the CKD group and the control group in terms of systolic blood pressure, fasting glycemic values, and CCI scores. Subjects in the CKD group were less likely to exhibit a normal weight and more likely to have higher systolic blood pressure, fasting glycemic values, and CCI scores compared to the controls.

Table 1.
Baseline characteristics of participants before and after propensity score overlap weighting.
After overlap propensity score weighting, standardized differences for all variables were reduced to ≤0.20, achieving an equal distribution of traits between the CKD and control groups. The standardized differences for weight status categories, systolic blood pressure, fasting glycemic values, and CCI scores were reduced to 0.00, with similar reductions observed for other covariates, indicating effective adjustment for confounding variables.
3.2. Association Between CKD and Osteoporosis Likelihood
The incidence rates of osteoporosis in the CKD and control counterpart groups were 11.10 and 13.40 per 1000 person-years, respectively (difference in incidence rates: HR −2.30, 95% CI: −3.39–−1.33). After overlap-weighting adjustment for all demographics and medical comorbidities, Cox regression analysis disclosed that CKD participants exhibited a lower incidence of osteoporosis (HR = 0.82, 95% CI: 0.77–0.87, p < 0.001) (Table 2). The Kaplan–Meier analysis with log-rank test displayed a more reduced chance of osteoporosis in patients suffering CKD than in those without CKD during a 16-year period commencing from the index date (p < 0.0001; Figure 2).

Table 2.
Crude and propensity score overlap-weighted HRs and 95% CIs of CKD for osteoporosis, with subgroup analyses according to age, sex, income, and region of residence.
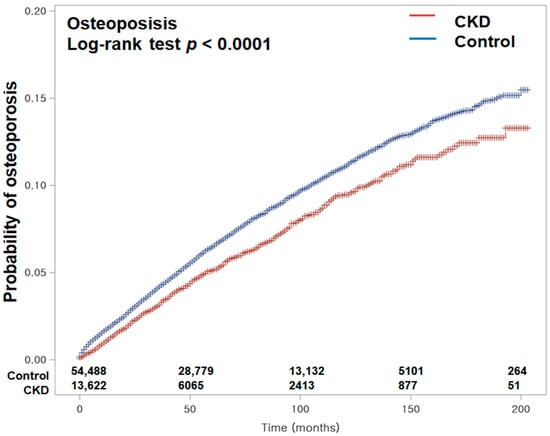
Figure 2.
Kaplan–Meier curves depicting the cumulative incidence of osteoporosis in patients with chronic kidney disease (CKD) and in control subjects over a 16-year period from the index date. The Kaplan–Meier analysis with the log-rank test demonstrated a significantly lower incidence of osteoporosis in the CKD group compared to the control group during the 16-year follow-up period.
3.3. Subgroup Analyses
Subgroup analyses (Table 3) revealed that the reduced risk of osteoporosis associated with CKD was consistent across various demographic and clinical factors. Female CKD patients exhibited a markedly reduced risk of developing osteoporosis (HR = 0.75, 95% CI: 0.69–0.82, p < 0.001), indicating a protective association. Conversely, in male CKD patients, the association was not substantial (HR = 0.95, 95% CI: 0.86–1.05, p = 0.348).

Table 3.
Subgroup analyses of the crude and propensity score overlap-weighted hazard ratios and 95% confidence intervals of CKD for osteoporosis.
Non-smokers with CKD demonstrated a substantially lower likelihood of osteoporosis (HR = 0.78, 95% CI: 0.72–0.84, p < 0.001) compared to smokers, where the association lacked statistical significance (HR = 1.00, 95% CI: 0.87–1.15, p = 0.956). Similarly, CKD patients who consumed alcohol minimally (less than once per week) showed a greater protective effect (HR = 0.79, 95% CI: 0.73–0.85, p < 0.001) relative to those who consumed alcohol more frequently (HR = 0.99, 95% CI: 0.86–1.15, p = 0.898).
CKD individuals with higher comorbidity scores (CCI ≥ 1) exhibited a more pronounced reduction in osteoporosis risk, whereas patients with low comorbidity burdens showed no association (HR = 0.92, 95% CI: 0.84–1.02, p = 0.103).
Variations were also observed across age groups, income levels, and rural versus urban residency, weight status, total cholesterol levels, blood pressure, fasting glycemic values, or generally demonstrating a slightly protective effect.
4. Discussion
This longitudinal cohort study, utilizing nationwide large-scale data, demonstrated no notable rise in the overall occurrence of osteoporosis among CKD patients compared to controls throughout the 16-year observation period. Notably, CKD patients demonstrated an 18% reduction in the likelihood of developing osteoporosis (95% CI = 0.77–0.87), with this effect being particularly pronounced among women, non-smokers, individuals with minimal alcohol intake, and those with a high comorbidity burden. These findings highlight the complex interplay between CKD and osteoporosis, suggesting potential protective factors in CKD patients that may lower osteoporosis risk. They emphasize the importance of subgroup-specific analyses and the consideration of demographic and clinical characteristics when evaluating osteoporosis risk in the Korean population aged over 40.
Previous studies have identified CKD as a contributing factor to bone mineral density loss due to disrupted calcium–phosphorus metabolism, systemic inflammation, and secondary hyperparathyroidism [9]. However, our findings indicated a more nuanced relationship between CKD and osteoporosis risk. Our results appear to contrast with prior reviews reporting an increased osteoporosis risk in CKD, largely driven by advanced stages and dialysis populations [11,28]. This discrepancy may reflect differences in study populations, disease severity, methodologies, and healthcare systems [12,14,15]. Notably, many earlier studies focused on patients with late-stage CKD, where disturbances in mineral metabolism are more pronounced [9,10,11,28]. In contrast, our study included individuals across all CKD stages, likely capturing a broader clinical spectrum, including early-stage patients with milder biochemical abnormalities. Additionally, the reduced osteoporosis risk identified within our study population could be partially attributed to the widespread implementation of early medical interventions and nationwide osteoporosis screening programs in Korea, which promote early detection and management under the National Health Insurance Service [5,6]. These public health efforts may be particularly effective in mitigating osteoporosis risk in specific subgroups. Taken together, our findings suggest that the association between CKD and osteoporosis is not uniform but rather may vary depending on demographic, clinical, and healthcare-related factors. By incorporating comorbidities, socioeconomic status, and lifestyle characteristics into an overlap-weighted Cox regression model, our study showed inverse estimates of the relationship between CKD and osteoporosis risk in certain subgroups. The use of propensity score overlap weighting may strengthen causal inference by minimizing confounding and improving covariate balance, offering a more reliable assessment of this complex association [25].
In the present study, subgroup analyses revealed that demographic, lifestyle, and clinical factors significantly influenced osteoporosis risk among patients with CKD. A more pronounced inverse association between CKD and osteoporosis was observed in females, non-smokers, individuals with minimal alcohol consumption, and those with a higher comorbidity burden. While reduced exposure to conventional osteoporosis risk factors likely contributes to these findings [11], additional biological mechanisms may also be involved. Healthy lifestyle behaviors, including smoking cessation and low alcohol intake, may lower systemic inflammation and oxidative stress—key drivers of bone loss—and are often associated with greater engagement in preventive healthcare [11]. In addition, sex- and hormone-related differences may underlie the stronger protective effect observed in women [12]. Estrogen has well-documented bone-preserving effects, and in early CKD stages, some degree of estrogenic activity may be preserved, potentially helping delaying bone loss [12,14,29]. This preservation of estrogenic activity can act as a protective factor against the bone loss that is commonly seen in later stages of CKD and in conditions like osteoporosis [12,29]. Although other risk modifiers include older age, postmenopausal status, low body mass index, and comorbidities such as vascular or chronic inflammatory diseases [10,11,30,31], women with CKD may also experience unique hormonal adaptations that influence bone turnover differently than in men [29]. In addition, CKD patients with comorbidities often receive regular monitoring and treatment for mineral and bone disorders, including vitamin D supplementation, phosphate binders, and parathyroid hormone modulators [13,32]. These interventions may mitigate bone deterioration and lower the likelihood of osteoporosis [12,32]. In dialysis patients, targeted treatments such as parathyroidectomy have also demonstrated protective effects, supporting the importance of individualized management strategies in modifying osteoporosis risk [30].
Genetic factors also contribute significantly to osteoporosis susceptibility, particularly among Korean women [33]. Using whole-genome comparative expression profiling, gene expression analysis, and association studies, a significant relationship was identified between single-nucleotide polymorphisms in five genes—ANXA6, COL5A1, ENO1, MYOF, and SCARA5—and bone mineral density and/or osteoporosis in a cohort of 3570 Korean women [33]. In addition, the ApaI polymorphism in the vitamin D receptor gene—associated with bone mineral density and fracture risk in Korean postmenopausal women—may influence individual responsiveness to vitamin D [34]. Together, these findings may suggest the multifactorial nature of osteoporosis risk in CKD, involving genetic predisposition, behavioral, hormonal, and metabolic pathways. Subgroup-specific differences should be considered when developing targeted prevention strategies in clinical practice.
This study leverages representative, nationwide data from the Korean National Health Insurance Service Health Screening Cohort, offering an in-depth analysis that accounts for economic background, behavioral risk factors, and concurrent health conditions. By incorporating complete medical histories from healthcare facilities nationwide, the findings are both highly precise and broadly generalizable. To the best of our knowledge, this is the most extensive nationwide follow-up analysis examining the association between osteoporosis and CKD risk in Korean adults. The study’s robustness is further bolstered by the use of overlap-weighted propensity score matching, which minimizes selection bias and enables subgroup analyses comparable to randomized clinical trials [25]. With a 16-year follow-up period, this long-term observational study offers important perspectives on the relationship between CKD and osteoporosis, with adjustments for potential confounders further enhancing the credibility and applicability of the findings [7].
This study has several limitations that should be considered. First, its observational design precludes causal inference, and residual confounding may persist despite robust statistical adjustments using exact matching and propensity score overlap weighting. Second, the use of ICD-10 codes and claims data may introduce misclassification bias, as undiagnosed or inaccurately coded cases of CKD and osteoporosis could affect classification. Third, the lack of detailed clinical data, such as estimated glomerular filtration rate or albuminuria, prevented stratification by CKD severity and limited our ability to assess stage-specific associations with osteoporosis risk. Although dialysis claim codes enabled the identification of end-stage renal disease, earlier CKD stages could not be distinguished. Fourth, healthy user bias may have influenced our findings. CKD patients often engage more frequently with the healthcare system, potentially leading to earlier detection and better management of osteoporosis, which could partially explain the lower observed risk in this group. Fifth, although we discussed potential effects of medications such as vitamin D, phosphate binders, and parathyroid hormone modulators, detailed information on medication use—including dosage, frequency, and adherence—was not available in the Korean National Health Insurance Service dataset. Thus, the role of pharmacologic treatment in modifying osteoporosis risk could not be directly assessed. Sixth, genetic factors, such as vitamin D receptor gene polymorphisms, which may influence bone metabolism and treatment response, were not included due to the absence of genomic data. Seventh, treatment protocols for CKD and osteoporosis in Korea, including standardized national screening and early intervention programs, may differ from those in other countries. These system-level healthcare differences could affect treatment patterns and osteoporosis detection rates, potentially limiting the generalizability of our findings across international settings. Lastly, our study population consisted of Korean adults aged ≥40 years, which may restrict generalizability to younger individuals or populations with different ethnic or geographic backgrounds.
5. Conclusions
In conclusion, this nationwide study suggests that overall, CKD patients in Korea may not experience a heightened risk of osteoporosis compared to individuals without CKD and even exhibit a slightly lower likelihood, particularly among females, non-smokers, those with minimal alcohol consumption, and individuals with a high comorbidity burden. These findings emphasize the need for individualized risk assessments and targeted prevention strategies, particularly for CKD patients with minimal lifestyle risk factors. Health professionals need to focus on early screening and tailored interventions to optimize bone health in this population. By identifying subgroup-specific variations in osteoporosis risk, this study may provide valuable insights for guiding targeted prevention strategies and improving clinical outcomes, potentially contributing to better public health policies in Korea.
Author Contributions
M.J.K.: investigation, funding acquisition, writing—original draft, review and editing; H.S.K.: writing—original draft, review and editing; J.-H.K.: formal analysis; W.J.B. and H.Y.P.: methodology; D.M.Y. and K.M.H.: investigation and methodology; N.Y.K.: validation; H.G.C.: conceptualization, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant (RS-2025-00556614) from the National Research Foundation of Korea, funded by the Korean Ministry of Science and ICT to M.J.K. The APC was funded by RS-2025-00556614.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Hallym University (code: 2022-12-005, approved on 22 December 2022). The requirement for written informed consent was waived by the Institutional Review Board due to secondary data.
Informed Consent Statement
This study used the Korean National Health Insurance Service Health Screening Cohort data from 2002 through 2019, which were collected from the Korean National Health Insurance Service. Therefore, the requirement for written informed consent was waived by the Institutional Review Board due to the fact that the study utilized secondary data.
Data Availability Statement
All data are available from the database of National Health Insurance Sharing Service (NHISS) https://nhiss.nhis.or.kr/ (accessed on 1 October 2024). NHISS allows access to all of this data for any researcher who promises to follow the research ethics at some processing charge. If you want to access the data of this article, you can download it from the website after promising to follow the research ethics.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
References
- Park, J.I.; Baek, H.; Jung, H.H. Prevalence of Chronic Kidney Disease in Korea: The Korean National Health and Nutritional Examination Survey 2011–2013. J. Korean Med. Sci. 2016, 31, 915–923. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Albertus, P.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Cao, J.; Chen, J.L.; et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2017, 69, A7–A8. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, S4–S9. [Google Scholar] [CrossRef]
- Yu, T.Y.; Cho, H.; Kim, T.Y.; Ha, Y.C.; Jang, S.; Kim, H.Y. Utilization of Osteoporosis-Related Health Services: Use of Data from the Korean National Health Insurance Database 2008–2012. J. Korean Med. Sci. 2018, 33, e20. [Google Scholar] [CrossRef]
- Choi, Y.J.; Oh, H.J.; Kim, D.J.; Lee, Y.; Chung, Y.S. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: The Korea National Health and Nutrition Examination Survey 2008–2009. J. Bone Miner. Res. 2012, 27, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Dingpeng, L.; Bihui, B.; Ruixuan, X.; Fei, Y.; Xingwen, X.; Demin, L. Distribution and diagnostic modeling of osteoporosis and comorbidities across demographic factors: A cross-sectional study of 2224 female patients. Exp. Gerontol. 2024, 198, 112638. [Google Scholar] [CrossRef] [PubMed]
- Iimori, S.; Mori, Y.; Akita, W.; Kuyama, T.; Takada, S.; Asai, T.; Kuwahara, M.; Sasaki, S.; Tsukamoto, Y. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—A single-center cohort study. Nephrol. Dial. Transplant. 2012, 27, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Lu, C.L.; Lu, K.C. Mineral bone disorders in chronic kidney disease. Nephrology 2018, 23 (Suppl. S4), 88–94. [Google Scholar] [CrossRef]
- Stehman-Breen, C. Osteoporosis and chronic kidney disease. Semin. Nephrol. 2004, 24, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.S.; David, K.; Salam, S.; Evenepoel, P.; European Renal Osteodystrophy (EUROD) Workgroup; CKD-MBD Working Group of the ERA-EDTA. Traditional and Non-traditional Risk Factors for Osteoporosis in CKD. Calcif. Tissue Int. 2021, 108, 496–511. [Google Scholar] [CrossRef]
- Kuang, C.; Shang, J.; Ma, M.; Huang, S.; Yan, B.; Zhong, Y.; Guan, B.; Gong, J.; Liu, F.; Chen, L. Risk factors and clinical prediction models for osteoporosis in pre-dialysis chronic kidney disease patients. Ren. Fail. 2024, 46, 2361802. [Google Scholar] [CrossRef]
- Smith, S.M.; Wallace, E.; O’Dowd, T.; Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst. Rev. 2021, 1, CD006560. [Google Scholar] [CrossRef]
- Wu, X.P.; Liao, E.Y.; Huang, G.; Dai, R.C.; Zhang, H. A comparison study of the reference curves of bone mineral density at different skeletal sites in native Chinese, Japanese, and American Caucasian women. Calcif. Tissue Int. 2003, 73, 122–132. [Google Scholar] [CrossRef]
- Mai, H.T.; Tran, T.S.; Ho-Le, T.P.; Center, J.R.; Eisman, J.A.; Nguyen, T.V. Two-Thirds of All Fractures Are Not Attributable to Osteoporosis and Advancing Age: Implications for Fracture Prevention. J. Clin. Endocrinol. Metab. 2019, 104, 3514–3520. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, C.H.; Kim, H.W.; Park, J.T.; Han, S.H.; Kim, J.; Jeong, J.C.; Kim, Y.; Kim, S.W.; Oh, K.-H.; et al. Kidney function and bone mineral density in chronic kidney disease patients. Clin. Kidney J. 2024, 17, sfae248. [Google Scholar] [CrossRef]
- Baek, J.Y.; Lee, E.; Jung, H.W.; Jang, I.Y. Geriatrics Fact Sheet in Korea 2021. Ann. Geriatr. Med. Res. 2021, 25, 65–71. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, J.K.; Kim, M.J.; Yoo, D.M.; Lee, N.E.; Han, K.M.; Kim, N.Y.; Kang, H.S.; Choi, H.G.; Kim, E.S. Associations between Chronic Kidney Disease and Migraine Incidence: Findings from a Korean Longitudinal Big Data Study. J. Pers. Med. 2024, 14, 356. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.J.; Min, C.; Choi, H.G. Association between benign paroxysmal positional vertigo and osteoporosis: Two nested case-control studies. Osteoporos. Int. 2020, 31, 2017–2024. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, J.H.; Kim, J.H.; Cho, S.J.; Nam, E.S.; Choi, H.G. The Occurrence of Alzheimer’s Disease and Parkinson’s Disease in Individuals with Osteoporosis: A Longitudinal Follow-Up Study Using a National Health Screening Database in Korea. Front. Aging Neurosci. 2021, 13, 786337. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Yang, D.; Dalton, J.E. A unified approach to measuring the effect size between two groups using SAS. In Proceedings of the SAS Global Forum 2012, Orlando, FL, USA, 22–25 April 2012; pp. 335–2012. [Google Scholar]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, L.R.; Chen, K.H. Osteoporosis in Patients with Chronic Kidney Diseases: A Systemic Review. Int. J. Mol. Sci. 2020, 21, 6846. [Google Scholar] [CrossRef]
- Sampson, H.W. Alcohol and other factors affecting osteoporosis risk in women. Alcohol. Res. Health 2002, 26, 292–298. [Google Scholar]
- Stein, M.S.; Packham, D.K.; Ebeling, P.R.; Wark, J.D.; Becker, G.J. Prevalence and risk factors for osteopenia in dialysis patients. Am. J. Kidney Dis. 1996, 28, 515–522. [Google Scholar] [CrossRef]
- Jorgensen, H.S.; Winther, S.; Bottcher, M.; Hauge, E.M.; Rejnmark, L.; Svensson, M.; Ivarsen, P. Bioavailable Testosterone is Positively Associated with Bone Mineral Density in Male Kidney Transplantation Candidates. Kidney Int. Rep. 2018, 3, 661–670. [Google Scholar] [CrossRef]
- Yamada, S.; Tsuruya, K.; Kitazono, T.; Nakano, T. Emerging cross-talks between chronic kidney disease-mineral and bone disorder (CKD-MBD) and malnutrition-inflammation complex syndrome (MICS) in patients receiving dialysis. Clin. Exp. Nephrol. 2022, 26, 613–629. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Kim, D.-W.; Park, E.; Kim, J.; Lee, C.-G.; Jin, H.-S.; Jeong, S.-Y. Identification of novel susceptibility genes associated with bone density and osteoporosis in Korean women. J. Genet. Med. 2022, 19, 63–75. [Google Scholar] [CrossRef]
- Choi, Y.M.; Jun, J.K.; Choe, J.; Hwang, D.; Park, S.H.; Ku, S.Y.; Kang, D.; Kim, J.G.; Moon, S.Y.; Lee, J.Y. Association of the vitamin D receptor start codon polymorphism (FokI) with bone mineral density in postmenopausal Korean women. J. Hum. Genet. 2000, 45, 280–283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).