Early-Onset Versus Late-Onset Preeclampsia in Bogotá, Colombia: Differential Risk Factor Identification and Evaluation Using Traditional Statistics and Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Variable Definitions and Main Outcomes
2.3. Traditional Statistical Analysis
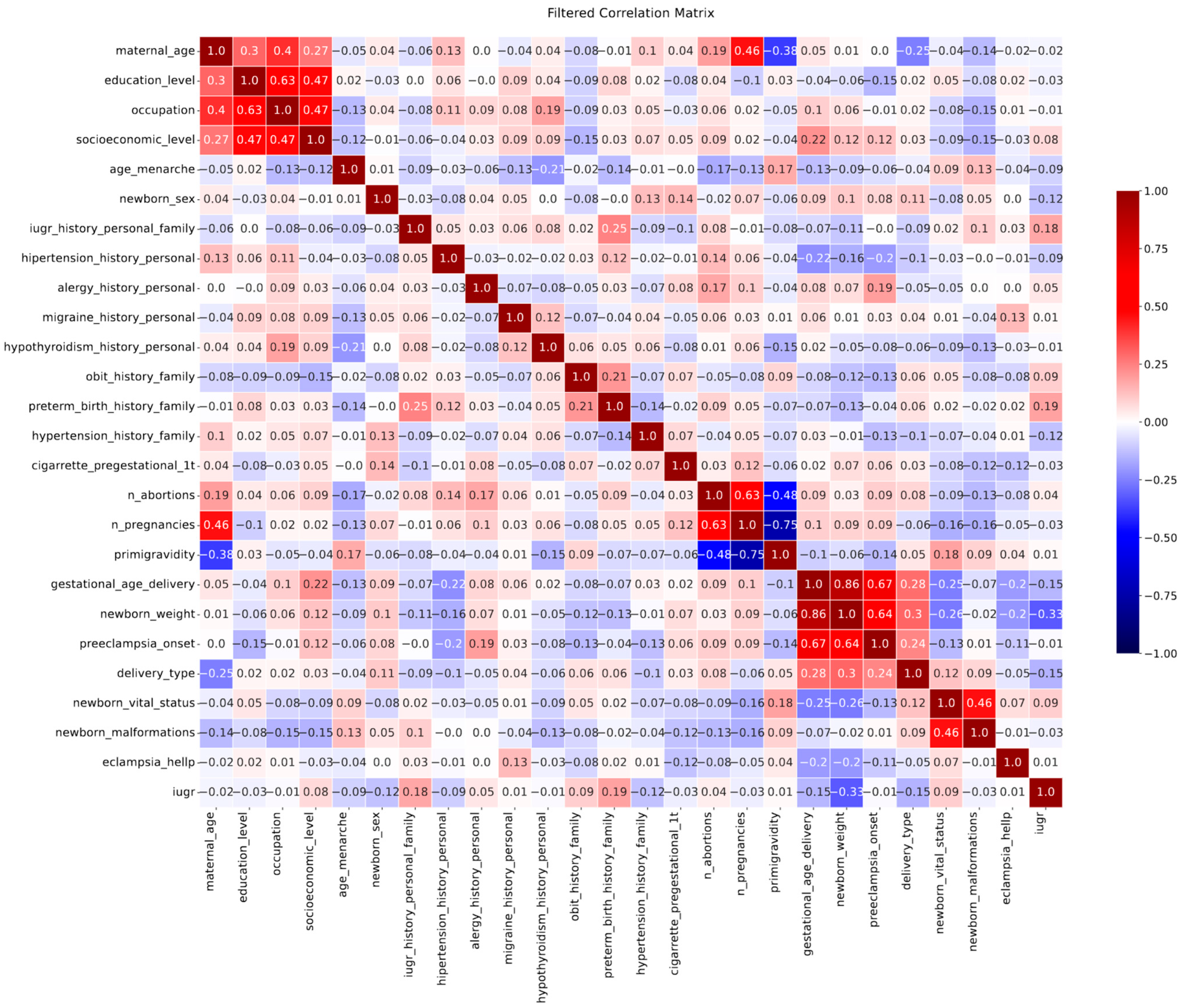
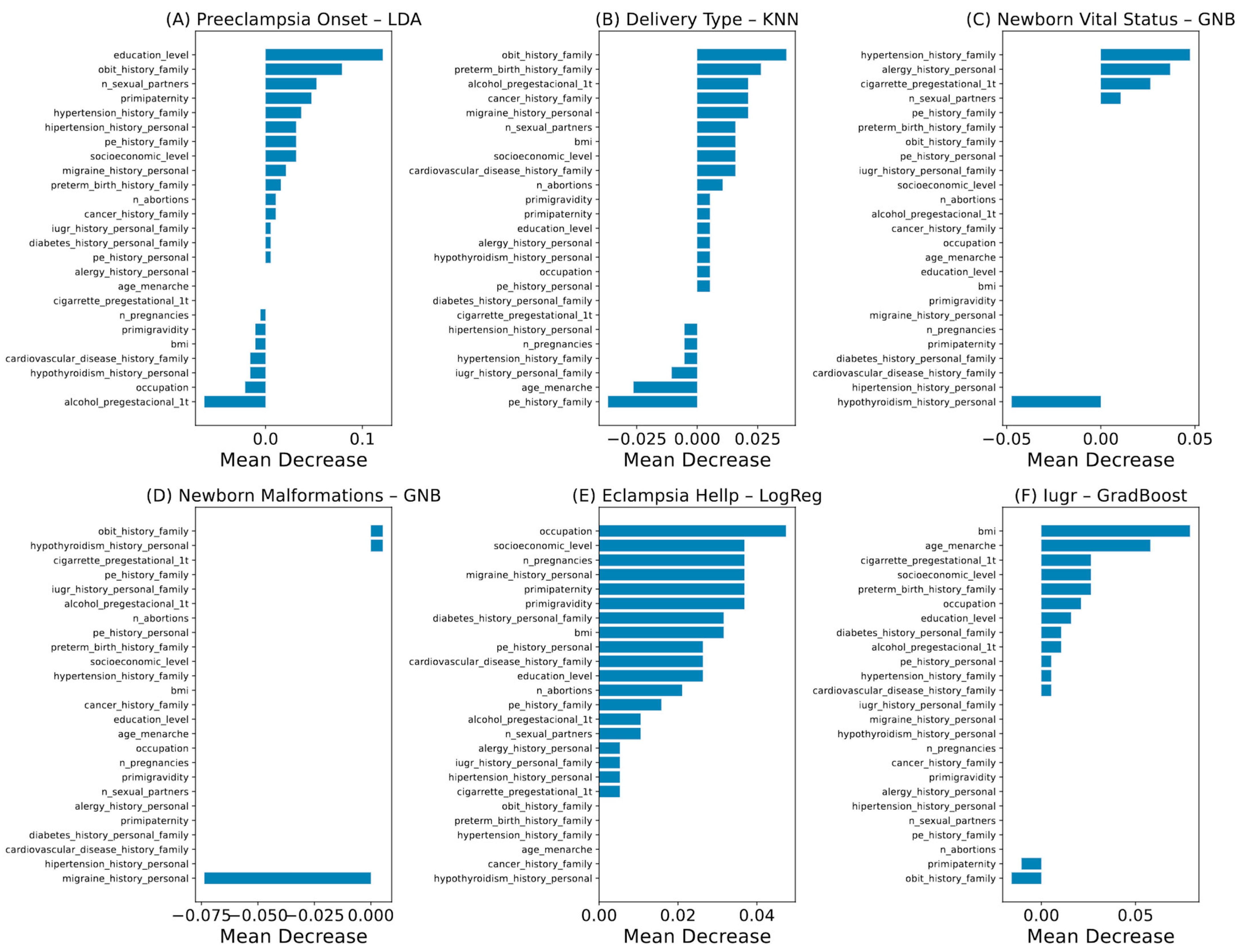
2.4. Machine Learning Analysis
3. Results
3.1. Population Characterization by Univariate Analysis
3.2. Outcome Prediction by Machine Learning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 202. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of Preeclampsia: Risk Factors and Outcomes Associated with Early-versus Late-Onset Disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Protocolo de Vigilancia En Salud Pública Morbilidad Materna Extrema; Instituto Nacional de Salud: Bogota, Colombia, 2024.
- Garcia Bedoya, A.M. Informe de Evento Mortalidad Materna; Instituto Nacional de Salud: Bogota, Colombia, 2024. [Google Scholar]
- Pinilla Saraza, M.E. Factores Identificados En Las Unidades de Análisis de Los Casos de Mortalidad Materna En Colombia, 2017. Inf. Quinc. Epidemiológico Nac. 2018, 23, 261–273. [Google Scholar] [CrossRef]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Cheng, W. Comparison of Indications of Pregnancy Termination and Prognosis of Mothers and Neonates in Early- and Late-Onset Preeclampsia. Hypertens. Pregnancy 2016, 35, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Iacobelli, S.; Bonsante, F.; Robillard, P.-Y. Comparison of Risk Factors and Perinatal Outcomes in Early Onset and Late Onset Preeclampsia: A Cohort Based Study in Reunion Island. J. Reprod. Immunol. 2017, 123, 12–16. [Google Scholar] [CrossRef]
- Kucukgoz Gulec, U.; Ozgunen, F.T.; Buyukkurt, S.; Guzel, A.B.; Urunsak, I.F.; Demir, S.C.; Evruke, I.C. Comparison of Clinical and Laboratory Findings in Early- and Late-Onset Preeclampsia. J. Matern.-Fetal Neonatal Med. 2013, 26, 1228–1233. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, W.; Zhang, S.; Feng, S.; Feng, F.; Dai, J.; Zhang, X.; Tian, P.; Wang, S.; Zhao, Z.; et al. Non-Invasive Prediction of Preeclampsia Using the Maternal Plasma Cell-Free DNA Profile and Clinical Risk Factors. Front. Med. 2024, 11, 1254467. [Google Scholar] [CrossRef]
- Ali Mahamda, H. Chronic Chlamydia Pneumonia Infection and Risk of Early-Onset Versus Late-Onset Preeclampsia. Arch. Razi Inst. 2022, 77, 1729–1735. [Google Scholar] [CrossRef]
- Procopciuc, L.M.; Nemeti, G.; Buzdugan, E.; Iancu, M.; Stamatian, F.; Caracostea, G. Renin-Angiotensin System Gene Variants and Risk of Early- and Late-Onset Preeclampsia: A Single Center Case-Control Study. Pregnancy Hypertens. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Kang, F.; Li, X.; Du, X.; Yang, Y. Comparison of Characteristics Between Early-Onset and Late-Onset Severe Preeclampsia: A Retrospective Cohort Study from a Tertiary Hospital in China. Reprod. Sci. 2024, 32, 139–149. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q.; Sun, G.; Jia, R.; Yang, L.; Liu, G.; Hao, D.; Zhang, S.; Yang, Y.; Li, X.; et al. Dynamic Gestational Week Prediction Model for Pre-Eclampsia Based on ID3 Algorithm. Front. Physiol. 2022, 13, 1035726. [Google Scholar] [CrossRef]
- Melinte-Popescu, A.S.; Vasilache, I.A.; Socolov, D.; Melinte-Popescu, M. Predictive Performance of Machine Learning-Based Methods for the Prediction of Preeclampsia—A Prospective Study. J. Clin. Med. 2023, 12, 418. [Google Scholar] [CrossRef]
- Vasilache, I.A.; Scripcariu, I.S.; Doroftei, B.; Bernad, R.L.; Cărăuleanu, A.; Socolov, D.; Melinte-Popescu, A.S.; Vicoveanu, P.; Harabor, V.; Mihalceanu, E.; et al. Prediction of Intrauterine Growth Restriction and Preeclampsia Using Machine Learning-Based Algorithms: A Prospective Study. Diagnostics 2024, 14, 453. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhang, X.; Xu, W.; Qiu, Z.; Yan, S.; Zhao, W.; Zhao, Z.; Tian, P.; Zhao, Q.; et al. Predicting Preeclampsia in Early Pregnancy Using Clinical and Laboratory Data via Machine Learning Model. BMC Med. Inform. Decis. Mak. 2025, 25, 178. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.C.; Quintero-Lesmes, D.C.; Becerra-Bayona, S.; Guio, E.; Beltran, M.; Paez, M.C.; Ortiz, R.; Saldarriaga, W.; Diaz, L.A.; Monterrosa, Á.; et al. Association of Pre-Eclampsia Risk with Maternal Levels of Folate, Homocysteine and Vitamin B12 in Colombia: A Case-Control Study. PLoS ONE 2018, 13, e0208137. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.C.; Guío, E.; Quintero-Lesmes, D.C.; Becerra-Bayona, S.; Luna-Gonzalez, M.L.; Herrera, V.M.; Prada, C.E. Vitamin D Deficiency and Pre-Eclampsia in Colombia: PREVitD Study. Pregnancy Hypertens. 2018, 14, 240–244. [Google Scholar] [CrossRef]
- Maldonado Gómez, H.; Sepúlveda, C.E.; Subdirector, R.; Abad, A.V.; Efraín, E.; Delgado, F.; Alberto, J.; López, G.; Lozano, B.G.; Victoria, A.; et al. La Visibilización Estadística de Los Grupos Étnicos Colombianos; Departamento Administrativo Nacional de Estadística: Bogotá, Colombia, 2015. [Google Scholar]
- Michita, R.T.; de Kaminski, V.L.; Chies, J.A.B. Genetic Variants in Preeclampsia: Lessons From Studies in Latin-American Populations. Front. Physiol. 2018, 9, 415120. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Montoya-Restrepo, N.E.; Correa-Morales, J.C. Curvas de Peso al Nacer. Revista Salud Pública 2007, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vatti, R.R.; Teuber, S.S. Asthma and Pregnancy. Clin. Rev. Allergy Immunol. 2012, 43, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.I.; Kinanti, H.; Ernawati, E.E.; Lestari, P. Maternal and Perinatal Outcomes of Early-Onset and Late-Onset Preeclampsia at a Tertiary Center Hospital. J. South Asian Fed. Obstet. Gynaecol. 2021, 13, 338–342. [Google Scholar] [CrossRef]
- Zheng, D.; Hao, X.; Khan, M.; Wang, L.; Li, F.; Xiang, N.; Kang, F.; Hamalainen, T.; Cong, F.; Song, K.; et al. Comparison of Machine Learning and Logistic Regression as Predictive Models for Adverse Maternal and Neonatal Outcomes of Preeclampsia: A Retrospective Study. Front. Cardiovasc. Med. 2022, 9, 959649. [Google Scholar] [CrossRef]
- Jhee, J.H.; Lee, S.; Park, Y.; Lee, S.E.; Kim, Y.A.; Kang, S.-W.; Kwon, J.-Y.; Park, J.T. Prediction Model Development of Late-Onset Preeclampsia Using Machine Learning-Based Methods. PLoS ONE 2019, 14, e0221202. [Google Scholar] [CrossRef]
- Albu, A.R.; Anca, A.F.; Horhoianu, V.V.; Horhoianu, I.A. Predictive Factors for Intrauterine Growth Restriction. J. Med. Life 2014, 7, 165. [Google Scholar]
- Yang, L.; Feng, L.; Huang, L.; Li, X.; Qiu, W.; Yang, K.; Qiu, J.; Li, H. Maternal Factors for Intrauterine Growth Retardation: Systematic Review and Meta-Analysis of Observational Studies. Reprod. Sci. 2023, 30, 1737–1745. [Google Scholar] [CrossRef]
- Reshetnikov, E.; Churnosova, M.; Reshetnikova, Y.; Stepanov, V.; Bocharova, A.; Serebrova, V.; Trifonova, E.; Ponomarenko, I.; Sorokina, I.; Efremova, O.; et al. Maternal Age at Menarche Genes Determines Fetal Growth Restriction Risk. Int. J. Mol. Sci. 2024, 25, 2647. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bhuiyan, A.; Iqbal, J.; Islam, A.; Taslima, A.; Maher, A.; Karim, M.; Islam, Z.; Faruk, A.; Sihan, N. Association between Prenatal Maternal Tobacco Use and Intrauterine Growth Restriction: A Case-Control Study. Sch J App Med Sci 2022, 10. [Google Scholar] [CrossRef]
- Delcroix-Gomez, C.; Delcroix, M.H.; Jamee, A.; Gauthier, T.; Marquet, P.; Aubard, Y. Fetal Growth Restriction, Low Birth Weight, and Preterm Birth: Effects of Active or Passive Smoking Evaluated by Maternal Expired CO at Delivery, Impacts of Cessation at Different Trimesters. Tob. Induc. Dis. 2022, 20, 70. [Google Scholar] [CrossRef]
- Sadovsky, A.D.I.; Matijasevich, A.; Santos, I.S.; Barros, F.C.; Miranda, A.E.; Silveira, M.F. LBW and IUGR Temporal Trend in 4 Population-Based Birth Cohorts: The Role of Economic Inequality. BMC Pediatr. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Muglia, L.J.; Katz, M. The Enigma of Spontaneous Preterm Birth. N. Engl. J. Med. 2010, 362, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, P.C.F.M.; Leão, M.D.; Queiroz, J.W.; Melo, E.M.D.; Pereira, F.V.M.; Nóbrega, M.H.; Jeronimo, A.K.; Ferreira, L.C.; Jerônimo, S.M.B.; De Araújo, A.C.P.F. Family History of Hypertension as an Important Risk Factor for the Development of Severe Preeclampsia. Acta Obstet. Gynecol. Scand. 2010, 89, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Yokomichi, H.; Akiyama, Y.; Ooka, T.; Miyake, K.; Horiuchi, S.; Shinohara, R.; Yamagata, Z.; Kamijima, M.; Yamazaki, S.; et al. Association between Preterm Birth and Maternal Allergy Considering IgE Level. Pediatr. Int. 2021, 63, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Källén, B.; Norstedt Wikner, B. Maternal Hypothyroidism in Early Pregnancy and Infant Structural Congenital Malformations. J. Thyroid. Res. 2014, 2014, 160780. [Google Scholar] [CrossRef]
- Dolan, S.M.; Moore, C. Linking Family History in Obstetric and Pediatric Care: Assessing Risk for Genetic Disease and Birth Defects. Pediatrics 2007, 120, S66–S70. [Google Scholar] [CrossRef]
- Luo, Z.C.; An, N.; Xu, H.R.; Larante, A.; Audibert, F.; Fraser, W.D. The Effects and Mechanisms of Primiparity on the Risk of Pre-Eclampsia: A Systematic Review. Paediatr. Perinat. Epidemiol. 2007, 21, 36–45. [Google Scholar] [CrossRef]

| Risk Factor or Secondary Outcome | Unit | EOP (n = 80) | LOP (n = 110) | p Value | All (n = 190) | |
|---|---|---|---|---|---|---|
| Maternal age | years | 27.50 (24–33) | 28 (23–34.25) | 0.8712 | NS | 28 (23–34) |
| Marital status | % | 0.6876 | NS | |||
| Single, widowed, divorced, or separated | 17.5 (14/80) | 14.55 (16/110) | 15.79 (30/190) | |||
| Married or cohabitating with the infant’s father | 82.5 (66/80) | 85.45 (94/110) | 84.21 (160/190) | |||
| Education level | % | 0.0420 | * | |||
| None | 0.00 (0/80) | 0.00 (0/110) | 0 (0/190) | |||
| Incomplete primary education | 0.00 (0/80) | 2.73 (3/110) | 1.579 (3/190) | |||
| Complete primary education | 1.25 (1/80) | 3.64 (4/110) | 2.632 (5/190) | |||
| Incomplete secondary education | 8.75 (7/80) | 8.18 (9/110) | 8.421 (16/190) | |||
| Complete secondary education | 53.75 (43/80) | 62.73 (69/110) | 58.947 (112/190) | |||
| Incomplete university studies | 8.75 (7/80) | 3.64 (4/110) | 5.789 (11/190) | |||
| Complete university studies | 25.00 (20/80) | 16.36 (18/110) | 20 (38/190) | |||
| Postgraduate | 2.50 (2/80) | 2.72 (3/110) | 2.632 (5/190) | |||
| Age at menarche | years | 13 (12–14) | 13 (12–14) | 0.5277 | NS | 13 (12–14) |
| Sex of newborn | % | 0.3032 | NS | |||
| Male | 57.5 (46/80) | 49.1 (54/110) | 52.632 (100/190) | |||
| Female | 42.5 (34/80) | 50.9 (56/110) | 47.368 (90/190) | |||
| Occupation | % | 0.5194 | NS | |||
| Housewife | 28.75 (23/80) | 29.09 (32/110) | 28.947 (55/190) | |||
| Student | 7.5 (6/80) | 4.54 (5/110) | 5.789 (11/190) | |||
| Non-qualified technician | 8.75 (7/80) | 16.36 (18/110) | 13.158 (25/190) | |||
| Qualified technician | 32.5 (26/80) | 26.36 (29/110) | 28.947 (55/190) | |||
| Independent | 2.5 (2/80) | 5.45 (6/110) | 4.211 (8/190) | |||
| Executive professional | 20 (16/80) | 19.18 (20/110) | 18.947 (36/190) | |||
| BMI | Kg/m2 | 25.18 (22.50–27.89) | 24.32 (22.63–27.52) | 0.5375 | NS | 24.45 (22.63–27.66) |
| Personal PE background | % | 10.0 (8/80) | 10.0 (11/110) | >0.999 | NS | 10 (19/190) |
| Family history of PE | % | 31.25 (25/80) | 20.91 (23/110) | 0.1284 | NS | 25.263 (48/190) |
| Personal or family history of IUGR | % | 7.50 (6/80) | 7.27 (8/110) | >0.999 | NS | 7.368 (14/190) |
| Personal history of chronic hypertension | % | 23.75 (19/80) | 9.09 (10/110) | 0.0075 | ** | 15.263 (29/190) |
| Personal history of allergy | % | 0 (0/80) | 8.18 (9/110) | 0.0110 | * | 4.737 (9/190) |
| Personal history of migraine | % | 7.5 (6/80) | 9.09 (10/110) | 0.7951 | NS | 8.421 (16/190) |
| Personal history of hypothyroidism | % | 15 (12/80) | 10 (11/110) | 0.3688 | NS | 12.105 (23/190) |
| Family history of cardiovascular disease | % | 17.5 (14/80) | 19.09 (21/110) | 0.8509 | NS | 18.421 (35/190) |
| Family history of spontaneous abortion | % | 12.5 (10/80) | 14.55 (16/110) | 0.8313 | NS | 13.684 (26/190) |
| Family history of obit or perinatal death | % | 8.75 (7/80) | 2.73 (3/110) | 0.0983 | NS | 5.263 (10/190) |
| Family history of preterm birth | % | 18.75 (15/80) | 15.45 (17/110) | 0.5618 | NS | 16.842 (32/190) |
| Personal and family history of diabetes | % | 36.25 (29/80) | 32.73 (36/110) | 0.6443 | NS | 34.211 (65/190) |
| Family history of cancer | % | 12.5 (10/80) | 20 (22/110) | 0.2386 | NS | 16.842 (32/190) |
| Family history of hypertension | % | 12.5 (10/80) | 5.45 (6/110) | 0.1124 | NS | 8.421 (16/190) |
| Pre-pregnancy and first trimester cigarette exposure | % | 8.75 (7/80) | 12.73 (14/110) | 0.4849 | NS | 11.053 (21/190) |
| Pre-pregnancy and first trimester alcohol consumption | % | 17.50 (14/80) | 14.55 (16/110) | 0.6876 | NS | 15.789 (30/190) |
| Primigravidity | % | 50 (40/80) | 36.36 (40/110) | 0.0743 | NS | 42.105 (80/190) |
| Primipaternity | % | 65 (52/80) | 53.64 (59/110) | 0.1368 | NS | 58.421 (11/190) |
| Number of abortions | 0 (0–0) | 0 (0–1) | 0.1332 | NS | 0 (0–1) | |
| Number of pregnancies | 1.5 (1–2) | 2 (1–2.25) | 0.0904 | NS | 2 (1–2) | |
| Number of sexual partners | 1 (1–1) | 1 (1–1) | 0.1928 | NS | 1(1–1) | |
| Socioeconomic status | % | 0.0986 | NS | |||
| 1 (lowest) | 21.25 (17/80) | 11.82 (13/110) | 15.789 (30/190) | |||
| 2 | 41.25 (33/80) | 45.45 (50/110) | 43.684 (83/190) | |||
| 3 | 33.75 (27/80) | 34.54 (38/110) | 34.211 (65/190) | |||
| 4 | 3.75 (3/80) | 7.27 (8/110) | 5.789 (11/190) | |||
| 5 (highest) | 0.00 (0/80) | 0.91 (1/110) | 0.526 (1/190) | |||
| Time in relationship with the infant’s father | Months | 36 (18–93) | 36 (14.5–108) | 0.6951 | NS | 36 (18–96) |
| Gestational age at delivery | Weeks | 31 (28.25–35.75) | 37 (36–38.25) | <0.0001 | **** | 36 (33–38) |
| Newborn weight | g | 1418 (995–1974) | 2668 (2259–3056) | <0.0001 | **** | 2285 (1575–2880) |
| Vital status of the newborn | % | 0.0983 | NS | |||
| Alive | 91.25 (73/80) | 97.27 (107/110) | 94.736 (180/190) | |||
| Death | 8.75 (7/80) | 2.72 (3/110) | 5.263 (10/190) | |||
| Type of delivery | % | 0.0009 | *** | |||
| C-section | 91.25 (73/80) | 71.81 (79/110) | 80 (152/190) | |||
| Eutocic delivery | 8.75 (7/80) | 28.18 (31/110) | 20 (38/190) | |||
| Malformations of the newborn | % | 10 (8/80) | 10.90 (12/110) | >0.9999 | NS | 10.526 (20/190) |
| IUGR | % | 23.75 (19/80) | 22.72 (25/110) | 0.8638 | NS | 23.157 (44/190) |
| Eclampsia or HELLP | % | 15 (12/80) | 8.18 (9/110) | 0.1631 | NS | 11.052 (21/190) |
| Risk Factor | Unit | Live Newborn (n = 180) | Stillbirth (n = 10) | p-Value | |
|---|---|---|---|---|---|
| Number of pregnancies | 2 (1–2) | 1 (1–1250) | 0.0127 | * | |
| Primigravidity | % | 40 (72/180) | 80 (8/10) | 0.0187 | * |
| Without malformations (n = 170) | With malformations (n = 20) | ||||
| Maternal age | Years | 28 (24–34) | 25.5 (22.25–28.75) | 0.0491 | * |
| Socioeconomic status | % | 0.0359 | * | ||
| 1 (lowest) | 13.529 (23/170) | 35 (7/20) | |||
| 2 | 44.706 (76/170) | 35 (7/20) | |||
| 3 | 34.706 (59/170) | 30 (6/20) | |||
| 4 | 6.471 (11/170) | 0 (0/20) | |||
| 5 (highest) | 0.588 (1/170) | 0 (0/20) | |||
| Number of pregnancies | 2 (1–2.25) | 1 (1–2) | 0.0461 | * | |
| Without IUGR (n = 146) | With IUGR (n = 44) | ||||
| Personal or family history of IUGR | % | 5 (7/146) | 16 (7/44) | 0.0211 | * |
| Family history of preterm birth | % | 13 (19/146) | 30 (13/44) | 0.0195 | * |
| Cesarean (n = 152) | Vaginal delivery (n = 38) | ||||
| Maternal age | Years | 29 (24–35) | 26 (21–30) | 0.0010 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paola, A.-R.; Daniela, M.; Carlos, F.; Enrique, G.-G.; Eduardo, R.-F.; Nancy, S.-G.; Diego, R.; Manuel, V.; Matias, C.-M.; Yanitza, G.-M.; et al. Early-Onset Versus Late-Onset Preeclampsia in Bogotá, Colombia: Differential Risk Factor Identification and Evaluation Using Traditional Statistics and Machine Learning. Biomedicines 2025, 13, 1958. https://doi.org/10.3390/biomedicines13081958
Paola A-R, Daniela M, Carlos F, Enrique G-G, Eduardo R-F, Nancy S-G, Diego R, Manuel V, Matias C-M, Yanitza G-M, et al. Early-Onset Versus Late-Onset Preeclampsia in Bogotá, Colombia: Differential Risk Factor Identification and Evaluation Using Traditional Statistics and Machine Learning. Biomedicines. 2025; 13(8):1958. https://doi.org/10.3390/biomedicines13081958
Chicago/Turabian StylePaola, Ayala-Ramírez, Mennickent Daniela, Farkas Carlos, Guzmán-Gutiérrez Enrique, Retamal-Fredes Eduardo, Segura-Guzmán Nancy, Roca Diego, Venegas Manuel, Carrillo-Muñoz Matias, Gutierrez-Monsalve Yanitza, and et al. 2025. "Early-Onset Versus Late-Onset Preeclampsia in Bogotá, Colombia: Differential Risk Factor Identification and Evaluation Using Traditional Statistics and Machine Learning" Biomedicines 13, no. 8: 1958. https://doi.org/10.3390/biomedicines13081958
APA StylePaola, A.-R., Daniela, M., Carlos, F., Enrique, G.-G., Eduardo, R.-F., Nancy, S.-G., Diego, R., Manuel, V., Matias, C.-M., Yanitza, G.-M., Doris, S., Catalina, O., Jaime, S., Mercedes, O.-C., & Reggie, G.-R. (2025). Early-Onset Versus Late-Onset Preeclampsia in Bogotá, Colombia: Differential Risk Factor Identification and Evaluation Using Traditional Statistics and Machine Learning. Biomedicines, 13(8), 1958. https://doi.org/10.3390/biomedicines13081958





