Functional and Emotional Impact of Dry Eye and Meibomian Gland Dysfunction in Keratoconus
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Analysis
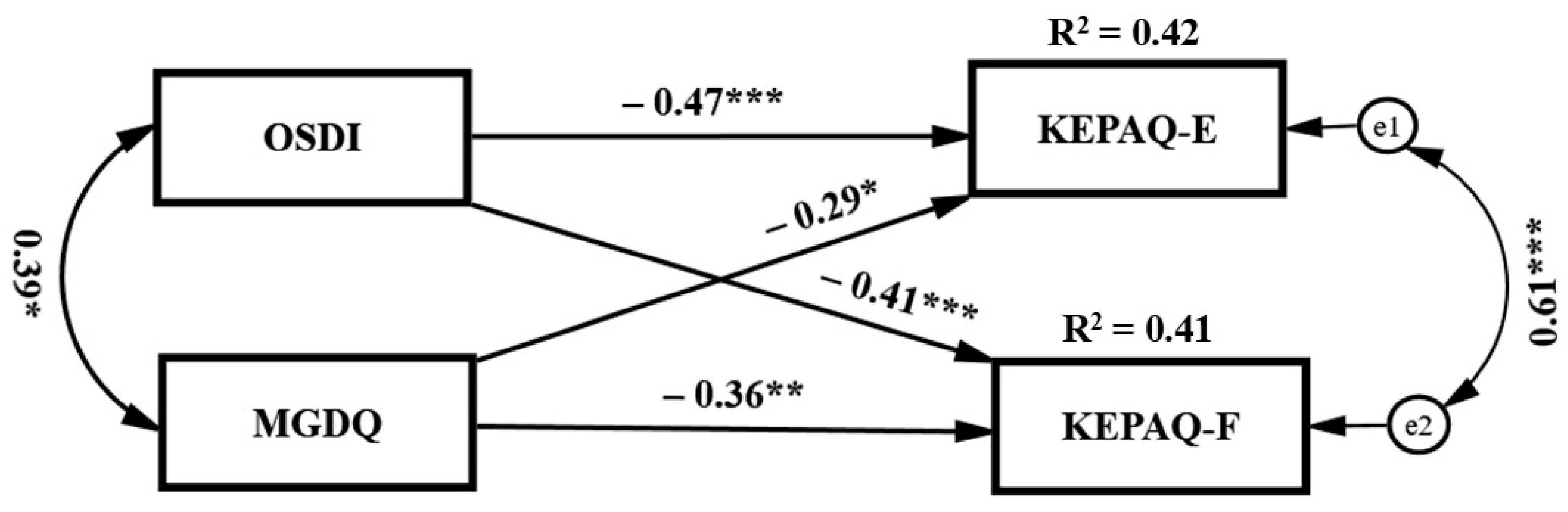
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Outcome Measure | Stage | Dry Eye (N = 22) | Non-Dry Eye (N = 23) | p | MGD (N = 15) | Non-MGD (N = 30) | p | All (N = 45) |
|---|---|---|---|---|---|---|---|---|
| Belin A | 0 | 11 (58%) | 15 (71%) | 0.70 | 5 (42%) | 21 (75%) | 0.09 | 26 (65%) |
| 1 | 1 (5.3%) | 1 (4.8%) | 1 (8.3%) | 1 (3.6%) | 2 (5.0%) | |||
| 2 | 4 (21%) | 3 (14%) | 3 (25%) | 4 (14%) | 7 (18%) | |||
| 3 | 0 (0%) | 1 (4.8%) | 0 (0%) | 1 (3.6%) | 1 (2.5%) | |||
| 4 | 3 (16%) | 1 (4.8%) | 3 (25%) | 1 (3.6%) | 4 (10%) | |||
| Belin B | 0 | 13 (68%) | 13 (62%) | 0.33 | 6 (50%) | 20 (71%) | 0.10 | 26 (65%) |
| 1 | 0 (0%) | 1 (4.8%) | 0 (0%) | 1 (3.6%) | 1 (2.5%) | |||
| 2 | 1 (5.3%) | 5 (24%) | 1 (8.3%) | 5 (18%) | 6 (15%) | |||
| 3 | 2 (11%) | 1 (4.8%) | 2 (17%) | 1 (3.6%) | 3 (7.5%) | |||
| 4 | 3 (16%) | 1 (4.8%) | 3 (25%) | 1 (3.6%) | 4 (10%) | |||
| Belin C | 0 | 10 (53%) | 12 (57%) | 0.69 | 4 (33%) | 18 (64%) | 0.04 | 22 (55%) |
| 1 | 2 (11%) | 4 (19%) | 2 (17%) | 4 (14%) | 6 (15%) | |||
| 2 | 5 (26%) | 3 (14%) | 5 (42%) | 3 (11%) | 8 (20%) | |||
| 3 | 1 (5.3%) | 2 (9.5%) | 0 (0%) | 3 (11%) | 3 (7.5%) | |||
| 4 | 1 (5.3%) | 10 (45%) | 1 (8.3%) | 0 (0%) | 1 (2.5%) | |||
| Belin D | 0 | 4 (18%) | 9 (41%) | 0.06 | 1 (6.7%) | 13 (45%) | 0.01 | 14 (32%) |
| 1 | 9 (41%) | 2 (9.1%) | 6 (40%) | 12 (41%) | 18 (41%) | |||
| 2 | 6 (27%) | 1 (4.5%) | 5 (33%) | 3 (10%) | 8 (18%) | |||
| 3 | 3 (14%) | 10 (45%) | 3 (20%) | 1 (3.4%) | 4 (9.1%) | |||
| 4 | 0 (0.0%) | 0 (0.0%) | 0 (0%) | 0 (0.0%) | 0 (0.0%) |
References
- Wolffsohn, J.S.; Benítez-Del-Castillo, J.; Loya-Garcia, D.; Inomata, T.; Iyar, G.; Liang, L.; Pult, H.; Sabater, A.L.; Starr, C.E.; Vehof, J.; et al. TFOS DEWS III Diagnostic Methodology. Am. J. Ophthalmol. 2025, in press. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- O’nEil, E.C.; Henderson, M.; Massaro-Giordano, M.; Bunya, V.Y. Advances in dry eye disease treatment. Curr. Opin. Ophthalmol. 2019, 30, 166–178. [Google Scholar] [CrossRef]
- Ngo, W.; Srinivasan, S.; Schulze, M.; Jones, L. Repeatability of grading meibomian gland dropout using two infrared systems. Optom. Vis. Sci. 2014, 91, 658–667. [Google Scholar] [CrossRef]
- Nelson, J.D.; Shimazaki, J.; Benitez-Del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Opthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef]
- Farrand, K.F.; Fridman, M.; Stillman, I.Ö.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef]
- Pflugfelder, S.C. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am. J. Manag. Care 2008, 14, S102–S106. [Google Scholar]
- Patel, V.; Watanabe, J.; Strauss, J.; Dubey, A.T. Work productivity loss in patients with dry eye disease: An online survey. Curr. Med Res. Opin. 2011, 27, 1041–1048. [Google Scholar] [CrossRef]
- Viso, E.; Gude, F.; Rodríguez-Ares, M.T. The association of meibomian gland dysfunction and other common ocular diseases with dry eye: A population-based study in Spain. Cornea 2011, 30, 1–6. [Google Scholar] [CrossRef]
- Mostovoy, D.; Vinker, S.; Mimouni, M.; Goldich, Y.; Levartovsky, S.; Kaiserman, I. The association of keratoconus with blepharitis. Clin. Exp. Optom. 2018, 101, 339–344. [Google Scholar] [CrossRef]
- McMonnies, C.W. Abnormal rubbing and keratectasia. Eye Contact Lens Sci. Clin. Pract. 2007, 33, 265–271. [Google Scholar] [CrossRef]
- E Davidson, A.; Hayes, S.; Hardcastle, A.J.; Tuft, S.J. The pathogenesis of keratoconus. Eye 2014, 28, 189–195. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Gordon-Shaag, A.; Millodot, M.; Ifrah, R.; Shneor, E. Aberrations and topography in normal, keratoconus-suspect, and keratoconic eyes. Optom. Vis. Sci. 2012, 89, 411–418. [Google Scholar] [CrossRef]
- Millodot, M.; Shneor, E.; Albou, S.; Atlani, E.; Gordon-Shaag, A. Prevalence and associated factors of keratoconus in Jerusalem: A cross-sectional study. Ophthalmic Epidemiol. 2011, 18, 91–97. [Google Scholar] [CrossRef]
- Dogru, M.; Karakaya, H.; Özçetin, H.; Ertürk, H.; Yücel, A.; Özmen, A.; Baykara, M.; Tsubota, K. Tear function and ocular surface changes in keratoconus. Ophthalmology 2003, 110, 1110–1118. [Google Scholar] [CrossRef]
- Carracedo, G.; Recchioni, A.; Alejandre-Alba, N.; Martin-Gil, A.; Crooke, A.; Morote, I.J.-A.; Pintor, J. Signs and Symptoms of Dry Eye in Keratoconus Patients: A Pilot Study. Curr. Eye Res. 2015, 40, 1088–1094. [Google Scholar] [CrossRef]
- Balparda, K.; Herrera-Chalarca, T.; Silva-Quintero, L.A.; Torres-Soto, S.A.; Vanegas-Ramirez, C.M. Development and Validation of the “Keratoconus End-Points Assessment Questionnaire” (KEPAQ), a Disease-Specific Instrument for Evaluating Subjective Emotional Distress and Visual Function Through Rasch Analysis. Clin. Ophthalmol. 2020, 14, 1287–1296. [Google Scholar] [CrossRef]
- Kandel, H.; Pesudovs, K.; Watson, S.L. Measurement of Quality of Life in Keratoconus. Cornea 2020, 39, 386–393. [Google Scholar] [CrossRef]
- Moschos, M.M.; Gouliopoulos, N.S.; Kalogeropoulos, C.; Androudi, S.; Kitsos, G.; Ladas, D.; Tsatsos, M.; Chatziralli, I. Psychological Aspects and Depression in Patients with Symptomatic Keratoconus. J. Ophthalmol. 2018, 2018, 7314308. [Google Scholar] [CrossRef]
- Mannis, M.J.; Ling, J.J.; Kyrillos, R.; Barnett, M. Keratoconus and Personality-A Review. Cornea 2018, 37, 400–404. [Google Scholar] [CrossRef]
- Balparda, K.; Herrera-Chalarca, T.; Torres-Soto, S.A.; Silva-Quintero, L.A. Both sub-scales of the “Keratoconus End-Points Assessment Questionnaire” (KEPAQ) are unidimensional and reliable. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2233–2239. [Google Scholar] [CrossRef]
- Braithwaite, T.; Calvert, M.; Gray, A.; Pesudovs, K.; Denniston, A.K. The use of patient-reported outcome research in modern ophthalmology: Impact on clinical trials and routine clinical practice. Patient Relat. Outcome Meas. 2019, 10, 9–24. [Google Scholar] [CrossRef]
- Belin, M.W.; Duncan, J.K. Keratoconus: The ABCD Grading System. Klin. Monatsblatter Augenheilkd. 2016, 233, 701–707. [Google Scholar] [CrossRef]
- Harbiyeli, I.I.; Bozkurt, B.; Erdem, E.; Ozcan, H.G.; Cam, B.; Sertdemir, Y.; Yagmur, M. Associations with meibomian gland loss in soft and rigid contact lens wearers. Contact Lens Anterior Eye 2022, 45, 101400. [Google Scholar] [CrossRef]
- Balparda, K.; Herrera-Chalarca, T.; Silva-Quintero, L.A.; Torres-Soto, S.A.; Segura-Muñoz, L.; Vanegas-Ramírez, C.M. Both Subjective Emotional Distress and Visual Handicap Correlate with Belin ABCD Classification in the Worse Eye as Measured with the “Keratoconus End-Points Assessment Questionnaire” (KEPAQ). Clin. Ophthalmol. 2020, 14, 1839–1845. [Google Scholar] [CrossRef]
- Pult, H.; Riede-Pult, B.H.; Nichols, J.J. Relation between upper and lower lids’ meibomian gland morphology, tear film, and dry eye. Optom. Vis. Sci. 2012, 89, E310–E315. [Google Scholar] [CrossRef]
- Bron, A.J.F.; Evans, V.E.B.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Bron, A.J. The Doyne Lecture: Reflections on the tears. Eye 1997, 11, 583–602. [Google Scholar] [CrossRef]
- Ifrah, R.; Quevedo, L.; Gantz, L. Repeatability and reproducibility of Cobra HD fundus camera meibography in young adults with and without symptoms of dry eye. Ophthalmic Physiol. Opt. 2022, 43, 183–194. [Google Scholar] [CrossRef]
- Pult, H. Relationships Between Meibomian Gland Loss and Age, Sex, and Dry Eye. Eye Contact Lens Sci. Clin. Pract. 2018, 44 (Suppl. S2), S318–S324. [Google Scholar] [CrossRef]
- Machalińska, A.; Zakrzewska, A.; Adamek, B.; Safranow, K.; Wiszniewska, B.; Parafiniuk, M.; Machaliński, B. Comparison of Morphological and Functional Meibomian Gland Characteristics Between Daily Contact Lens Wearers and Nonwearers. Cornea 2015, 34, 1098–1104. [Google Scholar] [CrossRef]
- Shapiro, A.; Merin, S. Schirmer test and break-up time of tear film in normal subjects. Am. J. Ophthalmol. 1979, 88, 752–757. [Google Scholar] [CrossRef]
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Investig. Opthalmology Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef]
- Robin, M.; Liang, H.; Rabut, G.; Augstburger, E.; Baudouin, C.; Labbé, A. The role of meibography in the diagnosis of meibomian gland dysfunction in ocular surface diseases. Transl. Vis. Sci. Technol. 2019, 8, 6. [Google Scholar] [CrossRef]
- Radwan, M.A.; Sherif, O.M.; Othman, T.M. Efficacy of Thermo Mechanical Pulsations in Management of Meibomien Gland Dysfunction: A Comparative Study. Acta Sci. Ophthalmol. 2020, 3, 12–19. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Machalińska, A.; Zakrzewska, A.; Safranow, K.; Wiszniewska, B.; Machaliński, B. Risk Factors and Symptoms of Meibomian Gland Loss in a Healthy Population. J. Ophthalmol. 2016, 2016, 7526120. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Sevim, D.G.; Gumus, K.; Unlu, M. Reliable, Noncontact Imaging Tool for the Evaluation of Meibomian Gland Function: Sirius Meibography. Eye Contact Lens Sci. Clin. Pract. 2020, 46 (Suppl. S2), S135–S140. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Tan, J.C.K.; Nguyen, V.; Fenwick, E.; Ferdi, A.; Dinh, A.; Watson, S.L. Vision-Related Quality of Life in Keratoconus: A Save Sight Keratoconus Registry Study. Cornea 2019, 38, 600–604. [Google Scholar] [CrossRef]
- Duncan, J.K.; Belin, M.W.; Borgstrom, M. Assessing progression of keratoconus: Novel tomographic determinants. Eye Vis. 2016, 3, 6. [Google Scholar] [CrossRef]
- Sahebjada, S.; Fenwick, E.K.; Xie, J.; Snibson, G.R.; Daniell, M.D.; Baird, P.N. Impact of Keratoconus in the Better Eye and the Worse Eye on Vision-Related Quality of Life. Investig. Opthalmology Vis. Sci. 2014, 55, 412–416. [Google Scholar] [CrossRef]
- Arbuckle, J.L. Amos, Version 29.0; Computer Program; IBM Corporation: Armonk, New York, USA, 2023. [Google Scholar]
- Tatematsu-Ogawa, Y.; Yamada, M.; Kawashima, M.; Yamazaki, Y.; Bryce, T.; Tsubota, K. The disease burden of keratoconus in patients’ lives: Comparisons to a Japanese normative sample. Eye Contact Lens. Sci. Clin. Pract. 2008, 34, 13–16. [Google Scholar] [CrossRef]
- (PDF) A Clinically Useful Tool to Determine an Effective Snellen Fraction: Details n.d. Available online: https://www.researchgate.net/publication/235119744_A_Clinically_Useful_Tool_to_Determine_an_Effective_Snellen_Fraction_Details (accessed on 23 June 2025).
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Carrel, H.; Wang, I.-J.; Lin, P.-J.; Hu, F.-R. Effect of tear film break-up on higher order aberrations of the anterior cornea in normal, dry, and post-LASIK eyes. J. Refract. Surg. 2005, 21, S525–S529. [Google Scholar] [CrossRef]
- Golden, M.I.; Meyer, J.J.; Zeppieri, M.; Patel, B.C. Dry Eye Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jones-Jordan, L.A.; Walline, J.J.O.; Sinnott, L.T.; Kymes, S.M.; Zadnik, K.O. Asymmetry in keratoconus and vision-related quality of life. Cornea 2013, 32, 267–272. [Google Scholar] [CrossRef]
- Ifrah, R.; Quevedo, L.; Hazrati, G.; Maman, S.; Mangisto, H.; Shmuel, E.; Gantz, L. Contact lens wear and follow-up and its association with signs and symptoms of meibomian gland dysfunction. Ophthalmic Physiol. Opt. 2024, 44, 153–167. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Inoue, K.; Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008, 115, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Shorter, E.O.; Harthan, J.O.; Nau, A.O.; Fogt, J.O.; Cao, D.; Schornack, M.O.; Nau, C.O. Dry Eye Symptoms in Individuals With Keratoconus Wearing Contact Lenses. Eye Contact Lens Sci. Clin. Pract. 2021, 47, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, L.; Viso, E.; Touriño, R.; Gude, F.; Rodríguez-Ares, M.T. Clinical evaluation of meibomian gland dysfunction in patients with keratoconus. Contact Lens Anterior Eye 2022, 45, 101495. [Google Scholar] [CrossRef]
- Wallace, S.; Edmond, J. In Support of Ophthalmology-Specific Patient-Reported Outcome Measures. Ophthalmology 2019, 126, 690–691. [Google Scholar] [CrossRef]
- Boon, J.; Goh, J.S.; Rojas-Carabali, W.; Puah, M.; Lee, B.; Rajagopalan, R.; Ang, B.; Agrawal, R. Web-based vs. conventional: A comprehensive analysis of visual acuity assessment using the PocDoc tool in a tertiary eye care centre. Eye 2024, 38, 3554–3561. [Google Scholar] [CrossRef] [PubMed]
| Outcome Measure | Dry Eye (N = 22) | Non Dry Eye (N = 23) | p | MGD (N = 15) | Non-MGD (N = 30) | p | All (N = 45) | ||
|---|---|---|---|---|---|---|---|---|---|
| Distance Visual Acuity (Snellen Decimal) | Mean ± SD Range | 0.61 ± 0.35 0.05–1.00 | 0.79 ± 0.27 0.10–1.00 | 0.07 | 0.50 ± 0.34 0.05–1.00 | 0.81 ± 0.26 0.10–1.00 | 0.003 | 0.70 ± 0.32 0.05–1.00 | |
| Median (Q1,Q3) | 0.75 (0.30, 0.90) | 0.85 (0.70, 1.00) | 0.40 (0.20, 0.90) | 0.90 (0.70, 1.00) | 0.80 (0.40, 1.00) | ||||
| Spherical Refraction (D) | Mean ± SD Range | −1.55 ± 3.81 −10.25–6.00 | −1.64 ± 2.91 −7.25–3.75 | 0.93 | −1.57 ± 4.12 −10.25–6.00 | −1.61 ± 2.96 −7.25–3.75 | 0.97 | −1.59 ± 3.34 −10.25–6.00 | |
| Median (Q1,Q3) | −0.75 (−4.25, 1.00) | −1.00 (−3.50, 0.75) | −0.75 (−4.25, 1.25) | −0.88 (−3.50, 0.75) | −0.75 (−4.00, 0.75) | ||||
| Cylindrical Refraction (D) | Mean ± SD Range | −4.36 ± 3.34 −11.75–−0.75 | −3.66 ± 4.65 −18.75–−0.25 | 0.12 | −5.20 ± 3.52 −11.75–−0.75 | −3.41 ± 4.19 −18.75–−0.25 | 0.02 | −4.01 ± 4.03 −18.75–−0.25 | |
| Median (Q1,Q3) | −3.50 (−6.75, −1.75) | −2.00 (−3.00, −1.25) | −4.50 (−7.75, −2.75) | −1.88 (−3.00, −1.25) | −2.50 (−4.75, −1.25) | ||||
| Keratometry (mm) | Mean ± SD Range | 7.56 ± 0.92 6.32–10.89 | 7.52 ± 0.57 6.32–8.47 | 0.32 | 7.56 ± 1.08 6.32–10.89 | 7.53 ± 0.55 6.32–8.55 | 0.29 | 7.54 ± 0.75 6.32–10.89 | |
| Median (Q1,Q3) | 7.44 (7.14, 7.71) | 7.62 (7.26, 7.81) | 7.44 (7.11, 7.71) | 7.58 (7.36, 7.77) | 7.57 (7.26, 7.76) | ||||
| NITBUT (sec) | Mean ± SD Range | 7.70 ± 4.78 1.70–17.00 | 10.84 ± 5.42 2.70–18.00 | 8.09 ± 4.88 1.70–17.00 | 9.91 ± 5.48 2.70–18.00 | 9.30 ± 5.30 1.70–18.00 | |||
| Median (Q1,Q3) | 6.50 (4.20, 9.40) | 9.80 (5.20, 17.00) | 7.00 (4.20, 11.20) | 8.50 (5.20, 17.00) | 7.70 (5.20, 14.30) | ||||
| Schirmer test (mm) | Mean ± SD Range | 13.91 ± 10.54 2.00–35.00 | 11.87 ± 11.68 0.00–35.00 | 0.52 | 13.33 ± 9.48 2.00–30.00 | 12.63 ± 11.92 0.00–35.00 | 0.78 | 12.87 ± 11.06 0.00–35.00 | |
| Median (Q1,Q3) | 13.00 (3.00, 22.00) | 8.00 (4.00, 20.00) | 14.00 (3.00, 22.00) | 8.00 (4.00, 22.00) | 9.00 (4.00, 22.00) | ||||
| MG loss (0–100%) | Upper | Mean ± SD Range | 27.11 ± 19.16 8.33–93.47 | 28.12 ± 13.38 5.50–59.67 | 32.96 ± 20.07 10.23–93.47 | 24.97 ± 13.49 5.50–59.67 | 27.63 ± 16.19 5.50–93.47 | ||
| Median (Q1,Q3) | 23.88 (14.87, 31.75) | 28.55 (18.03, 34.30) | 28.78 (23.27, 34.03) | 24.97 (15.05, 33.60) | 27.07 (16.67, 34.03) | ||||
| Lower | Mean ± SD Range | 30.17 ± 19.44 8.13–70.20 | 26.67 ± 14.73 7.17–65.63 | 37.11 ± 18.93 8.13–70.20 | 23.71 ± 14.24 7.17–65.63 | 28.38 ± 17.07 7.17–70.20 | |||
| Median (Q1,Q3) | 25.57 (13.03, 42.00) | 21.92 (16.33, 39.77) | 29.27 (25.13, 56.33) | 18.38 (13.95, 31.92) | 24.50 (14.67, 39.94) | ||||
| OSDI score (0–100) | Mean ± SD Range | 45.57 ± 23.00 16.67–100.00 | 8.19 ± 14.38 0.00–70.83 | 47.67 ± 23.12 18.75–100.00 | 15.86 ± 21.79 0.00–81.25 | 26.46 ± 26.70 0.00–100.00 | |||
| Median (Q1,Q3) | 45.74 (22.92, 59.37) | 6.25 (0.00, 10.42) | 47.73 (22.92, 60.00) | 8.71 (2.08, 16.67) | 16.67 (6.25, 47.73) | ||||
| MGD symptoms questionnaire (0–11) | Mean ± SD Range | 4.14 ± 2.38 0.00–8.00 | 2.57 ± 1.75 0.00–7.00 | 0.02 | 4.33 ± 2.53 0.00–8.00 | 2.83 ± 1.88 0.00–7.00 | 0.05 | 3.33 ± 2.21 0.00–8.00 | |
| Median (Q1,Q3) | 4.00 (2.00, 6.00) | 2.00 (1.00, 4.00) | 4.00 (2.00, 6.00) | 2.00 (1.00, 4.00) | 3.00 (1.00, 5.00) | ||||
| Outcome Measure | Dry Eye (N = 22) | Non-Dry Eye (N = 23) | p | MGD (N = 15) | Non-MGD (N = 30) | p | All (N = 45) | |
|---|---|---|---|---|---|---|---|---|
| KEPAQ-E | Mean ± SD Range | 1.84 ± 2.72 −5.47–6.40 | 3.82 ± 3.03 −2.64–6.40 | 0.05 | 1.31 ± 2.69 −5.47–4.89 | 3.62 ± 2.91 −2.64–6.40 | 0.03 | 2.85 ± 3.02 −5.47–6.40 |
| Median (Q1,Q3) | 2.22 (0.14, 3.72) | 4.89 (1.26, 6.40) | 2.22 (−0.09, 3.72) | 3.88 (1.26, 6.40) | 2.87 (0.55, 6.40) | |||
| KEPAQ-F | Mean ± SD Range | 0.85 ± 2.60 −2.98–6.97 | 2.58 ± 3.25 −5.43–6.97 | 0.17 | 0.23 ± 2.43 −2.98–5.49 | 2.49 ± 3.07 −5.43–6.97 | 0.09 | 1.74 ± 3.04 −5.43–6.97 |
| Median (Q1,Q3) | −0.02 (−0.58, 2.12) | 2.47 (0.54, 5.49) | −0.52 (−0.97, 2.00) | 2.00 (0.47, 5.49) | 1.21 (−0.40, 4.41) |
| KEPAQ-E R (p) | KEPAQ-F R (p) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Dry Eye (N = 22) | Non-Dry Eye (N = 23) | MGD (N = 15) | Non-MGD (N = 30) | All (N = 45) | Dry Eye (N = 22) | Non-Dry Eye (N = 23) | MGD (N = 15) | Non-MGD (N = 30) | All (N = 45) | |
| A | −0.36 (0.13) | −0.41 (0.07) | 0.26 (0.35) | −0.50 (0.01) | −0.43 (0.006) | −0.29 (0.22) | −0.51 (0.02) | −0.18 (0.52) | −0.50 (0.01) | −0.35 (0.03) | |
| B | −0.48 (0.04) | −0.45 (0.04) | −0.39 (0.16) | −0.34 (0.09) | −0.34 (0.03) | −0.55 (0.01) | −0.49 (0.03) | −0.39 (0.15) | −0.36 (0.08) | −0.32 (0.04) | |
| C | −0.13 (0.60) | −0.45 (0.04) | −0.15 (0.59) | −0.62 (0.001) | −0.47 (0.002) | −0.20 (0.41) | −0.38 (0.09) | −0.15 (0.59) | −0.48 (0.02) | −0.37 (0.02) | |
| D | −0.04 (0.87) | −0.06 (0.79) | −0.14 (0.60) | −0.46 (0.02) | −0.37 (0.01) | −0.05 (0.84) | −0.49 (0.02) | −0.34 (0.19) | −0.51 (0.007) | −0.44 (0.003) | |
| Schirmer test | −0.42 (0.05) | 0.11 (0.63) | −0.05 (0.86) | −0.11 (0.57) | −0.06 (0.70) | −0.17 (0.44) | 0.18 (0.42) | −0.18 (0.50) | −0.05 (0.82) | −0.01 (0.93) | |
| MG loss | Upper | −0.38 (0.10) | −0.26 (0.25) | −0.17 (0.29) | −0.15 (0.52) | −0.08 (0.71) | −0.11 (0.49) | ||||
| Lower | −0.44 (0.04) | −0.10 (0.68) | −0.06 (0.69) | −0.44 (0.04) | 0.10 (0.66) | −0.20 (0.20) | |||||
| Meibum Expressibility | −0.25 (0.27) | −0.33 (0.15) | −0.28 (0.34) | −0.25 (0.21) | −0.24 (0.13) | −0.22 (0.33) | −0.11 (0.65) | −0.42 (0.14) | 0.05 (0.79) | −0.09 (0.57) | |
| Meibum Quality | −0.10 (0.66) | −0.24 (0.30) | −0.11 (0.70) | −0.21 (0.29) | −0.16 (0.32) | −0.03 (0.91) | −0.16 (0.50) | −0.06 (0.85) | −0.07 (0.71) | −0.07 (0.65) | |
| Tear Meniscus Height | 0.31 (0.18) | −0.38 (0.07) | 0.26 (0.37) | −0.24 (0.21) | −0.12 (0.44) | −0.08 (0.72) | −0.25 (0.25) | −0.08 (0.80) | −0.19 (0.31) | −0.18 (0.24) | |
| Corneal Staining | −0.17 (0.46) | 0.11 (0.63) | 0.09 (0.76) | −0.02 (0.92) | −0.09 (0.57) | −0.22 (0.34) | −0.11 (0.60) | −0.17 (0.54) | −0.16 (0.39) | −0.21 (0.17) | |
| Conjunctival Staining | 0.20 (0.37) | 0.00 (1.00) | 0.10 (0.73) | 0.14 (0.45) | 0.10 (0.51) | 0.00 (1.00) | −0.23 (0.28) | −0.006 (0.98) | −0.08 (0.66) | −0.08 (0.62) | |
| MGD questionnaire score | −0.50 (0.02) | −0.32 (0.14) | −0.14 (0.59) | −0.47 (0.01) | −0.44 (0.003) | −0.53 (0.01) | −0.43 (0.04) | −0.22 (0.40) | −0.59 (0.001) | −0.56 (<0.001) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gantz, L.; Besser, A.; Bloom, R.; Ifrah, R. Functional and Emotional Impact of Dry Eye and Meibomian Gland Dysfunction in Keratoconus. Biomedicines 2025, 13, 1918. https://doi.org/10.3390/biomedicines13081918
Gantz L, Besser A, Bloom R, Ifrah R. Functional and Emotional Impact of Dry Eye and Meibomian Gland Dysfunction in Keratoconus. Biomedicines. 2025; 13(8):1918. https://doi.org/10.3390/biomedicines13081918
Chicago/Turabian StyleGantz, Liat, Avi Besser, Rivki Bloom, and Reut Ifrah. 2025. "Functional and Emotional Impact of Dry Eye and Meibomian Gland Dysfunction in Keratoconus" Biomedicines 13, no. 8: 1918. https://doi.org/10.3390/biomedicines13081918
APA StyleGantz, L., Besser, A., Bloom, R., & Ifrah, R. (2025). Functional and Emotional Impact of Dry Eye and Meibomian Gland Dysfunction in Keratoconus. Biomedicines, 13(8), 1918. https://doi.org/10.3390/biomedicines13081918






