Efficacy and Safety of the Combination of Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: A Real-World Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
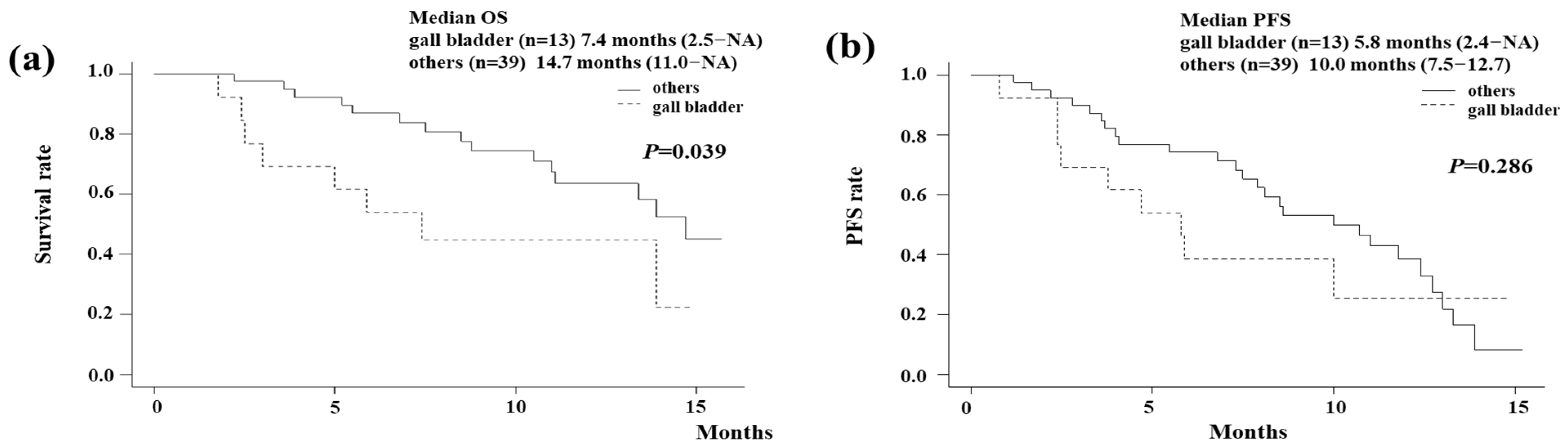
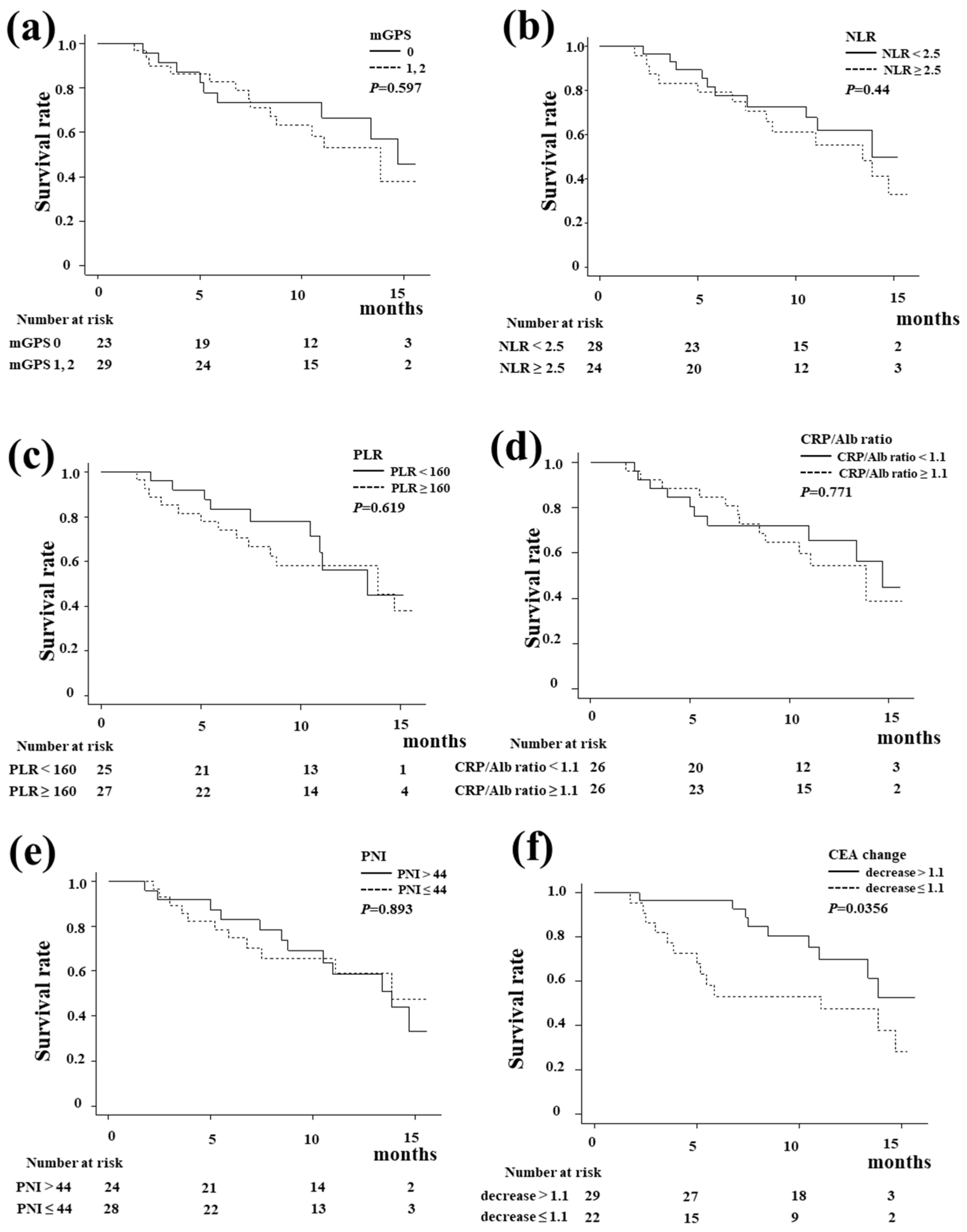
3.1. The OS and PFS
3.2. The ORR and DCR
3.3. AEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD-L1 | programmed death ligand 1 |
| GCD | durvalumab plus gemcitabine and cisplatin |
| mGPS | modified Glasgow Prognostic Score |
| NLR | neutrophil–lymphocyte ratio |
| PLR | platelet–lymphocyte ratio |
| ORR | overall response rate |
| DCR | disease control rate |
| PFS | progression-free survival |
| OS | overall survival |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| AEs | adverse events |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| PNI | prognostic nutritional index |
| BMI | body mass index |
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Lee, W.-S.; Lee, K.-W.; Heo, J.-S.; Kim, S.-J.; Choi, S.-H.; Kim, Y.-I.; Joh, J.-W. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg. Today 2006, 36, 892–897. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Sabbatino, F.; Villani, V.; Yearley, J.H.; Deshpande, V.; Cai, L.; Konstantinidis, I.T.; Moon, C.; Nota, S.; Wang, Y.; Al-Sukaini, A.; et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2016, 22, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. N. Engl. J. Med. Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label single-center phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Plain language summary of the TOPAZ-1 study: Durvalumab and chemotherapy for advanced biliary tract cancer. Futur. Oncol. 2023, 19, 2277–2289. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. NCI Common Terminology Criteria for Adverse Events (CTCAE) ver 5.0. 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 1 June 2024).
- McMillan, D.C.; Crozier, J.E.; Canna, K.; Angerson, W.J.; McArdle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Color. Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef]
- Inoue, Y.; Iwata, T.; Okugawa, Y.; Kawamoto, A.; Hiro, J.; Toiyama, Y.; Tanaka, K.; Uchida, K.; Mohri, Y.; Miki, C.; et al. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology 2013, 84, 100–107. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Ozaka, M.; Verslype, C.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomized double-blind placebo-controlled phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef]
- Smith, R.A.; Bosonnet, L.; Raraty, M.; Sutton, R.; Neoptolemos, J.P.; Campbell, F.; Ghaneh, P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am. J. Surg. 2009, 197, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Haruki, K.; Shiba, H.; Shirai, Y.; Horiuchi, T.; Iwase, R.; Fujiwara, Y.; Furukawa, K.; Misawa, T.; Yanaga, K. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J. Surg. 2016, 40, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, M.; Hanaki, T.; Endo, K.; Suzuki, K.; Nakamura, S.; Sawata, T.; Shimizu, T. C-reactive protein/albumin ratio and prognostic nutritional index are strong prognostic indicators of survival in resected pancreatic ductal adenocarcinoma. J. Pancreat. Cancer 2017, 3, 31–36. [Google Scholar] [CrossRef]
- Hou, S.; Song, D.; Zang, Y.; Hao, R.; Li, L.; Zhu, J. Prognostic relevance of platelet lymphocyte ratio (PLR) in gastric cancer patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1367990. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow. Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelet microparticles and miRNA transfer in cancer progression: Many targets, modes of action, and effects across cancer stages. Front. Cardiovasc. Med. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Petrie, H.T.; Klassen, L.W.; Kay, H.D. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J. Immunol. 1985, 134, 230–234. [Google Scholar] [CrossRef]
- El-Hag, A.; Clark, R.A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987, 139, 2406–2413. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Number (%) or Median (IQR) | |
|---|---|---|
| Patients, n (%) | 52 (100) | |
| Sex, n (%) | Male | 36 (69.2) |
| Female | 16 (30.8) | |
| Age, years (IQR) | 73.0 (67.8–77.3) | |
| BMI, kg/m2 (IQR) | 21.9 (19.7–23.7) | |
| Primary tumor type, n (%) | Intrahepatic cholangiocarcinoma | 7 (13.5) |
| Extrahepatic cholangiocarcinoma (distal/perihilar) | 10 (19.2)/19 (36.5) | |
| Gall bladder | 13 (25.0) | |
| Ampullary carcinoma | 3 (5.8) | |
| Cancer stage, n (%) | Local/locally advanced | 30 (57.7) |
| Metastatic | 22 (42.3) | |
| Performance status | 0/1/2 | 43 (82.7)/7 (13.5)/2 (3.8) |
| Alb, g/dL (IQR) | 3.7 (3.4–4.0) | |
| CRP (IQR) | 0.39 (0.17–2.08) | |
| mGPS (%) | 0/1/2 | 23 (44.2)/18 (34.6)/11 (21.2) |
| NLR (IQR) | 2.4 (1.7–3.9) | |
| PLR (IQR) | 162.5 (118.3–242.8) | |
| CRP–Alb ratio (IQR) | 0.12 (0.04–0.60) | |
| PNI (IQR) | 44.5 (40.3–48.1) | |
| CEA (IQR) | 4.8 (2.6–10.5) | |
| CA19-9 (IQR) | 255.9 (40.8–1014.3) | |
| Line of therapy at which GCD was administered (%) | 1st | 38 (73.1) |
| 2nd | 12 (23.1) | |
| 3rd | 2 (3.8) | |
| First-line cancer therapy (%) | GCD | 38 (73.1) |
| GC | 12 (23.1) | |
| GCS | 1 (1.9) | |
| GEM | 1 (1.9) |
| All Patients (n = 52) | |
|---|---|
| Complete response, n (%) | 2 (3.8) |
| Partial response, n (%) | 11 (21.2) |
| Stable disease, n (%) | 28 (53.8) |
| Progressive disease, n (%) | 10 (19.2) |
| Not evaluable, n (%) | 1 (1.9) |
| Overall response rate (%) | 13/52 (25.0%) |
| Disease control rate (%) | 41/52 (78.8%) |
| Toxicity | All | Grade 3/4 |
|---|---|---|
| Neutropenia | 22 (42.3) | 15 (28.8) |
| Anemia | 13 (25.0) | 3 (5.8) |
| Thrombocytopenia | 7 (13.5) | 0 (0) |
| Appetite loss | 5 (9.6) | 0 (0) |
| General fatigue | 4 (7.7) | 2 (3.8) |
| Dysgeusia | 3 (5.8) | 0 (0) |
| Nausea | 1 (1.9) | 0 (0) |
| Cholangitis | 2 (3.8) | 1 (1.9) * |
| Colitis | 1 (1.9) | 1 (1.9) * |
| Constipation | 2 (3.8) | 0 (0) |
| Abdominal infection | 1 (1.9) | 1 (1.9) |
| Hypothyroidism | 2 (3.8) | 2 (3.8) * |
| Eruption | 2 (3.8) | 0 (0) |
| AST increased | 6 (11.5) | 1 (1.9) |
| ALT increased | 5 (9.6) | 1 (1.9) |
| Creatinine increased | 2 (3.8) | 0 (0) |
| Hypersensitivity for cisplatin | 2 (3.8) | 1 (1.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurihara, E.; Kakizaki, S.; Ijima, M.; Hatanaka, T.; Kubo, N.; Suzuki, Y.; Yasuoka, H.; Hoshino, T.; Naganuma, A.; Tani, N.; et al. Efficacy and Safety of the Combination of Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: A Real-World Retrospective Cohort Study. Biomedicines 2025, 13, 1915. https://doi.org/10.3390/biomedicines13081915
Kurihara E, Kakizaki S, Ijima M, Hatanaka T, Kubo N, Suzuki Y, Yasuoka H, Hoshino T, Naganuma A, Tani N, et al. Efficacy and Safety of the Combination of Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: A Real-World Retrospective Cohort Study. Biomedicines. 2025; 13(8):1915. https://doi.org/10.3390/biomedicines13081915
Chicago/Turabian StyleKurihara, Eishin, Satoru Kakizaki, Masashi Ijima, Takeshi Hatanaka, Norio Kubo, Yuhei Suzuki, Hidetoshi Yasuoka, Takashi Hoshino, Atsushi Naganuma, Noriyuki Tani, and et al. 2025. "Efficacy and Safety of the Combination of Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: A Real-World Retrospective Cohort Study" Biomedicines 13, no. 8: 1915. https://doi.org/10.3390/biomedicines13081915
APA StyleKurihara, E., Kakizaki, S., Ijima, M., Hatanaka, T., Kubo, N., Suzuki, Y., Yasuoka, H., Hoshino, T., Naganuma, A., Tani, N., Yamazaki, Y., & Uraoka, T. (2025). Efficacy and Safety of the Combination of Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: A Real-World Retrospective Cohort Study. Biomedicines, 13(8), 1915. https://doi.org/10.3390/biomedicines13081915







