Research Strategies and Methods of Hydrogels for Antitumor Drug Delivery
Abstract
1. Introduction
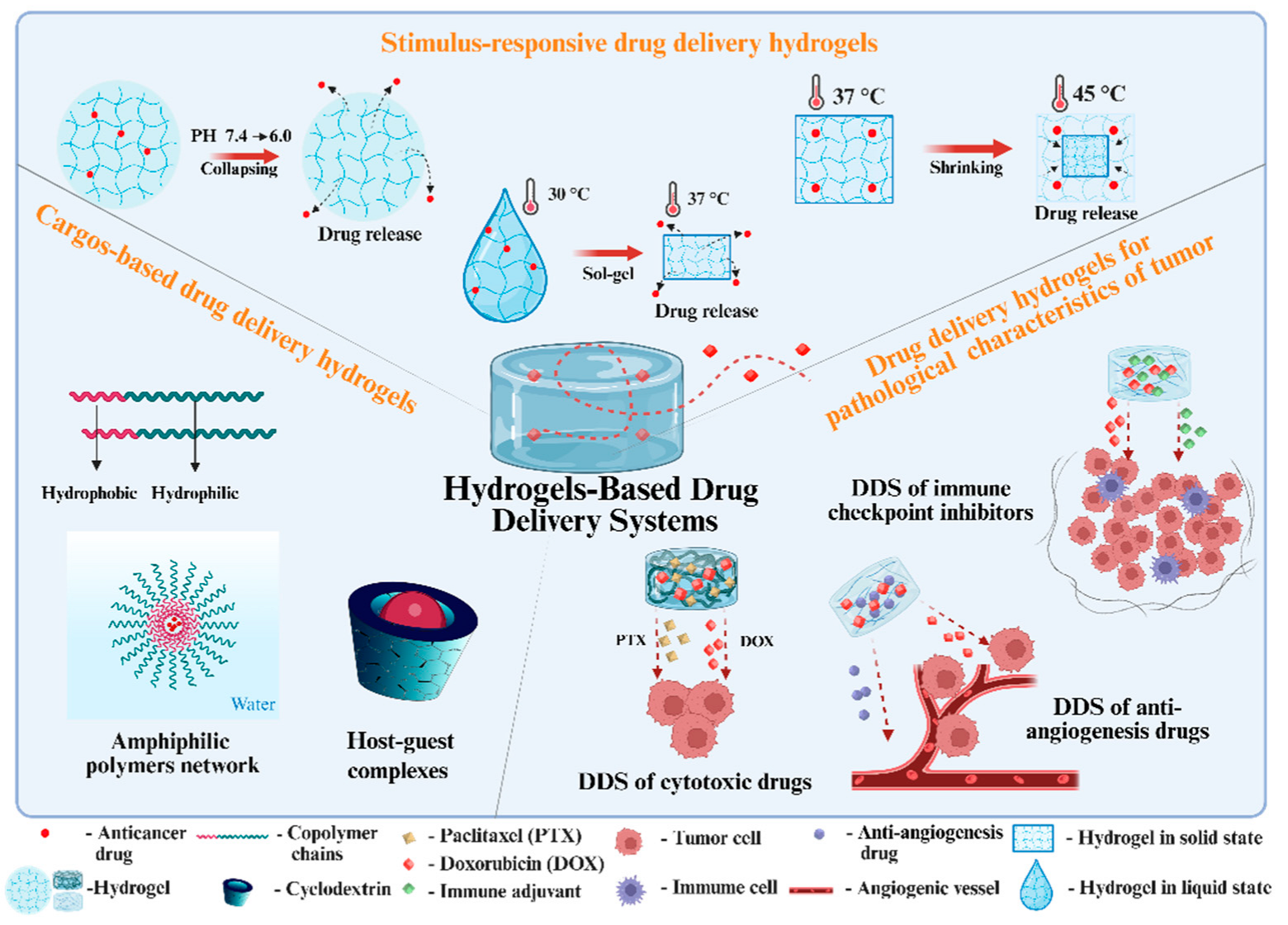
2. Design of Hydrogel-Based DDSs
| Polymers | Loading Drugs | Preparing Method | Degradation and Stability | Sterilization Method | In Vitro/In Vivo Analysis | Biocompatibility | Reference | |
|---|---|---|---|---|---|---|---|---|
| Naturally derived polymers | Gelatin and alginate | Doxorubicin (DOX) | Chemically crosslinking by “Shift-Base” condensation reaction | / | No description while Schiff base has part of antibacterial effect | In vitro | Good biocompatibility (less than 10% decrease in cell viability of no-drug-loading hydrogel) | [30] |
| Bisphosphonate-functionalized hyaluronic acid | DOX | Chemical interaction of bisphosphonate–zinc | 60% mass decrease in drug-loading hydrogel after 316 h in medium | / | In vitro and in vivo | Good biocompatibility (less than 5% decrease in cell viability of no-drug-loading hydrogel) | [31] | |
| Alginate sodium carboxymethyl cellulose | Methotrexate (MTX) and aspirin (AP) | Physical crosslinking | 42% mass decrease after 8 days in PBS | / | In vitro | Good biocompatibility (less than 5% decrease in cell viability of no-drug-loading hydrogel) | [32] | |
| Alginate and magnetic hydroxyapatite | 5-fluorouracil (5-FU) | Physical crosslinking | Increasing light and temperature reduces the storage stability | Filtration | In vitro | Good biocompatibility (less than 15% decrease in cell viability of no-drug-loading hydrogel) | [33] | |
| Synthetic polymers | Poly (D, L-lactide-co-glycolide)-poly(ethylene-glycol)-poly (D, L-lactide-co-glycolide), beta-cyclodextrin | DOX and curcumin | Physical crosslinking | 13.3% mass decrease after 24 h in PBS | / | In vitro and in vivo | Good biocompatibility (less than 10% decrease in cell viability of no-drug-loading hydrogel) | [34] |
| Methacrylic acid (MAA) | Camptothecin (CPT) | Chemically crosslinking through disulfide linkage between CPT and MAA | Stable in normal physiological environment; quickly shrinks to a smaller volume in low pH environment of tumor tissue or tumor cells | / | In vitro and in vivo | Good biocompatibility (less than 20% decrease in cell viability of no-drug-loading hydrogel) | [35] | |
| Polyethylene glycol (PEG) | DOX and curcumin | Chemically crosslinking | Instability | / | In vitro | Good biocompatibility (less than 10% decrease in cell viability of no-drug-loading hydrogel) | [36] | |
| Polyacrylamide (PAM) and carbon nanotube | DOX | Physically crosslinking by hydrogen bond | Stable in PBS after 80 h | / | In vitro | Good biocompatibility (less than 20% decrease in cell viability of no-drug-loading hydrogel) | [37] | |
| Combination and/or modified polymers | Chitosan, polypyrrole | DOX | Chemically crosslinking by “Shift-Base” condensation reaction | Stable in PBS (both pH 7.4 and 6.5) after 7 days | / | In vitro and in vivo | Good biocompatibility (less than 3% decrease in cell viability of no-drug-loading hydrogel) | [38] |
| PEG-modified bovine serum albumin | Paclitaxel (PTX) | Physical crosslinking of PEG-BSA | 46% mass loss in PBS after 50 days. Degraded completely within 200 days in vivo | / | In vitro and in vivo | No toxicity of hydrogel | [39] | |
| Carboxymethyl arabinoxylan | 5-FU | Physically crosslinking | 5 to 15% mass decrease after 7 days in PBS, 37 °C | The nanocomposite hydrogel has antibacterial activity | In vitro | Degradation leads to cell death | [40] | |
| Carboxymethyl chitosan and polyvinyl alcohol | Oxaliplatin | Chemically crosslinking by free-radical polymerization | / | / | In vitro and in vivo | Good biocompatibility by acute oral toxicity assay | [41] | |
| Ultrasmall peptide | DOX | Dimerization of the PyKC peptide | High stability after 18 days in vivo | / | In vitro and in vivo | Good biocompatibility (less than 5% decrease in cell viability of no-drug-loading hydrogel) | [42] | |
| Thiol-modified hyaluronic acid (HASH) and vinyl sulfone-modified β-cyclodextrin | DOX | Chemically crosslinking of a “click reaction” between thiol and vinyl sulfone groups | Keep stable after 15 days in PBS while degraded after 5 days in enzymatic conditions | / | In vitro | Good biocompatibility | [43] | |
| N-isopropylacrylamide (NIPAAm) and maleic anhydride (MA) copolymer (poly (NIPAAm-co-MA)) chitosan | MTX and curcumin | Physical crosslinking | Over 80% weight loss after 28 days in PBS | / | In vitro | Good biocompatibility (less than 10% decrease in cell viability of no-drug-loading hydrogel) | [44] | |
2.1. Stimulus-Responsive Drug Delivery Hydrogels
| Loading Drugs | Polymers | DDS Mechanism | Drug Release pH | Degradation and Stability | Sterilization Method | In Vitro/In Vivo Analysis | Reference |
|---|---|---|---|---|---|---|---|
| DOX | Nitrogen-doped carbon quantum dots and hydroxyapatite | Disintegration of the Schiff base bond and dissolution of hydroxyapatite in acidic conditions | 6.5–4.0 | / | The introduction of HA by either chemical bonding or physical incorporation can result in hydrogels with enhanced antimicrobial activity | In vitro | [56] |
| DOX | N-carboxyethyl chitosan (CEC) and Di benzaldehyde-terminated poly (ethylene glycol) (PEGDA) | Disintegration of the Schiff base bond | 6.8–4.0 | Around 30% mass loss in PBS 7.4 and 50% mass loss in PBS 5.5 after 200 h | / | In vitro | [51] |
| DOX | Sericin, rice bran albumin, and gellan gum | Degradation in the ester bond and increase in the swelling ratio of the hydrogel | 5.0–4.0 | / | / | In vitro | [53] |
| Prospidine | Dextran phosphate | Diffusion of the cytostatic bond and the destruction of the polymer | 4.0–1.2 | 100% mass loss after 30 days in PBS | / | In vitro and in vivo | [54] |
| DOX | Glycol chitosan–Pluronic F127 and α-CD | Dissociation of the hydrogel | 5.0 | 100% mass loss after 220 to 260 h in PBS | / | In vitro and in vivo | [55] |
| Bortezomib | A ‘ABA’ triblock copolymer of phenylboronic acid-functionalized polycarbonate/poly (ethylene glycol) | Dissociations of boronated ester bond | 5.8 | At pH 7.4, the BTZ release from the micelle/hydrogel composite remained low at 7%, an acidic environment, ∼85% of BTZ was released over 9 days | / | In vitro and in vivo | [57] |
| DOX | Dextran-based nanogel | Disintegration of the Schiff base bond | 5.0–2.0 | / | / | In vitro | [58] |
| DOX | Carboxymethyl chitosan | Hydrolysis of ortho ester bond | 6.5–5.0 | Around 30% mass loss after 300 h in PBS 7.4 and around 40% mass loss after 300 h in PBS 5.0 | / | In vitro | [59] |
| Gemcitabine, paclitaxel (PTX) | OE peptide (VKVKVOVK-VDPPT-KVEVKVKV-NH2) | Disruption of the 3D network of peptide from beta-sheet to random coil | 5.8 | / | / | In vitro and in vivo | [60] |
2.2. Cargo-Based Drug Delivery Hydrogels
| Hydrophobic Drugs | Materials to Synthesize Hydrogels | Drug-Loading Mechanism | Drug Entrapment Efficiency | Drug-Loading Efficiency | Degradation and Stability | Sterilization Method | In Vitro/In Vivo Analysis | References |
|---|---|---|---|---|---|---|---|---|
| PTX | Mixed polymeric micelle composed of PF127, tocopherol polyethylene glycol 1000 succinate (TPGS), and hyaluronic acid (HA) | Micelle formation by hydrophobic interaction | ~51% | ~12% | Hydrogel maintained 75% of its original weight over 5 days at 37 °C, low viscosity at 4 °C converted to a semisolid upon heating to 35 °C | / | [76] In vitro [77] In vitro and in vivo | [79,80] |
| PTX | Gellan gum | Formed CD: drug complex | / | / | / | Sterilized by UV radiation | In vitro | [85] |
| PTX | PEGylated star polymer and PNIPAAm | Formed CD: drug complex | ~90% | ~3% | Complex nanoparticles experienced fast size broadening under physiological salt conditions (150 mM) within 30 min | / | In vitro and in vivo | [95] |
| PTX | Gellan gum modified by prednisolone | Hydrophobic interaction by prednisolone | ~40% | / | Good stability | / | In vitro | [96] |
| PTX | IC1-R peptide | Beta-folded structure formation by hydrophobic interaction | ~98% | / | Parameters set to a frequency of 0.1–100 rad/s, a sheer force of 1%, and a test time of 15 min. Fixed frequency of 6.28 rad/s, 0–5 min shear force set to 1%, and 5–7 min set to 100%. The shear force was set to 1% at 7–37 min, and the process was repeated at 37–70 min | Filtration via 0.22 μm filter | In vitro and in vivo | [97] |
| PTX, epirubicin | HA and poly (e-caprolactone)-poly (ethylene glycol)-poly-(e-caprolactone) (PCL-PEG-PCL) | Loading PTX to PCL-PEG-PCL nanoparticles | ~90% | ~10% | The HA-Gel embedded subcutaneously in mice gradually degraded 3 days post-implantation, and the extent of degradation was significant on day 5. Almost no residual hydrogel on day 9 | / | In vitro and in vivo | [98] |
| PTX | PEG-PCL-PEG/DDP + MPEG-PCL/PTX | Loading PTX by monomethoxy PEG-PCL | ~99% | ~4% | When the temperature exceeded 25 °C, the samples changed from a liquid to an elastic gel-like substance at the crossover point of G and G; the composite’s viscosity was the strongest at about 43 °C | The prepared PDMP hydrogel composite was sterilized using CO-60 (20 Gy) before injection | In vitro and in vivo | [99] |
| Curcumin | Thiolated chitosan | Encapsulated by liposomes | ~88% | ~4% | [2] The deformation loss ratio was 20.06% (CSSH Gel), 23.92% (100 μM), 20.95% (150 μM), and 17.95 % (200 μM) after five cycles of compression [19]. The best compressive performance is at 5%, the compressive modulus of 5% is 27.44 kPa, and the maximum compressive strength is 32 kPa | / | In vitro and in vivo | [2,19] |
| Curcumin | Peptide (MAX8) | Beta-hairpin structure formation by hydrophobic interaction | / | / | Stable colloidal dispersion system and good mechanical properties | / | In vivo | [100] |
| Curcumin | Polyethylene glycol (PEG) and polycaprolactone (PCL) polymer PCL-PEG-PCL | Hydrophobic interaction by hydrophobic PCL | 47~74% | 2~9% | / | / | In vitro | [101] |
| Curcumin | Alginate/chitosan hydrogel microparticles | Loading curcumin by hyaluronic acid/zein nanoparticles | ~70% | / | Cur/MPS in simulated gastric fluid (SGF) (pH 2) has no obvious change; NMPs can decompose rapidly in colonic fluid | / | In vitro and in vivo | [102] |
| Camptothecin (CPT) | Pluronic F127 and alpha-CDs | Micelle formation by hydrophobic interaction | / | / | / | Filtration via 0.45 μm filter | In vitro and in vivo | [92] |
| CPT | Folic acid and beta-CDs | Formed CD: drug complex | / | / | Good rheological mechanical property and thermodynamic stability | / | In vitro | [86] |
| Triptolide | PNIPAm-g-pluronic F68 | Micelle formation by hydrophobic interaction | ~84% | ~5% | 0.45 mg/kg TPL-equivalent dose three times over 14 days in 4T1 tumor-bearing mice | Filtration via 0.45 μm filter | In vitro and in vivo | [62] |
| DOX (DOX·HCl deprotonated at pH 9.6) | PEG-b-PCL and alpha-CDs | Micelle formation by hydrophobic interaction | 72~74% | ~15% | / | / | In vitro | [103] |
| DOC | Mixed polymeric micelle composed of PF127, PL121, and hyaluronic acid (HA) | Micelle formation by hydrophobic interaction | ~99% | ~2% | / | / | In vivo | [104] |
2.3. Drug Delivery Hydrogels for Pathological Characteristics of Tumors
2.3.1. DDSs of Cytotoxic Drugs
| Tumor | Drugs | Materials to Synthesize Hydrogels | Effects | Reference |
|---|---|---|---|---|
| Melanoma | DOX and PTX | Glycol chitosan and benzaldehyde-terminated polymer | High antitumor efficacy and safety | [133] |
| Colon carcinoma | DOX and DOC | Micelles by PL121, PF127, and HA | High antitumor efficacy and safety | [104] |
| Bladder carcinoma | DOX and CDDP | PEG-b-PCL and alpha-CDs | Controllable drug release and injectable ability | [103] |
| Hepatoma | DOX and Curcumin | PCL-PEG-PCL | High antitumor efficacy and safety | [101] |
| Lung tumor | PTX and CDDP | PEG-PCL-PEG and PMEG-PCL | High antitumor efficacy, controllable drug release, and safety | [99] |
| Ovarian tumor | PTX and CDDP | Bi-mPEG-PLGA-Pt (IV) | High antitumor efficacy and safety | [139] |
| Breast tumor | PTX and Epirubicin (EPI) | PCL-PEG-PCL and HA | High antitumor efficacy, controllable drug release, and safety | [98] |
| Breast tumor | PTX and Gemcitabine (GEM) | OE peptide hydrogel | High antitumor efficacy | [60] |
| Breast tumor | PTX and Honokiol | PLGA-PEG-PLGA | High antitumor efficacy and safety | [146] |
| Ovarian tumor | PTX, Rapamaycin, LS301 | PLGA-b-PEG-b-PLGA | High antitumor efficacy | [147] |
| Colorectal peritoneal carcinomatosis | CDDP and 5-FU | Ring-opening copolymerization to synthesize ε-CL and PEG and chitosan | High antitumor efficacy and safety | [145] |
| Gastric tumor | CDDP and 5-FU | PDLLA-PEG-PDLLA | High antitumor efficacy, safety, and high efficacy to inhibit tumor recurrence | [138] |
| Pancreatic tumor | CDDP and GEM | PDLLA-PEG-PDLLA | High antitumor efficacy | [148] |
| Colorectal tumor | Oxaliplatin and Hesperetin | Cationic Okra gum | High cytotoxicity | [149] |
| Brain tumor | Carmustine and Curcumin | PCL-PEG | High efficacy to inhibit tumor recurrence | [150] |
2.3.2. DDSs of Anti-Angiogenesis Drugs
2.3.3. DDSs of Immune Checkpoint Inhibitors
3. Methods for In Vitro and In Vivo Evaluations
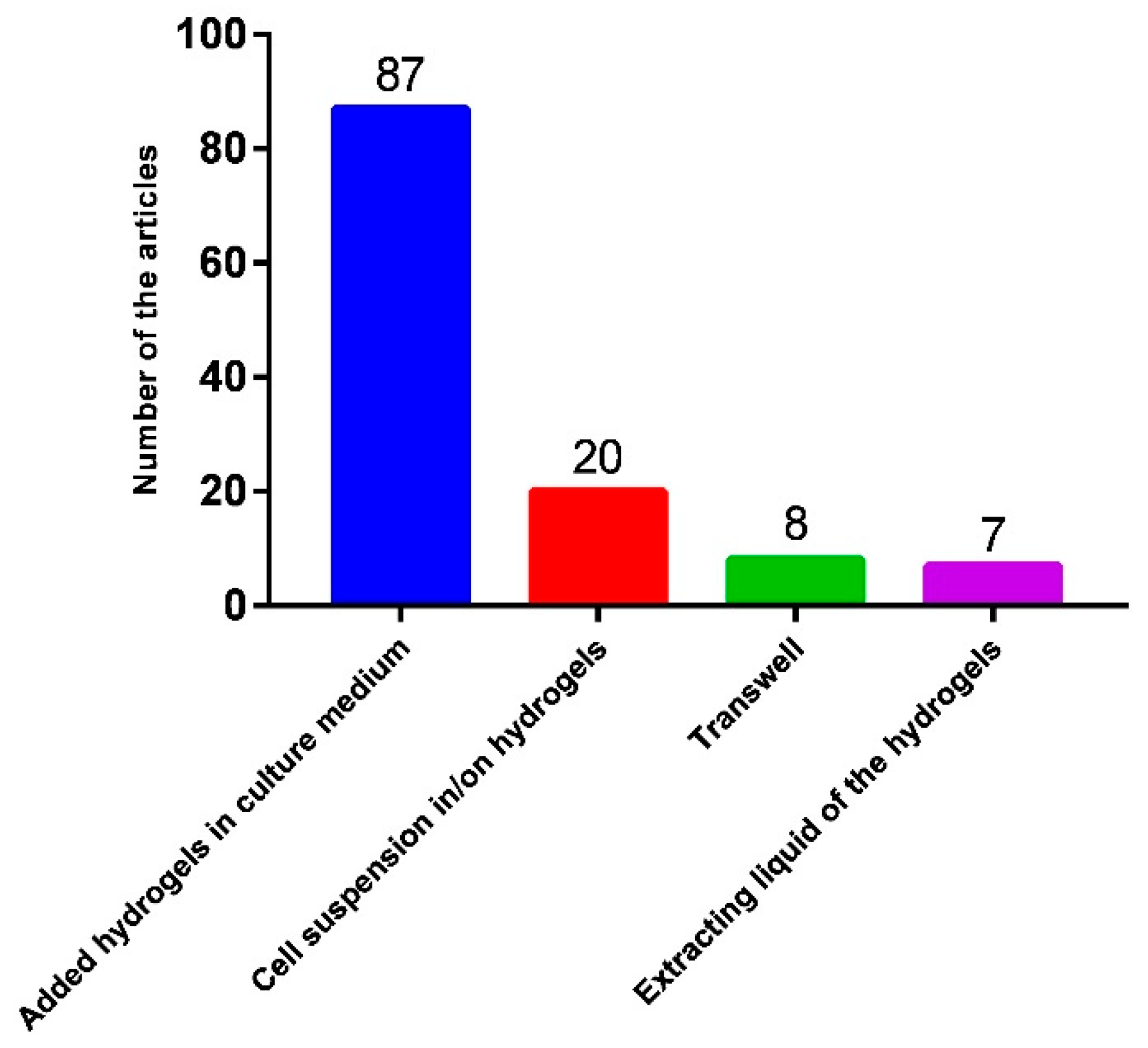
3.1. Method for In Vitro Antitumor Effect Evaluation
3.2. Method for In Vivo Antitumor Effect Evaluation
4. Challenges and Developments of Hydrogel-Based DDSs
4.1. Preclinical Evaluation and Clinical Trials
4.2. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bruschi, M.L. Modification of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 15–28. [Google Scholar]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and In Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef]
- Xu, L.; Cooper, R.C.; Wang, J.; Yeudall, W.A.; Yang, H. Synthesis and Application of Injectable Bioorthogonal Dendrimer Hydrogels for Local Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 1641–1653. [Google Scholar] [CrossRef]
- Shukla, A.; Singh, A.P.; Maiti, P. Injectable hydrogels of newly designed brush biopolymers as sustained drug-delivery vehicle for melanoma treatment. Signal Transduct. Target. Ther. 2021, 6, 63. [Google Scholar] [CrossRef]
- Sheykhisarem, R.; Dehghani, H. In Vitro biocompatibility evaluations of pH-sensitive Bi(2)MoO(6)/NH(2)-GO conjugated polyethylene glycol for release of daunorubicin in cancer therapy. Colloids Surf. B Biointerfaces 2023, 221, 113006. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Poon, R.T.; Borys, N. Lyso-thermosensitive liposomal doxorubicin: A novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin. Pharmacother. 2009, 10, 333–343. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Sun, R.; Wang, M.; Zeng, T.; Chen, H.; Yoshitomi, T.; Takeguchi, M.; Kawazoe, N.; Yang, Y.; Chen, G. Scaffolds functionalized with matrix metalloproteinase-responsive release of miRNA for synergistic magnetic hyperthermia and sensitizing chemotherapy of drug-tolerant breast cancer. Bioact. Mater. 2025, 44, 205–219. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Hsu, H.J.; Bugno, J.; Lee, S.R.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2017, 9, e1409. [Google Scholar] [CrossRef]
- Parra-Nieto, J.; Del Cid, M.A.G.; de Carcer, I.A.; Baeza, A. Inorganic Porous Nanoparticles for Drug Delivery in Antitumoral Therapy. Biotechnol. J. 2021, 16, e2000150. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Gong, Y.; Zhu, W.; Zhu, J.; Pan, F.; Chao, Y.; Xiao, Z.; Liu, Y.; Wang, X.; et al. Vitamin C supramolecular hydrogel for enhanced cancer immunotherapy. Biomaterials 2022, 287, 121673. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, C.; Liu, J.; Fan, C.; Yin, J.; Wu, T. An oral hydrogel carrier for delivering resveratrol into intestine-specific target released with high encapsulation efficiency and loading capacity based on structure-selected alginate and pectin. Food Funct. 2022, 13, 12051–12066. [Google Scholar] [CrossRef]
- Li, R.; Lyu, Y.; Luo, S.; Wang, H.; Zheng, X.; Li, L.; Ao, N.; Zha, Z. Fabrication of a multi-level drug release platform with liposomes, chitooligosaccharides, phospholipids and injectable chitosan hydrogel to enhance anti-tumor effectiveness. Carbohydr. Polym. 2021, 269, 118322. [Google Scholar] [CrossRef]
- Thiele, J.; Ma, Y.; Bruekers, S.M.; Ma, S.; Huck, W.T. 25th anniversary article: Designer hydrogels for cell cultures: A materials selection guide. Adv. Mater. 2014, 26, 125–147. [Google Scholar] [CrossRef]
- Balbir, S.; Anjali, S.; Amit, K. Hydrogels: Synthesis, classification, properties and potential applications-a brief review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef]
- Masters, K.S.; Shah, D.N.; Walker, G.; Leinwand, L.A.; Anseth, K.S. Designing scaffolds for valvular interstitial cells: Cell adhesion and function on naturally derived materials. J. Biomed. Mater. Res. Part. A 2004, 71, 172–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 12374. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020, 17, 373–391. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, C.; Du, D.; Li, Y.; Sun, L.; Han, Y.; He, X.; Dai, J.; Shi, L. Metal-organic framework-based hydrogel with structurally dynamic properties as a stimuli-responsive localized drug delivery system for cancer therapy. Acta Biomater. 2022, 145, 43–51. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-drug delivery system based on the hydrogels of alginate and sodium carboxymethyl cellulose for colorectal cancer treatment. Carbohydr. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Mohammadzadeh, A.; Javanbakht, S.; Mohammadi, R.; Ghorbani, M. Alginate/magnetic hydroxyapatite bio-nanocomposite hydrogel bead as a pH-responsive oral drug carrier for potential colon cancer therapy. Results Chem. 2025, 15, 102177. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Lu, Y. Doxorubicin and CD-CUR inclusion complex co-loaded in thermosensitive hydrogel PLGA-PEG-PLGA localized administration for osteosarcoma. Int. J. Oncol. 2020, 57, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chu, B.; Wei, X.; Lei, M.; Hu, D.; Zha, R.; Zhong, L.; Wang, M.; Wang, F.; Qian, Z. Redox/pH dual-stimuli responsive camptothecin prodrug nanogels for “on-demand” drug delivery. J. Control. Release 2019, 296, 93–106. [Google Scholar] [CrossRef]
- Lin, J.-T.; Ye, Q.-B.; Yang, Q.-J.; Wang, G.-H. Hierarchical bioresponsive nanocarriers for codelivery of curcumin and doxorubicin. Colloids Surf. B Biointerfaces 2019, 180, 93–101. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ramazani, A.; Sillanpaa, M.; Ghasemzadeh, H.; Mohammadi, E. Biocompatible porous PAM/CNT nanocomposite hydrogel films for sustained drug delivery and cancer therapy. Sci. Rep. 2025, 15, 22387. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Zhang, C.; Pich, A.; Xing, L.; Shi, X. Intelligent nanogels with self-adaptive responsiveness for improved tumor drug delivery and augmented chemotherapy. Bioact. Mater. 2021, 6, 3473–3484. [Google Scholar] [CrossRef]
- Qian, H.; Qian, K.; Cai, J.; Yang, Y.; Zhu, L.; Liu, B. Therapy for Gastric Cancer with Peritoneal Metastasis Using Injectable Albumin Hydrogel Hybridized with Paclitaxel-Loaded Red Blood Cell Membrane Nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 1100–1112. [Google Scholar] [CrossRef]
- Nazir, S.; Umar Aslam Khan, M.; Shamsan Al-Arjan, W.; Izwan Abd Razak, S.; Javed, A.; Rafiq Abdul Kadir, M. Nanocomposite hydrogels for melanoma skin cancer care and treatment: In-vitro drug delivery, drug release kinetics and anti-cancer activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Ullah, K.; Sohail, M.; Murtaza, G.; Khan, S.A. Natural and synthetic materials based CMCh/PVA hydrogels for oxaliplatin delivery: Fabrication, characterization, In-Vitro and In-Vivo safety profiling. Int. J. Biol. Macromol. 2019, 122, 538–548. [Google Scholar] [CrossRef]
- Halder, S.; Das, T.; Kushwaha, R.; Misra, A.K.; Jana, K.; Das, D. Targeted and precise drug delivery using a glutathione-responsive ultra-short peptide-based injectable hydrogel as a breast cancer cure. Mater. Horiz. 2025, 12, 987–1001. [Google Scholar] [CrossRef]
- Sathi Devi, L.; Gigliobianco, M.R.; Gabrielli, S.; Agas, D.; Sabbieti, M.G.; Morelli, M.B.; Amantini, C.; Casadidio, C.; Di Martino, P.; Censi, R. Localized Cancer Treatment Using Thiol–Ene Hydrogels for Dual Drug Delivery. Biomacromolecules 2025, 26, 3234–3254. [Google Scholar] [CrossRef]
- Ahmadi, S.; Olad, A.; Fathi, M.; Molavi, O. An injectable chitosan based dual thermo/pH-responsive fast gelling hydrogel loaded by methotrexate/curcumin as local drug delivery system of breast cancer. J. Drug Deliv. Sci. Technol. 2025, 104, 106540. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Progress. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chemistry 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of physiological pH and chemical pKa under the umbrella of physiologically based pharmacokinetic modeling of drug absorption, distribution, metabolism, excretion, and toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, L.; Wang, T.; Wang, H. A pH-responsive hydrogel system based on cellulose and dopamine with controlled hydrophobic drug delivery ability and long-term bacteriostatic property. Colloid. Polym. Sci. 2019, 297, 705–717. [Google Scholar] [CrossRef]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Sericin/RBA embedded gellan gum based smart nanosystem for pH responsive drug delivery. Int. J. Biol. Macromol. 2018, 120, 1561–1571. [Google Scholar] [CrossRef]
- Solomevich, S.O.; Bychkovsky, P.M.; Yurkshtovich, T.L.; Golub, N.V.; Mirchuk, P.Y.; Revtovich, M.Y.; Shmak, A.I. Biodegradable pH-sensitive prospidine-loaded dextran phosphate based hydrogels for local tumor therapy. Carbohydr. Polym. 2019, 226, 115308. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, G.; Wang, C.; Li, C.; Yao, P. Shear-responsive injectable supramolecular hydrogel releasing doxorubicin loaded micelles with pH-sensitivity for local tumor chemotherapy. Int. J. Pharm. 2017, 530, 53–62. [Google Scholar] [CrossRef]
- Turk, S.; Altinsoy, I.; Efe, G.C.; Ipek, M.; Ozacar, M.; Bindal, C. A novel multifunctional NCQDs-based injectable self-crosslinking and in situ forming hydrogel as an innovative stimuli responsive smart drug delivery system for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111829. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Voo, Z.X.; Chin, W.; Ono, R.J.; Yang, C.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable Coacervate Hydrogel for Delivery of Anticancer Drug-Loaded Nanoparticles in vivo. ACS Appl. Mater. Interfaces 2018, 10, 13274–13282. [Google Scholar] [CrossRef]
- Su, H.; Zhang, W.; Wu, Y.; Han, X.; Liu, G.; Jia, Q.; Shan, S. Schiff base-containing dextran nanogel as pH-sensitive drug delivery system of doxorubicin: Synthesis and characterization. J. Biomater. Appl. 2018, 33, 170–181. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, P.; Wang, X.; Cheng, X.; Qin, J.; Tang, R. pH-sensitive carboxymethyl chitosan hydrogels via acid-labile ortho ester linkage for potential biomedical applications. Carbohydr. Polym. 2017, 178, 166–179. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M.; et al. pH-Sensitive Peptide Hydrogels as a Combination Drug Delivery System for Cancer Treatment. Pharmaceutics 2022, 14, 652. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Hu, Y.; Gao, F.; Pak-Heng Leung, G.; Geng, F.; Fu, C.; Zhang, J. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on “two strikes” effects. Acta Pharm. Sin. B 2020, 10, 2227–2245. [Google Scholar] [CrossRef]
- Wei, L.; Chen, J.; Zhao, S.; Ding, J.; Chen, X. Thermo-sensitive polypeptide hydrogel for locally sequential delivery of two-pronged antitumor drugs. Acta Biomater. 2017, 58, 44–53. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Chou, H.Y.; Hsu, W.H.; Lai, J.Y.; Lin, S.Y.; Tsai, H.C. Localized controlled release of bevacizumab and doxorubicin by thermo-sensitive hydrogel for normalization of tumor vasculature and to enhance the efficacy of chemotherapy. Int. J. Pharm. 2019, 572, 118799. [Google Scholar] [CrossRef]
- Hu, Y.; Darcos, V.; Monge, S.; Li, S.; Zhou, Y.; Su, F. Thermo-responsive release of curcumin from micelles prepared by self-assembly of amphiphilic P(NIPAAm-co-DMAAm)-b-PLLA-b-P(NIPAAm-co-DMAAm) triblock copolymers. Int. J. Pharm. 2014, 476, 31–40. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Zhang, J.; Ju, J. Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers 2020, 12, 389. [Google Scholar] [CrossRef]
- Li, W.; Huang, L.; Ying, X.; Jian, Y.; Hong, Y.; Hu, F.; Du, Y. Antitumor Drug Delivery Modulated by A Polymeric Micelle with an Upper Critical Solution Temperature. Angew. Chem. Int. Ed. 2015, 54, 3126–3131. [Google Scholar] [CrossRef]
- Le, M.; Huang, W.; Chen, K.-F.; Lin, C.; Cai, L.; Zhang, H.; Jia, Y.-G. Upper critical solution temperature polymeric drug carriers. Chem. Eng. J. 2022, 432, 134354. [Google Scholar] [CrossRef]
- Park, T.G.; Hoffman, A.S. Deswelling characteristics of poly(N-isopropylacrylamide) hydrogel. J. Appl. Polym. Sci. 1994, 52, 85–89. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Xu, F.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery. Carbohydr. Polym. 2017, 174, 904–914. [Google Scholar] [CrossRef]
- Burger, K.N.; Staffhorst, R.W.; de Vijlder, H.C.; Velinova, M.J.; Bomans, P.H.; Frederik, P.M.; de Kruijff, B. Nanocapsules: Lipid-coated aggregates of cisplatin with high cytotoxicity. Nat. Med. 2002, 8, 81–84. [Google Scholar] [CrossRef]
- Zhang, P.; Ling, G.; Sun, J.; Zhang, T.; Yuan, Y.; Sun, Y.; Wang, Z.; He, Z. Multifunctional nanoassemblies for vincristine sulfate delivery to overcome multidrug resistance by escaping P-glycoprotein mediated efflux. Biomaterials 2011, 32, 5524–5533. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, M.; Reddy, A.; Yavvari, P.S.; Srivastava, A.; Bajaj, A. Interactions governing the entrapment of anticancer drugs by low-molecular-weight hydrogelator for drug delivery applications. RSC Adv. 2016, 6, 19751–19757. [Google Scholar] [CrossRef]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Wang, Z.; Jing, X. Ultrafine PEG-PLA fibers loaded with both paclitaxel and doxorubicin hydrochloride and their in vitro cytotoxicity. Eur. J. Pharm. Biopharm. 2009, 72, 18–25. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Shah, B.; Dong, X. Design and Evaluation of Two-Step Biorelevant Dissolution Methods for Docetaxel Oral Formulations. AAPS PharmSciTech 2022, 23, 113. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Akbari, V.; Amuaghae, E.; Emami, J. Preparation and characterization of an injectable thermosensitive hydrogel for simultaneous delivery of paclitaxel and doxorubicin. Res. Pharm. Sci. 2018, 13, 181–191. [Google Scholar] [CrossRef]
- Akbari, V.; Hejazi, E.; Minaiyan, M.; Emami, J.; Lavasanifar, A.; Rezazadeh, M. An injectable thermosensitive hydrogel/nanomicelles composite for local chemo-immunotherapy in mouse model of melanoma. J. Biomater. Appl. 2022, 551–562. [Google Scholar] [CrossRef]
- Yu, A.C.; Stapleton, L.M.; Mann, J.L.; Appel, E.A. Self-assembled biomaterials using host-guest interactions. In Self-Assembling Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 205–231. [Google Scholar]
- Angelova, S.; Nikolova, V.; Pereva, S.; Spassov, T.; Dudev, T. alpha-Cyclodextrin: How Effectively Can Its Hydrophobic Cavity Be Hydrated? J. Phys. Chem. B 2017, 121, 9260–9267. [Google Scholar] [CrossRef]
- Miranda, J.C.D.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Cabral, J.; Hanton, L.R.; Moratti, S.C. Cyclodextrin-polyhydrazine degradable gels for hydrophobic drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 144–153. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Rodríguez, V.; Esteban, P.P.; Martín del Valle, E.M. Biodegradable gellan gum hydrogels loaded with paclitaxel for HER2+ breast cancer local therapy. Carbohydr. Polym. 2022, 294, 119732. [Google Scholar] [CrossRef]
- Ma, M.; Shang, W.; Jia, R.; Chen, R.; Zhao, M.; Wang, C.; Tian, M.; Yang, S.; Hao, A. A novel folic acid hydrogel loading β-cyclodextrin/camptothecin inclusion complex with effective antitumor activity. J. Incl. Phenom. Macrocycl. Chem. 2019, 96, 169–179. [Google Scholar] [CrossRef]
- Jensen, G.; Hwang, J.; Schluep, T. Antitumor activity of IT-101, a cyclodextrin-containing polymer-camptothecin nanoparticle, in combination with various anticancer agents in human ovarian cancer xenografts. Cancer Res. 2008, 68, 767. [Google Scholar]
- Cheng, J.; Khin, K.T.; Davis, M.E. Antitumor activity of beta-cyclodextrin polymer-camptothecin conjugates. Mol. Pharm. 2004, 1, 183–193. [Google Scholar] [CrossRef]
- Davis, M.E. Design and development of IT-101, a cyclodextrin-containing polymer conjugate of camptothecin. Adv. Drug Deliv. Rev. 2009, 61, 1189–1192. [Google Scholar] [CrossRef]
- Schluep, T.; Hwang, J.; Cheng, J.; Heidel, J.D.; Bartlett, D.W.; Hollister, B.; Davis, M.E. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin. Cancer Res. 2006, 12, 1606–1614. [Google Scholar] [CrossRef]
- Schluep, T.; Cheng, J.; Khin, K.T.; Davis, M.E. Pharmacokinetics and biodistribution of the camptothecin-polymer conjugate IT-101 in rats and tumor-bearing mice. Cancer Chemother. Pharmacol. 2006, 57, 654–662. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Liu, T.; Ma, D.; Xue, W. Supramolecular hydrogels co-loaded with camptothecin and doxorubicin for sustainedly synergistic tumor therapy. J. Mater. Chem. B 2015, 3, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive Hydrogel Induced by Dual Supramolecular Assemblies and Its Controlled Release Property for Enhanced Anticancer Drug Delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef]
- Fu, C.; Lin, X.; Wang, J.; Zheng, X.; Li, X.; Lin, Z.; Lin, G. Injectable micellar supramolecular hydrogel for delivery of hydrophobic anticancer drugs. J. Mater. Sci. Mater. Med. 2016, 27, 73. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, X.; Wang, X.; Ye, E.; Loh, X.J.; Li, Z.; Wu, Y.L. Hierarchically Self-Assembled Supramolecular Host-Guest Delivery System for Drug Resistant Cancer Therapy. Biomacromolecules 2018, 19, 1926–1938. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Navarro, G.; Di Meo, C.; Matricardi, P.; Torchilin, V. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: A multi-drug delivery system for a combination therapy in cancer treatment. Eur. J. Pharm. Biopharm. 2014, 87, 208–216. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Li, Y.; Huang, Z.; Luo, S.; He, Y.; Han, H.; Raza, F.; Wu, J.; Ge, L. Injectable pH and redox dual responsive hydrogels based on self-assembled peptides for anti-tumor drug delivery. Biomater. Sci. 2020, 8, 5415–5426. [Google Scholar] [CrossRef]
- Leng, Q.; Li, Y.; Zhou, P.; Xiong, K.; Lu, Y.; Cui, Y.; Wang, B.; Wu, Z.; Zhao, L.; Fu, S. Injectable hydrogel loaded with paclitaxel and epirubicin to prevent postoperative recurrence and metastasis of breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112390. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, X.; Yang, L.; Lin, S.; Fan, J.; Yang, B.; Sun, X.; Wan, Q.; Chen, Y.; Fu, S. Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer. Colloids Surf. B Biointerfaces 2014, 122, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Wei, C.; Ma, T.; Zhang, Y.; Zhu, D.; Dong, X.; Lv, F. Multiplex fluorescence imaging-guided programmed delivery of doxorubicin and curcumin from a nanoparticles/hydrogel system for synergistic chemotherapy. J. Polym. Sci. 2021, 60, 1557–1570. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Xiao, M.; Ma, J.; Qu, Y.; Zou, L.; Zhang, J. Nano-in-micro alginate/chitosan hydrogel via electrospray technology for orally curcumin delivery to effectively alleviate ulcerative colitis. Mater. Des. 2022, 221, 110894. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Liu, L.; Chen, Y.; Xi, F. Supramolecular hydrogels as a universal scaffold for stepwise delivering Dox and Dox/cisplatin loaded block copolymer micelles. Int. J. Pharm. 2012, 437, 11–19. [Google Scholar] [CrossRef]
- Sheu, M.T.; Jhan, H.J.; Su, C.Y.; Chen, L.C.; Chang, C.E.; Liu, D.Z.; Ho, H.O. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointerfaces 2016, 143, 260–270. [Google Scholar] [CrossRef]
- Suri, A.; Campos, R.; Rackus, D.G.; Spiller, N.J.S.; Richardson, C.; Pålsson, L.-O.; Kataky, R. Liposome-doped hydrogel for implantable tissue. Soft Matter 2011, 7, 7071–7077. [Google Scholar] [CrossRef]
- Mauri, E.; Negri, A.; Rebellato, E.; Masi, M.; Perale, G.; Rossi, F. Hydrogel-Nanoparticles Composite System for Controlled Drug Delivery. Gels 2018, 4, 74. [Google Scholar] [CrossRef]
- Zeng, T.; Xu, M.; Zhang, W.; Gu, X.; Zhao, F.; Liu, X.; Zhang, X. Autophagy inhibition and microRNA-199a-5p upregulation in paclitaxel-resistant A549/T lung cancer cells. Oncol. Rep. 2021, 46, 149. [Google Scholar] [CrossRef]
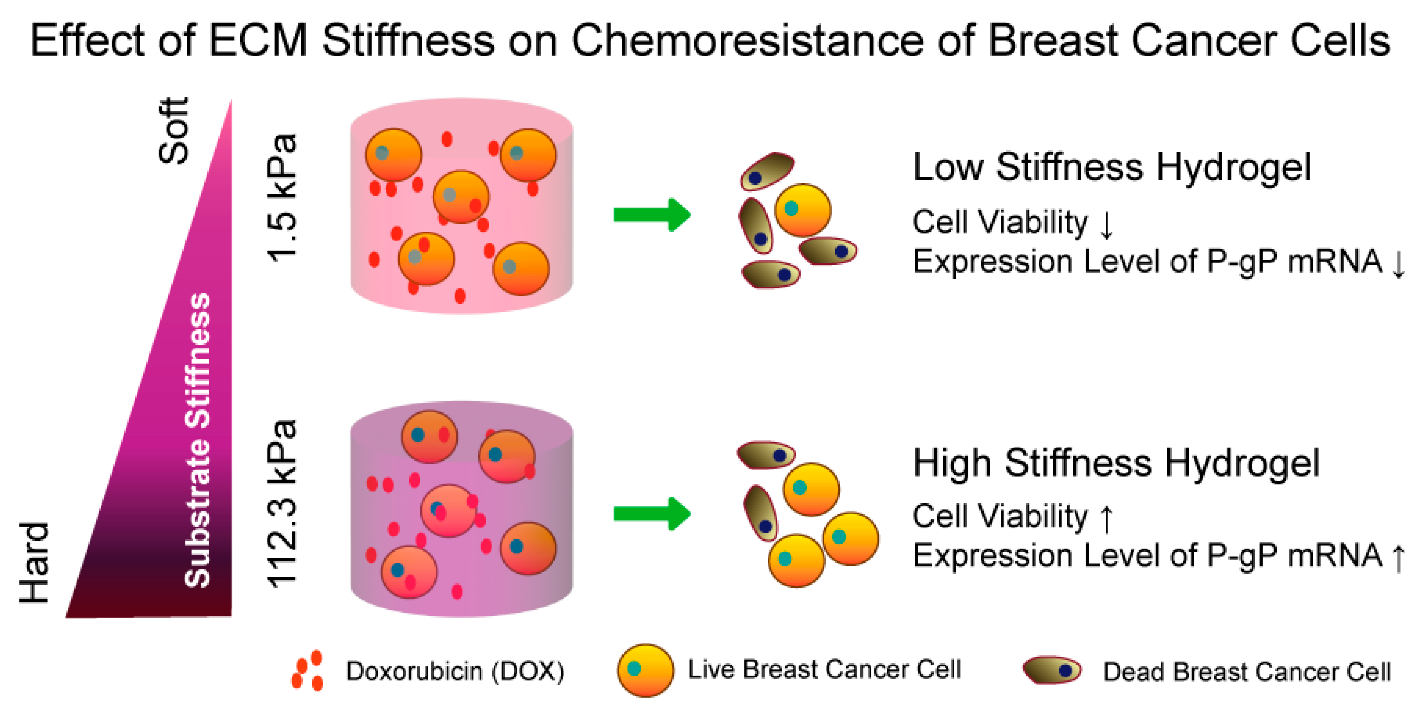
- Zeng, T.; Chen, H.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Effect of Hydrogel Stiffness on Chemoresistance of Breast Cancer Cells in 3D Culture. Gels 2024, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Lu, C.; Wang, M.; Chen, H.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. The effect of microenvironmental viscosity on the emergence of colon cancer cell resistance to doxorubicin. J. Mater. Chem. B 2025, 13, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Mohelnikova-Duchonova, B.; Melichar, B.; Soucek, P. FOLFOX/FOLFIRI pharmacogenetics: The call for a personalized approach in colorectal cancer therapy. World J. Gastroenterol. 2014, 20, 10316–10330. [Google Scholar] [CrossRef] [PubMed]
- Linschoten, M.; Kamphuis, J.A.M.; van Rhenen, A.; Bosman, L.P.; Cramer, M.J.; Doevendans, P.A.; Teske, A.J.; Asselbergs, F.W. Cardiovascular adverse events in patients with non-Hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (R-CHOP): A systematic review and meta-analysis. Lancet Haematol. 2020, 7, e295–e308. [Google Scholar] [CrossRef]
- Chua, W.; Goldstein, D.; Lee, C.K.; Dhillon, H.; Michael, M.; Mitchell, P.; Clarke, S.J.; Iacopetta, B. Molecular markers of response and toxicity to FOLFOX chemotherapy in metastatic colorectal cancer. Br. J. Cancer 2009, 101, 998–1004. [Google Scholar] [CrossRef]
- Herold, M.; Hieke, K. Costs of toxicity during chemotherapy with CHOP, COP/CVP, and fludarabine. Eur. J. Health Econ. 2002, 3, 166–172. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, C.; Sun, T.; Wang, L.; Chen, C. Tumor Therapy Strategies Based on Microenvironment-Specific Responsive Nanomaterials. Adv. Healthc. Mater. 2023, 12, 2300153. [Google Scholar] [CrossRef]
- Zhou, S.; Zheng, J.; Zhai, W.; Chen, Y. Spatio-temporal heterogeneity in cancer evolution and tumor microenvironment of renal cell carcinoma with tumor thrombus. Cancer Lett. 2023, 572, 216350. [Google Scholar] [CrossRef]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor heterogeneity: Preclinical models, emerging technologies, and future applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef] [PubMed]
- Reitan, N.K.; Thuen, M.; Goa, P.L.E.; de Lange Davies, C. Characterization of tumor microvascular structure and permeability: Comparison between magnetic resonance imaging and intravital confocal imaging. J. Biomed. Opt. 2010, 15, 036004. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y. Spatial Heterogeneity in the Tumor Microenvironment. Cold Spring Harb. Perspect. Med. 2016, 6, a026583. [Google Scholar] [CrossRef]
- Zhou, K.I.; Peterson, B.; Serritella, A.; Thomas, J.; Reizine, N.; Moya, S.; Tan, C.; Wang, Y.; Catenacci, D.V.T. Spatial and Temporal Heterogeneity of PD-L1 Expression and Tumor Mutational Burden in Gastroesophageal Adenocarcinoma at Baseline Diagnosis and after Chemotherapy. Clin. Cancer Res. 2020, 26, 6453–6463. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N.; Sakurai, Y.; Hida, Y.; Harashima, H. Heterogeneity of tumor endothelial cells and drug delivery. Adv. Drug Deliv. Rev. 2016, 99, 140–147. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, Q.; Cheng, J.-N.; Jia, Q.; Zhu, B. Heterogeneity of the tumor immune microenvironment and clinical interventions. Front. Med. 2023, 17, 617–648. [Google Scholar] [CrossRef]
- Wang, B.; Song, B.; Li, Y.; Zhao, Q.; Tan, B. Mapping spatial heterogeneity in gastric cancer microenvironment. Biomed. Pharmacother. 2024, 172, 116317. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liu, X.; Li, J.; Zou, H.; Wang, J.; Yan, Z.; Liu, Y.; Lyu, Y.; Feng, N. Hybrid NIR-responsive liposome/hydrogel platform mediating chemo-photothermal therapy of retinoblastoma enhanced by quercetin as an adjuvant. Theranostics 2025, 15, 3995–4015. [Google Scholar] [CrossRef]
- Arvejeh, P.M.; Chermahini, F.A.; Marincola, F.; Taheri, F.; Mirzaei, S.A.; Alizadeh, A.; Deris, F.; Jafari, R.; Amiri, N.; Soltani, A.; et al. A novel approach for the co-delivery of 5-fluorouracil and everolimus for breast cancer combination therapy: Stimuli-responsive chitosan hydrogel embedded with mesoporous silica nanoparticles. J. Transl. Med. 2025, 23, 382. [Google Scholar] [CrossRef]
- Feng, C.; Xiao, S.; Mu, M.; Wang, X.; Yu, W.; Pan, S.; Li, H.; Fan, R.; Han, B.; Guo, G. In situ TLR7 activation with biocompatible injectable hydrogel for synergistic chemo-immunotherapy. J. Mater. Sci. Technol. 2026, 241, 93–106. [Google Scholar] [CrossRef]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Bielack, S.S.; Erttmann, R.; Winkler, K.; Landbeck, G. Doxorubicin: Effect of different schedules on toxicity and anti-tumor efficacy. Eur. J. Cancer Clin. Oncol. 1989, 25, 873–882. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Wang, X.; Zhu, D.; Liu, L.; Leng, X. Simultaneous monitoring of the drug release and antitumor effect of a novel drug delivery system-MWCNTs/DOX/TC. Drug Deliv. 2017, 24, 143–151. [Google Scholar] [CrossRef]
- Ogawara, K.; Un, K.; Tanaka, K.; Higaki, K.; Kimura, T. In vivo anti-tumor effect of PEG liposomal doxorubicin (DOX) in DOX-resistant tumor-bearing mice: Involvement of cytotoxic effect on vascular endothelial cells. J. Control. Release 2009, 133, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, W.; Bian, S.; Huang, Y.; Tan, Y.; Liang, J.; Fan, Y.; Zhang, X. Bioreducible PAA-g-PEG graft micelles with high doxorubicin loading for targeted antitumor effect against mouse breast carcinoma. Biomaterials 2013, 34, 6818–6828. [Google Scholar] [CrossRef]
- Rihova, B.; Srogl, J.; Jelinkova, M.; Hovorka, O.; Buresova, M.; Subr, V.; Ulbrich, K. HPMA-based biodegradable hydrogels containing different forms of doxorubicin. Antitumor effects and biocompatibility. Ann. N. Y. Acad. Sci. 1997, 831, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.; Lee, S.M.; Kim, C.W.; Hong, K.Y.; Kim, S.Y.; Yang, H.K.; Song, S.C. Doxorubicin-polyphosphazene conjugate hydrogels for locally controlled delivery of cancer therapeutics. Biomaterials 2009, 30, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, L.; Liu, F.; Liu, C.; Shan, D.; Wang, Q.; Zhang, C.; Li, J.; Liu, J.; Qu, X.; et al. pH triggered injectable amphiphilic hydrogel containing doxorubicin and paclitaxel. Int. J. Pharm. 2011, 410, 83–91. [Google Scholar] [CrossRef]
- Wang, W.; Song, H.; Zhang, J.; Li, P.; Li, C.; Wang, C.; Kong, D.; Zhao, Q. An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J. Control. Release 2015, 203, 57–66. [Google Scholar] [CrossRef]
- Wensing, K.U.; Ciarimboli, G. Saving ears and kidneys from cisplatin. Anticancer Res. 2013, 33, 4183–4188. [Google Scholar] [PubMed]
- Ciarimboli, G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014, 34, 547–550. [Google Scholar] [CrossRef]
- Taguchi, T.; Nazneen, A.; Abid, M.R.; Razzaque, M.S. Cisplatin-associated nephrotoxicity and pathological events. Contrib. Nephrol. 2005, 148, 107–121. [Google Scholar] [CrossRef]
- Chen, W.; Shi, K.; Liu, J.; Yang, P.; Han, R.; Pan, M.; Yuan, L.; Fang, C.; Yu, Y.; Qian, Z. Sustained co-delivery of 5-fluorouracil and cis-platinum via biodegradable thermo-sensitive hydrogel for intraoperative synergistic combination chemotherapy of gastric cancer. Bioact. Mater. 2023, 23, 1–15. [Google Scholar] [CrossRef]
- Shen, W.; Chen, X.; Luan, J.; Wang, D.; Yu, L.; Ding, J. Sustained Codelivery of Cisplatin and Paclitaxel via an Injectable Prodrug Hydrogel for Ovarian Cancer Treatment. ACS Appl. Mater. Interfaces 2017, 9, 40031–40046. [Google Scholar] [CrossRef]
- Peng, H.; Huang, Q.; Yue, H.; Li, Y.; Wu, M.; Liu, W.; Zhang, G.; Fu, S.; Zhang, J. The antitumor effect of cisplatin-loaded thermosensitive chitosan hydrogel combined with radiotherapy on nasopharyngeal carcinoma. Int. J. Pharm. 2019, 556, 97–105. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.; Feng, X.; Zhou, X.; Xu, X.; Xie, L.; Wang, R.; Zhang, D.; Wang, H.; Deng, P.; et al. Biodegradable thermosensitive hydrogel for SAHA and DDP delivery: Therapeutic effects on oral squamous cell carcinoma xenografts. PLoS ONE 2012, 7, e33860. [Google Scholar] [CrossRef] [PubMed]
- Sharon Gabbay, R.; Rubinstein, A. Synchronizing the release rates of topotecan and paclitaxel from a self-eroding crosslinked chitosan—PLGA platform. Int. J. Pharm. 2022, 623, 121945. [Google Scholar] [CrossRef] [PubMed]
- Cocarta, A.I.; Hobzova, R.; Trchova, M.; Svojgr, K.; Kodetova, M.; Pochop, P.; Uhlik, J.; Sirc, J. 2-Hydroxyethyl Methacrylate Hydrogels for Local Drug Delivery: Study of Topotecan and Vincristine Sorption/Desorption Kinetics and Polymer-Drug Interaction by ATR-FTIR Spectroscopy. Macromol. Chem. Phys. 2021, 222, 2100086. [Google Scholar] [CrossRef]
- Bai, R.; Deng, X.; Wu, Q.; Cao, X.; Ye, T.; Wang, S. Liposome-loaded thermo-sensitive hydrogel for stabilization of SN-38 via intratumoral injection: Optimization, characterization, and antitumor activity. Pharm. Dev. Technol. 2018, 23, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.; Wang, S.S.; Xu, S.; Yang, J.P.; Fan, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; Wu, J.B. Use of 5-Fluorouracil Loaded Micelles and Cisplatin in Thermosensitive Chitosan Hydrogel as an Efficient Therapy against Colorectal Peritoneal Carcinomatosis. Macromol. Biosci. 2017, 17, 1600262. [Google Scholar] [CrossRef]
- Lu, X.; Lu, X.; Yang, P.; Zhang, Z.; Lv, H. Honokiol nanosuspensions loaded thermosensitive hydrogels as the local delivery system in combination with systemic paclitaxel for synergistic therapy of breast cancer. Eur. J. Pharm. Sci. 2022, 175, 106212. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Tang, R.; Liang, K.; Achilefu, S.; Kwon, G.S.; Cho, H. Proof-of-Concept of Polymeric Sol-Gels in Multi-Drug Delivery and Intraoperative Image-Guided Surgery for Peritoneal Ovarian Cancer. Pharm. Res. 2016, 33, 2298–2306. [Google Scholar] [CrossRef]
- Shi, K.; Xue, B.; Jia, Y.; Yuan, L.; Han, R.; Yang, F.; Peng, J.; Qian, Z. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. 2019, 12, 1389–1399. [Google Scholar] [CrossRef]
- Hodaei, M.; Varshosaz, J. Cationic Okra gum coated nanoliposomes as a pH-sensitive carrier for co-delivery of hesperetin and oxaliplatin in colorectal cancers. Pharm. Dev. Technol. 2022, 27, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Wanjale, M.V.; Sunil Jaikumar, V.; Sivakumar, K.C.; Ann Paul, R.; James, J.; Kumar, G.S.V. Supramolecular Hydrogel Based Post-Surgical Implant System for Hydrophobic Drug Delivery Against Glioma Recurrence. Int. J. Nanomed. 2022, 17, 2203–2224. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumor Stroma, Tumor Blood Vessels, and Antiangiogenesis Therapy. Cancer J. 2015, 21, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.C.; Harriss-Phillips, W.M.; Douglass, M.J.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef]
- Jain, R.K. Determinants of Tumor Blood Flow: A Review1. Cancer Res. 1988, 48, 2641–2658. [Google Scholar]
- Tozer, G.M.; Kanthou, C.; Baguley, B.C. Disrupting tumour blood vessels. Nat. Rev. Cancer 2005, 5, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, S.; Pages, G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie 2013, 95, 1110–1119. [Google Scholar] [CrossRef]
- Maj, E.; Papiernik, D.; Wietrzyk, J. Antiangiogenic cancer treatment: The great discovery and greater complexity (Review). Int. J. Oncol. 2016, 49, 1773–1784. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef]
- Pasquier, E.; Kavallaris, M.; Andre, N. Metronomic chemotherapy: New rationale for new directions. Nat. Rev. Clin. Oncol. 2010, 7, 455–465. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, Y.J.; Siriwon, N.; Rohrs, J.A.; Yu, Z.; Wanga, P. Combination drug delivery via multilamellar vesicles enables targeting of tumor cells and tumor vasculature. Biotechnol. Bioeng. 2018, 115, 1403–1415. [Google Scholar] [CrossRef]
- Li, F.; Shao, X.; Liu, D.; Jiao, X.; Yang, X.; Yang, W.; Liu, X. Vascular Disruptive Hydrogel Platform for Enhanced Chemotherapy and Anti-Angiogenesis through Alleviation of Immune Surveillance. Pharmaceutics 2022, 14, 1809. [Google Scholar] [CrossRef]
- Westin, S.N.; Sood, A.K.; Coleman, R.L. Targeted Therapy and Molecular Genetics. In Clinical Gynecologic Oncology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 539–560.e536. [Google Scholar]
- Drzyzga, A.; Cichon, T.; Czapla, J.; Jarosz-Biej, M.; Pilny, E.; Matuszczak, S.; Wojcieszek, P.; Urbas, Z.; Smolarczyk, R. The Proper Administration Sequence of Radiotherapy and Anti-Vascular Agent-DMXAA Is Essential to Inhibit the Growth of Melanoma Tumors. Cancers 2021, 13, 3924. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, L.; Xie, Y.; Cheng, S.; Xiong, M.; Luo, X. An injectable hydrogel/staple fiber composite for sustained release of CA4P and doxorubicin for combined chemotherapy of xenografted breast tumor in mice. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Montemagno, C.; Pages, G. Resistance to Anti-angiogenic Therapies: A Mechanism Depending on the Time of Exposure to the Drugs. Front. Cell Dev. Biol. 2020, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, A. Long-term survival of patients with advanced non-small cell lung cancer treated using immune checkpoint inhibitors. Respir. Investig. 2024, 62, 85–89. [Google Scholar] [CrossRef]
- Chao, Y.; Liang, C.; Tao, H.; Du, Y.; Wu, D.; Dong, Z.; Jin, Q.; Chen, G.; Xu, J.; Xiao, Z.; et al. Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses. Sci. Adv. 2020, 6, eaaz4204. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Fu, C.; Liu, H.; Yin, Y.; Cao, Y.; Feng, J.; Zhang, A.; Wang, W. Chemoimmunotherapeutic Nanogel for Pre- and Postsurgical Treatment of Malignant Melanoma by Reprogramming Tumor-Associated Macrophages. Nano Lett. 2024, 24, 1717–1728. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-Responsive Smart Hydrogel Releasing Immune Adjuvant Synchronized with Repeated Chemotherapy or Radiotherapy to Boost Antitumor Immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Nakajima, T.; Hourdet, D.; Marcellan, A.; Creton, C.; Hong, W.; Kurokawa, T.; Gong, J.P. Hydrophobic Hydrogels with Fruit-Like Structure and Functions. Adv. Mater. 2019, 31, 1900702. [Google Scholar] [CrossRef]
- Cui, W.; Zhu, R.; Zheng, Y.; Mu, Q.; Pi, M.; Chen, Q.; Ran, R. Transforming non-adhesive hydrogels to reversible tough adhesives via mixed-solvent-induced phase separation. J. Mater. Chem. A 2021, 9, 9706–9718. [Google Scholar] [CrossRef]
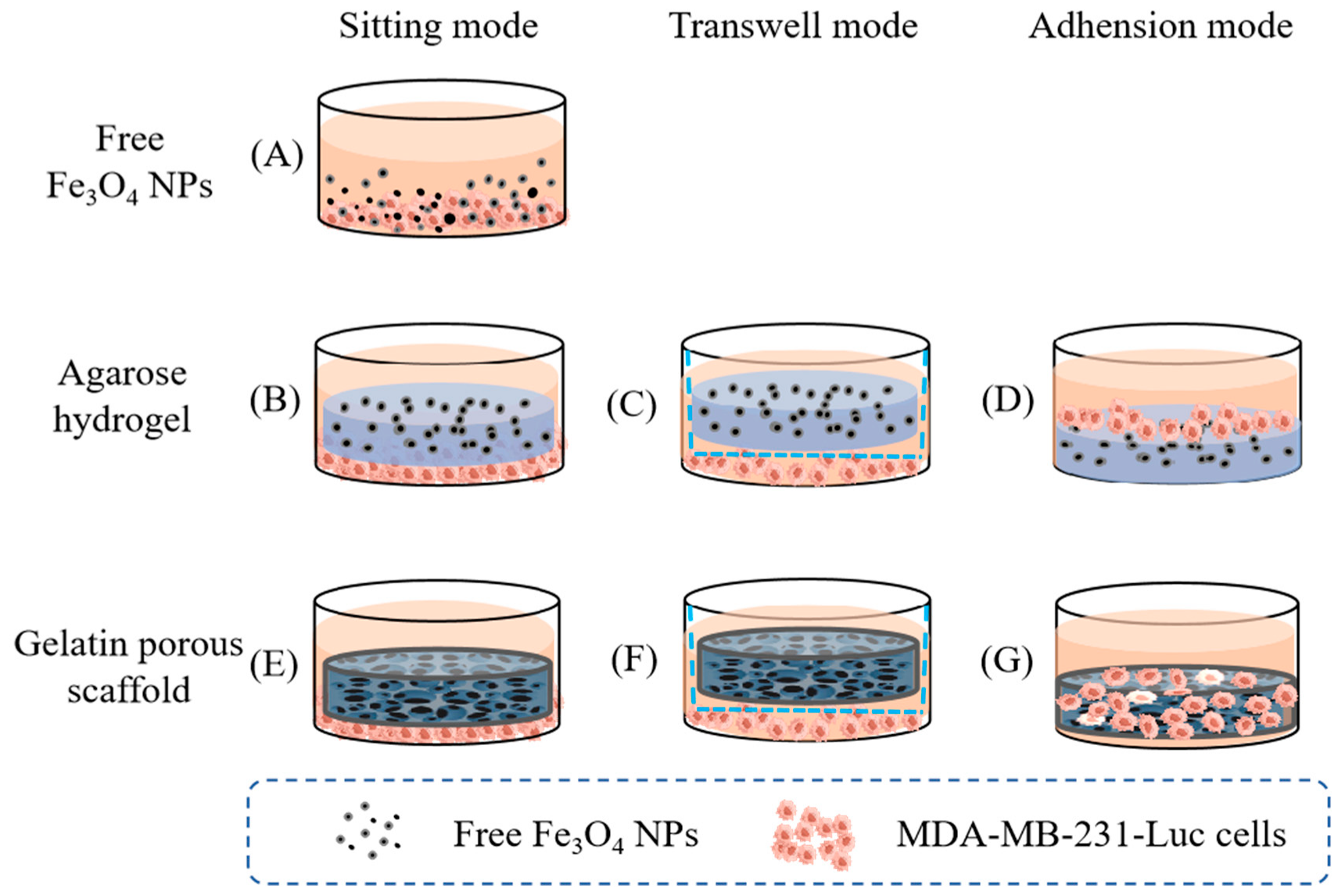
- Wang, M.; Sun, R.; Chen, H.; Liu, X.; Yoshitomi, T.; Takeguchi, M.; Kawazoe, N.; Yang, Y.; Chen, G. Influence of hydrogel and porous scaffold on the magnetic thermal property and anticancer effect of Fe3O4 nanoparticles. Microstructures 2023, 3, 2023042. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Georgiou, T.K. Pre-clinical and clinical applications of thermoreversible hydrogels in biomedical engineering: A review. Polym. Int. 2021, 70, 1433–1448. [Google Scholar] [CrossRef]
- Xu, S.; Wang, W.; Li, X.; Liu, J.; Dong, A.; Deng, L. Sustained release of PTX-incorporated nanoparticles synergized by burst release of DOXHCl from thermosensitive modified PEG/PCL hydrogel to improve anti-tumor efficiency. Eur. J. Pharm. Sci. 2014, 62, 267–273. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, F.; Ding, B.; Sun, N.; Liu, X.; Ding, X.; Gao, S. A thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxel. J. Drug Target. 2011, 19, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Turabee, M.H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr. Polym. 2019, 203, 302–309. [Google Scholar] [CrossRef]
- Zhu, Y.; Jia, J.; Zhao, G.; Huang, X.; Wang, L.; Zhang, Y.; Zhang, L.; Konduru, N.; Xie, J.; Yu, R.; et al. Multi-responsive nanofibers composite gel for local drug delivery to inhibit recurrence of glioma after operation. J. Nanobiotechnol. 2021, 19, 198. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, J.; Zhang, L.; Wang, L.; Xu, H.; Han, Y.; Jia, J.; Lu, Y.; Yu, R.; Liu, H. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater. Sci. 2020, 8, 5306–5316. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle Drug Delivery System for Glioma and Its Efficacy Improvement Strategies: A Comprehensive Review. Int. J. Nanomed. 2020, 15, 2563–2582. [Google Scholar] [CrossRef]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020, 11, 5687. [Google Scholar] [CrossRef]
- Kim, J.; Francis, D.M.; Thomas, S.N. In Situ Crosslinked Hydrogel Depot for Sustained Antibody Release Improves Immune Checkpoint Blockade Cancer Immunotherapy. Nanomaterials 2021, 11, 471. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, S.; Huang, L.; Li, Y.; Lu, Y.; Li, H.; Chen, G.; Meng, F.; Liu, G.L.; Yang, X.; et al. Reactive oxygen species-responsive and Raman-traceable hydrogel combining photodynamic and immune therapy for postsurgical cancer treatment. Nat. Commun. 2022, 13, 4553. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wan, C.; Zou, Z.; Zhao, G.; Zhang, L.; Geng, Y.; Chen, T.; Huang, A.; Jiang, F.; Feng, J.P.; et al. Tumor Ablation and Therapeutic Immunity Induction by an Injectable Peptide Hydrogel. ACS Nano 2018, 12, 3295–3310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Deng, L.; Xu, S.; Zhao, X.; Lv, N.; Zhang, G.; Gu, N.; Hu, R.; Zhang, J.; Liu, J.; et al. A reconstituted “two into one” thermosensitive hydrogel system assembled by drug-loaded amphiphilic copolymer nanoparticles for the local delivery of paclitaxel. J. Mater. Chem. B 2013, 1, 552–563. [Google Scholar] [CrossRef]
- Jin, J.F.; Zhu, L.L.; Chen, M.; Xu, H.M.; Wang, H.F.; Feng, X.Q.; Zhu, X.P.; Zhou, Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 9, 923–942. [Google Scholar] [CrossRef]
- Melero, I.; Castanon, E.; Alvarez, M.; Champiat, S.; Marabelle, A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 2021, 18, 558–576. [Google Scholar] [CrossRef]
- Marabelle, A.; Tselikas, L.; de Baere, T.; Houot, R. Intratumoral immunotherapy: Using the tumor as the remedy. Ann. Oncol. 2017, 28, xii33–xii43. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Shive, M.; Bichara, A.; Berrada, M.; Le Garrec, D.; Chenite, A.; Leroux, J.-C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004, 57, 53–63. [Google Scholar] [CrossRef]
- Duffy, C.V.; David, L.; Crouzier, T. Covalently-crosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater. 2015, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, P.; Ng, W.C.; Yan, W.C.; Srinivasan, M.P.; Wang, C.H. Double-Walled Microparticles-Embedded Self-Cross-Linked, Injectable, and Antibacterial Hydrogel for Controlled and Sustained Release of Chemotherapeutic Agents. ACS Appl. Mater. Interfaces 2016, 8, 22785–22800. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef]
- Tahir, D.; Ardiansyah, A.; Heryanto, H.; Noor, E.E.M.; Mohamed, M.A. Chitosan-based hydrogels: A comprehensive review of transdermal drug delivery. Int. J. Biol. Macromol. 2025, 298, 140010. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef]
- Frumento, D.; Țălu, Ș. Immunomodulatory Potential and Biocompatibility of Chitosan–Hydroxyapatite Biocomposites for Tissue Engineering. J. Compos. Sci. 2025, 9, 305. [Google Scholar] [CrossRef]
- Pagar, R.R.; Musale, S.R.; Pawar, G.; Kulkarni, D.; Giram, P.S. Comprehensive Review on the Degradation Chemistry and Toxicity Studies of Functional Materials. ACS Biomater. Sci. Eng. 2022, 8, 2161–2195. [Google Scholar] [CrossRef]
- Witherel, C.E.; Abebayehu, D.; Barker, T.H.; Spiller, K.L. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Healthc. Mater. 2019, 8, 1801451. [Google Scholar] [CrossRef]
- Taylor, M.S.; Daniels, A.U.; Andriano, K.P.; Heller, J. Six bioabsorbable polymers: In vitro acute toxicity of accumulated degradation products. J. Appl. Biomater. 1994, 5, 151–157. [Google Scholar] [CrossRef]
- Kim, M.-N.; Lee, B.-Y.; Lee, I.-M.; Lee, H.-S.; Yoon, J.-S. TOXICITY AND BIODEGRADATION OF PRODUCTS FROM POLYESTER HYDROLYSIS. J. Environ. Sci. Health Part. A 2001, 36, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Innovative Therapeutic Treatments to Inhibit Perineural Invasion in Pancreatic Adenocarcinoma. 2024. Available online: https://www.clinicaltrials.gov/study/NCT06616688?term=inhibit&viewType=Table&checkSpell=&rank=7 (accessed on 31 July 2025).
- Fluorouracil Treatment Via Colon for Colorectal Cancer: An Exploratory Study. 2024. Available online: https://clinicaltrials.gov/study/NCT06385418 (accessed on 31 July 2025).
- Harris, E. Industry update: The latest developments in the field of therapeutic delivery, January 2025. Ther. Deliv. 2025, 16, 407–414. [Google Scholar] [CrossRef]
- Prasad, S.M.; Shishkov, D.; Mihaylov, N.V.; Khuskivadze, A.; Genov, P.; Terzi, V.; Kates, M.; Huang, W.C.; Louie, M.J.; Raju, S.; et al. Primary Chemoablation of Recurrent Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer with UGN-102: A Single-Arm, Open-Label, Phase 3 Trial (ENVISION). J. Urol. 2025, 213, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.H.; Power, J.W.; Dempsey, P.J.; Agnew, A.; Murphy, B.D.; Moore, R.; El Bassiouni, M.; McNicholas, M.M.J. SpaceOAR hydrogel distribution and early complications in patients undergoing radiation therapy for prostate cancer. Br. J. Radiol. 2023, 96, 20220947. [Google Scholar] [CrossRef]
- A Randomized, Controlled, Open-Label Study of the Efficacy, Durability, and Safety of UGN-102 with or Without TURBT in Patients With Low-Grade Intermediate-Risk Non-Muscle Invasive Bladder Cancer (LG-IR-NMIBC). 2020. Available online: https://clinicaltrials.gov/study/NCT04688931 (accessed on 31 July 2025).
- Feasibility and Safety of Local Immunomodulation Combined With Radiofrequency Ablation for Unresectable Colorectal Liver Metastases: A Monocentric Phase I Trial. 2019. Available online: https://clinicaltrials.gov/study/NCT04062721 (accessed on 31 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, T.; Chen, L.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Research Strategies and Methods of Hydrogels for Antitumor Drug Delivery. Biomedicines 2025, 13, 1899. https://doi.org/10.3390/biomedicines13081899
Zeng T, Chen L, Yoshitomi T, Kawazoe N, Yang Y, Chen G. Research Strategies and Methods of Hydrogels for Antitumor Drug Delivery. Biomedicines. 2025; 13(8):1899. https://doi.org/10.3390/biomedicines13081899
Chicago/Turabian StyleZeng, Tianjiao, Lusi Chen, Toru Yoshitomi, Naoki Kawazoe, Yingnan Yang, and Guoping Chen. 2025. "Research Strategies and Methods of Hydrogels for Antitumor Drug Delivery" Biomedicines 13, no. 8: 1899. https://doi.org/10.3390/biomedicines13081899
APA StyleZeng, T., Chen, L., Yoshitomi, T., Kawazoe, N., Yang, Y., & Chen, G. (2025). Research Strategies and Methods of Hydrogels for Antitumor Drug Delivery. Biomedicines, 13(8), 1899. https://doi.org/10.3390/biomedicines13081899








