Morphofunctional Profile Focusing on Strength and Ultrasound of the Upper Limbs in Female Breast Cancer Survivors: A Comparative Cross-Sectional Study Between Groups with and Without Lymphoedema and Between Ipsilateral and Contralateral Limbs
Abstract
1. Introduction
2. Patients and Methods
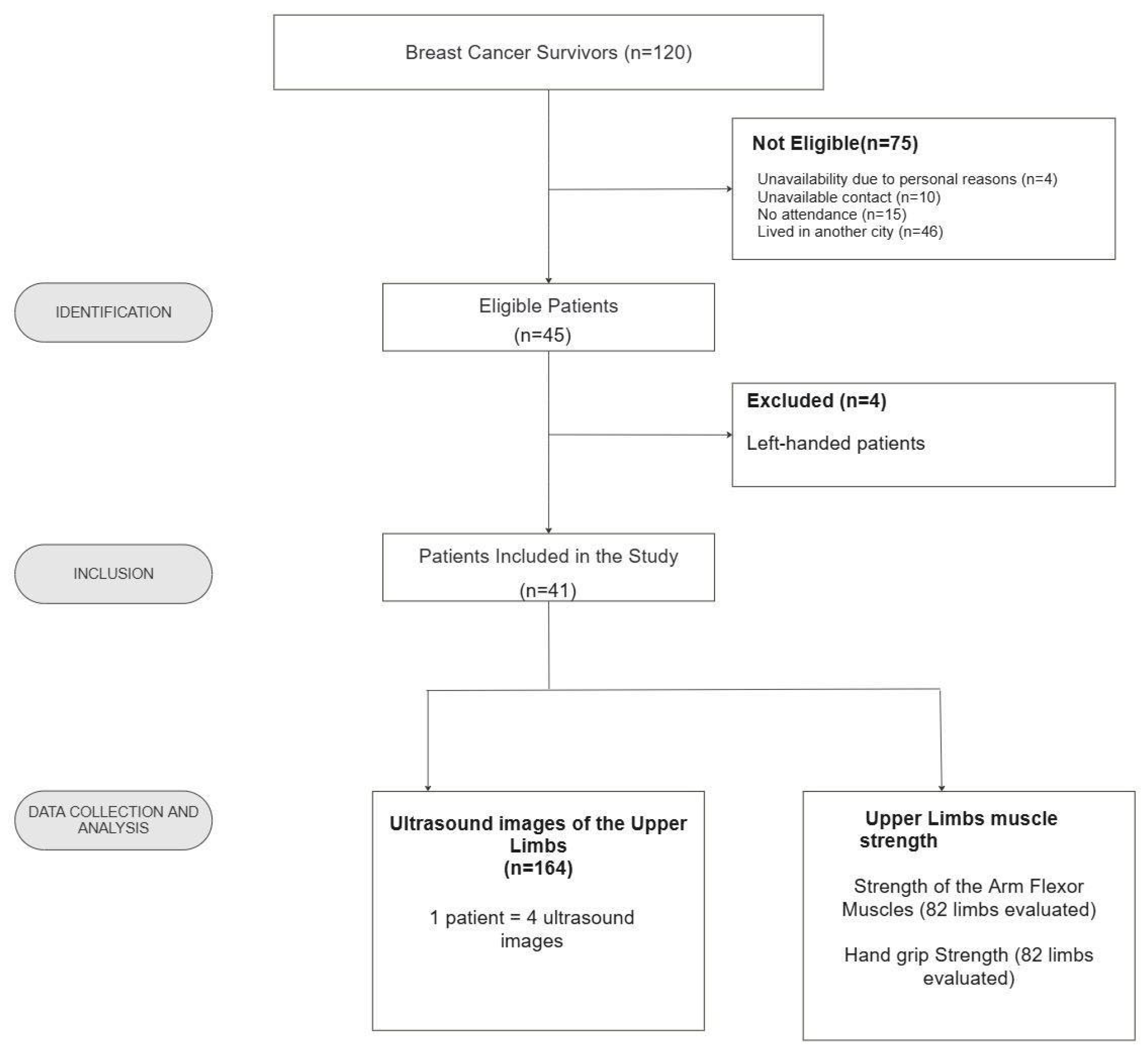
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analyses
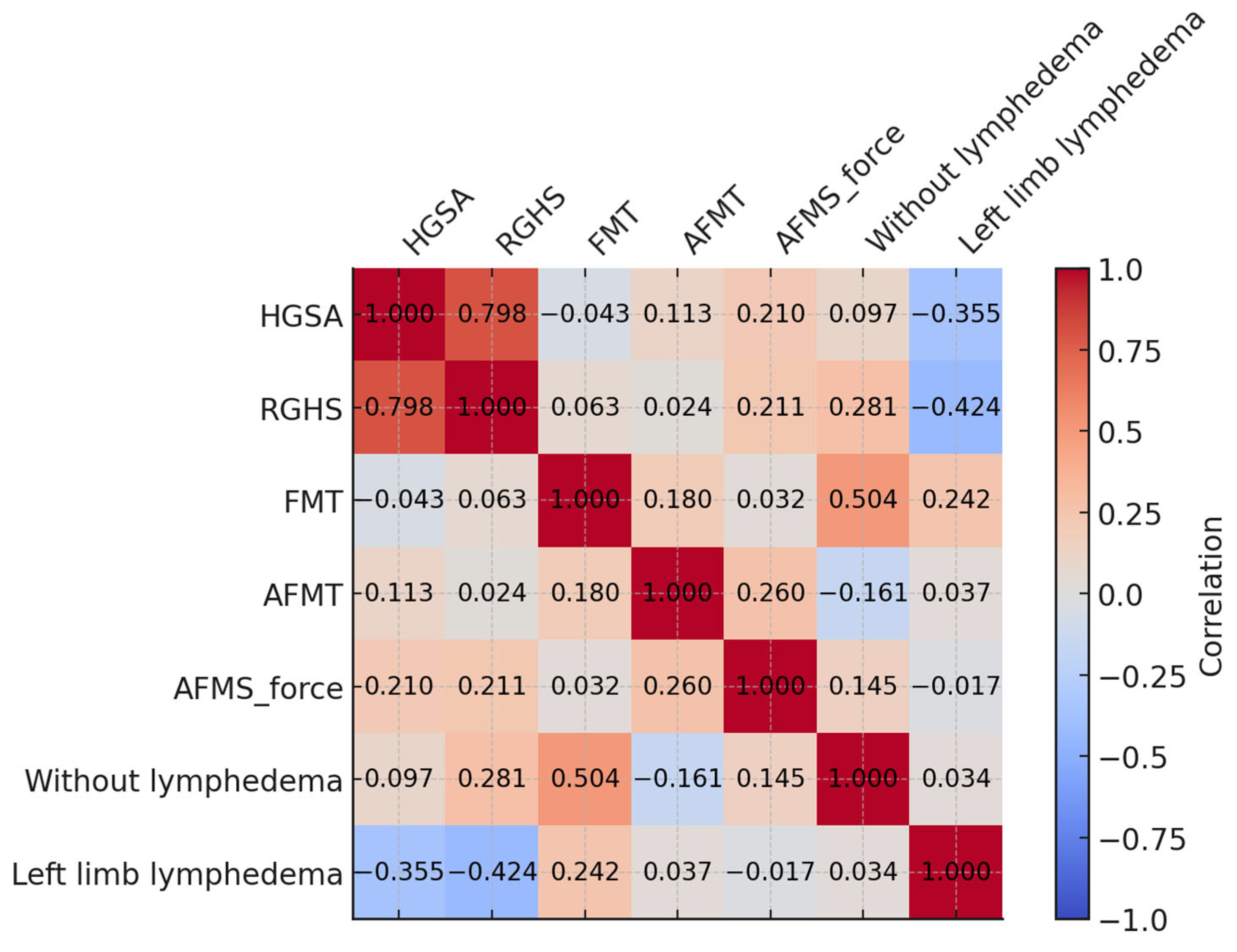
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHGS | absolute handgrip strength |
| BCRL | breast cancer-related lymphoedema |
| BMI | body mass index |
| DEC | dermal–epidermal complex |
| RHGS | relative handgrip strength |
| SCLC | secondary breast cancer lymphoedema |
| SUB | subcutaneous tissue |
| FMT | forearm flexors |
| AFMT | arm flexor muscle thickness |
| MT | muscle thickness |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Instituto Nacional de Câncer—INCA. Estimativa 2023: Incidência de câncer no Brasil; Instituto Nacional De Câncer: Rio de Janeiro, RJ, USA, 2023; ISBN 9786588517093. [Google Scholar]
- Peng, X.; Lu, Z. Development and Validation of Upper Limb Lymphedema in Patients After Breast Cancer Surgery Using a Practicable Machine Learning Model: A Retrospective Cohort Study. Int. J. Gen. Med. 2024, 17, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, B.-E.; Muñoz-García, M.-G.; José-Ochoa, L.-L.; Quiroga-Morales, L.-A.; Cervántes-González, L.-M.; Mireles-Ramírez, M.-A.; Delgadillo-Cristerna, R.; Nuño-Guzmán, C.-M.; Leal-Cortés, C.-A.; Portilla-de-Buen, E.; et al. Role of Incretins in Muscle Functionality, Metabolism, and Body Composition in Breast Cancer: A Metabolic Approach to Understanding This Pathology. Biomedicines 2024, 12, 280. [Google Scholar] [CrossRef]
- Alstrup, M.; Johannessen, A.L.; Mohanakumar, S.; Offersen, B.V.; Hjortdal, V.E. Lymphatic Function in the Arms of Breast Cancer Patients—A Prospective Cohort Study. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3779. [Google Scholar] [CrossRef]
- Mistry, S.; Ali, T.; Qasheesh, M.; Beg, R.A.; Shaphe, M.A.; Ahmad, F.; Kashoo, F.Z.; Shalaby, A.S. Assessment of Hand Function in Women with Lymphadenopathy after Radical Mastectomy. PeerJ 2021, 9, e11252. [Google Scholar] [CrossRef]
- Leonardis, J.M.; Lulic-Kuryllo, T.; Lipps, D.B. The Impact of Local Therapies for Breast Cancer on Shoulder Muscle Health and Function. Crit. Rev. Oncol. Hematol. 2022, 177, 103759. [Google Scholar] [CrossRef]
- Fuentes-Abolafio, I.J.; Roldán-Jiménez, C.; Campos, M.I.; Pajares-Hachero, B.I.; Alba-Conejo, E.; Cuesta-Vargas, A. Forearm Muscle Activity During the Handgrip Test in Breast Cancer Survivors: A Cross-Sectional Study. Clin. Breast Cancer 2023, 23, e175–e181. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Fan, J.; Murakami, S.; Xie, H.; Huo, M. The Factors Influencing Shoulder Mobility Disorders in Patients after Radical Breast Cancer Surgery: A Cross-Sectional Study. Breast Care 2024, 19, 43–48. [Google Scholar] [CrossRef]
- Ramirez-Parada, K.; Gonzalez-Santos, A.; Riady-Aleuy, L.; Pinto, M.P.; Ibañez, C.; Merino, T.; Acevedo, F.; Walbaum, B.; Fernández-Verdejo, R.; Sanchez, C. Upper-Limb Disability and the Severity of Lymphedema Reduce the Quality of Life of Patients with Breast Cancer-Related Lymphedema. Curr. Oncol. Tor. Ont. 2023, 30, 8068–8077. [Google Scholar] [CrossRef] [PubMed]
- Hasanah, U.; Ahmad, M.; Prihantono, P.; Usman, A.N.; Arsyad, A.; Agustin, D.I. The Quality of Life Assessment of Breast Cancer Patients. Breast Dis. 2024, 43, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.S.; Mestriner, C.; Ribeiro, L.T.N.; Grillo, F.W.; Lemos, T.W.; Carneiro, A.A.; Guirro, R.R.D.J.; Guirro, E.C.O. Relationship between lymphedema after breast cancer treatment and biophysical characteristics of the affected tissue. PLoS ONE 2022, 17, e0264160. [Google Scholar] [CrossRef]
- Klein, I.; Friger, M.; David, M.B.; Shahar, D. Risk Factors for Long-Term Arm Morbidities Following Breast Cancer Treatments: A Systematic Review. Oncotarget 2023, 14, 921–942. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE Guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Perez, C.S.; das Neves, L.M.S.; Vacari, A.L.; de Cássia Registro Fonseca, M.; de Jesus Guirro, R.R.; de Oliveira Guirro, E.C. Reduction in Handgrip Strength and Electromyographic Activity in Women with Breast Cancer. J. Back. Musculoskelet. Rehabil. 2018, 31, 447–452. [Google Scholar] [CrossRef]
- International Society of Lymphology, 2023. Diagnostic Criteria for Breast Cancer-Related Lymphedema of the Upper Extremity: The Need for Universal Agreement|Annals of Surgical Oncology. Available online: https://link.springer.com/article/10.1245/s10434-021-10645-3 (accessed on 21 October 2024).
- Levenhagen, K.; Davies, C.; Perdomo, M.; Ryans, K.; Gilchrist, L. Diagnosis of Upper Quadrant Lymphedema Secondary to Cancer: Clinical Practice Guideline From the Oncology Section of the American Physical Therapy Association. Phys. Ther. 2017, 97, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, S.; Pai, G.M.; Verma, V.; Rajappa, S.; Bhat, A.; Gaba, S.; Thatte, M. Normative Data of Grip Strength and Pinch Strength in the Indian Population. Indian. J. Plast. Surg. Off. Publ. Assoc. Plast. Surg. India 2024, 57, 256–262. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.F.; Vieira, M.C.A.; do Nascimento, R.A.; Moreira, M.A.; da Câmara, S.M.A.; Maciel, Á.C.C. Relationship between Strength and Muscle Mass in Middle-Aged and Elderly Women: A Cross-Sectional Study. Rev. Bras. Geriatr. Gerontol. Online 2017, 20, 660–669. [Google Scholar] [CrossRef]
- Choquette, S.; Bouchard, D.R.; Doyon, C.Y.; Sénéchal, M.; Brochu, M.; Dionne, I.J. Relative Strength as a Determinant of Mobility in Elders 67-84 Years of Age. a Nuage Study: Nutrition as a Determinant of Successful Aging. J. Nutr. Health Aging 2010, 14, 190–195. [Google Scholar] [CrossRef]
- Esteban-Simón, A.; Díez-Fernández, D.M.; Artés-Rodríguez, E.; Casimiro-Artés, M.Á.; Rodríguez-Pérez, M.A.; Moreno-Martos, H.; Casimiro-Andújar, A.J.; Soriano-Maldonado, A. Absolute and Relative Handgrip Strength as Indicators of Self-Reported Physical Function and Quality of Life in Breast Cancer Survivors: The EFICAN Study. Cancers 2021, 13, 5292. [Google Scholar] [CrossRef]
- Nepomuceno Júnior, B.R.V.; Menezes, M.P.D.S.; Santos, K.R.B.D.; Gomes Neto, M. Comparison of Methods for Evaluating Upper Limb Strength by Hand-Held Dynamometry. Rev. Bras. Med. Esporte 2021, 27, 42–48. [Google Scholar] [CrossRef]
- Jeon, Y.; Beom, J.; Ahn, S.; Bok, S.-K. Ultrasonographic Evaluation of Breast Cancer-Related Lymphedema. J. Vis. Exp. JoVE 2017, 119, 54996. [Google Scholar] [CrossRef]
- Devoogdt, N.; Pans, S.; De Groef, A.; Geraerts, I.; Christiaens, M.-R.; Neven, P.; Vergote, I.; Van Kampen, M. Postoperative Evolution of Thickness and Echogenicity of Cutis and Subcutis of Patients With and Without Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2014, 12, 23–31. [Google Scholar] [CrossRef]
- Suehiro, K.; Morikage, N.; Yamashita, O.; Harada, T.; Samura, M.; Takeuchi, Y.; Mizoguchi, T.; Nakamura, K.; Hamano, K. Skin and Subcutaneous Tissue Ultrasonography Features in Breast Cancer-Related Lymphedema. Ann. Vasc. Dis. 2016, 9, 312–316. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, C.-H.; Heo, S.J.; Moon, M.-H. The Clinical Usefulness of Lymphedema Measurement Technique Using Ultrasound. Lymphat. Res. Biol. 2021, 19, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Meza-Valderrama, D.; Sánchez- Rodríguez, D.; Perkisas, S.; Duran, X.; Bastijns, S.; Dávalos-Yerovi, V.; Da Costa, E.; Marco, E. The Feasibility and Reliability of Measuring Forearm Muscle Thickness by Ultrasound in a Geriatric Inpatient Setting: A Cross-Sectional Pilot Study. BMC Geriatr. 2022, 22, 137. [Google Scholar] [CrossRef]
- Hadda, V.; Kumar, R.; Hussain, T.; Khan, M.A.; Madan, K.; Mohan, A.; Khilnani, G.C.; Guleria, R. Reliability of Ultrasonographic Arm Muscle Thickness Measurement by Various Levels of Health Care Providers in ICU. Clin. Nutr. ESPEN 2018, 24, 78–81. [Google Scholar] [CrossRef]
- Nelson, C.M.; Dewald, J.P.A.; Murray, W.M. In Vivo Measurements of Biceps Brachii and Triceps Brachii Fascicle Lengths Using Extended Field-of-View Ultrasound. J. Biomech. 2016, 49, 1948–1952. [Google Scholar] [CrossRef]
- Fisher, M.I.; Capilouto, G.; Malone, T.; Bush, H.; Uhl, T.L. Comparison of Upper Extremity Function in Women With and Women Without a History of Breast Cancer. Phys. Ther. 2020, 100, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Dancey, C.P.; Reidy, J. Estatistica Sem Matematica Para Psicologia: Usando SPSS Para Windows; Artmed: Porto Alegre, RS, Brazil, 2006. [Google Scholar]
- Zhang, X.-M.; Zhang, Z.-B.; Chen, W.; Wu, X. The Association between Handgrip Strength and Depression in Cancer Survivors: A Cross-Sectional Study. BMC Geriatr. 2022, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.; Silva, A.C.; Bergmann, A.; Araujo, C.M.; Montenegro, A.K.S.; Tenorio, A.D.S.; Dantas, D. Association of Handgrip Strength with Quality of Life in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 3237–3245. [Google Scholar] [CrossRef]
- Baklaci, M.; Eyigör, S.; Tanıgör, G.; Özgür İnbat, M.; Çalışkan Kabayel, S. Assessment of Muscle Strength and Volume Changes in Patients with Breast Cancer-Related Lymphedema. Oncol. Res. Treat. 2020, 43, 584–591. [Google Scholar] [CrossRef]
- Fretta, T.D.B.; Boing, L.; Leite, B.; Vieira, M.D.C.S.; Moratelli, J.; Klen, J.A.; Campeiz, E.; Machado, Z.; Guimarães, A.C.D.A. Physical Functionality of the Upper Limb after Breast Cancer Surgery in Southern Brazilian Survivors: Cross-Sectional Study. Rev. Bras. Cancerol. 2021, 67, e-021168. [Google Scholar] [CrossRef]
- Kang, S.; Yoo, S.; Baek, H.; Lee, J.; Choi, Y.; Kim, H.; Yi, H.; Yang, E.J. Potentials of Smart Dynamometer Use for Clinical and Self-Management of Rehabilitation in Breast Cancer Survivors: A Feasibility Study. Biomed. Eng. Lett. 2019, 9, 211–219. [Google Scholar] [CrossRef]
- Gomes, P.R.L.; Freitas Junior, I.F.; da Silva, C.B.; Gomes, I.C.; Rocha, A.P.R.; Salgado, A.S.I.; do Carmo, E.M. Short-Term Changes in Handgrip Strength, Body Composition, and Lymphedema Induced by Breast Cancer Surgery. Rev. Bras. Ginecol. E Obstet. Rev. Fed. Bras. Soc. Ginecol. E Obstet. 2014, 36, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Leray, H.; Malloizel-Delaunay, J.; Lusque, A.; Chantalat, E.; Bouglon, L.; Chollet, C.; Chaput, B.; Garmy-Susini, B.; Yannoutsos, A.; Vaysse, C. Body Mass Index as a Major Risk Factor for Severe Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2020, 18, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Yusof, K.M.; Avery-Kiejda, K.A.; Ahmad Suhaimi, S.; Ahmad Zamri, N.; Rusli, M.E.F.; Mahmud, R.; Saini, S.M.; Abdul Wahhab Ibraheem, S.; Abdullah, M.; Rosli, R. Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors. Diagnostics 2021, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; von Atzigen, J.; Kaiser, B.; Grünherz, L.; Kim, B.-S.; Giovanoli, P.; Lindenblatt, N.; Gousopoulos, E. Is Lymphedema a Systemic Disease? A Paired Molecular and Histological Analysis of the Affected and Unaffected Tissue in Lymphedema Patients. Biomolecules 2022, 12, 1667. [Google Scholar] [CrossRef]
- Ricci, V.; Ricci, C.; Gervasoni, F.; Giulio, C.; Farì, G.; Andreoli, A.; Özçakar, L. From Physical to Ultrasound Examination in Lymphedema: A Novel Dynamic Approach. J. Ultrasound 2022, 25, 757–763. [Google Scholar] [CrossRef]
- Han, N.; Cho, Y.; Hwang, J.; Kim, H.; Cho, G. Usefulness of Ultrasound Examination in Evaluation of Breast Cancer-Related Lymphedema. J. Korean Acad. Rehabil. Med. 2011, 35, 101–109. [Google Scholar]
- Alabadi, B.; Bastijns, S.; Cock, A.-M.D.; Civera, M.; Real, J.T.; Perkisas, S. Relation Between Ultrasonographic Measurements of the Biceps Brachii and Total Muscle Mass in Older Hospitalized Persons: A Pilot Study. J. Frailty Sarcopenia Falls 2024, 9, 25–31. [Google Scholar] [CrossRef]
- Baran, E.; Özçakar, L.; Özgül, S.; Aksoy, S.; Akbayrak, T. Upper Limb Sensory Evaluations and Ultrasonographic Skin Measurements in Breast Cancer–Related Lymphedema Receiving Complex Decongestive Physiotherapy. Support. Care Cancer 2021, 29, 6545–6553. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, S.; Whyte, J.; Boily, M.; Towers, A.; Kilgour, R.D.; Rivaz, H. Segmentation of Arm Ultrasound Images in Breast Cancer-Related Lymphedema: A Database and Deep Learning Algorithm. IEEE Trans. Biomed. Eng. 2023, 70, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Azhar, S.H.; Lim, H.Y.; Tan, B.-K.; Angeli, V. The Unresolved Pathophysiology of Lymphedema. Front. Physiol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Polat, A.V.; Ozturk, M.; Polat, A.K.; Karabacak, U.; Bekci, T.; Murat, N. Efficacy of Ultrasound and Shear Wave Elastography for the Diagnosis of Breast Cancer-Related Lymphedema. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2020, 39, 795–803. [Google Scholar] [CrossRef]
- Laett, C.; Gavilão, U.; do Rio, J.; Cossich, V.; de Oliveira, C.G. Relationship between Upper and Lower Limbs Muscle Explosive Strength with the Vastus Lateralis and Biceps Brachii Architecture. Rev. Bras. Ciênc. Esporte 2021, 43, e012820. [Google Scholar] [CrossRef]
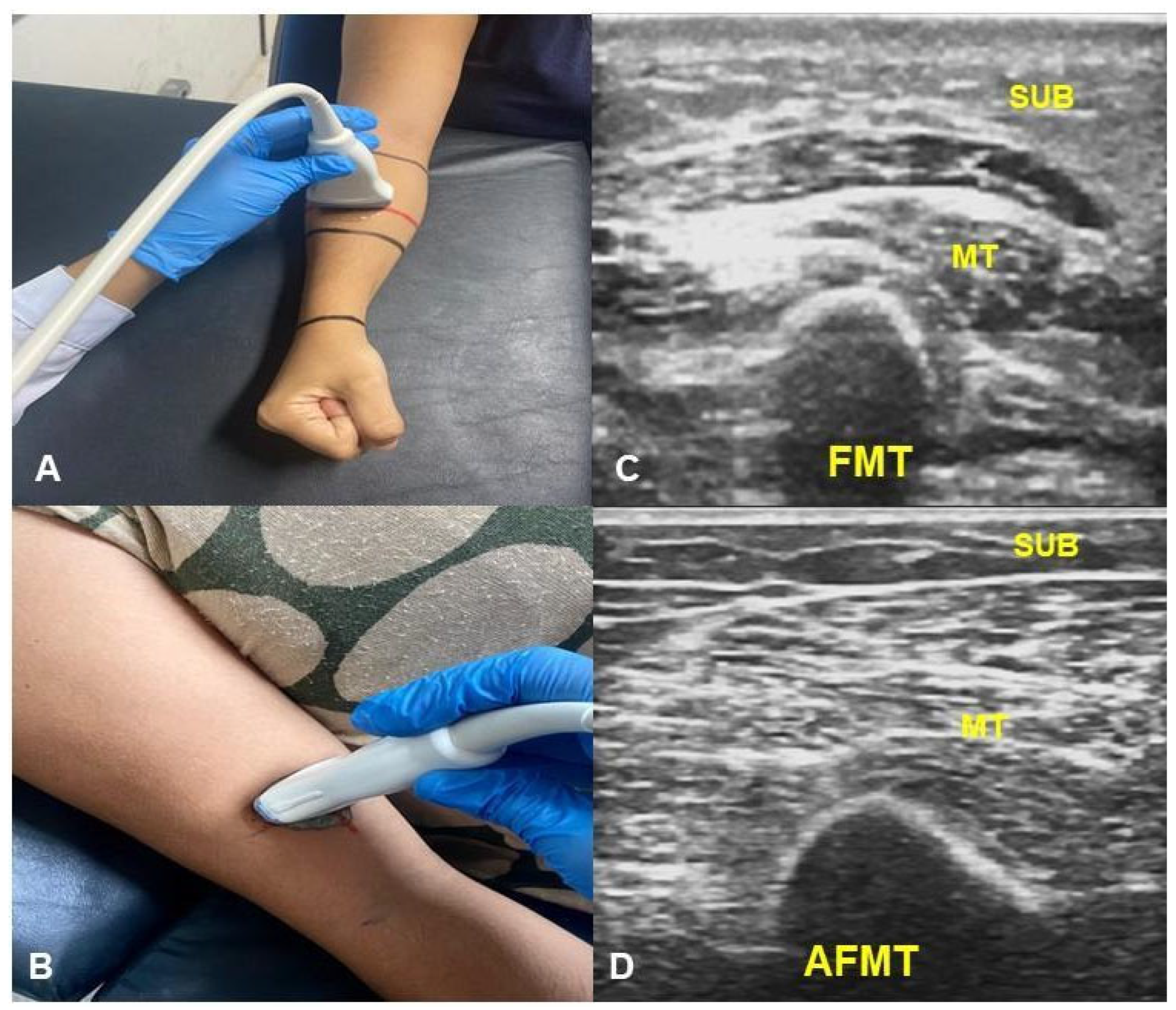
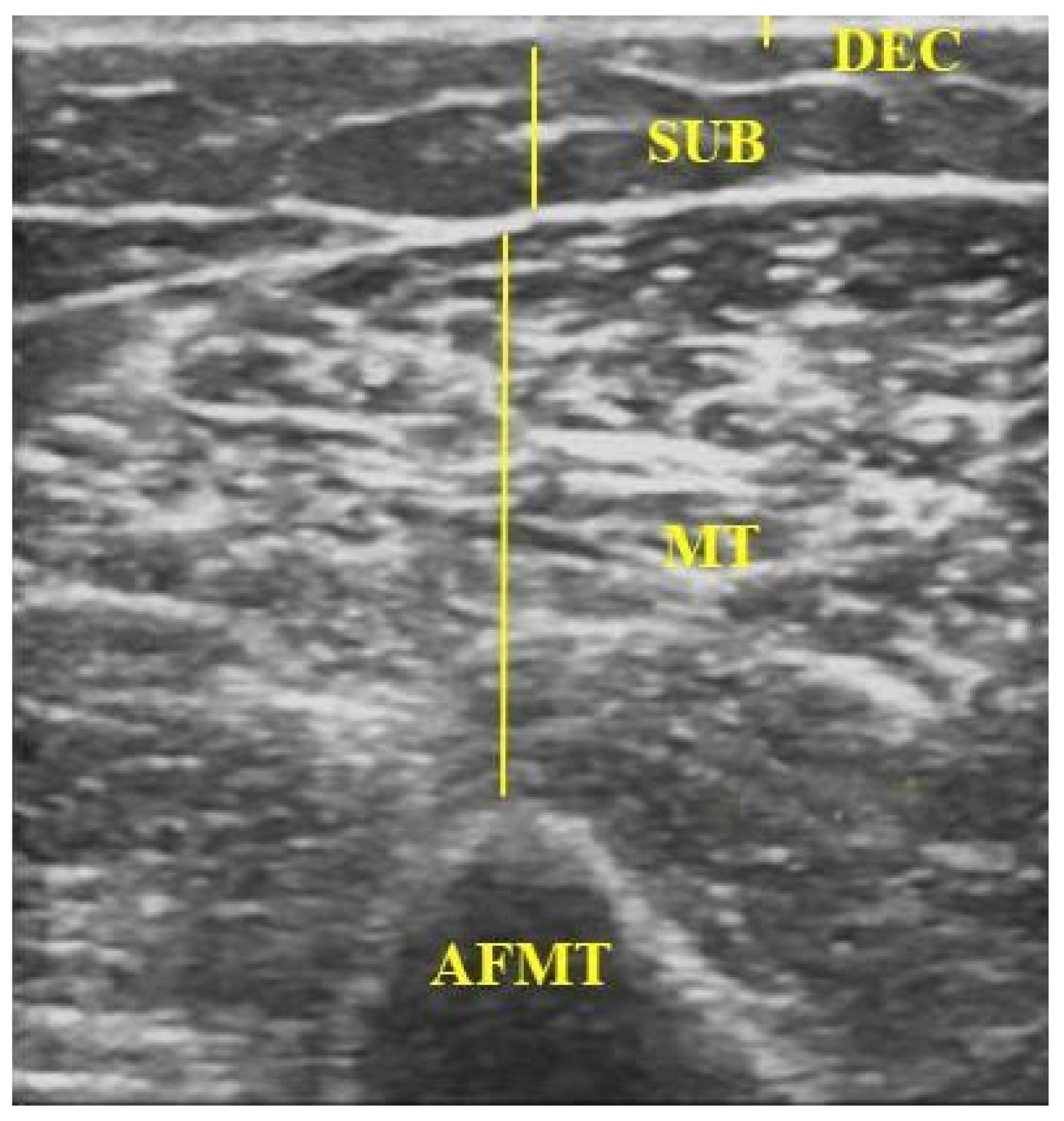
| Variable | n (%) | Mean (SD) |
|---|---|---|
| Age (years) | 53.8 (7.5) | |
| BMI (kg/m2) | 27.7 (5.0) | |
| Limb with lymphoedema | ||
| Right (dominant) | 23 (56.1) | |
| Left | 18 (43.9) | |
| Time since surgery (months) | 76.2 (75.6) | |
| Type of mastectomy | ||
| Simple | 25 (61.0) | |
| Modified | 2 (2.5) | |
| Radical | 14 (36.5) | |
| Therapies received | ||
| Chemotherapy | 38 (92.7) | |
| Radiotherapy | 37 (90.2) | |
| Hormonal | 16 (39.0) | |
| Lymphoedema | ||
| Yes | 19 (46.3) | |
| No | 22 (53.7) |
| Variables | IL Mean (SD) | CL Mean (SD) | p-Value | Mean Difference (95% CI) |
|---|---|---|---|---|
| AFMS | ||||
| Mean force (N) | 73.23 (25.61) | 83.97 (41.85) | <0.001 | −5.91 (−9.77 to −2.50) |
| Peak force (N) | 92.84 (34.97) | 101.83 (35.21) | <0.001 | −7.55 (−12.25 to −3.18) |
| Time to peak (s) | 2.35 (0.53) | 2.31 (0.53) | 0.936 | 0.01 (−0.12 to 0.15) |
| HGS | ||||
| AHGS (kg) * | 22.48 (5.49) | 25.14 (5.83) | <0.001 | −2.66 (−4.10 to −1.22) |
| RHGS (kg/m2) * | 0.81 (0.24) | 0.90 (0.24) | <0.001 | −0.09 (−0.15 to −0.04) |
| US | ||||
| FMT (mm) | ||||
| DEC | 1.40 (1.10) | 1.00 (0.20) | 0.022 | 0.20 (<0.01 to 0.55) |
| SUB | 5.25 (2.01) | 4.51 (1.87) | 0.011 | 0.65 (0.15 to 1.10) |
| MT | 11.17 (4.04) | 12.87(5.13) | 0.002 | −1.80 (−2.75 to −0.60) |
| AFMT (mm) | ||||
| DEC | 1.25 (0.45) | 1.02 (0.24) | <0.001 | 0.25 (0.15 to 0.40) |
| SUB | 6.12 (3.16) | 5.79 (2.43) | 0.075 | 0.30 (<−0.01 to 0.60) |
| MT | 15.00 (4.34) | 14.18 (5.00) | 0.937 | 2.73 × 10−5 (−0.85 to 1.15) |
| Variables | Lymphoedema (n = 19) | Without Lymphoedema (n = 22) | p-Value | Mean Difference (95% CI) |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| AFMS | ||||
| Mean force (N) | 72.63 (24.17) | 87.36 (51.49) | 0.367 | 14.72 (−11.37 to 40.82) |
| Peak force (N) | 91.98 (33.71) | 101.57 (96.03) | 0.302 | 9.59 (−11.25 to 30.44) |
| Time to peak (s) | 2.11 (0.54) | 2.43 (0.52) | 0.204 | 0.31 (−0.02 to 0.65) |
| HGS | ||||
| AHGS (kg) * | 22.03 (5.22) | 22.88 (5.81) | 0.628 * | 0.85 (−2.67 to 4.37) |
| RHGS (kg/m2) * | 0.75 (0.23) | 0.87 (0.25) | 0.128 | 0.12 (−0.04 to 0.27) |
| US | ||||
| FMT (mm) | ||||
| DEC | 1.96 (1.42) | 0.94 (0.35) | <0.001 | 0.70 (0.40 to 1.1) |
| SUB | 5.76 (2.29) | 4.77 (1.69) | 0.374 | 0.37 (−0.60 to 2.00) |
| MT | 9.27 (3.22) | 12.80 (4.12) | 0.001 | −3.71 (−5.70 to −0.90) |
| AFMT (mm) | ||||
| DEC * | 1.53 (0.46) | 1.01 (0.27) | <0.001 | 0.51 (0.27 to 0.75) |
| SUB | 7.07 (3.94) | 5.30 (2.12) | 0.084 | 1.2 (−0.20 to 2.70) |
| MT * | 15.76 (5.39) | 14.49 (3.20) | 0.357 | 1.27 (−1.48 to 4.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.R.C.D.; Maia, J.N.; Gomes, V.M.D.S.A.; Tenório, N.; Barbosa, J.F.d.S.; Silva, A.C.S.d.; Sousa, V.P.S.d.; Barbosa, L.M.A.; Andrade, A.d.F.D.d.; Dantas, D. Morphofunctional Profile Focusing on Strength and Ultrasound of the Upper Limbs in Female Breast Cancer Survivors: A Comparative Cross-Sectional Study Between Groups with and Without Lymphoedema and Between Ipsilateral and Contralateral Limbs. Biomedicines 2025, 13, 1884. https://doi.org/10.3390/biomedicines13081884
Silva ARCD, Maia JN, Gomes VMDSA, Tenório N, Barbosa JFdS, Silva ACSd, Sousa VPSd, Barbosa LMA, Andrade AdFDd, Dantas D. Morphofunctional Profile Focusing on Strength and Ultrasound of the Upper Limbs in Female Breast Cancer Survivors: A Comparative Cross-Sectional Study Between Groups with and Without Lymphoedema and Between Ipsilateral and Contralateral Limbs. Biomedicines. 2025; 13(8):1884. https://doi.org/10.3390/biomedicines13081884
Chicago/Turabian StyleSilva, Ana Rafaela Cardozo Da, Juliana Netto Maia, Vanessa Maria Da Silva Alves Gomes, Naiany Tenório, Juliana Fernandes de Souza Barbosa, Ana Claudia Souza da Silva, Vanessa Patrícia Soares de Sousa, Leila Maria Alvares Barbosa, Armèle de Fátima Dornelas de Andrade, and Diego Dantas. 2025. "Morphofunctional Profile Focusing on Strength and Ultrasound of the Upper Limbs in Female Breast Cancer Survivors: A Comparative Cross-Sectional Study Between Groups with and Without Lymphoedema and Between Ipsilateral and Contralateral Limbs" Biomedicines 13, no. 8: 1884. https://doi.org/10.3390/biomedicines13081884
APA StyleSilva, A. R. C. D., Maia, J. N., Gomes, V. M. D. S. A., Tenório, N., Barbosa, J. F. d. S., Silva, A. C. S. d., Sousa, V. P. S. d., Barbosa, L. M. A., Andrade, A. d. F. D. d., & Dantas, D. (2025). Morphofunctional Profile Focusing on Strength and Ultrasound of the Upper Limbs in Female Breast Cancer Survivors: A Comparative Cross-Sectional Study Between Groups with and Without Lymphoedema and Between Ipsilateral and Contralateral Limbs. Biomedicines, 13(8), 1884. https://doi.org/10.3390/biomedicines13081884





