Mechanism of RCD and the Role of Different Death Signaling Pathways in Cancer
Abstract
1. Introduction
2. Diversity and Pathway of RCD
3. Cancer Regulation with RCD
3.1. Autophagy
3.1.1. Tumor-Suppressive Mechanisms of Autophagy
- a.
- Clearance of Damaged Mitochondria (Mitophagy)
- b.
- Degradation of Misfolded Proteins
3.1.2. Induction of Apoptosis and Senescence
- a.
- p53-Dependent Pathways
- b.
- Autophagic Cell Death
- c.
- Cell Cycle Regulation
3.1.3. Suppression of Protumor Inflammation
- a.
- Degradation of Inflammasomes
- b.
- Immunomodulation of the Tumor Microenvironment
3.1.4. Supporting Tumor Cell Survival
- a.
- Nutrient Recycling
- a.
- Resistance to Metabolic Stress
- Metabolic Stress
- Hypoxic Stress
- Oxidative Stress
- Endoplasmic Reticulum Stress
3.1.5. Drug Resistance and Immune Evasion
- a.
- Chemoresistance
- b.
- Immune Evasion
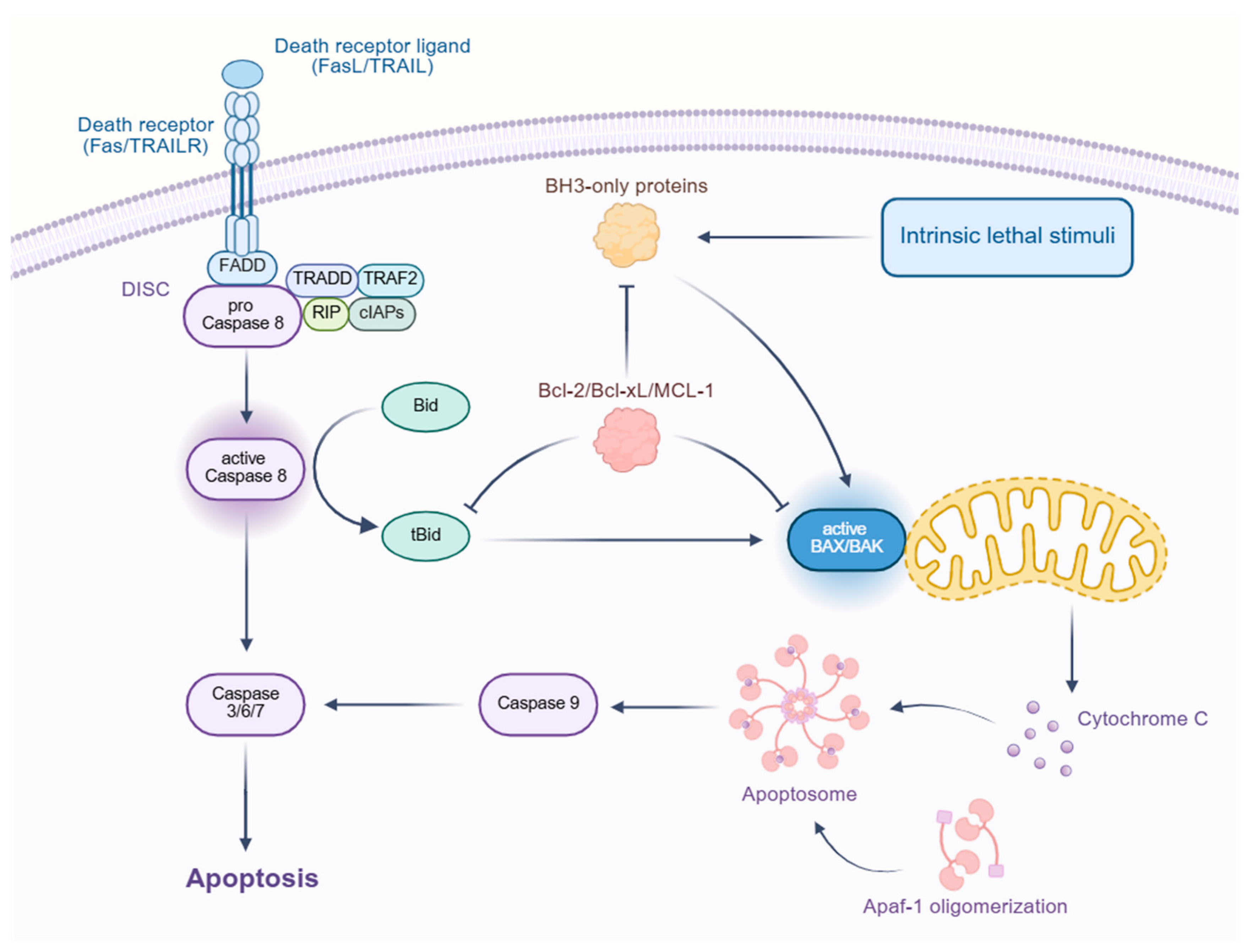
3.2. Apoptosis
3.2.1. Abnormalities in Apoptosis Proteins
3.2.2. Abnormalities in Apoptosis Signaling Pathways
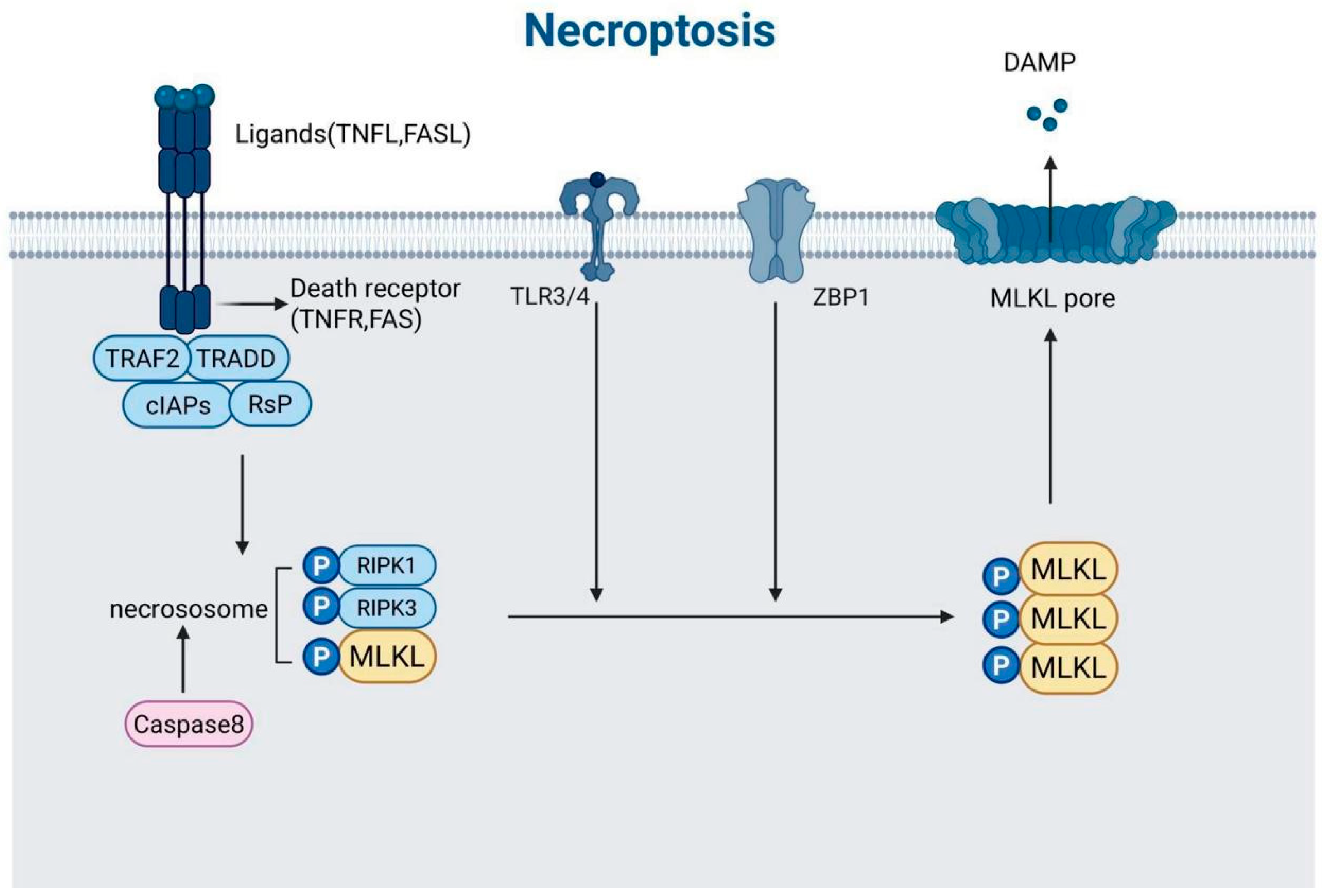
3.3. Necroptosis
3.3.1. Immune Regulation (RIPK3 Expression)
3.3.2. Tumor Metastasis
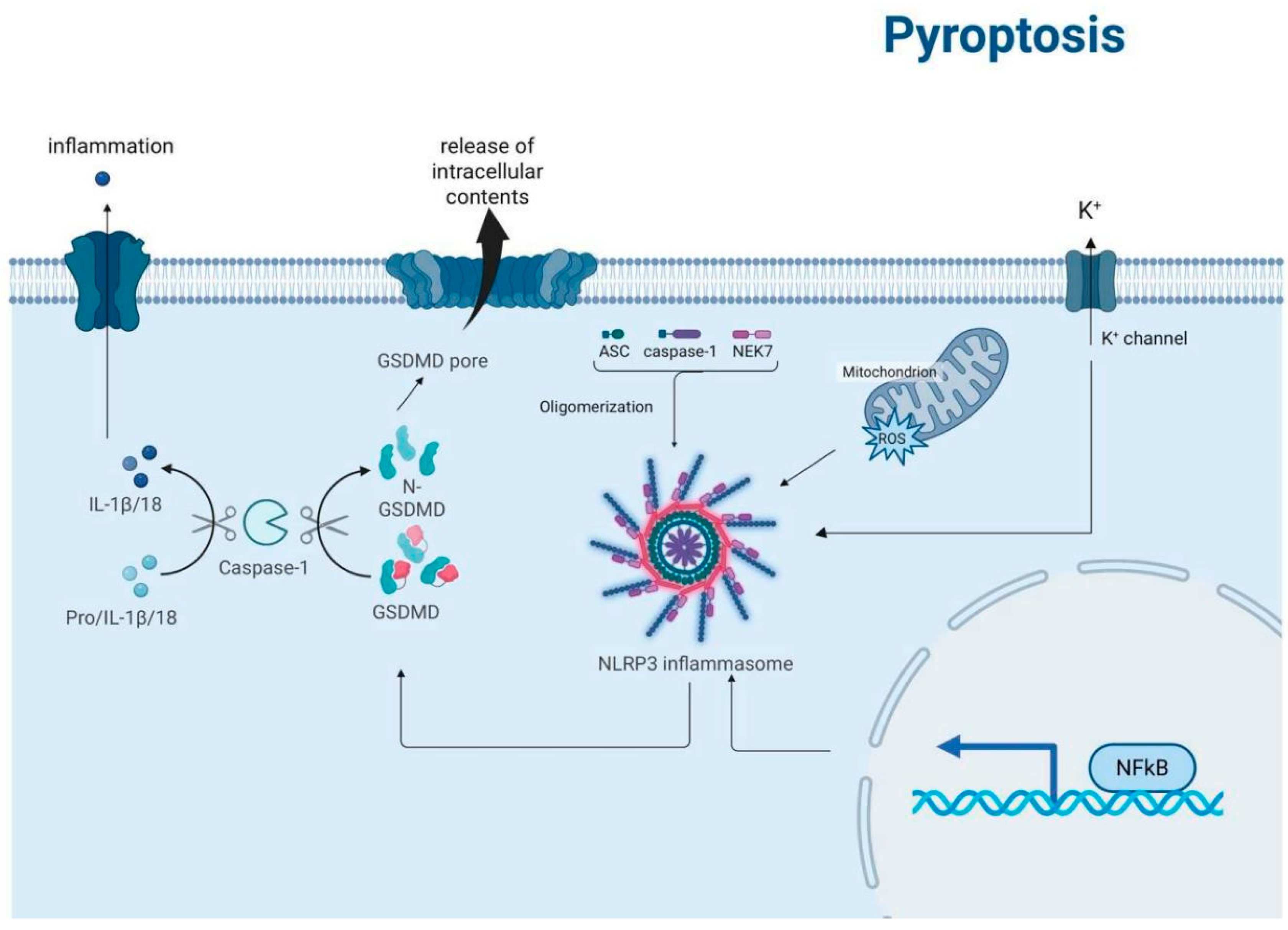
3.4. Pyroptosis
3.4.1. Tumor-Suppressing Effects
3.4.2. Tumor-Promoting Effects
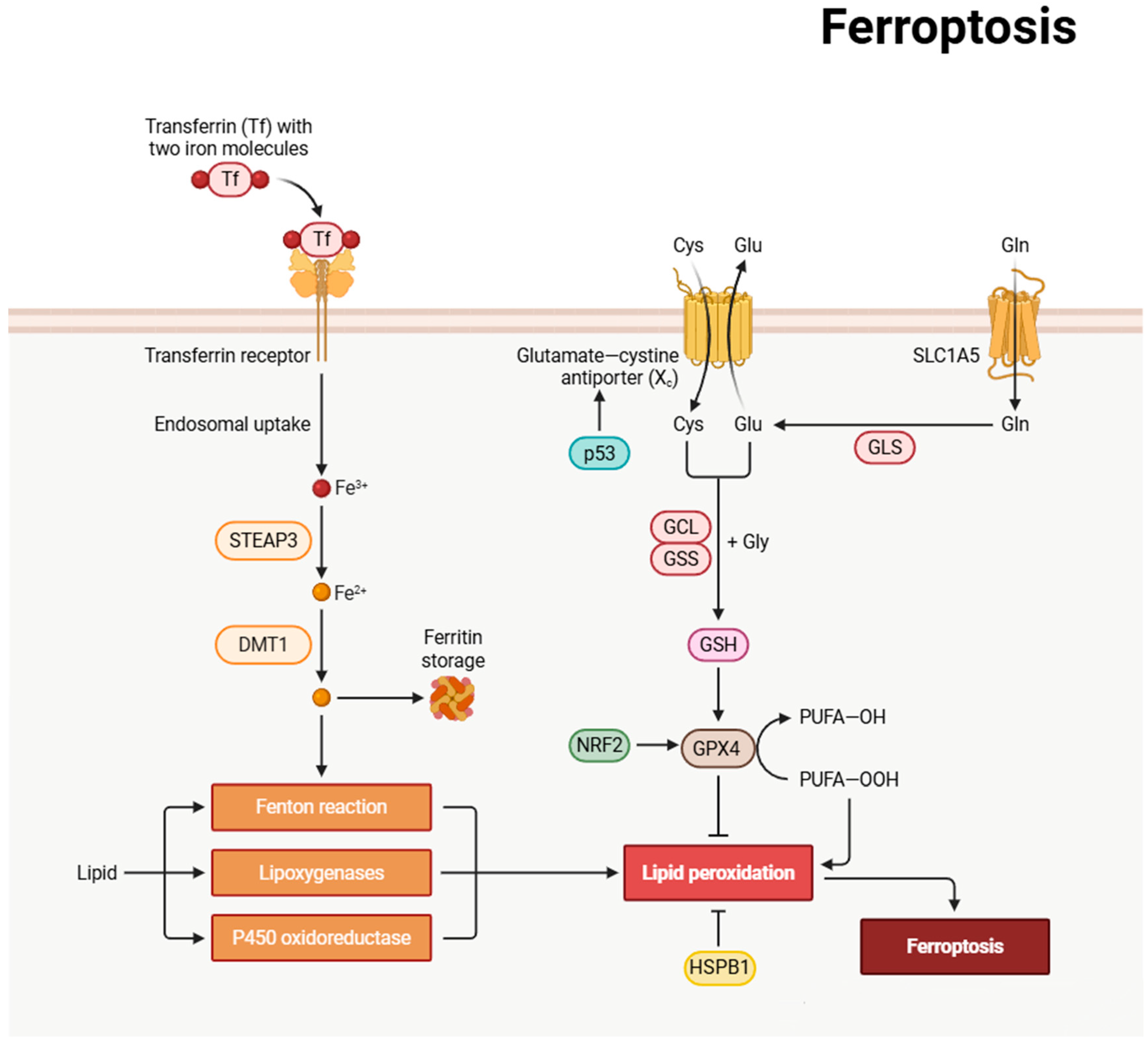
3.5. Ferroptosis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. Global cancer burden growing, amidst mounting need for services. Saudi Med. J. 2024, 45, 326–327. [Google Scholar]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer. 2025, 156, 1336–1346. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Morishita, H.; Mizushima, N. Diverse Cellular Roles of Autophagy. Annu. Rev. Cell Dev. Biol. 2019, 35, 453–475. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Hara, T.; Takamura, A.; Kishi, C.; Iemura, S.; Natsume, T.; Guan, J.L.; Mizushima, N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell Biol. 2008, 181, 497–510. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Nishimura, T.; Tooze, S.A. Emerging roles of ATG proteins and membrane lipids in autophagosome formation. Cell Discov. 2020, 6, 32. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 1743. [Google Scholar] [CrossRef]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [CrossRef]
- Nuñez-Olvera, S.I.; Gallardo-Rincón, D.; Puente-Rivera, J.; Salinas-Vera, Y.M.; Marchat, L.A.; Morales-Villegas, R.; López-Camarillo, C. Autophagy Machinery as a Promising Therapeutic Target in Endometrial Cancer. Front. Oncol. 2019, 9, 1326. [Google Scholar] [CrossRef]
- Liu, M.Y.; Liu, F.; Li, Y.J.; Yin, J.N.; Gao, Y.L.; Wang, X.Y.; Yang, C.; Liu, J.G.; Li, H.J. Ginsenoside Rg5 Inhibits Human Osteosarcoma Cell Proliferation and Induces Cell Apoptosis through PI3K/Akt/mTORC1-Related LC3 Autophagy Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5040326. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Q.; Luo, L.; Ning, B.; Fang, Y. β-asarone inhibited cell growth and promoted autophagy via P53/Bcl-2/Bclin-1 and P53/AMPK/mTOR pathways in Human Glioma U251 cells. J. Cell. Physiol. 2018, 233, 2434–2443. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar]
- Zhang, Y.; Liu, D.; Hu, H.; Zhang, P.; Xie, R.; Cui, W. HIF-1α/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2019, 120, 109464. [Google Scholar] [CrossRef]
- Cheng, Z. The FoxO-Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Chen, S.Y.; Ma, D.N.; Chen, Q.D.; Zhang, J.J.; Tian, Y.R.; Wang, Z.C.; Cai, H.; Lin, Y.; Sun, H.C. MicroRNA-200a inhibits cell growth and metastasis by targeting Foxa2 in hepatocellular carcinoma. J. Cancer 2017, 8, 617–625. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Wallach, D.; Kovalenko, A. Keeping inflammation at bay. Published 2014, 3, e02583. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, R.; Gandhi, V. Targeting the apoptosis pathway in hematologic malignancies. Leuk. Lymphoma 2014, 55, 1980–1992. [Google Scholar] [CrossRef]
- Nagata, S. Fas-mediated apoptosis. Adv. Exp. Med. Biol. 1996, 406, 119–124. [Google Scholar]
- Bruckheimer, E.M.; Cho, S.H.; Sarkiss, M.; Herrmann, J.; McDonnell, T.J. The Bcl-2 gene family and apoptosis. Adv. Biochem. Eng. Biotechnol. 1998, 62, 75–105. [Google Scholar]
- Zhang, Y.; Yang, X.; Ge, X.; Zhang, F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 2019, 109, 726–733. [Google Scholar] [CrossRef]
- Hutt, K.J. The role of BH3-only proteins in apoptosis within the ovary. Reproduction 2015, 149, R81–R89. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, J.; Lu, H.; Zhou, X.; Yao, X. The ARTS of p53-dependent mitochondrial apoptosis. J. Mol. Cell Biol. 2022, 14. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Fornace, A.J.J.R. Death and decoy receptors and p53-mediated apoptosis. Leukemia 2000, 14, 1509–1513. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepato-cellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Wang, Y.; Mao, X.; Zhang, Y.; Xia, J. Baicalein induces cervical cancer apoptosis through the NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 5088–5094. [Google Scholar] [CrossRef]
- Yan, J.; Wan, P.; Choksi, S.; Liu, Z.-G. Necroptosis and tumor progression. Trends Cancer 2021, 8, 21–27. [Google Scholar] [CrossRef]
- Shi, F.L.; Yuan, L.S.; Wong, T.S.; Li, Q.; Li, Y.P.; Xu, R.; You, Y.P.; Yuan, T.; Zhang, H.R.; Shi, Z.J.; et al. Dimethyl fumarate inhibits necroptosis and alleviates systemic inflammatory response syndrome by blocking the RIPK1-RIPK3-MLKL axis. Pharmacol. Res. 2023, 189, 106697. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Xu, L.; Ouyang, M.; Wang, D.; Tang, D.; Yang, L.; Xie, M.; Cao, L.; Yang, M. Extracellular HMGB1 prevents necroptosis in acute myeloid leukemia cells. Biomed. Pharmacother. 2019, 112, 108714. [Google Scholar] [CrossRef]
- Chen, C.; Xie, J.; Chen, Z.; Ye, K.; Wu, C.; Dai, X.; Yuan, Y.; Lin, Y.; Wang, Y.; Chen, H.; et al. Role of Z-DNA Binding Protein 1 Sensing Mitochondrial Z-DNA and Triggering Necroptosis in Oxalate-Induced Acute Kidney Injury. J. Am. Soc. Nephrol. 2024, 36, 361–377. [Google Scholar] [CrossRef]
- Newton, K.; Wickliffe, K.E.; Dugger, D.L.; Maltzman, A.; Roose-Girma, M.; Dohse, M.; Kőműves, L.; Webster, J.D.; Dixit, V.M. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 2019, 574, 428–431. [Google Scholar] [CrossRef]
- Nailwal, H.; Chan, F.K. Necroptosis in anti-viral inflammation. Cell Death Differ. 2019, 26, 4–13. [Google Scholar] [CrossRef]
- Cao, L.; Mu, W. Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications. Pharmacol. Res. 2021, 163, 105297. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, H.; Yang, R.; Ji, D.; Xia, X. GSK872 and necrostatin-1 protect retinal ganglion cells against necroptosis through inhibition of RIP1/RIP3/MLKL pathway in glutamate-induced retinal excitotoxic model of glaucoma. J. Neuroinflamm. 2022, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- Weindel, C.G.; Martinez, E.L.; Zhao, X.; Mabry, C.J.; Bell, S.L.; Vail, K.J.; Coleman, A.K.; VanPortfliet, J.J.; Zhao, B.; Wagner, A.R.; et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 2022, 185, 3214–3231.e23. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Song, M.; Song, Y.; Fang, Y.; Zhang, Q.; Li, X.; Song, N.; Ding, J.; Lu, M.; et al. NLRP3/caspase-1/GSDMD–mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. J. Clin. Investig. 2021, 6. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Li, Z.; Hua, Q.; Song, P.; Gao, L.; Zhou, L.; Cai, Q. Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway. Int. J. Mol. Sci. 2023, 24, 14771. [Google Scholar] [CrossRef]
- Rozario, P.; Pinilla, M.; Gorse, L.; Vind, A.C.; Robinson, K.S.; Toh, G.A.; Firdaus, M.J.; Martínez, J.F.; Kerk, S.K.; Lin, Z.; et al. Mechanistic basis for potassium efflux–driven activation of the human NLRP1 inflammasome. Proc. Natl. Acad. Sci. USA 2024, 121, e2309579121. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Wang, X.; Bu, Q.; Wang, Q.; Su, W.; Li, L.; Zhou, H.; Lu, L. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022, 52, 102305. [Google Scholar] [CrossRef]
- Sun, L.; Ma, W.; Gao, W.; Xing, Y.; Chen, L.; Xia, Z.; Zhang, Z.; Dai, Z. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. 2019, 10, 542. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Zhang, G.; Mi, W.; Wang, C.; Li, J.; Zhang, Y.; Liu, N.; Jiang, M.; Jia, G.; Wang, F.; Yang, G.; et al. Targeting AKT induced Ferroptosis through FTO/YTHDF2-dependent GPX4 m6A meth-ylation up-regulating and degradating in colorectal cancer. Cell Death Discov. 2023, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, J.; Hou, G.; Liu, H.; Zheng, S.; Wang, X.; Lin, Q.; Zhang, Y.; Lu, M.; Gong, Y.; et al. SMURF2 predisposes cancer cell toward ferroptosis in GPX4-independent manners by promoting GSTP1 degradation. Mol. Cell 2023, 83, 4352–4369.e8. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Kroemer, G.; Tang, D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021, 28, 1135–1148. [Google Scholar] [CrossRef]
- Awasthi, A.; Maparu, K.; Singh, S. Ferroptosis role in complexity of cell death: Unrevealing mechanisms in Parkinson’s disease and therapeutic approaches. Inflammopharmacology 2025, 33, 1271–1287. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Tian, X.; Miao, Y.; Ma, L.; Zhang, C.; Xu, X.; Wang, J.; Fang, W.; Zhang, X. LPCAT3 Is Transcriptionally Regulated by YAP/ZEB/EP300 and Collaborates with ACSL4 and YAP to Determine Ferroptosis Sensitivity. Antioxid. Redox Signal. 2023, 39, 491–511. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, e1904197. [Google Scholar] [CrossRef]
- Tang, L.J.; Zhou, Y.J.; Xiong, X.M.; Li, N.S.; Zhang, J.J.; Luo, X.J.; Peng, J. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic. Biol. Med. 2021, 162, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Zhang, Y.; Ye, F.; Luo, D.; Li, Y.; Jin, Y.; Han, D.; Wang, Z.; Chen, B.; et al. HSPB1 facilitates chemoresistance through inhibiting ferroptotic cancer cell death and regulating NF-κB signaling pathway in breast cancer. Cell Death Dis. 2023, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Aslkhodapasandhokmabad, H.; Libby, P.; Tuomilehto, J.; Lip, G.Y.; Penninger, J.M.; Richardson, D.R.; Tang, D.; Zhou, H.; Wang, S.; et al. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol. Metab. 2021, 32, 444–462. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, K.; Hu, H.; Zhang, X.; Zeng, S.; Li, J.; Dong, X.; Deng, X.; Zhang, J.; Zhang, Y. METTL17 coordinates ferroptosis and tumorigenesis by regulating mitochondrial translation in col-orectal cancer. Redox Biol. 2024, 71, 103087. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Interactions between reactive oxygen species and autophagy: Special issue: Death mechanisms in cellular homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119041. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Ma, R.X.; Li, J.; Zou, B.; Yang, H.; Ma, R.Y.; Wu, Z.Q.; Li, J.; Yao, Y. Review of PINK1-Parkin-mediated mitochondrial autophagy in Alzheimer’s disease. Eur. J. Pharmacol. 2023, 959, 176057. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; AKellum, J.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2022, 19, 401–414. [Google Scholar] [CrossRef]
- Narendra, D.P.; Youle, R.J. The role of PINK1–Parkin in mitochondrial quality control. Nat. Cell Biol. 2024, 26, 1639–1651. [Google Scholar] [CrossRef]
- Sharma, A.; Alswillah, T.; Kapoor, I.; Debjani, P.; Willard, B.; Summers, M.K.; Gong, Z.; Almasan, A. USP14 is a deubiquitinase for Ku70 and critical determinant of non-homologous end joining repair in autophagy and PTEN-deficient cells. Nucleic Acids Res. 2019, 48, 736–747. [Google Scholar] [CrossRef]
- Mao, L.; Liu, H.; Zhang, R.; Deng, Y.; Hao, Y.; Liao, W.; Yuan, M.; Sun, S. PINK1/Parkin-mediated mitophagy inhibits warangalone-induced mitochondrial apoptosis in breast cancer cells. Aging 2021, 13, 12955–12972. [Google Scholar] [CrossRef]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Münz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Norberg, E.; Vakifahmetoglu-Norberg, H. Mutant p53 as a Regulator and Target of Autophagy. Front. Oncol. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Luo, Y.; Liu, Y.; Yi, Y.; Li, J.; Pan, Y.; Li, W.; You, W.; Hu, Q.; et al. USP5-Beclin 1 axis overrides p53-dependent senescence and drives Kras-induced tumorigenicity. Nat. Commun. 2022, 13, 7799. [Google Scholar] [CrossRef]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar]
- He, W.; Liu, S.; Wei, W.; Qin, R.; Tan, J.; Tang, J.; Huang, Z.; Gao, M. mTOR inhibition by AZD2014 alleviates BCR::ABL1 independent imatinib resistance through enhancing autophagy in CML resistant cells. Am. J. Cancer Res. 2024, 14, 2770–2789. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, Y.; Li, Y.; Wang, Y.; Zhu, L.; Chen, P.; Tian, Z.; Qiu, Y.; Feng, R.; et al. DNA Damage-Regulated Autophagy Modulator 1 (DRAM1) Mediates Autophagy and Apoptosis of Intestinal Epithelial Cells in Inflammatory Bowel Disease. Dig. Dis. Sci. 2020, 66, 3375–3390. [Google Scholar] [CrossRef]
- Wang, J.; Cui, D.; Gu, S.; Chen, X.; Bi, Y.; Xiong, X.; Zhao, Y. Autophagy regulates apoptosis by targeting NOXA for degradation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Tracy, K.; Dibling, B.C.; Spike, B.T.; Knabb, J.R.; Schumacker, P.; Macleod, K.F. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol. Cell. Biol. 2007, 27, 6229–6242. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.B.; Chen, W.; Mai, J.; Wu, X.Q.; Sun, T.; Wu, R.Y.; Jiao, L.; Li, D.D.; Ji, J.; et al. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit au-tophagy and promote tumor progression. Autophagy 2021, 17, 4323–4340. [Google Scholar] [CrossRef]
- Huang, L.; Wang, S.; Li, S.-S.; Yang, X.-M. Prognostic Significance of Beclin-1 Expression in Laryngeal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2013, 19, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; You, L.; Qiao, J.; Han, W.; Pan, H. Autophagy in colitis-associated colon cancer: Exploring its potential role in reducing initiation and preventing IBD-Related CAC development. Autophagy 2023, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chen, Y.; Song, C.; Chen, Y.; Chen, Y.; Ferguson, D.; Yang, Y.; He, A. Autophagy modulates the stability of Wee1 and cell cycle G2/M transition. Biochem. Biophys. Res. Commun. 2023, 677, 63–69. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef] [PubMed]
- López, A.R.; Jørgensen, M.H.; Havelund, J.F.; Arendrup, F.S.; Kolapalli, S.P.; Nielsen, T.M.; Pais, E.; Beese, C.J.; Abdul-Al, A.; Vind, A.C.; et al. Autophagy-mediated control of ribosome homeostasis in onco-gene-induced senescence. Cell Rep. 2023, 42, 113381. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Liu, Y.; Zhao, Y.; Bi, Z.; Yao, Y.; Liu, Q.; Shi, H.; Wang, F.; Wang, Y. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy 2020, 16, 1221–1235. [Google Scholar] [CrossRef]
- Xie, M.; Yang, Z.; Liu, Y.; Zheng, M. The role of HBV-induced autophagy in HBV replication and HBV related-HCC. Life Sci. 2018, 205, 107–112. [Google Scholar] [CrossRef]
- Mommersteeg, M.; Simovic, I.; Yu, B.; van Nieuwenburg, S.; I, M.B.; Doukas, M.; Kuipers, E.; Spaander, M.; Peppelenbosch, M.; Castaño-Rodríguez, N.; et al. Autophagy mediates ER stress and inflammation in Helicobacter pylori -related gastric cancer. Gut Microbes 2021, 14, 2015238. [Google Scholar] [CrossRef]
- Pathak, S.O.; Manohar, S.M. Molecular Milieu of Autophagy in Cervical Cancer and its Therapeutic Implications. Curr. Cancer Drug Targets 2023, 23, 843–857. [Google Scholar] [CrossRef]
- Hu, F.; Song, D.; Yan, Y.; Huang, C.; Shen, C.; Lan, J.; Chen, Y.; Liu, A.; Wu, Q.; Sun, L.; et al. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat. Commun. 2021, 12, 3651. [Google Scholar] [CrossRef]
- Shen, Y.; Malik, S.A.; Amir, M.; Kumar, P.; Cingolani, F.; Wen, J.; Liu, Y.; Zhao, E.; Farris, A.B.; Raeman, R.; et al. Decreased Hepatocyte Autophagy Leads to Synergistic IL-1β and TNF Mouse Liver Injury and Inflammation. Hepatology 2020, 72, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef]
- Smith, A.G.; Macleod, K.F. Autophagy, cancer stem cells and drug resistance. J. Pathol. 2019, 247, 708–718. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Wei, X.; Yu, W.; Min, Z.; Ye, M. The SIRT6-Autophagy-Warburg Effect Axis in Papillary Thyroid Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, T.; Tu, B.; Yuan, M.; Shu, Z.; Fan, M.; Huo, S.; Guo, Y.; Wang, L.; Wang, H.; et al. Autophagy loss impedes cancer-associated fibroblast activation via downregulating proline biosyn-thesis. Autophagy 2023, 19, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, L.; Zhao, J.; Yuan, Y.; Wang, T.; Bian, J.; Liu, Z. MiR-155 promotes cadmium-induced autophagy in rat hepatocytes by suppressing Rheb expression. Ecotoxicol. Environ. Saf. 2021, 227, 112895. [Google Scholar] [CrossRef]
- Jing, Z.; He, X.; Jia, Z.; Sa, Y.; Yang, B.; Liu, P. NCAPD2 inhibits autophagy by regulating Ca(2+)/CAMKK2/AMPK/mTORC1 pathway and PARP-1/SIRT1 axis to promote colorectal cancer. Cancer Lett. 2021, 520, 26–37. [Google Scholar] [CrossRef]
- Gross, A.S.; Graef, M. Mechanisms of Autophagy in Metabolic Stress Response. J. Mol. Biol. 2020, 432, 28–52. [Google Scholar] [CrossRef]
- Hamidi, T.; Cano, C.E.; Grasso, D.; Garcia, M.N.; Sandi, M.J.; Calvo, E.L.; Dagorn, J.-C.; Lomberk, G.; Goruppi, S.; Urrutia, R.; et al. NUPR1 works against the metabolic stress-induced autophagy-associated cell death in pancreatic cancer cells. Autophagy 2013, 9, 95–97. [Google Scholar] [CrossRef]
- Hu, Y.L.; DeLay, M.; Jahangiri, A.; Molinaro, A.M.; Rose, S.D.; Carbonell, W.S.; Aghi, M.K. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012, 72, 1773–1783. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Cai, S.L.; Kim, J.; Nanez, A.; Sahin, M.; MacLean, K.H.; Inoki, K.; Guan, K.L.; Shen, J.; Person, M.D.; et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA 2010, 107, 4153–4158. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Deng, W.Q.; Lyu, W.Y.; Sun, Z.T.; Fan, S.R.; Qi, M.; Qiu, S.H.; Zhu, Y.R.; Lin, J.P.; Chen, M.F.; et al. Erianin inhibits the growth and metastasis through autophagy-dependent ferroptosis in KRAS(G13D) colorectal cancer. Free Radic. Biol Med. 2023, 204, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ming, S.L.; Zhang, S.; Wang, Q.; Zeng, L.; Zhou, L.Y.; Wang, M.D.; Ma, Y.X.; Han, L.Q.; Zhong, K.; Zhu, H.S.; et al. Inhibition of USP14 influences alphaherpesvirus proliferation by degrading viral VP16 protein via ER stress-triggered selective autophagy. Autophagy 2022, 18, 1801–1821. [Google Scholar] [CrossRef]
- Dou, H.; Yang, S.; Hu, Y.; Xu, D.; Liu, L.; Li, X. Sesamin induces ER stress-mediated apoptosis and activates autophagy in cervical cancer cells. Life Sci. 2018, 200, 87–93. [Google Scholar] [CrossRef]
- Jiang, X.; Li, G.; Zhu, B.; Yang, J.; Cui, S.; Jiang, R.; Wang, B. p20BAP31 Induces Autophagy in Colorectal Cancer Cells by Promoting PERK-Mediated ER Stress. Int. J. Mol. Sci. 2024, 25, 5101. [Google Scholar] [CrossRef]
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef]
- Jing, Y.; Liang, W.; Liu, J.; Zhang, L.; Wei, J.; Yang, J.; Zhang, Y.; Huang, Z. Autophagy-mediating microRNAs in cancer chemoresistance. Cell Biol. Toxicol. 2020, 36, 517–536. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Heng, Y.; Liang, Y.; Zhang, J.; Li, L.; Zhang, W.; Jiang, Y.; Wang, S.; Jia, L. Camptothecin Inhibits Neddylation to Activate the Protective Autophagy Through NF-κB/AMPK/mTOR/ULK1 Axis in Human Esophageal Cancer Cells. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- O’donovan, T.R.; Rajendran, S.; O’reilly, S.; O’sullivan, G.C.; McKenna, S.L.; Ulasov, I. Lithium Modulates Autophagy in Esophageal and Colorectal Cancer Cells and Enhances the Efficacy of Therapeutic Agents In Vitro and In Vivo. PLoS ONE 2015, 10, e0134676. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Yang, M.F.; Yu, W.; Tao, H.M. Molecular mechanisms of estrogen receptor β-induced apoptosis and autophagy in tumors: Implication for treating osteosarcoma. J. Int. Med. Res. 2019, 47, 4644–4655. [Google Scholar] [CrossRef]
- Gowda Saralamma, V.V.; Lee, H.J.; Raha, S.; Lee, W.S.; Kim, E.H.; Lee, S.J.; Heo, J.D.; Won, C.; Kang, C.K.; Kim, G.S. Inhibition of IAP’s and activation of p53 leads to caspase-dependent apoptosis in gastric cancer cells treated with Scutellarein. Oncotarget 2018, 9, 5993–6006. [Google Scholar] [CrossRef]
- Cocco, S.; Leone, A.; Roca, M.S.; Lombardi, R.; Piezzo, M.; Caputo, R.; Ciardiello, C.; Costantini, S.; Bruzzese, F.; Sisalli, M.J.; et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J. Transl. Med. 2022, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.E.; Kakiuchi, M.; Gupta, S.; Sohn, A.S.W.; Mukhopadhyay, S.; Lin, E.Y.; Parker, S.J.; et al. Autophagy Promotes Immune Evasion of Pancreatic Cancer by Degrading MHC-I; Springer: Berlin/Heidelberg, Germany, 2020; Volume 581, ISBN 0000007587. [Google Scholar]
- Cheung, P.F.; Yang, J.; Fang, R.; Borgers, A.; Krengel, K.; Stoffel, A.; Althoff, K.; Yip, C.W.; Siu, E.H.L.; Ng, L.W.C.; et al. Progranulin mediates immune evasion of pancreatic ductal adenocarcinoma through regulation of MHCI expression. Nat. Commun. 2022, 13, 156. [Google Scholar] [CrossRef]
- Auzmendi-Iriarte, J.; Matheu, A. Intrinsic role of chaperone-mediated autophagy in cancer stem cell maintenance. Autophagy 2022, 18, 3035–3036. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.A.; Sottoriva, A. Measuring cancer evolution from the genome. J. Pathol. 2017, 241, 183–191. [Google Scholar] [CrossRef]
- Avery-Kiejda, K.A.; Bowden, N.A.; Croft, A.J.; Scurr, L.L.; Kairupan, C.F.; Ashton, K.A.; Talseth-Palmer, B.A.; Rizos, H.; Zhang, X.D.; Scott, R.J.; et al. P53 in human melanoma fails to regulate target genes associated with apoptosis and the cell cycle and may contribute to proliferation. BMC Cancer 2011, 11, 203. [Google Scholar] [CrossRef]
- Slatter, T.L.; Hung, N.; Campbell, H.; Rubio, C.; Mehta, R.; Renshaw, P.; Williams, G.; Wilson, M.; Engelmann, A.; Jeffs, A.; et al. Hyperproliferation, cancer, and inflammation in mice expressing a Δ133p53-like isoform. Blood 2011, 117, 5166–5177. [Google Scholar] [CrossRef] [PubMed]
- Vikhanskaya, F.; Lee, M.K.; Mazzoletti, M.; Broggini, M.; Sabapathy, K. Cancer-derived p53 mutants suppress p53-target gene expres-sion--potential mechanism for gain of function of mutant p53. Nucleic Acids Res. 2007, 35, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Zhang, J.; Liu, Y.; Zhao, B.; Yang, X.; Zhou, W.; Chen, B.; Zhang, S.; Huang, R.; et al. miR-19a-3p promotes the growth of hepatocellular carcinoma by regulating p53/SOX4. Heliyon 2024, 10, e36282. [Google Scholar] [CrossRef] [PubMed]
- Raffo, A.J.; Perlman, H.; Chen, M.W.; Day, M.L.; Streitman, J.S.; Buttyan, R. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995, 55, 4438–4445. [Google Scholar]
- Fulda, S.; Meyer, E.; Debatin, K.-M. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 2002, 21, 2283–2294. [Google Scholar] [CrossRef]
- Miquel, C.; Borrini, F.; Grandjouan, S.; Aupérin, A.; Viguier, J.; Velasco, V.; Duvillard, P.; Praz, F.; Sabourin, J.-C. Role of bax Mutations in Apoptosis in Colorectal Cancers with Microsatellite Instability. Am. J. Clin. Pathol. 2005, 123, 562–570. [Google Scholar] [CrossRef]
- Goolsby, C.; Paniagua, M.; Tallman, M.; Gartenhaus, R.B. Bcl-2 regulatory pathway is functional in chronic lymphocytic leukemia. Cytom. Part B Clin. Cytom. 2004, 63B, 36–46. [Google Scholar] [CrossRef]
- Pepper, C.; Hoy, T.; Bentley, D.P. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br. J. Cancer. 1997, 76, 935–938. [Google Scholar] [CrossRef]
- Lopes, R.B.; Gangeswaran, R.; McNeish, I.A.; Wang, Y.; Lemoine, N.R. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int. J. Cancer 2007, 120, 2344–2352. [Google Scholar] [CrossRef]
- Chen, Z.; Naito, M.; Hori, S.; Mashima, T.; Yamori, T.; Tsuruo, T. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochem. Biophys. Res. Commun. 1999, 264, 847–854. [Google Scholar] [CrossRef]
- Krepela, E.; Dankova, P.; Moravcikova, E.; Krepelova, A.; Prochazka, J.; Cermak, J.; Schützner, J.; Zatloukal, P. Benkova KIncreased expression of inhibitor of apoptosis proteins survivin XIAP, in non-small cell lung carcinoma. Int. J. Oncol. 2009, 35, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Lavrik, I.; Golks, A.; Krammer, P.H. Death receptor signaling. J. Cell Sci. 2005, 118, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.; Fulda, S.; Debatin, K.M. Deficient activation of the CD95 (APO-1/Fas) system in drug-resistant cells. Leukemia 1997, 11, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Los, M.; Friesen, C.; Debatin, K.M. Chemosensitivity of solid tumor cells in vitro is related to activation of the CD95 system. Int J Cancer. 1998, 76, 105–114. [Google Scholar] [CrossRef]
- Reesink-Peters, N.; Hougardy, B.M.; van den Heuvel, F.A.; Ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; de Vries, E.G.; de Jong, S.; van der Zee, A.G. Death receptors and ligands in cervical carcino-genesis: An immunohistochemical study. Gynecol. Oncol. 2005, 96, 705–713. [Google Scholar] [CrossRef]
- Morgan, M.J.; Kim, Y.S. Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med. 2022, 54, 1695–1704. [Google Scholar] [CrossRef]
- Liu, J.; Hong, M.; Li, Y.; Chen, D.; Wu, Y.; Hu, Y. Programmed Cell Death Tunes Tumor Immunity. Front. Immunol. 2022, 13, 847345. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer. 2015, 14, 48. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Peng, J.; Wang, T.; Yue, C.; Luo, X.; Xiao, P. PGAM5: A necroptosis gene associated with poor tumor prognosis that promotes cutaneous melanoma progression. Front. Oncol. 2022, 12, 1004511. [Google Scholar] [CrossRef]
- Meier, P.; Legrand, A.J.; Adam, D.; Silke, J. Immunogenic cell death in cancer: Targeting necroptosis to induce antitumour immunity. Nat. Rev. Cancer 2024, 24, 299–315. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Liu, C.; Kang, Y.; Qin, D.; Chen, S.; Zhou, J.; Liu, H.J.; Ferdows, B.E.; Patel, D.N.; et al. In situ Engineering of Tumor-Associated Macrophages via a Nanodrug-Delivering-Drug (β-Elemene@Stanene) Strategy for Enhanced Cancer Chemo-Immunotherapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202308413. [Google Scholar] [CrossRef]
- Rodriguez, D.A.; Quarato, G.; Liedmann, S.; Tummers, B.; Zhang, T.; Guy, C.; Crawford, J.C.; Palacios, G.; Pelletier, S.; Kalkavan, H.; et al. Caspase-8 and FADD prevent spontaneous ZBP1 expression and necroptosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2207240119. [Google Scholar] [CrossRef]
- Hänggi, K.; Li, J.; Gangadharan, A.; Liu, X.; Celias, D.P.; Osunmakinde, O.; Keske, A.; Davis, J.; Ahmad, F.; Giron, A.; et al. Interleukin-1α release during necrotic-like cell death generates myeloid-driven immunosuppression that restricts anti-tumor immunity. Cancer Cell 2024, 42, 2015–2031.e11. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Xu, W.; Ding, J.; Yin, F.; Xu, J.; Sun, W.; Wang, H.; Sun, M.; Cai, Z.; et al. Sarcoma-Targeting Peptide-Decorated Polypeptide Nanogel Intracellularly Delivers Shikonin for Upregulated Osteosarcoma Necroptosis and Diminished Pulmonary Metastasis. Theranostics 2018, 8, 1361–1375. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Q.; Liao, H.; Fu, H.; Wang, Q.; Yu, J.; Zhang, W.; Chen, C.; Dong, Y.; Yang, X.; et al. Multi-Arm PEG/Peptidomimetic Conjugate Inhibitors of DR6/APP Interaction Block Hematogenous Tumor Cell Extravasation. Adv. Sci. 2021, 8, 2003558. [Google Scholar] [CrossRef]
- Ye, Y.; Dai, Q.; Qi, H. A novel defined pyroptosis-related gene signature for predicting the prognosis of ovarian cancer. Cell Death Discov. 2021, 7, 71. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Mao, L.; Guo, Y.; Hao, Y.; Deng, Y.; Han, X.; Li, Q.; Liao, W.; Yuan, M. Nobiletin Triggers Reactive Oxygen Species-Mediated Pyroptosis through Regulating Autophagy in Ovarian Cancer Cells. J. Agric. Food Chem. 2020, 68, 1326–1336. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Ding, J.; Wang, C.; Zhou, X.; Gao, W.; Huang, H.; Shao, F.; Liu, Z. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 2020, 579, 421–426. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef]
- Gao, J.; Qiu, X.; Xi, G.; Liu, H.; Zhang, F.; Lv, T.; Song, Y. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncol. Rep. 2018, 40, 1971–1984. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef]
- Tan, C.; Liu, W.; Zheng, Z.H.; Wan, X.G. LncRNA HOTTIP inhibits cell pyroptosis by targeting miR-148a-3p/AKT2 axis in ovarian cancer. Cell Biol. Int. 2021, 45, 1487–1497. [Google Scholar] [CrossRef]
- Wang, S.J.; Li, D.; Ou, Y.; Jiang, L.; Chen, Y.; Zhao, Y.; Gu, W. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016, 17, 366–373. [Google Scholar] [CrossRef]
- Han, C.; Ge, M.; Xing, P.; Xia, T.; Zhang, C.; Ma, K.; Ma, Y.; Li, S.; Li, W.; Liu, X.; et al. Cystine deprivation triggers CD36-mediated ferroptosis and dysfunction of tumor infiltrating CD8+ T cells. Cell Death Dis. 2024, 15, 145. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Chen, Q.; Cheng, J.; Chen, X.; Mao, Y.; Chen, W.; Liu, C.; Wu, H.; Lv, Y.; et al. MicroRNA-200a and microRNA-141 have a synergetic effect on the suppression of epi-thelial-mesenchymal transition in liver cancer by targeting STAT4. Oncol. Lett. 2021, 21, 137. [Google Scholar] [CrossRef]
- Lang, X.; Green, M.D.; Wang, W.; Yu, J.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Xue, Y.; Álvarez-Benedicto, E.; Shi, Y.; Chen, W.; Koo, S.; Siegwart, D.J.; Dong, Y.; Tao, W. Nanotechnology-based mRNA vaccines. Nat. Rev. Methods Primers 2023, 3, 63. [Google Scholar] [CrossRef]


| Interaction Type | Description | Location |
|---|---|---|
| Autophagy and Apoptosis | Autophagy can degrade inhibitors of pro-apoptotic factors, indirectly activating apoptosis. p53 exerts dual roles in autophagy regulation based on its subcellular localization: cytoplasmic p53 inhibits autophagy via mTORC1 activation, while nuclear p53 activates autophagy via AMPK/mTOR. | Section 3.1.2 |
| Autophagy and Apoptosis | Excessive autophagy can lead to cell death via over-degradation of critical components, which is a form of autophagic cell death. This process can inhibit tumor progression. | Section 3.1.2 |
| Autophagy and Apoptosis | Autophagy can induce cell cycle arrest and senescence, which can suppress tumor proliferation. This is related to the regulation of the autophagy and apoptosis pathways. | Section 3.1.3 |
| Autophagy and Inflammation (Necroptosis) | Autophagy can degrade inflammasomes and damaged lysosomes, preventing the release of proinflammatory cytokines and thus suppressing protumor inflammation. This is part of the “chronic inflammation–fibrosis–cancer” axis. | Section 3.1.4 |
| Autophagy and Inflammation (Necroptosis) | Autophagy can clear damaged mitochondria and inflammasomes, which helps to prevent infection-related cancers. This is related to the degradation of inflammasomes by autophagy. | Section 3.1.4 |
| Autophagy and Viral Carcinogenesis (Necroptosis) | Autophagy can degrade oncogenic viruses (e.g., HBV, HPV), but these viruses can evade clearance by encoding autophagy inhibitors. This interaction is important in viral carcinogenesis. | Section 3.1.4 |
| Autophagy and Pyroptosis | Autophagy can negatively regulate pyroptosis by eliminating damaged mitochondria and inflammasome components. This interaction helps to control inflammatory responses. | Section 3.4 |
| Autophagy and Ferroptosis | Autophagy can promote ferroptosis through ferritin degradation (ferritinophagy). This interaction can influence cellular sensitivity to ferroptosis. | Section 3.5 |
| Apoptosis and Necroptosis | Caspase-8 can suppress necroptosis through RIPK1/RIPK3 cleavage while also executing apoptotic signaling. This dual functionality of caspase-8 links the two pathways. | Section 3.3 and Section 3.4 |
| Necroptosis and Pyroptosis | Synergistic interactions between MLKL (necroptosis effector) and gasdermin D (pyroptosis effector) can amplify inflammatory responses. This interaction highlights the crosstalk between these two forms of cell death. | Section 3.3 and Section 3.4 |
| Ferroptosis and Apoptosis | p53, a key regulator of apoptosis, also plays a dual role in ferroptosis regulation. Nuclear p53 can inhibit ferroptosis by upregulating SLC7A11, while under certain stress conditions, p53 may promote ferroptosis. | Section 3.5 |
| Ferroptosis and Apoptosis | Anti-apoptotic Bcl-2 family proteins may influence ferroptosis progression by modulating mitochondrial function. This interaction shows the crosstalk between the apoptosis and ferroptosis pathways. | Section 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Huang, R.; Aimaiti, M.; Zhou, Q.; Wu, X.; Zhu, J.; Ma, X.; Qian, K.; Zhou, Q.; Hu, L.; et al. Mechanism of RCD and the Role of Different Death Signaling Pathways in Cancer. Biomedicines 2025, 13, 1880. https://doi.org/10.3390/biomedicines13081880
Zhou J, Huang R, Aimaiti M, Zhou Q, Wu X, Zhu J, Ma X, Qian K, Zhou Q, Hu L, et al. Mechanism of RCD and the Role of Different Death Signaling Pathways in Cancer. Biomedicines. 2025; 13(8):1880. https://doi.org/10.3390/biomedicines13081880
Chicago/Turabian StyleZhou, Jianming, Ruotong Huang, Maidinai Aimaiti, Qingyu Zhou, Xiang Wu, Jiajun Zhu, Xiangyi Ma, Ke Qian, Qi Zhou, Lianlong Hu, and et al. 2025. "Mechanism of RCD and the Role of Different Death Signaling Pathways in Cancer" Biomedicines 13, no. 8: 1880. https://doi.org/10.3390/biomedicines13081880
APA StyleZhou, J., Huang, R., Aimaiti, M., Zhou, Q., Wu, X., Zhu, J., Ma, X., Qian, K., Zhou, Q., Hu, L., Yang, X., Tang, Y., Lin, Y., & Chen, S. (2025). Mechanism of RCD and the Role of Different Death Signaling Pathways in Cancer. Biomedicines, 13(8), 1880. https://doi.org/10.3390/biomedicines13081880






