Clinical and Cognitive Improvement Following Treatment with a Hemp-Derived, Full-Spectrum, High-Cannabidiol Product in Patients with Anxiety: An Open-Label Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Participants
2.3. Study Product
2.4. Clinical Scales
2.5. Cognitive Assessments
2.6. Statistics
3. Results
3.1. Participant Flow and Demographics
3.2. Study Product Use
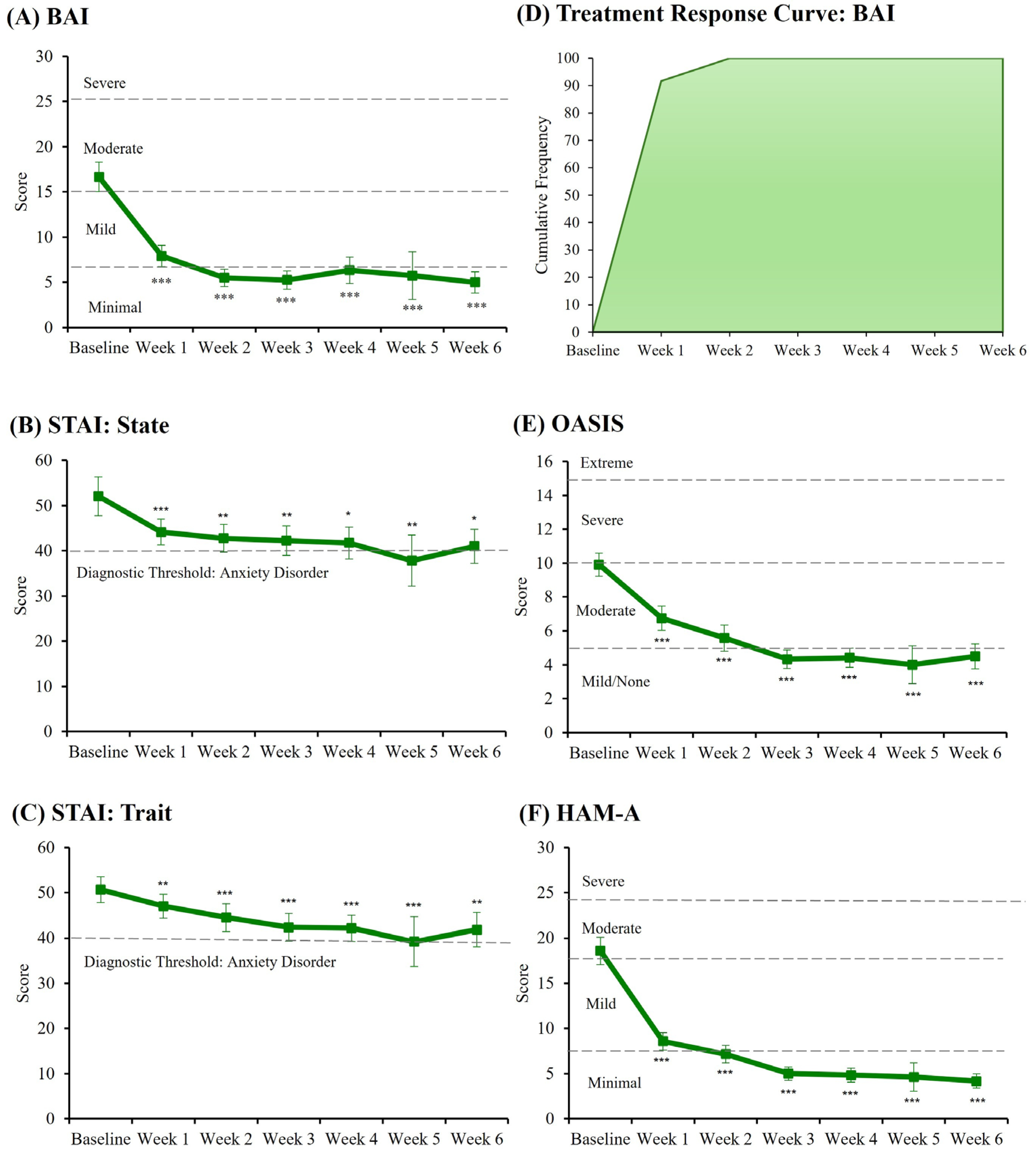
3.3. Clinical State and Treatment Response
3.4. Expectancy and Perceived Effects
3.5. Side Effects and Tolerability
3.6. Cognitive Tasks
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALR | Abuse Liability Rating |
| BAI | Beck Anxiety Inventory |
| BDI | Beck Depression Inventory |
| BID | Twice daily |
| BVRT | Benton Visual Retention Test |
| CBD | Cannabidiol |
| COWAT | Controlled Oral Word Association Test |
| CW | Charlotte’s Web |
| D9-THC | Delta-9-tetryhydrocannabinol |
| GC-MS | Gas chromatography-mass spectrometry |
| HAM-A | Hamilton Anxiety Rating Scale |
| LC | Liquid chromatography |
| LMM | Linear mixed model |
| LNS | Letter-Number Sequencing |
| MC | Medical cannabis |
| MCT | Medium chain triglyceride |
| MSIT | Multi-Source Interference Test |
| OASIS | Overall Anxiety Severity and Impairment Scale |
| PANAS | Positive and Negative Affect Scale |
| PGIC | Patient’s Global Impression of Change |
| POMS | Profile of Mood States |
| PSQI | Pittsburgh Sleep Quality Index |
| RAVLT | Rey Auditory Verbal Learning Test |
| SAD | Social anxiety disorder |
| SCID | Structured Clinical Interview—DSM-5 |
| SEQ | Side Effects Questionnaire |
| SF-36 | Medical Outcomes Study Questionnaire Short Form 36 Item |
| SPST | Simulated public speaking test |
| SSRI | Selective serotonin reuptake inhibitor |
| STAI | State-Trait Anxiety Inventory |
| TID | Three times per day |
| TMD | Total Mood Disturbance |
| TMT | Trail Making Test |
| U.S. | United States |
| WAIS-R | Wechsler Adult Intelligence Scale-Revised |
| WASI-II | Wechsler Abbreviated Scale of Intelligence—II |
References
- Walsh, Z.; Callaway, R.; Belle-Isle, L.; Capler, R.; Kay, R.; Lucas, P.; Holtzman, S. Cannabis for Therapeutic Purposes: Patient Characteristics, Access, and Reasons for Use. Int. J. Drug Policy 2013, 24, 511–516. [Google Scholar] [CrossRef]
- Sagar, K.A.; Gruber, S.A. The Complex Relationship Between Cannabis Use and Mental Health: Considering the Influence of Cannabis Use Patterns and Individual Factors. CNS Drugs 2025, 39, 113–125. [Google Scholar] [CrossRef]
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve-Month and Lifetime Prevalence and Lifetime Morbid Risk of Anxiety and Mood Disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef]
- Katzman, M.A.; Bleau, P.; Blier, P.; Chokka, P.; Kjernisted, K.; Van Ameringen, M.; the Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles anxieux and McGill University. Canadian Clinical Practice Guidelines for the Management of Anxiety, Posttraumatic Stress and Obsessive-Compulsive Disorders. BMC Psychiatry 2014, 14, S1. [Google Scholar] [CrossRef]
- Fava, M.; Graves, L.M.; Benazzi, F.; Scalia, M.J.; Iosifescu, D.V.; Alpert, J.E.; Papakostas, G.I. A Cross-Sectional Study of the Prevalence of Cognitive and Physical Symptoms during Long-Term Antidepressant Treatment. J. Clin. Psychiatry 2006, 67, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Springer, K.S.; Levy, H.C.; Tolin, D.F. Remission in CBT for Adult Anxiety Disorders: A Meta-Analysis. Clin. Psychol. Rev. 2018, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W. History of Cannabis as a Medicine: A Review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Groce, E. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. J. Med. Regul. 2018, 104, 32. [Google Scholar] [CrossRef]
- 115th United States Congress. Hemp Farming Act, H.R.5485. Available online: https://www.congress.gov/bill/115th-congress/house-bill/5485 (accessed on 20 July 2021).
- Kritikos, A.F.; Pacula, R.L. Characterization of Cannabis Products Purchased for Medical Use in New York State. JAMA Netw. Open 2022, 5, e2227735. [Google Scholar] [CrossRef]
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef]
- Han, K.; Wang, J.-Y.; Wang, P.-Y.; Peng, Y.-C.-H. Therapeutic Potential of Cannabidiol (CBD) in Anxiety Disorders: A Systematic Review and Meta-Analysis. Psychiatry Res. 2024, 339, 116049. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimarães, F.S. Effects of Ipsapirone and Cannabidiol on Human Experimental Anxiety. J. Psychopharmacol. 1993, 7 (Suppl. 1), 82–88. [Google Scholar] [CrossRef]
- Crippa, J.A.S.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural Basis of Anxiolytic Effects of Cannabidiol (CBD) in Generalized Social Anxiety Disorder: A Preliminary Report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Chagas, M.H.N.; de Oliveira, D.C.G.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef]
- Masataka, N. Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers with Social Anxiety Disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Li, E.; Rice, S.; Davey, C.G.; Ratheesh, A.; Adams, S.; Jackson, H.; Hetrick, S.; Parker, A.; Spelman, T.; et al. Cannabidiol for Treatment-Resistant Anxiety Disorders in Young People: An Open-Label Trial. J. Clin. Psychiatry 2022, 83, 21m14130. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacol. Pharm. 2015, 6, 75–85. [Google Scholar] [CrossRef]
- Berthold, E.C.; Kamble, S.H.; Kanumuri, S.R.R.; Kuntz, M.A.; Senetra, A.S.; Chiang, Y.H.; McMahon, L.R.; McCurdy, C.R.; Sharma, A. Comparative Pharmacokinetics of Commercially Available Cannabidiol Isolate, Broad-Spectrum, and Full-Spectrum Products. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 427–435. [Google Scholar] [CrossRef]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential Clinical Benefits of CBD-Rich Cannabis Extracts over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-Analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef]
- Dahlgren, M.K.; Lambros, A.M.; Smith, R.T.; Sagar, K.A.; El-Abboud, C.; Gruber, S.A. Clinical and Cognitive Improvement Following Full-Spectrum, High-Cannabidiol Treatment for Anxiety: Open-Label Data from a Two-Stage, Phase 2 Clinical Trial. Commun. Med. 2022, 2, 139. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A. Manual for the Beck Anxiety Inventory; Psychological Corporation: San Antonio, TX, USA, 1990. [Google Scholar]
- Campbell-Sills, L.; Norman, S.B.; Craske, M.G.; Sullivan, G.; Lang, A.J.; Chavira, D.A.; Bystritsky, A.; Sherbourne, C.; Roy-Byrne, P.; Stein, M.B. Validation of a Brief Measure of Anxiety-Related Severity and Impairment: The Overall Anxiety Severity and Impairment Scale (OASIS). J. Affect. Disord. 2009, 112, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence—Second Edition (WASI-II); NCS Pearson: San Antonio, TX, USA, 2011. [Google Scholar]
- Fraser, A.D.; Worth, D. Urinary Excretion Profiles of 11-nor-9-Carboxy-Δ9-Tetrahydrocannabinol. Forensic Sci. Int. 2003, 137, 196–202. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version SCID-5-RV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Norman, S.B.; Cissell, S.H.; Means-Christensen, A.J.; Stein, M.B. Development and Validation of an Overall Anxiety Severity and Impairment Scale (OASIS). Depress. Anxiety 2006, 23, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Hamilton, M. The Assessment of Anxiety States by Rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Pollock, V.; Cho, D.W.; Reker, D.; Volavka, J. Profile of Mood States: The Factors and Their Physiological Correlates. J. Nerv. Ment. Dis. 1979, 167, 612–614. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-Item Short-Form Health Survey (SF-36): Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global Rating of Change Scales: A Review of Strengths and Weaknesses and Considerations for Design. J. Man. Manip. Ther. 2009, 17, 163–170. [Google Scholar] [CrossRef]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An Evidence-Based Review of Acute and Long-Term Effects of Cannabis Use on Executive Cognitive Functions. J. Addict. Med. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Sagar, K.A.; Gruber, S.A. Marijuana Matters: Reviewing the Impact of Marijuana on Cognition, Brain Structure and Function, & Exploring Policy Implications and Barriers to Research. Int. Rev. Psychiatry Abingdon Engl. 2018, 30, 251–267. [Google Scholar] [CrossRef]
- MacLeod, C.M. Half a Century of Research on the Stroop Effect: An Integrative Review. Psychol. Bull. 1991, 109, 163–203. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessments, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Bush, G.; Shin, L.M. The Multi-Source Interference Task: An fMRI Task That Reliably Activates the Cingulo-Frontal-Parietal Cognitive/Attention Network. Nat. Protoc. 2006, 1, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Manual for the Wechsler Memory Scale-Revised; Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Benton, A.L. Benton Visual Retention Test Manual, 5th ed.; Psychological Corporation: San Antonio, TX, USA, 1992. [Google Scholar]
- Schmidt, M. Rey Auditory Verbal Learning Test; Western Psychological Services: Torrance, CA, USA, 2016. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Johnco, C.; De Nadai, A.S.; Lewin, A.B.; Ehrenreich-May, J.; Wood, J.J.; Storch, E.A. Defining Treatment Response and Symptom Remission for Anxiety Disorders in Pediatric Autism Spectrum Disorders Using the Pediatric Anxiety Rating Scale. J. Autism Dev. Disord. 2015, 45, 3232–3242. [Google Scholar] [CrossRef]
- Johnco, C.J.; Salloum, A.; Lewin, A.B.; Storch, E.A. Refining Clinical Judgment of Treatment Response and Symptom Remission Identification in Childhood Anxiety Using a Signal Detection Analysis on the Pediatric Anxiety Rating Scale. J. Child Adolesc. Psychopharmacol. 2015, 25, 674–683. [Google Scholar] [CrossRef]
- Dammann, I.; Rohleder, C.; Leweke, F.M. Cannabidiol and Its Potential Evidence-Based Psychiatric Benefits—A Critical Review. Pharmacopsychiatry 2024, 57, 115–132. [Google Scholar] [CrossRef]
- Jakubovski, E.; Johnson, J.A.; Nasir, M.; Müller-Vahl, K.; Bloch, M.H. Systematic Review and Meta-Analysis: Dose–Response Curve of SSRIs and SNRIs in Anxiety Disorders. Depress. Anxiety 2019, 36, 198–212. [Google Scholar] [CrossRef]
- Dahlgren, M.K.; Sagar, K.A.; Lambros, A.M.; Smith, R.T.; Gruber, S.A. Urinary Tetrahydrocannabinol after 4 Weeks of a Full-Spectrum, High-Cannabidiol Treatment in an Open-Label Clinical Trial. JAMA Psychiatry 2021, 78, 335–337. [Google Scholar] [CrossRef]
- Gruber, S.A.; Sagar, K.A.; Dahlgren, M.K.; Racine, M.T.; Smith, R.T.; Lukas, S.E. Splendor in the Grass? A Pilot Study Assessing the Impact of Medical Marijuana on Executive Function. Front. Pharmacol. 2016, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.A.; Sagar, K.A.; Dahlgren, M.K.; Gonenc, A.; Smith, R.T.; Lambros, A.M.; Cabrera, K.B.; Lukas, S.E. The Grass Might Be Greener: Medical Marijuana Patients Exhibit Altered Brain Activity and Improved Executive Function after 3 Months of Treatment. Front. Pharmacol. 2018, 8, 983. [Google Scholar] [CrossRef]
- Sagar, K.A.; Dahlgren, M.K.; Lambros, A.M.; Smith, R.T.; El-Abboud, C.; Gruber, S.A. An Observational, Longitudinal Study of Cognition in Medical Cannabis Patients over the Course of 12 Months of Treatment: Preliminary Results. J. Int. Neuropsychol. Soc. 2021, 27, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Burdinski, D.C.L.; Kodibagkar, A.; Potter, K.; Schuster, R.M.; Evins, A.E.; Ghosh, S.S.; Gilman, J.M. Year-Long Cannabis Use for Medical Symptoms and Brain Activation During Cognitive Processes. JAMA Netw. Open 2024, 7, e2434354. [Google Scholar] [CrossRef] [PubMed]
- Wieghorst, A.; Roessler, K.K.; Hendricks, O.; Andersen, T.E. The Effect of Medical Cannabis on Cognitive Functions: A Systematic Review. Syst. Rev. 2022, 11, 210. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Shirakawa, I.; Finkelfarb, E.; Karniol, I.G. Action of Cannabidiol on the Anxiety and Other Effects Produced by Δ9-THC in Normal Subjects. Psychopharmacology 1982, 76, 245–250. [Google Scholar] [CrossRef]
- Lisdahl, K.M.; Wright, N.E.; Medina-Kirchner, C.; Maple, K.E.; Shollenbarger, S. Considering Cannabis: The Effects of Regular Cannabis Use on Neurocognition in Adolescents and Young Adults. Curr. Addict. Rep. 2014, 1, 144–156. [Google Scholar] [CrossRef]
- Asnaani, A.; Richey, J.A.; Dimaite, R.; Hinton, D.E.; Hofmann, S.G. A Cross-Ethnic Comparison of Lifetime Prevalence Rates of Anxiety Disorders. J. Nerv. Ment. Dis. 2010, 198, 551–555. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal Properties of Terpenes Found in Cannabis Sativa and Humulus Lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
| Demographics | Patients (n = 12) | ||
|---|---|---|---|
| n (%) or Mean ± SD | Minimum | Maximum | |
| Sex: | |||
| Female | 9 (75.0%) | - | - |
| Male | 3 (25.0%) | - | - |
| Age | 38.00 ± 12.14 | 22 | 64 |
| Estimated IQ (WASI) | 109.75 ± 9.12 | 89 | 123 |
| Body Mass Index (BMI) | 26.40 ± 4.64 | 20.80 | 39.31 |
| Race/Ethnicity: | |||
| White, Non-Hispanic | 12 (100.0%) | - | - |
| High-Cannabidiol (CBD) Study Product Use | |||
| Treatment Days | 44.92 ± 6.23 | 39 | 56 |
| Product Use (mL/day) | 0.97 ± 0.20 | 0.73 | 1.38 |
| Exposure to Specific Cannabinoids a (mg/day): | |||
| Cannabidiol (CBD) | 31.29 ± 6.32 | 23.66 | 44.64 |
| ∆9-Tetrahydrocannabinol (d9-THC) | 0.59 ± 0.12 | 0.45 | 0.85 |
| Cannabichromene (CBC) | 1.47 ± 0.30 | 1.11 | 2.09 |
| Cannabigerol (CBG) | 0.38 ± 0.08 | 0.29 | 0.54 |
| Cannabidivarin (CBDV) | 0.24 ± 0.05 | 0.18 | 0.35 |
| Cannabidiolic acid (CBDA) | 0.12 ± 0.02 | 0.09 | 0.17 |
| Abuse Liability Rating Scale (ALR) Following 6 Weeks of Treatment b | |||
| Strength of Product | 1.75 ± 0.75 | 1 | 3 |
| Good Effects of Product | 2.67 ± 1.07 | 1 | 4 |
| Bad Effects of Product | 0.25 ± 0.45 | 0 | 1 |
| Liking Product | 3.42 ± 0.67 | 2 | 4 |
| Willingness to Take Again | 3.83 ± 0.39 | 3 | 4 |
| Feelings of Intoxication c | 0.17 ± 0.58 | 0 | 2 |
| Baseline n = 12 | 2 Weeks n = 12 | 4 Weeks n = 12 | 6 Weeks n = 12 | |
|---|---|---|---|---|
| Urine Cup Assay | n (%) | n (%) | n (%) | n (%) |
| THC Positive a | 0 (0.0%) | 3 (25.0%) | 3 (25.0%) | 4 (33.3%) |
| Gas Chromatography-Mass Spectrometry Urinalyses (Quest Laboratories) | n (%) | n (%) | n (%) | n (%) |
| 0 (0.0%) | 6 (50.0%) | 6 (50.0%) | 6 (50.0%) | |
| THC-COOH/Creatinine Ratio (ng/mg) | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| - | 12.00 ± 3.29 | 12.17 ± 4.96 | 11.33 ± 5.54 |
| Mixed Model | Baseline n = 12 | 6 Weeks n = 12 | |
|---|---|---|---|
| Fixed Effects | Mean ± SD | Mean ± SD | |
| Multi-Source Interference Task (MSIT) | |||
| Control Response Time (ms) | F(1,11) = 8.204, p = 0.015 | 579.00 ± 69.45 | 542.73 ± 57.13 |
| Control Accuracy (%) | F(1,11) = 6.557, p = 0.026 | 98.79 ± 1.07 | 99.74 ± 0.47 |
| Interference Response Time (ms) | F(1,11) = 26.694, p < 0.001 | 873.74 ± 84.81 | 828.06 ± 81.99 |
| Interference Accuracy (%) | F(1,11) = 15.775, p = 0.002 | 88.37 ± 6.65 | 95.06 ± 3.42 |
| Stroop Color Word Test | |||
| Interference Time (s) | F(1,11) = 1.798, p = 0.207 | 95.33 ± 26.41 | 91.92 ± 21.34 |
| Interference Accuracy (%) | F(1,11) = 0.186, p = 0.674 | 98.17 ± 2.44 | 97.92 ± 2.23 |
| Trail Making Test (TMT) | |||
| Trails A Time (s) | F(1,11) = 5.872, p = 0.034 | 28.50 ± 9.24 | 23.50 ± 4.56 |
| Trails B Time (s) | F(1,11) = 4.066, p = 0.069 | 60.92 ± 21.47 | 49.25 ± 22.00 |
| Letter-Number Sequencing (LNS) | |||
| LNS Total | F(1,11) = 0.011, p = 0.919 | 12.67 ± 2.77 | 12.75 ± 2.80 |
| Controlled Oral Word Association Task (COWAT) | |||
| Phonemic Fluency | F(1,11) = 0.006, p = 0.938 | 44.17 ± 9.71 | 44.33 ± 7.92 |
| Benton Visual Retention Task (BVRT) | |||
| BVRT Total | F(1,11) = 1.941, p = 0.191 | 7.42 ± 1.51 | 7.92 ± 1.38 |
| Rey Auditory Verbal Learning Task (RAVLT) | |||
| Trials 1–5: Total Correct | F(1,11) = 0.004, p = 0.953 | 49.92 ± 10.33 | 50.08 ± 7.08 |
| Trials 1–5: Total Perseverations | F(1,11) = 4.538, p = 0.057 | 3.58 ± 3.23 | 2.00 ± 2.04 |
| Short Delay: Correct | F(1,11) = 2.883, p = 0.118 | 9.25 ± 3.77 | 10.75 ± 2.83 |
| Short Delay: Perseverations | F(1,11) = 0.186, p = 0.674 | 0.25 ± 0.45 | 0.17 ± 0.39 |
| Long Delay: Correct | F(1,11) = 0.100, p = 0.758 | 9.67 ± 3.85 | 10.00 ± 2.73 |
| Long Delay: Perseverations | F(1,11) = 0.314, p = 0.586 | 0.17 ± 0.39 | 0.08 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, R.T.; Dahlgren, M.K.; Sagar, K.A.; Kosereisoglu, D.; Gruber, S.A. Clinical and Cognitive Improvement Following Treatment with a Hemp-Derived, Full-Spectrum, High-Cannabidiol Product in Patients with Anxiety: An Open-Label Pilot Study. Biomedicines 2025, 13, 1874. https://doi.org/10.3390/biomedicines13081874
Smith RT, Dahlgren MK, Sagar KA, Kosereisoglu D, Gruber SA. Clinical and Cognitive Improvement Following Treatment with a Hemp-Derived, Full-Spectrum, High-Cannabidiol Product in Patients with Anxiety: An Open-Label Pilot Study. Biomedicines. 2025; 13(8):1874. https://doi.org/10.3390/biomedicines13081874
Chicago/Turabian StyleSmith, Rosemary T., Mary Kathryn Dahlgren, Kelly A. Sagar, Deniz Kosereisoglu, and Staci A. Gruber. 2025. "Clinical and Cognitive Improvement Following Treatment with a Hemp-Derived, Full-Spectrum, High-Cannabidiol Product in Patients with Anxiety: An Open-Label Pilot Study" Biomedicines 13, no. 8: 1874. https://doi.org/10.3390/biomedicines13081874
APA StyleSmith, R. T., Dahlgren, M. K., Sagar, K. A., Kosereisoglu, D., & Gruber, S. A. (2025). Clinical and Cognitive Improvement Following Treatment with a Hemp-Derived, Full-Spectrum, High-Cannabidiol Product in Patients with Anxiety: An Open-Label Pilot Study. Biomedicines, 13(8), 1874. https://doi.org/10.3390/biomedicines13081874





