GSTM5 as a Potential Biomarker for Treatment Resistance in Prostate Cancer
Abstract
1. Introduction
2. Methods
2.1. In Silico Analysis of GSTM5 Role in PC
2.2. In Silico Characterization of the rs3768490 Variant (GSTM5)
2.3. Study Design
2.4. DNA Extraction from Blood Samples and Buccal Swabs
2.5. Genotyping Analysis by qPCR
2.6. RNA Extraction and Reverse Transcription from Fresh Prostatic Tissue
2.7. Gene Expression Analysis by qPCR
2.8. Statistical Analysis
3. Results
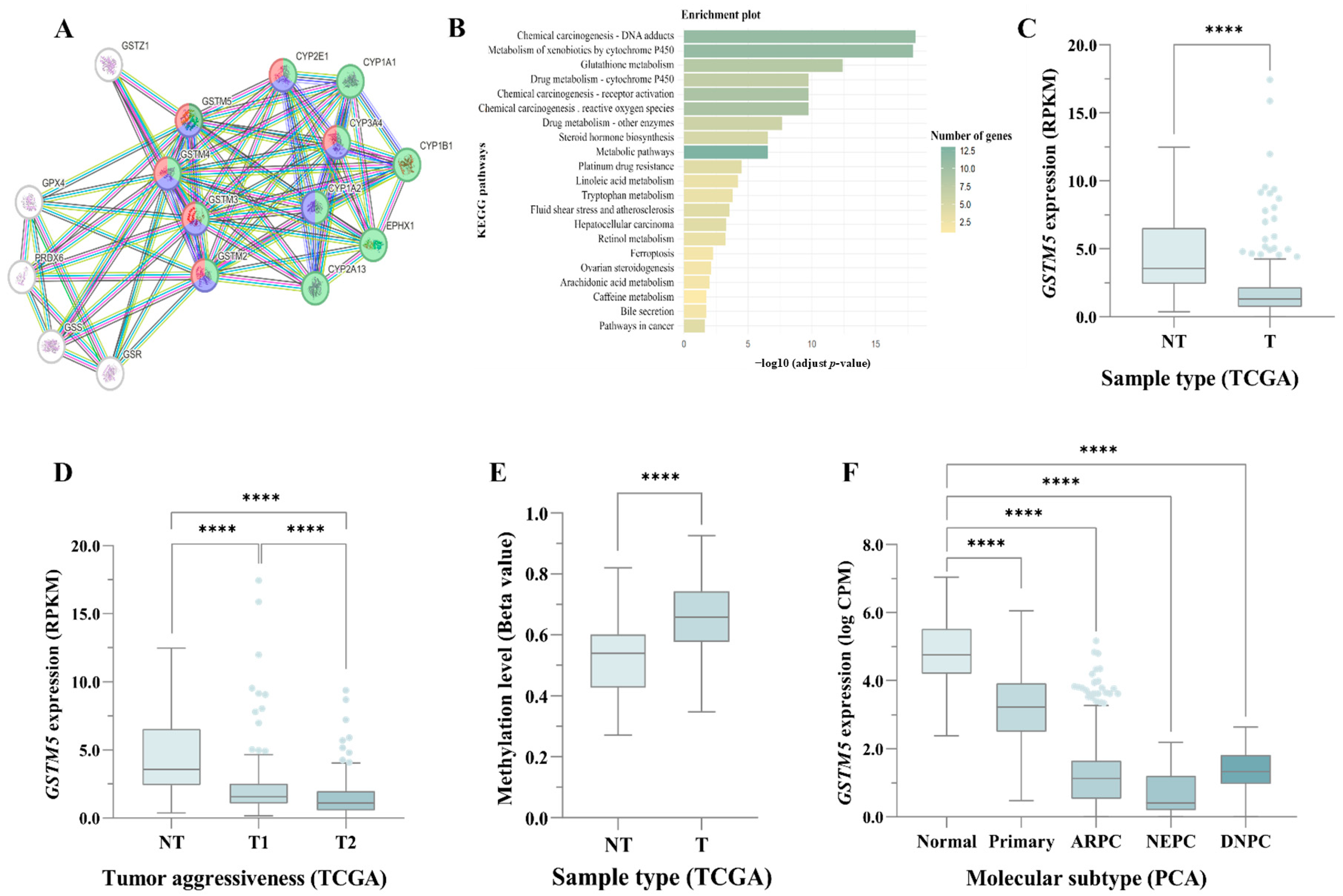
3.1. Expression and Functional Role of GSTM5 in PC
3.2. Functional Impact of rs3768490 (GSTM5)
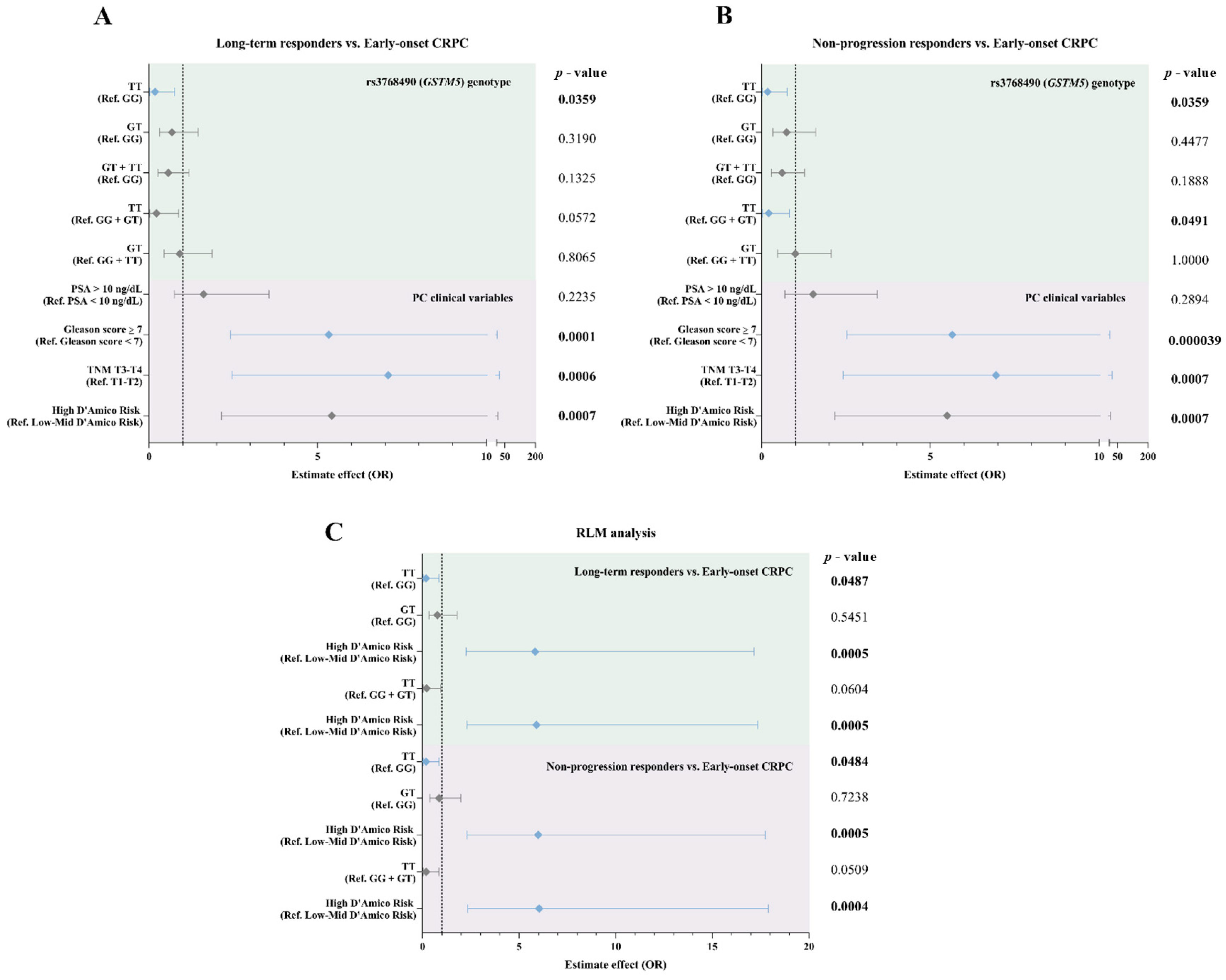
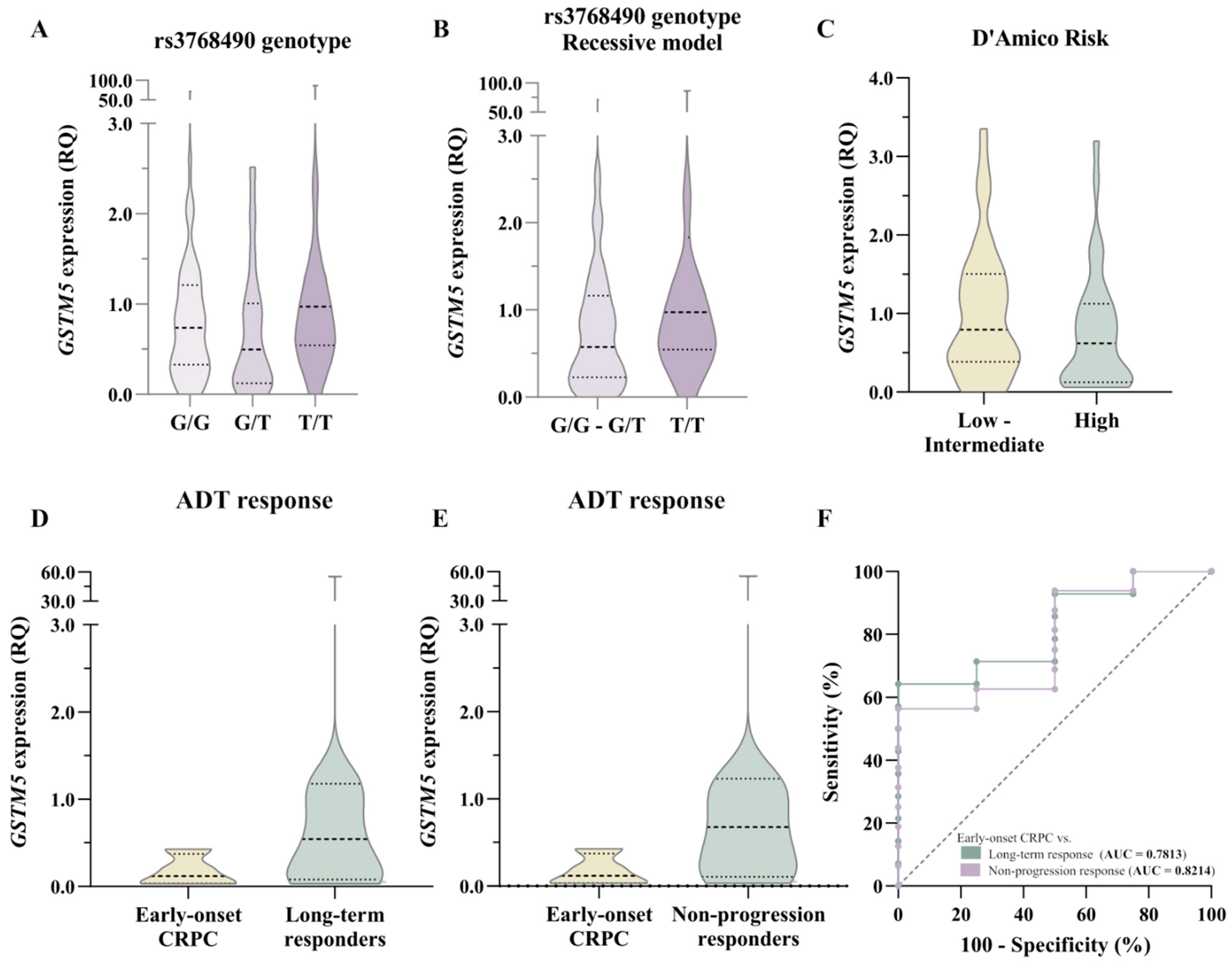
3.3. Association of rs3768490 Genotype with ADT Response
3.4. Impact of rs3768490 on GSTM5 Expression and ADT Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vellky, J.E.; Ricke, W.A. Development and Prevalence of Castration-Resistant Prostate Cancer Subtypes. Neoplasia 2020, 22, 566–575. [Google Scholar] [CrossRef]
- Wadosky, K.M.; Koochekpour, S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016, 7, 64447–64470. [Google Scholar] [CrossRef] [PubMed]
- Congregado Ruiz, B.; Rivero Belenchón, I.; Lendínez Cano, G.; Medina López, R.A. Strategies to Re-Sensitize Castration-Resistant Prostate Cancer to Antiandrogen Therapy. Biomedicines 2023, 11, 1105. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Dawson, N.A. Castration-resistant prostate cancer: Treatments targeting the androgen pathway. In UpToDate; UpToDate Inc.: Waltham, MA, USA, 2025. [Google Scholar]
- Le, T.K.; Duong, Q.H.; Baylot, V.; Fargette, C.; Baboudjian, M.; Colleaux, L.; Taïeb, D.; Rocchi, P. Castration-Resistant Prostate Cancer: From Uncovered Resistance Mechanisms to Current Treatments. Cancers 2023, 15, 5047. [Google Scholar] [CrossRef]
- Rodrigo-Aliaga, M.; Alvarez-Ossorio, J.L.; Rodríguez-Alonso, A.; García-Porrero, Á.; Quesada-García, A.; del Toro, J.M.; Rodríguez-Antolín, A. Prevalence and Management of Castration-Resistant Prostate Cancer of Unknown Metastatic Status in the Real-World Setting: The AfroDiTA Study. Urol. Oncol. Semin. Orig. Investig. 2025, 43, 64.e11–64.e18. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, Z.S.; Spratt, D.E.; Pei, I.; Zhang, Z.; Yamada, Y.; Kollmeier, M.; Zelefsky, M.J. A New Risk Classification System for Therapeutic Decision Making with Intermediate-Risk Prostate Cancer Patients Undergoing Dose-Escalated External-Beam Radiation Therapy. Eur. Urol. 2013, 64, 895–902. [Google Scholar] [CrossRef]
- Rodrigues, G.; Warde, P.; Pickles, T.; Crook, J.; Brundage, M.; Souhami, L.; Lukka, H. Pre-Treatment Risk Stratification of Prostate Cancer Patients: A Critical Review. Can. Urol. Assoc. J. 2012, 6, 121–127. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Urabe, F.; Sumiyoshi, T.; Tashiro, K.; Goto, T.; Kimura, T.; Kobayashi, T. Prostate Cancer and Liquid Biopsies: Clinical Applications and Challenges. Int. J. Urol. 2024, 31, 617–626. [Google Scholar] [CrossRef]
- Imamura, J.; Ganguly, S.; Muskara, A.; Liao, R.S.; Nguyen, J.K.; Weight, C.; Wee, C.E.; Gupta, S.; Mian, O.Y. Lineage Plasticity and Treatment Resistance in Prostate Cancer: The Intersection of Genetics, Epigenetics, and Evolution. Front. Endocrinol. 2023, 14, 1191311. [Google Scholar] [CrossRef]
- Maitland, N.J. Resistance to Antiandrogens in Prostate Cancer: Is It Inevitable, Intrinsic or Induced? Cancers 2021, 13, 327. [Google Scholar] [CrossRef]
- Shore, N.D.; Morgans, A.K.; Ryan, C.J. Resetting the Bar of Castration Resistance—Understanding Androgen Dynamics in Therapy Resistance and Treatment Choice in Prostate Cancer. Clin. Genitourin. Cancer 2021, 19, 199–207. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 2011, 51, 1320–1328. [Google Scholar] [CrossRef]
- Khurana, N.; Sikka, S.C. Targeting Crosstalk between Nrf-2, NF-κB and Androgen Receptor Signaling in Prostate Cancer. Cancers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Baldeiras, I.; Proença, T.; Alves, V.; Mota-Pinto, A.; Sarmento-Ribeiro, A. Oxidative Stress Adaptation in Aggressive Prostate Cancer May Be Counteracted by the Reduction of Glutathione Reductase. FEBS Open Bio 2012, 2, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D. The Role of Glutathione-S-Transferase in Anti-Cancer Drug Resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; He, D.; Mao, F.; Rao, X.; Zhao, Y.; Lanman, N.A.; Kazemian, M.; Farah, E.; Liu, J.; et al. GSTM2 Is a Key Molecular Determinant of Resistance to SG-ARIs. Oncogene 2022, 41, 4498–4511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, X.; Ma, Z.; Sun, Z.; Wang, Z. Association of Androgen-Receptor Gene Mutations with the Copy Number of Androgen-Receptor Silk Protein A Complex and Glutathione-S-Transferases T1 and M1 in Prostate Cancer Patients. Genet. Res. 2023, 2023, 5956951. [Google Scholar] [CrossRef]
- Jou, Y.C.; Wang, S.C.; Dia, Y.C.; Wang, S.T.; Yu, M.H.; Yang, H.Y.; Chen, L.C.; Shen, C.H.; Liu, Y.W. Anti-cancer Effects and Tumor Marker Role of Glutathione S-transferase Mu 5 in Human Bladder Cancer. Int. J. Mol. Sci. 2021, 22, 3056. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, J.; Chen, G.; Cao, W.; Chen, H.; Chen, S. Aberrant Expression of GSTM5 in Lung Adenocarcinoma Is Associated with DNA Hypermethylation and Poor Prognosis. BMC Cancer 2022, 22, 685. [Google Scholar] [CrossRef]
- Garcia-Moreno, A.; López-Domínguez, R.; Villatoro-García, J.A.; Ramirez-Mena, A.; Aparicio-Puerta, E.; Hackenberg, M.; Pascual-Montano, A.; Carmona-Saez, P. Functional Enrichment Analysis of Regulatory Elements. Biomedicines 2022, 10, 590. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. Mutationtaster2: Mutation Prediction for the Deep-Sequencing Age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Freeman, B.; Smith, N.; Curtis, C.; Huckett, L.; Mill, J.; Craig, I.W. DNA from Buccal Swabs Recruited by Mail: Evaluation of Storage Effects on Long-Term Stability and Suitability for Multiplex Polymerase Chain Reaction Genotyping. Behav. Genet. 2003, 33, 67–72. [Google Scholar] [CrossRef]
- Gómez-Martín, A.; Hernández, A.F.; Martínez-González, L.J.; González-Alzaga, B.; Rodríguez-Barranco, M.; López-Flores, I.; Aguilar-Garduno, C.; Lacasana, M. Polymorphisms of Pesticide-Metabolizing Genes in Children Living in Intensive Farming Communities. Chemosphere 2015, 139, 534–540. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (NCBI). Primer-BLAST. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 10 October 2024).
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione Transferases: Substrates, Inihibitors and pro-Drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Akkus, E.; Arslan, Ç.; Ürün, Y. Advancements in Platinum Chemotherapy for Metastatic Castration-Resistant Prostate Cancer: Insights and Perspectives. Cancer Treat. Rev. 2024, 130, 102818. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Celeste Simon, M. Glutathione Metabolism in Cancer Progression and Treatment Resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Santric, V.; Djokic, M.; Suvakov, S.; Pljesa-Ercegovac, M.; Nikitovic, M.; Radic, T.; Acimovic, M.; Stankovic, V.; Bumbasirevic, U.; Milojevic, B.; et al. GSTP1 RS1138272 Polymorphism Affects Prostate Cancer Risk. Medicina 2020, 56, 128. [Google Scholar] [CrossRef]
- Cao, D.L.; Ye, D.W.; Dai, B.; Zhang, H.L.; Shen, Y.J.; Zhu, Y.; Zhu, Y.P.; Shi, G.H.; Ma, C.G.; Xiao, W.J.; et al. Association of Glutathione S-Transferase T1 and M1 Polymorphisms with Prostate Cancer Susceptibility in Populations of Asian Descent: A Meta-Analysis. Oncotarget 2015, 6, 35843–35850. [Google Scholar] [CrossRef]
- Yu, K.D.; Fan, L.; Di, G.H.; Yuan, W.T.; Zheng, Y.; Huang, W.; Chen, A.X.; Yang, C.; Wu, J.; Shen, Z.Z.; et al. Genetic Variants in GSTM3 Gene within GSTM4-GSTM2-GSTM1-GSTM5-GSTM3 Cluster Influence Breast Cancer Susceptibility Depending on GSTM1. Breast Cancer Res. Treat. 2010, 121, 485–496. [Google Scholar] [CrossRef]
- Gohari, N.; Abbasi, E.; Akrami, H. Comprehensive Analysis of the Prognostic Value of Glutathione S-transferases Mu Family Members in Breast Cancer. Cell Biol. Int. 2024, 48, 1313–1325. [Google Scholar] [CrossRef]
- Bulger, M.; Groudine, M. Functional and Mechanistic Diversity of Distal Transcription Enhancers. Cell 2011, 144, 327–339. [Google Scholar] [CrossRef]
- Chiaradonna, F.; Barozzi, I.; Miccolo, C.; Bucci, G.; Palorini, R.; Fornasari, L.; Botrugno, O.A.; Pruneri, G.; Masullo, M.; Passafaro, A.; et al. Redox-Mediated Suberoylanilide Hydroxamic Acid Sensitivity in Breast Cancer. Antioxid. Redox Signal. 2015, 23, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Zou, J.; Lai, C.-T.; Zeng, T.; Peng, J.; Zou, W.-D.; Cao, B.; Liu, D.; Zhu, L.-Y.; et al. Comprehensive analysis of the glutathione S-transferase Mu (GSTM) gene family in ovarian cancer identifies prognostic and expression significance. Front. Oncol. 2022, 12, 968547. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.; Gerke, T.; Markt, S.C.; Peisch, S.F.; Wilson, K.M.; Ahearn, T.U.; Giovannucci, E.L.; Parmigiani, G.; Mucci, L.A. Family history of breast or prostate cancer and prostate cancer risk. Clin. Cancer Res. 2018, 24, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Sundquist, J.; Hemminki, A.; Hemminki, K. Familial Associations Between Prostate Cancer and Other Cancers. Eur. Urol. 2017, 71, 162–165. [Google Scholar] [CrossRef]
| Clinical Variable | N (%) |
|---|---|
| PSA serum levels: | |
| PSA < 10 ng/dL | 135 (40.79%) |
| PSA ≥ 10 ng/dL | 196 (59.21%) |
| Gleason score: | |
| Gleason < 7 | 107 (31.66%) |
| Gleason ≥ 7 | 231 (68.34%) |
| T Stage (TNM) | |
| T1–T2 | 209 (83.60%) |
| T3–T4 | 41 (16.40%) |
| D’Amico Risk | |
| Low-Mid | 157 (45.77%) |
| High | 186 (54.23%) |
| Metastasis | |
| Yes | 133 (51.55%) |
| No | 125 (48.45%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porras-Quesada, P.; Chica-Redecillas, L.; Álvarez-González, B.; Gutiérrez-Tejero, F.; Arrabal-Martín, M.; Rios-Pelegrina, R.; Martínez-González, L.J.; Álvarez-Cubero, M.J.; Vázquez-Alonso, F. GSTM5 as a Potential Biomarker for Treatment Resistance in Prostate Cancer. Biomedicines 2025, 13, 1872. https://doi.org/10.3390/biomedicines13081872
Porras-Quesada P, Chica-Redecillas L, Álvarez-González B, Gutiérrez-Tejero F, Arrabal-Martín M, Rios-Pelegrina R, Martínez-González LJ, Álvarez-Cubero MJ, Vázquez-Alonso F. GSTM5 as a Potential Biomarker for Treatment Resistance in Prostate Cancer. Biomedicines. 2025; 13(8):1872. https://doi.org/10.3390/biomedicines13081872
Chicago/Turabian StylePorras-Quesada, Patricia, Lucía Chica-Redecillas, Beatriz Álvarez-González, Francisco Gutiérrez-Tejero, Miguel Arrabal-Martín, Rosa Rios-Pelegrina, Luis Javier Martínez-González, María Jesús Álvarez-Cubero, and Fernando Vázquez-Alonso. 2025. "GSTM5 as a Potential Biomarker for Treatment Resistance in Prostate Cancer" Biomedicines 13, no. 8: 1872. https://doi.org/10.3390/biomedicines13081872
APA StylePorras-Quesada, P., Chica-Redecillas, L., Álvarez-González, B., Gutiérrez-Tejero, F., Arrabal-Martín, M., Rios-Pelegrina, R., Martínez-González, L. J., Álvarez-Cubero, M. J., & Vázquez-Alonso, F. (2025). GSTM5 as a Potential Biomarker for Treatment Resistance in Prostate Cancer. Biomedicines, 13(8), 1872. https://doi.org/10.3390/biomedicines13081872











