Unlocking the Role of OCT4 in Cancer Lineage Plasticity: A Cross-Cancer Perspective with an Emphasis on Prostate Cancer
Abstract
1. Introduction
2. OCT4 in Stemness and Plasticity
2.1. OCT4′s Function in Embryonic Stem Cells and Normal Tissue Development
2.2. OCT4 in Cancer Stem-like Cells and Lineage Plasticity
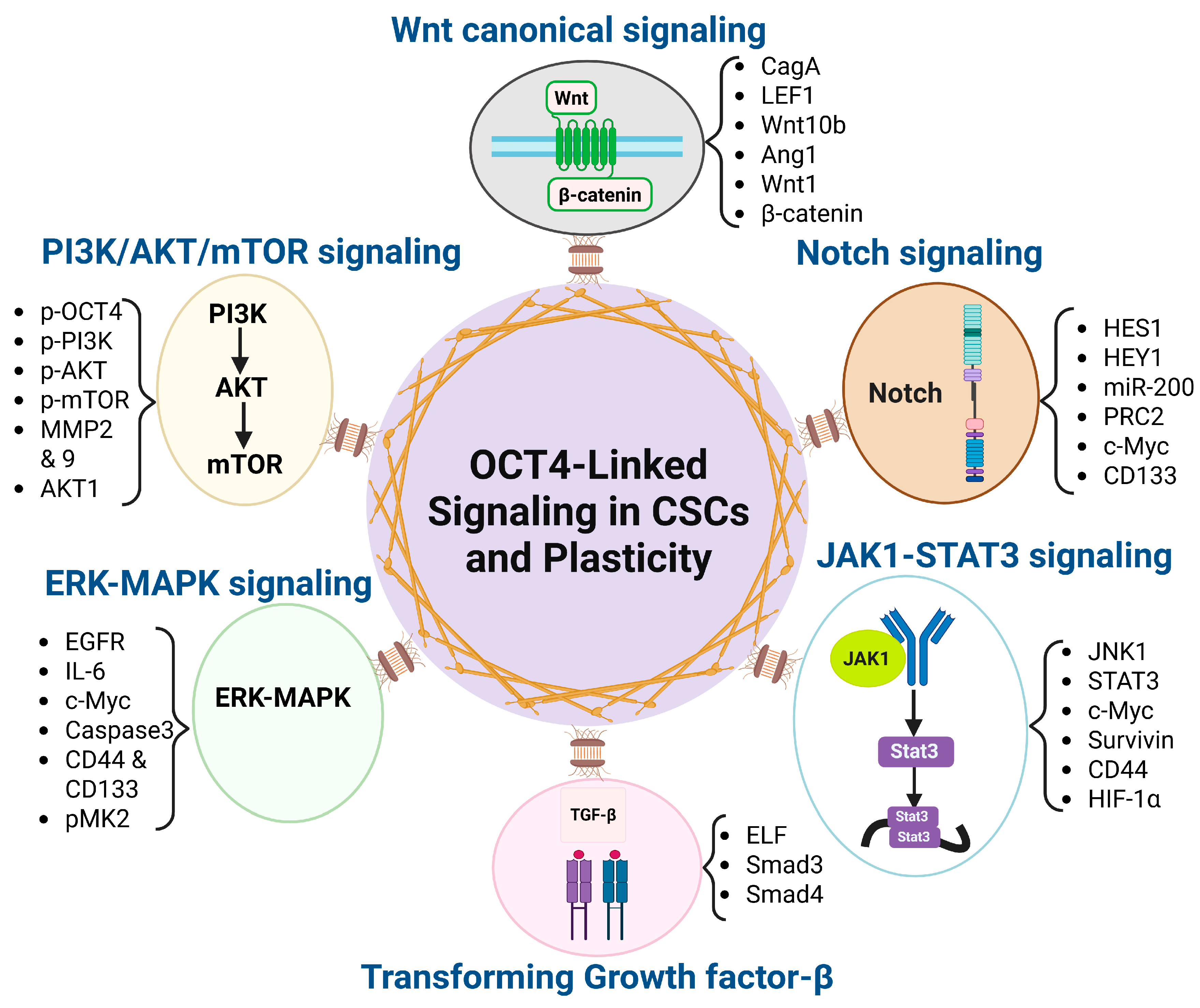
2.3. OCT4-Associated Signaling Pathways in CSC and Plasticity
2.3.1. Wnt/β-Catenin
2.3.2. TGF-β
2.3.3. PI3K/AKT/mTOR
2.3.4. Notch
2.3.5. JAK1-STAT3
2.3.6. ERK-MAPK
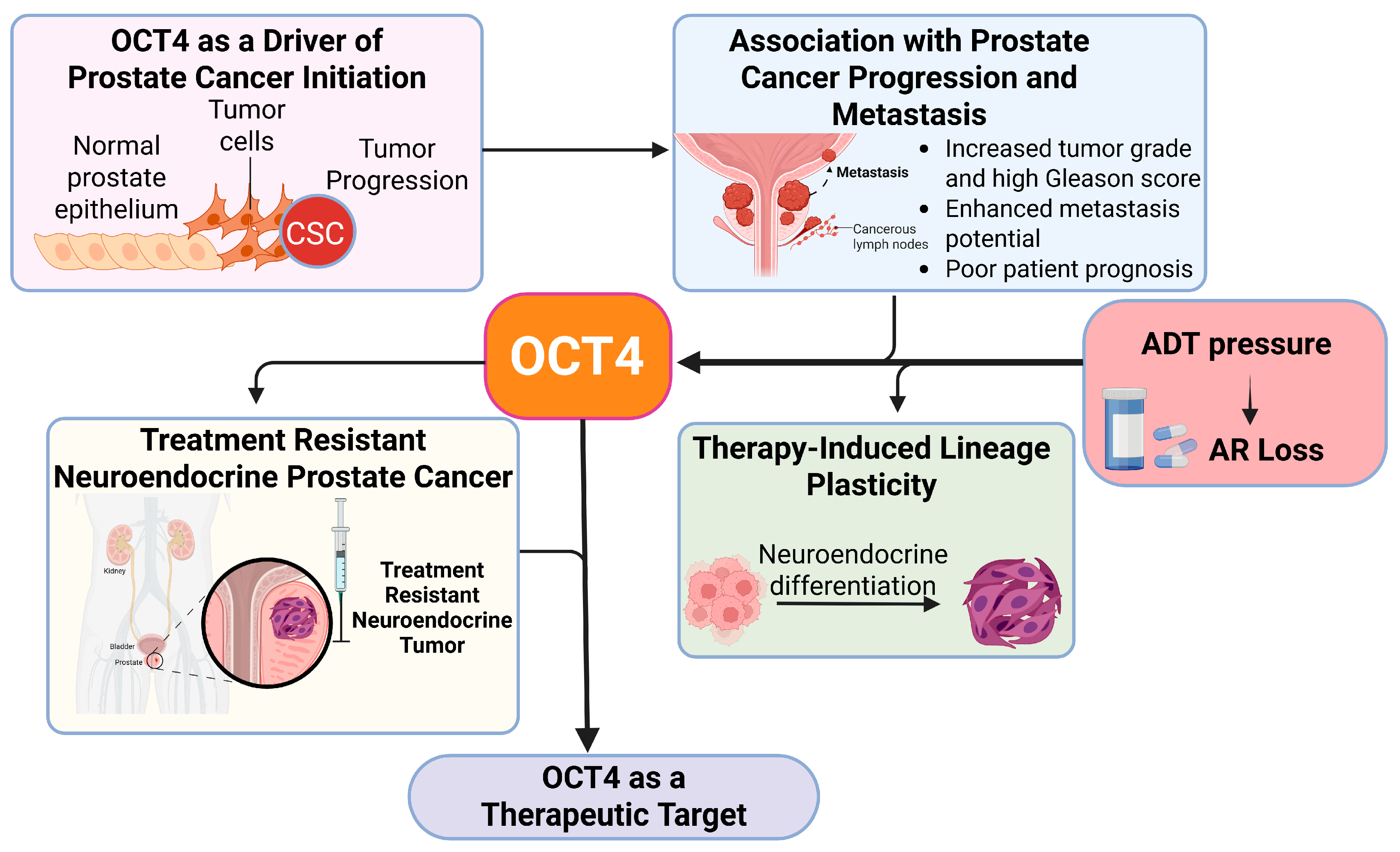
3. OCT4 in Prostate Cancer Progression and Lineage Plasticity
3.1. OCT4 as a Driver of Prostate Cancer Initiation
3.2. Association of OCT4 with Prostate Cancer Progression and Metastasis
- Increased Tumor Grade and High Gleason Score
- Enhanced Metastatic Potential
- Poor Patient Prognosis
3.3. OCT4 and Therapy-Induced Lineage Plasticity in Prostate Cancer
3.3.1. The Role of AR-Targeted Therapy in Driving Stemness and Plasticity
3.3.2. Chromatin Modifications in Driving Stemness and Plasticity in Prostate Cancer Contributing to Drug Resistance
3.4. OCT4 as a Therapeutic Target in Prostate Cancer
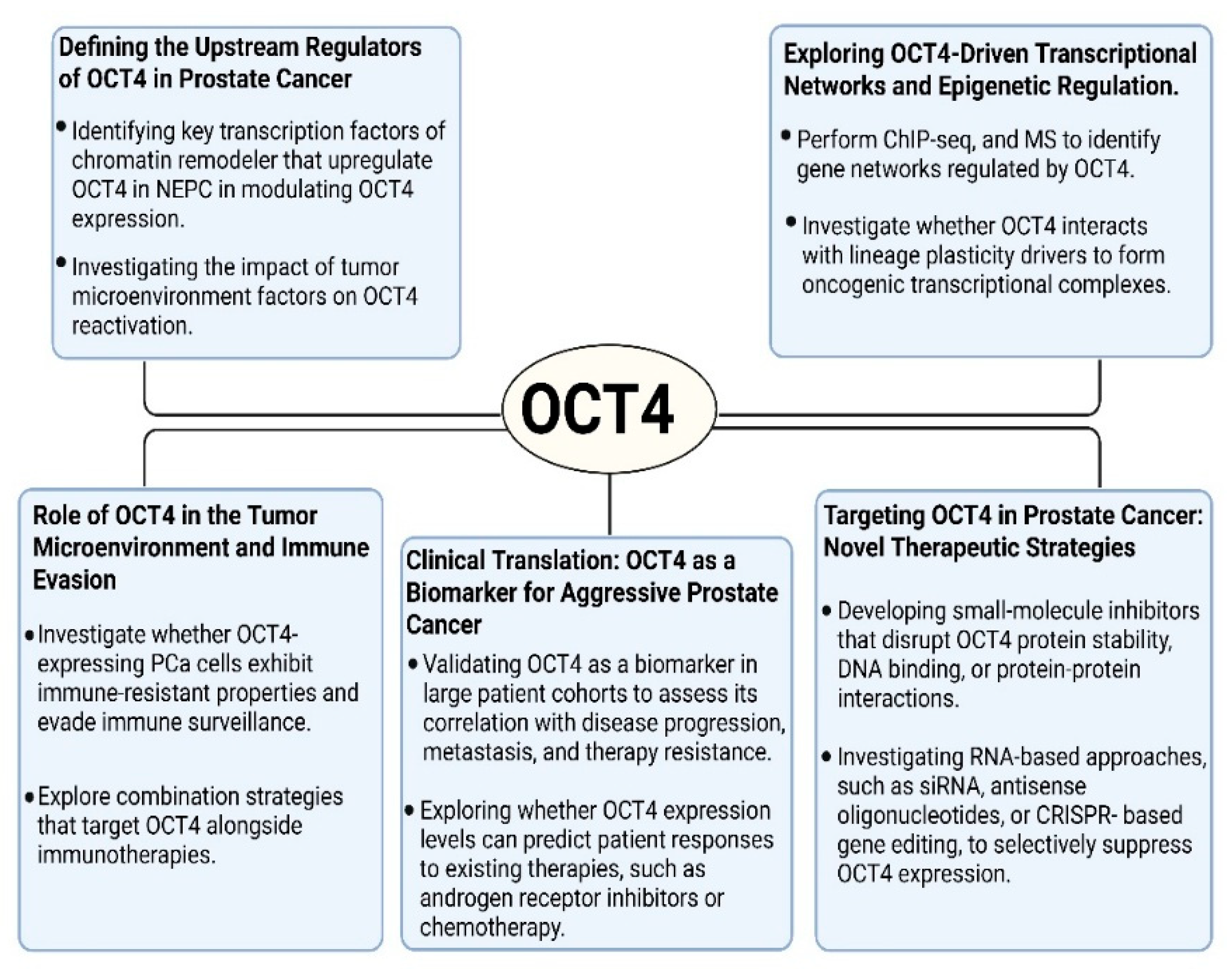
4. Glimpse into the Future
- 1.
- Defining the Upstream Regulators of OCT4 in Prostate Cancer
- Identifying key transcription factors or chromatin remodelers that upregulate OCT4 in CRPC and NEPC.
- Investigating the role of long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) in modulating the OCT4 expression.
- Investigating the impact of tumor microenvironment factors, including hypoxia, inflammatory cytokines, and stromal interactions, on the OCT4 reactivation.
- 2.
- Exploring OCT4-Driven Transcriptional Networks and Epigenetic Regulation
- Perform chromatin immunoprecipitation sequencing (ChIP-seq), RNA sequencing (RNA-seq), and mass spectrometry (MS) to identify the gene networks regulated by OCT4 in PCa.
- Investigate whether OCT4 interacts with other lineage plasticity drivers, such as SOX2, NANOG, EZH2, or AURKA, to form oncogenic transcriptional complexes.
- Examine the role of super-enhancers (SEs) in sustaining OCT4 expression and whether disrupting SEs could be a viable therapeutic approach.
- 3.
- Role of OCT4 in the Tumor Microenvironment and Immune Evasion
- Investigate whether OCT4-expressing PCa cells exhibit immune-resistant properties and evade immune surveillance.
- Determine whether OCT4 influences immune checkpoint expression (e.g., PD-L1) or modulates tumor-associated macrophages, myeloid-derived suppressor cells (MDSCs), or T cells in the tumor microenvironment.
- Explore combination strategies that target OCT4 and other key master regulators—such as SOX2, MYC, EZH2, and BRN2—alongside immunotherapies, to more effectively disrupt the stemness and immune-evasive phenotypes associated with lineage plasticity in prostate cancer.
- 4.
- Clinical Translation: OCT4 as a Biomarker for Aggressive Prostate Cancer
- Validating OCT4 as a biomarker in large patient cohorts to assess its correlation with disease progression, metastasis, and therapy resistance.
- Developing non-invasive diagnostic tools (e.g., CTCs, exosomal OCT4 detection) to monitor disease progression in CRPC and NEPC patients.
- Exploring whether OCT4 expression levels can predict patient responses to existing therapies, such as androgen receptor inhibitors or chemotherapy.
- 5.
- Targeting OCT4 in Prostate Cancer: Novel Therapeutic Strategies
- Developing small-molecule inhibitors that disrupt OCT4 protein stability, DNA binding, or protein–protein interactions.
- Investigating RNA-based approaches, such as siRNA, antisense oligonucleotides, or CRISPR-based gene editing, to selectively suppress OCT4 expression.
- Identifying upstream regulatory pathways (e.g., NFκB, FGFR, or Wnt/β-catenin) that indirectly modulate OCT4 and could be targeted with existing inhibitors.
- Exploring the potential of targeted protein degradation strategies, such as PROTACs (proteolysis-targeting chimeras), to selectively degrade OCT4 in PCa cells.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA 2025, 75, 10. [Google Scholar] [CrossRef]
- Shih, H.J.; Fang, S.C.; An, L.; Shao, Y.H.J. Early-onset prostate cancer is associated with increased risks of disease progression and cancer-specific mortality. Prostate 2021, 81, 118–126. [Google Scholar] [CrossRef]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of advanced prostate cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef]
- Swami, U.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N. Advanced prostate cancer: Treatment advances and future directions. Trends Cancer 2020, 6, 702–715. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Yu, W.; Huang, H.; Wang, Y.; Niu, Y. Investigating High-risk Factors, Precise Diagnosis, and Treatment of Castration-Resistant Prostate Cancer (CRPC). Comb. Chem. High Throughput Screen. 2024, 27, 2598–2608. [Google Scholar] [CrossRef]
- Tashiro, K.; Kimura, S.; Tsuzuki, S.; Urabe, F.; Fukuokaya, W.; Mori, K.; Aikawa, K.; Murakami, M.; Sasaki, H.; Miki, K. Radiographic Progression at Castration-Resistant Prostate Cancer Diagnosis: A Prognostic Indicator of Metastatic Hormone-Sensitive Prostate Cancer. Clin. Genitourin. Cancer 2024, 22, 102075. [Google Scholar] [CrossRef]
- Yamada, Y.; Beltran, H. Clinical and biological features of neuroendocrine prostate cancer. Curr. Oncol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Liu, S.; Alabi, B.R.; Yin, Q.; Stoyanova, T. Molecular mechanisms underlying the development of neuroendocrine prostate cancer. Semin. Cancer Biol. 2022, 86, 57–68. [Google Scholar] [CrossRef]
- Storck, W.K.; May, A.M.; Westbrook, T.C.; Duan, Z.; Morrissey, C.; Yates, J.A.; Alumkal, J.J. The role of epigenetic change in therapy-induced neuroendocrine prostate cancer lineage plasticity. Front. Endocrinol. 2022, 13, 926585. [Google Scholar] [CrossRef]
- Beltran, H.; Hruszkewycz, A.; Scher, H.I.; Hildesheim, J.; Isaacs, J.; Yu, E.Y.; Kelly, K.; Lin, D.; Dicker, A.; Arnold, J. The role of lineage plasticity in prostate cancer therapy resistance. Clin. Cancer Res. 2019, 25, 6916–6924. [Google Scholar] [CrossRef]
- Ferguson, A.M.; Rubin, M.A. Lineage plasticity in prostate cancer: Looking beyond intrinsic alterations. Cancer Lett. 2022, 548, 215901. [Google Scholar] [CrossRef]
- Zhu, J.; Liang, X.; Wu, D.; Chen, S.; Yang, B.; Mao, W.; Shen, D. Clinicopathological characteristics and survival outcomes in neuroendocrine prostate cancer: A population-based study. Medicine 2021, 100, e25237. [Google Scholar] [CrossRef]
- Yao, J.; Liu, Y.; Liang, X.; Shao, J.; Zhang, Y.; Yang, J.; Zheng, M. Neuroendocrine carcinoma as an independent prognostic factor for patients with prostate cancer: A population-based study. Front. Endocrinol. 2021, 12, 778758. [Google Scholar] [CrossRef]
- Rubin, M.A.; Bristow, R.G.; Thienger, P.D.; Dive, C.; Imielinski, M. Impact of lineage plasticity to and from a neuroendocrine phenotype on progression and response in prostate and lung cancers. Mol. Cell 2020, 80, 562–577. [Google Scholar] [CrossRef]
- Takayama, K.-i.; Kosaka, T.; Suzuki, T.; Hongo, H.; Oya, M.; Fujimura, T.; Suzuki, Y.; Inoue, S. Subtype-specific collaborative transcription factor networks are promoted by OCT4 in the progression of prostate cancer. Nat. Commun. 2021, 12, 3766. [Google Scholar] [CrossRef]
- Imamura, J.; Ganguly, S.; Muskara, A.; Liao, R.S.; Nguyen, J.K.; Weight, C.; Wee, C.E.; Gupta, S.; Mian, O.Y. Lineage plasticity and treatment resistance in prostate cancer: The intersection of genetics, epigenetics, and evolution. Front. Endocrinol. 2023, 14, 1191311. [Google Scholar] [CrossRef]
- Swain, N.; Thakur, M.; Pathak, J.; Swain, B. SOX2, OCT4 and NANOG: The core embryonic stem cell pluripotency regulators in oral carcinogenesis. J. Oral Maxillofac. Pathol. 2020, 24, 368–373. [Google Scholar] [CrossRef]
- Esch, D.; Vahokoski, J.; Groves, M.R.; Pogenberg, V.; Cojocaru, V.; Vom Bruch, H.; Han, D.; Drexler, H.C.; Arauzo-Bravo, M.J.; Ng, C.K. A unique Oct4 interface is crucial for reprogramming to pluripotency. Nat. Cell Biol. 2013, 15, 295–301. [Google Scholar] [CrossRef]
- Robinson, M.; Gilbert, S.F.; Waters, J.A.; Lujano-Olazaba, O.; Lara, J.; Alexander, L.J.; Green, S.E.; Burkeen, G.A.; Patrus, O.; Sarwar, Z. Characterization of SOX2, OCT4 and NANOG in ovarian cancer tumor-initiating cells. Cancers 2021, 13, 262. [Google Scholar] [CrossRef]
- Mohiuddin, I.S.; Wei, S.-J.; Kang, M.H. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165432. [Google Scholar] [CrossRef]
- Kosaka, T.; Nagamatsu, G.; Saito, S.; Oya, M.; Suda, T.; Horimoto, K. Identification of drug candidate against prostate cancer from the aspect of somatic cell reprogramming. Cancer Sci. 2013, 104, 1017–1026. [Google Scholar] [CrossRef]
- Ma, Y. OCT4-positive circulating tumor cells may predict a poor prognosis in patients with metastatic castration-resistant prostate cancer treated with abiraterone plus prednisone therapy. Oncol. Lett. 2023, 26, 452. [Google Scholar] [CrossRef]
- Xie, W.; Yu, J.; Yin, Y.; Zhang, X.; Zheng, X.; Wang, X. OCT4 induces EMT and promotes ovarian cancer progression by regulating the PI3K/AKT/mTOR pathway. Front. Oncol. 2022, 12, 876257. [Google Scholar] [CrossRef]
- Bu, X.; Liu, Y.; Wang, L.; Yan, Z.; Xin, G.; Su, W. Oct4 promoted proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) in colon cancer cells by activating the SCF/c-Kit signaling pathway. Cell Cycle 2023, 22, 291–302. [Google Scholar] [CrossRef]
- Formaggio, N.; Rubin, M.A.; Theurillat, J.-P. Loss and revival of androgen receptor signaling in advanced prostate cancer. Oncogene 2021, 40, 1205–1216. [Google Scholar] [CrossRef]
- Nouruzi, S.; Ganguli, D.; Tabrizian, N.; Kobelev, M.; Sivak, O.; Namekawa, T.; Thaper, D.; Baca, S.C.; Freedman, M.L.; Aguda, A. ASCL1 activates neuronal stem cell-like lineage programming through remodeling of the chromatin landscape in prostate cancer. Nat. Commun. 2022, 13, 2282. [Google Scholar] [CrossRef]
- Kaarijärvi, R.; Kaljunen, H.; Ketola, K. Molecular and functional links between neurodevelopmental processes and treatment-induced neuroendocrine plasticity in prostate cancer progression. Cancers 2021, 13, 692. [Google Scholar] [CrossRef]
- Ko, J.; Meyer, A.N.; Haas, M.; Donoghue, D.J. Characterization of FGFR signaling in prostate cancer stem cells and inhibition via TKI treatment. Oncotarget 2021, 12, 22. [Google Scholar] [CrossRef]
- Guo, C.; Kadier, A.; Zhang, Z.; Mao, S.; Yang, B.; Zheng, J.; Yao, X. ADT increases prostate cancer cell invasion via altering AR/SALL4/SOX2-OCT4 stem cell signaling. Cell Biol. Toxicol. 2025, 41, 107. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Huang, L.; Niu, S.; Lu, Q.; Gong, D.; Huang, S.; Yuan, Y.; Chen, H. Prognostic value of association of OCT4 with LEF1 expression in esophageal squamous cell carcinoma and their impact on epithelial-mesenchymal transition, invasion, and migration. Cancer Med. 2018, 7, 3977–3987. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Shi, S.; Xu, Y.; Dai, X.; Li, H.; Wang, J.; Zhang, Q.; Wang, Y.; Sun, S. The prognostic and clinicopathologic characteristics of OCT4 and lung cancer: A meta-analysis. Curr. Mol. Med. 2019, 19, 54–75. [Google Scholar] [CrossRef]
- Kosaka, T.; Mikami, S.; Yoshimine, S.; Miyazaki, Y.; Daimon, T.; Kikuchi, E.; Miyajima, A.; Oya, M. The prognostic significance of OCT4 expression in patients with prostate cancer. Hum. Pathol. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Z.; Zhu, Y.; Chen, J.; Li, W. The role and specific mechanism of OCT4 in cancer stem cells: A review. Int. J. Stem Cells 2020, 13, 312–325. [Google Scholar] [CrossRef]
- Cui, Y.; Niu, Y.; Zhou, J.; Chen, Y.; Cheng, Y.; Li, S.; Ai, Z.; Chu, C.; Wang, H.; Zheng, B. Generation of a precise Oct4-hrGFP knockin cynomolgus monkey model via CRISPR/Cas9-assisted homologous recombination. Cell Res. 2018, 28, 383–386. [Google Scholar] [CrossRef]
- Gao, L.; Yang, Y.; Xu, H.; Liu, R.; Li, D.; Hong, H.; Qin, M.; Wang, Y. MiR-335 functions as a tumor suppressor in pancreatic cancer by targeting OCT4. Tumor Biol. 2014, 35, 8309–8318. [Google Scholar] [CrossRef]
- Vaddi, P.K.; Stamnes, M.A.; Cao, H.; Chen, S. Elimination of SOX2/OCT4-associated prostate cancer stem cells blocks tumor development and enhances therapeutic response. Cancers 2019, 11, 1331. [Google Scholar] [CrossRef]
- Fogarty, N.M.; McCarthy, A.; Snijders, K.E.; Powell, B.E.; Kubikova, N.; Blakeley, P.; Lea, R.; Elder, K.; Wamaitha, S.E.; Kim, D. Genome editing reveals a role for OCT4 in human embryogenesis. Nature 2017, 550, 67–73. [Google Scholar] [CrossRef]
- Hisey, E.; Ross, P.J.; Meyers, S.A. A review of OCT4 functions and applications to equine embryos. J. Equine Vet. Sci. 2021, 98, 103364. [Google Scholar] [CrossRef]
- Niwa, H.; Miyazaki, J.-i.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Z.; Wei, C.; Wu, M.; Zheng, W.; Zhang, H.; Liu, C.; Liu, L. Prognostic evaluation of NANOG and OCT4 expression for posttransplantation hepatocellular carcinoma recurrence. J. Cell. Biochem. 2019, 120, 8419–8429. [Google Scholar] [CrossRef]
- Noel, K.; Ibraheem, M.M.; Ahmed, B.S.; Hameed, A.F.; Khamees, N.H.; Akkila, S.S. Expression of OCT4 stem cell marker in benign prostatic hyperplasia and normal tissue around the prostatic carcinoma in a sample of Iraqi patients. Egypt. J. Histol. 2020, 43, 245–254. [Google Scholar]
- Su, B.-H.; Wang, C.-T.; Chang, J.-M.; Chen, H.-Y.; Huang, T.-H.; Yen, Y.-T.; Tseng, Y.-L.; Chang, M.-Y.; Lee, C.-H.; Cheng, L.-H. OCT4 promotes lung cancer progression through upregulation of VEGF-correlated chemokine-1. Int. J. Med. Sci. 2025, 22, 680. [Google Scholar] [CrossRef]
- Lambis-Anaya, L.; Fernández-Ruiz, M.; Liscano, Y.; Suarez-Causado, A. High OCT4 expression might be associated with an aggressive phenotype in rectal cancer. Cancers 2023, 15, 3740. [Google Scholar] [CrossRef]
- Pandian, J.; Panneerpandian, P.; Sekar, B.T.; Selvarasu, K.; Ganesan, K. OCT4-mediated transcription confers oncogenic advantage for a subset of gastric tumors with poor clinical outcome. Funct. Integr. Genom. 2022, 22, 1345–1360. [Google Scholar] [CrossRef]
- Ding, J.; Xu, H.; Faiola, F.; Ma’ayan, A.; Wang, J. Oct4 links multiple epigenetic pathways to the pluripotency network. Cell Res. 2012, 22, 155–167. [Google Scholar] [CrossRef]
- Ambrosetti, D.-C.; Basilico, C.; Dailey, L. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein-protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol. Cell. Biol. 1997, 17, 6321–6329. [Google Scholar] [CrossRef]
- Cho, Y.; Kang, H.G.; Kim, S.-J.; Lee, S.; Jee, S.; Ahn, S.G.; Kang, M.J.; Song, J.S.; Chung, J.-Y.; Yi, E.C. Post-translational modification of OCT4 in breast cancer tumorigenesis. Cell Death Differ. 2018, 25, 1781–1795. [Google Scholar] [CrossRef]
- Sohn, E.J.; Moon, H.J.; Lim, J.K.; Kim, D.S.; Kim, J.H. Regulation of the protein stability and transcriptional activity of OCT4 in stem cells. Adv. Biol. Regul. 2021, 79, 100777. [Google Scholar] [CrossRef]
- Dan, S.; Kang, B.; Duan, X.; Wang, Y.-J. A cell-free system toward deciphering the post-translational modification barcodes of Oct4 in different cellular contexts. Biochem. Biophys. Res. Commun. 2015, 456, 714–720. [Google Scholar] [CrossRef]
- MacLean, M.R.; Walker, O.L.; Arun, R.P.; Fernando, W.; Marcato, P. Informed by cancer stem cells of solid tumors: Advances in treatments targeting tumor-promoting factors and pathways. Int. J. Mol. Sci. 2024, 25, 4102. [Google Scholar]
- Weng, Z.; Lin, J.; He, J.; Gao, L.; Lin, S.; Tsang, L.L.; Zhang, H.; He, X.; Wang, G.; Yang, X. Human embryonic stem cell-derived neural crest model unveils CD55 as a cancer stem cell regulator for therapeutic targeting in MYCN-amplified neuroblastoma. Neuro-Oncology 2022, 24, 872–885. [Google Scholar] [CrossRef]
- Liu, H.-L.; Tang, H.-t.; Yang, H.-l.; Deng, T.-T.; Xu, Y.-P.; Xu, S.-Q.; Peng, L.; Wang, Z.; Fang, Q.; Kuang, X.-Y. Oct4 regulates the transition of cancer stem-like cells to tumor endothelial-like cells in human liver cancer. Front. Cell Dev. Biol. 2020, 8, 563316. [Google Scholar] [CrossRef]
- Smith, B.A.; Sokolov, A.; Uzunangelov, V.; Baertsch, R.; Newton, Y.; Graim, K.; Mathis, C.; Cheng, D.; Stuart, J.M.; Witte, O.N. A basal stem cell signature identifies aggressive prostate cancer phenotypes. Proc. Natl. Acad. Sci. USA 2015, 112, E6544–E6552. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Seifeldin, S.A.; Alshaghdali, K.; Siddiqui, S.; Abdelwadoud, M.E.; Vyas, M.; Saeed, M.; Mazumder, A.; Saeed, A. Targeting Wnt/β-catenin pathway by flavonoids: Implication for cancer therapeutics. Nutrients 2023, 15, 2088. [Google Scholar] [CrossRef]
- Leonardo-Sousa, C.; Barriga, R.; Florindo, H.F.; Acúrcio, R.C.; Guedes, R.C. Structural Insights and Development of Small Molecule Inhibitors Targeting TGF-β Receptor I: A Comprehensive Review of Clinical Advances. Mol. Ther. Oncol. 2025, 33, 200945. [Google Scholar] [CrossRef]
- Du, L.; Zhu, W. Research Progress on TGF-Β Gene Family. Octa J. Environ. Res. 2024, 12, 1–10. [Google Scholar]
- Ebrahimi, M.; Nourbakhsh, E.; Hazara, A.Z.; Mirzaei, A.; Shafieyari, S.; Salehi, A.; Hoseinzadeh, M.; Payandeh, Z.; Barati, G. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol.-Res. Pract. 2022, 237, 154010. [Google Scholar]
- Song, J.-x.; Dong, Y.-q.; Han, R.-l.; Xie, J.; Zhu, A.-y.; Chen, X.; Yang, Y.-y.; Sheng, C.-x.; Jiang, T.; Zhao, H.-y. PI3K/AKT/mTOR Activation is Associated with Malignant Severity and Poorer Prognosis in Parathyroid Carcinomas. J. Clin. Endocrinol. Metab. 2025, dgaf042. [Google Scholar] [CrossRef]
- Versari, I.; Salucci, S.; Bavelloni, A.; Battistelli, M.; Traversari, M.; Wang, A.; Sampaolesi, M.; Faenza, I. The Emerging Role and Clinical Significance of PI3K-Akt-mTOR in Rhabdomyosarcoma. Biomolecules 2025, 15, 334. [Google Scholar] [CrossRef]
- Iluta, S.; Nistor, M.; Buruiana, S.; Dima, D. Notch and Hedgehog Signaling Unveiled: Crosstalk, Roles, and Breakthroughs in Cancer Stem Cell Research. Life 2025, 15, 228. [Google Scholar] [CrossRef]
- Lou, L.; Peng, K.; Ouyang, S.; Ding, W.; Mo, J.; Yan, J.; Gong, X.; Liu, G.; Lu, J.; Yue, P. Periostin-mediated NOTCH1 activation between tumor cells and HSCs crosstalk promotes liver metastasis of small cell lung cancer. J. Exp. Clin. Cancer Res. 2025, 44, 6. [Google Scholar] [CrossRef]
- Chen, C.; Du, Y.; Nie, R.; Wang, S.; Wang, H.; Li, P. Notch signaling in cancers: Mechanism and potential therapy. Front. Cell Dev. Biol. 2025, 13, 1542967. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Wu, H.; Wang, Y.; Deng, Y.; Chang, Y.; Su, K.; Yang, L.; Tao, W.; Liu, W. Exploring the mechanism of Jianpi Lishi Jiedu Granules against postoperative recurrence of colorectal adenoma based on IL-6/JAK/STAT3 signaling pathway. Cell. Signal. 2025, 127, 111535. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Roopashree, R.; Kazmi, S.W.; Jasim, S.A.; Phaninder Vinay, K.; Fateh, A.; Yang, F.; Rajabivahid, M.; Dehghani-Ghorbi, M. Long non-coding RNAs (lncRNAs) in cancer development: New insight from STAT3 signaling pathway to immune evasion. Clin. Exp. Med. 2025, 25, 53. [Google Scholar] [CrossRef]
- Gao, Y.; Lan, L.; Wang, C.; Wang, Y.; Shi, L.; Sun, L. Selective JAK1 inhibitors and the therapeutic applications thereof: A patent review (2016–2023). Expert Opin. Ther. Pat. 2025, 35, 181–195. [Google Scholar] [CrossRef]
- Duan, J.; Wang, Y.; Chen, Y.; Wang, Y.; Li, Q.; Liu, J.; Fu, C.; Cao, C.; Cong, Z.; Su, M. Silencing LY6D expression inhibits colon cancer in xenograft mice and regulates colon cancer stem cells’ proliferation, stemness, invasion, and apoptosis via the MAPK pathway. Molecules 2023, 28, 7776. [Google Scholar] [CrossRef]
- Emelyanova, A.; Zolotovskaia, M.; Poddubskaya, E.; Modestov, A.; Buzdin, A.; Kuzmin, D. Activation of P38 MAPK Signaling Cascade is Linked with Clinical Outcomes and Therapeutic Responses in Human Cancers. Biochemistry 2024, 89, 2155–2173. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lin, T.-J.; Chong, K.-Y.; Chen, G.-Y.; Kuo, C.-Y.; Lin, Y.-Y.; Chang, C.-W.; Hsiao, T.-F.; Wang, C.-L.; Shih, Y.-C. Targeting the ERK1/2 and p38 MAPK pathways attenuates Golgi tethering factor golgin-97 depletion-induced cancer progression in breast cancer. Cell Commun. Signal. 2025, 23, 22. [Google Scholar] [CrossRef]
- Yong, X.; Tang, B.; Xiao, Y.-F.; Xie, R.; Qin, Y.; Luo, G.; Hu, C.-J.; Dong, H.; Yang, S.-M. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016, 374, 292–303. [Google Scholar] [CrossRef]
- Sun, L.; Liu, T.; Zhang, S.; Guo, K.; Liu, Y. Oct4 induces EMT through LEF1/β-catenin dependent WNT signaling pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 2599–2606. [Google Scholar] [CrossRef]
- Yuan, F.; Zhou, W.; Zou, C.; Zhang, Z.; Hu, H.; Dai, Z.; Zhang, Y. Expression of Oct4 in HCC and modulation to wnt/β-catenin and TGF-β signal pathways. Mol. Cell. Biochem. 2010, 343, 155–162. [Google Scholar] [CrossRef]
- Wang, P.; Deng, Z.; Li, A.; Li, R.; Huang, W.; Cui, J.; Chen, S.; Li, B.; Zhang, S. β-Catenin promotes long-term survival and angiogenesis of peripheral blood mesenchymal stem cells via the Oct4 signaling pathway. Exp. Mol. Med. 2022, 54, 1434–1449. [Google Scholar] [CrossRef]
- Bassiouny, A. Regulation of Oct4 signaling on tumorigenesis and modulation of wnt/β-catenin and TGF-β signal pathways in hepatocellular carcinoma cells. J. Clin. Oncol. 2011, 29 (Suppl. 15), e13551. [Google Scholar]
- Kelly, K.F.; Ng, D.Y.; Jayakumaran, G.; Wood, G.A.; Koide, H.; Doble, B.W. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell 2011, 8, 214–227. [Google Scholar]
- Guo, Y.; Li, B.; Yan, X.; Shen, X.; Ma, J.; Liu, S.; Zhang, D. Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere 2020, 246, 125775. [Google Scholar]
- Shen, W.; Zhang, X.; Tang, J.; Zhang, Z.; Du, R.; Luo, D.; Liu, X.; Xia, Y.; Li, Y.; Wang, S. CCL16 maintains stem cell-like properties in breast cancer by activating CCR2/GSK3β/β-catenin/OCT4 axis. Theranostics 2021, 11, 2297. [Google Scholar]
- Huang, H.; Wang, C.; Liu, F.; Li, H.-Z.; Peng, G.; Gao, X.; Dong, K.-Q.; Wang, H.-R.; Kong, D.-P.; Qu, M. Reciprocal network between cancer stem-like cells and macrophages facilitates the progression and androgen deprivation therapy resistance of prostate cancer. Clin. Cancer Res. 2018, 24, 4612–4626. [Google Scholar]
- Zhao, Q.W.; Zhou, Y.W.; Li, W.X.; Kang, B.; Zhang, X.Q.; Yang, Y.; ChENG, J.; Yin, S.Y.; Tong, Y.; He, J.Q. Akt-mediated phosphorylation of Oct4 is associated with the proliferation of stem-like cancer cells. Oncol. Rep. 2015, 33, 1621–1629. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Li, W.; Chen, Q.; Li, J.; Pan, X.; Zhou, L.; Liu, C.; Chen, C.; He, J. Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol. Cell 2012, 48, 627–640. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Zhang, X.; Yang, Y.; Dan, S.; Su, T.; She, S.; Dong, W.; Zhao, Q.; Jia, J. Dual inhibiting OCT4 and AKT potently suppresses the propagation of human cancer cells. Sci. Rep. 2017, 7, 46246. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, D. TLR5/7-mediated PI3K activation triggers epithelial-mesenchymal transition of ovarian cancer cells through WAVE3-dependent mesothelin or OCT4/SOX2 expression. Oncol. Rep. 2017, 38, 3167–3176. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zhang, Y.; Zhang, Z.; Peng, J.; Li, Z.; Han, L.; You, Q.; Chen, X.; Rao, X. Downregulation of cancer stem cell properties via mTOR signaling pathway inhibition by rapamycin in nasopharyngeal carcinoma. Int. J. Oncol. 2015, 47, 909–917. [Google Scholar] [CrossRef]
- Johnson, A.; Korleski, J.; Laterra, J.; Lopez-Bertoni, H. Abstract PR013: Oct4 and Sox2 induce cellular transition of glioma stem cells to an immune suppressive, regulatory T cell-like state. Cancer Res. 2022, 82 (Suppl. 10), PR013. [Google Scholar] [CrossRef]
- Hagiwara, M.; Yasumizu, Y.; Yamashita, N.; Rajabi, H.; Fushimi, A.; Long, M.D.; Li, W.; Bhattacharya, A.; Ahmad, R.; Oya, M. MUC1-C activates the BAF (mSWI/SNF) complex in prostate cancer stem cells. Cancer Res. 2021, 81, 1111–1122. [Google Scholar] [CrossRef]
- Kong, D.; Banerjee, S.; Ahmad, A.; Li, Y.; Wang, Z.; Sethi, S.; Sarkar, F.H. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE 2010, 5, e12445. [Google Scholar] [CrossRef]
- Zhang, L.; Sha, J.; Yang, G.; Huang, X.; Bo, J.; Huang, Y. Activation of Notch pathway is linked with epithelial-mesenchymal transition in prostate cancer cells. Cell Cycle 2017, 16, 999–1007. [Google Scholar] [CrossRef]
- Ibrahim, D.A.; Elsebai, E.A.; Fayed, A.; Abdelrahman, A.E. Prognostic value of NOTCH1 and OCT4 in gastric carcinoma. Indian J. Pathol. Microbiol. 2022, 65, 328–335. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, G.; Wang, Z.; Ding, X.; Qian, L.; Li, Y. Reck-notch1 signaling mediates miR-221/222 regulation of lung cancer stem cells in NSCLC. Front. Cell Dev. Biol. 2021, 9, 663279. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; Xu, S.; Zhang, L.; Zhang, X.; Deng, J.; Ye, W.; Liu, S. Targeting EGFR enriches stem cell-like properties in salivary adenoid cystic carcinoma by activating the Notch1 pathway. Cancer Manag. Res. 2020, 12, 6655–6663. [Google Scholar] [CrossRef]
- Cheng, J.-w.; Duan, L.-x.; Yu, Y.; Wang, P.; Feng, J.-l.; Feng, G.-z.; Liu, Y. Bone marrow mesenchymal stem cells promote prostate cancer cell stemness via cell–cell contact to activate the Jagged1/Notch1 pathway. Cell Biosci. 2021, 11, 87. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, Y.; Chen, W.; Peng, W.; Wang, Y.; Xiong, S.; Aruna; Li, Y.; Yang, Y.; Chen, S. The stromal-tumor amplifying STC1-Notch1 feedforward signal promotes the stemness of hepatocellular carcinoma. J. Transl. Med. 2023, 21, 236. [Google Scholar] [CrossRef]
- Zhao, L.; Lei, J.; Gu, S.; Zhang, Y.; Jing, X.; Wang, L.; Zhang, L.; Ning, Q.; Luo, M.; Qi, Y. A yes-associated protein 1-Notch1 receptor positive feedback loop promotes breast cancer lung metastasis by attenuating the bone morphogenetic protein 4-SMAD family member 1/5 signaling. Carcinogenesis 2022, 43, 1162–1175. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, H.; Gu, Z.; Gao, Q.; Shen, G. Oct4 promotes cancer cell proliferation and migration and leads to poor prognosis associated with the survivin/STAT3 pathway in hepatocellular carcinoma. Oncol. Rep. 2018, 40, 979–987. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kang, J.W.; Song, X.; Kim, B.K.; Yoo, Y.D.; Kwon, Y.T.; Lee, Y.J. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell. Signal. 2013, 25, 961–969. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Shi, L.-H.; Wang, X.-J.; Wang, S.-X.; Wan, X.-Q.; Liu, S.-R.; Wang, Y.-F.; Lu, Z.; Wang, L.-H.; Ding, Y. Stat3/Oct-4/c-Myc signal circuit for regulating stemness-mediated doxorubicin resistance of triple-negative breast cancer cells and inhibitory effects of WP1066. Int. J. Oncol. 2018, 53, 339–348. [Google Scholar] [CrossRef]
- Sun, S.; Yang, H.; Wang, F.; Zhao, S. Oct4 downregulation-induced inflammation increases the migration and invasion rate of oral squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2021, 53, 1440–1449. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, Q.; Dai, X.; Wen, X.; Luo, X.; Duan, Y.; Yang, Z.; Dai, Q. Alantolactone enhances the sensitivity of melanoma to MAPK pathway inhibitors by targeting inhibition of STAT3 activation and down-regulating stem cell markers. Cancer Cell Int. 2024, 24, 191. [Google Scholar] [CrossRef]
- Chen, M.; Ye, A.; Wei, J.; Wang, R.; Poon, K. Deoxycholic acid upregulates the reprogramming factors KFL4 and OCT4 through the IL-6/STAT3 pathway in esophageal adenocarcinoma cells. Technol. Cancer Res. Treat. 2020, 19, 1533033820945302. [Google Scholar] [CrossRef]
- Pandian, J.; Ganesan, K. Delineation of gastric tumors with activated ERK/MAPK signaling cascades for the development of targeted therapeutics. Exp. Cell Res. 2022, 410, 112956. [Google Scholar] [CrossRef]
- Jiang, P.; Li, F.; Liu, Z.; Hao, S.; Gao, J.; Li, S. BTB and CNC homology 1 (Bach1) induces lung cancer stem cell phenotypes by stimulating CD44 expression. Respir. Res. 2021, 22, 320. [Google Scholar] [CrossRef]
- Xu, H.; Du, Z.; Li, Z.; Liu, X.; Li, X.; Zhang, X.; Ma, J. MUC1-EGFR crosstalk with IL-6 by activating NF-κB and MAPK pathways to regulate the stemness and paclitaxel-resistance of lung adenocarcinoma. Ann. Med. 2024, 56, 2313671. [Google Scholar] [CrossRef]
- Wei, S.-J.; Nguyen, T.H.; Yang, I.-H.; Mook, D.G.; Makena, M.R.; Verlekar, D.; Hindle, A.; Martinez, G.M.; Yang, S.; Shimada, H. MYC transcription activation mediated by OCT4 as a mechanism of resistance to 13-cis RA-mediated differentiation in neuroblastoma. Cell Death Dis. 2020, 11, 368. [Google Scholar] [CrossRef]
- Emhemmed, F.; Azouaou, S.A.; Thuaud, F.; Schini-Kerth, V.; Désaubry, L.; Muller, C.D.; Fuhrmann, G. Selective anticancer effects of a synthetic flavagline on human Oct4-expressing cancer stem-like cells via a p38 MAPK-dependent caspase-3-dependent pathway. Biochem. Pharmacol. 2014, 89, 185–196. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar]
- Roy, A.; Mishra, J.; Chakraborty, S.; Singh, S.P.; Patra, S.K. Epigenetic regulation of pluripotency inducer genes NANOG and SOX2 in human prostate cancer. Prog. Mol. Biol. Transl. Sci. 2023, 197, 241–260. [Google Scholar]
- Costa, C.D.; Justo, A.A.; Kobayashi, P.E.; Story, M.M.; Palmieri, C.; Amorim, R.L.; Fonseca-Alves, C.E. Characterization of OCT3/4, Nestin, NANOG, CD44 and CD24 as stem cell markers in canine prostate cancer. Int. J. Biochem. Cell Biol. 2019, 108, 21–28. [Google Scholar] [CrossRef]
- Qi, Y.-F.; Wu, L.; Li, Z.-Q.; Wu, M.-L.; Wang, H.-F.; Chan, K.-Y.; Lu, L.-L.; Cai, S.-H.; Wang, H.-S.; Du, J. Nodal signaling modulates the expression of Oct-4 via nuclear translocation of β-catenin in lung and prostate cancer cells. Arch. Biochem. Biophys. 2016, 608, 34–41. [Google Scholar] [CrossRef]
- De Resende, M.F.; Chinen, L.T.D.; Vieira, S.; Jampietro, J.; Da Fonseca, F.P.; Vassallo, J.; Campos, L.C.; Guimarães, G.C.; Soares, F.A.; Rocha, R.M. Prognostication of OCT4 isoform expression in prostate cancer. Tumor Biol. 2013, 34, 2665–2673. [Google Scholar] [CrossRef]
- Nong, S.; Guan, Y.; Wang, Z.; Wei, Z.; Zhang, Y.; Ni, J.; He, C.; Ma, L.; Zhou, S.; Li, W. Significant association between IL-18 and OCT4 gene polymorphisms in susceptibility and clinical characteristics of prostate cancer. Oncol. Transl. Med. 2019, 5, 123–130. [Google Scholar] [CrossRef]
- Caputo, S.; Grioni, M.; Brambillasca, C.S.; Monno, A.; Brevi, A.; Freschi, M.; Piras, I.S.; Elia, A.R.; Pieri, V.; Baccega, T. Galectin-3 in prostate cancer stem-like cells is immunosuppressive and drives early metastasis. Front. Immunol. 2020, 11, 540641. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, W. Expression and significance of S100P, CD147, and OCT4 in different prostate cancer tissue TNM stages. Genet. Mol. Res. 2015, 14, 6844–6851. [Google Scholar] [CrossRef]
- Hepburn, A.; Steele, R.; Veeratterapillay, R.; Wilson, L.; Kounatidou, E.; Barnard, A.; Berry, P.; Cassidy, J.; Moad, M.; El-Sherif, A. The induction of core pluripotency master regulators in cancers defines poor clinical outcomes and treatment resistance. Oncogene 2019, 38, 4412–4424. [Google Scholar] [CrossRef]
- Miyashita, M.; Tomogane, M.; Nakamura, Y.; Shimizu, T.; Fujihara, A.; Ukimura, O.; Ashihara, E. Sphere-derived prostate cancer stem cells are resistant to γδ T cell cytotoxicity. Anticancer Res. 2020, 40, 5481–5487. [Google Scholar] [CrossRef]
- Federer-Gsponer, J.R.; Müller, D.C.; Zellweger, T.; Eggimann, M.; Marston, K.; Ruiz, C.; Seifert, H.H.; Rentsch, C.A.; Bubendorf, L.; Le Magnen, C. Patterns of stemness-associated markers in the development of castration-resistant prostate cancer. Prostate 2020, 80, 1108–1117. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, H.-Y.; Yuan, T.; Luo, J.; Zhou, W.-D.; Jiang, Q.-Q.; Wu, D. Enzalutamide-induced upregulation of PCAT6 promotes prostate cancer neuroendocrine differentiation by regulating miR-326/HNRNPA2B1 axis. Front. Oncol. 2021, 11, 650054. [Google Scholar] [CrossRef]
- Fujimoto, N.; Tsubonuma, Y.; Nagata, Y.; Minato, A.; Tomisaki, I.; Harada, K.; Miyamoto, H. Second-line systemic therapy for highly aggressive neuroendocrine prostate cancer. Anticancer Res. 2023, 43, 3841–3847. [Google Scholar] [CrossRef]
- Bishop, J.L.; Thaper, D.; Vahid, S.; Davies, A.; Ketola, K.; Kuruma, H.; Jama, R.; Nip, K.M.; Angeles, A.; Johnson, F. The master neural transcription factor BRN2 is an androgen receptor–suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 2017, 7, 54–71. [Google Scholar] [CrossRef]
- Sotomayor, P.; Godoy, A.; Smith, G.J.; Huss, W.J. Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate 2009, 69, 401–410. [Google Scholar] [CrossRef]
- Gillessen, S.; Turco, F.; Davis, I.D.; Efstathiou, J.A.; Fizazi, K.; James, N.D.; Shore, N.; Small, E.; Smith, M.; Sweeney, C.J. Management of patients with advanced prostate cancer. Report from the 2024 Advanced Prostate Cancer Consensus Conference (APCCC). Eur. Urol. 2025, 87, 157–216. [Google Scholar]
- Thomson, A.; Al Saffar, H.; Tempo, J.; Lawrentschuk, N.; Murphy, D.G.; Perera, M. Time to castrate the cost? the rising expense of chemical castration for the management of prostate cancer. Prostate Int. 2025. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Puzanov, I.; Goodrich, D.W.; Chatta, G.; Tang, D.G. Prostate cancer as a dedifferentiated organ: Androgen receptor, cancer stem cells, and cancer stemness. Essays Biochem. 2022, 66, 291–303. [Google Scholar]
- Sanchez, B.G.; Bort, A.; Vara-Ciruelos, D.; Diaz-Laviada, I. Androgen deprivation induces reprogramming of prostate cancer cells to stem-like cells. Cells 2020, 9, 1441. [Google Scholar] [CrossRef]
- Quintero, J.C.; Díaz, N.F.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Cancer stem cells and androgen receptor signaling: Partners in disease progression. Int. J. Mol. Sci. 2023, 24, 15085. [Google Scholar] [CrossRef]
- Tiwari, R.; Manzar, N.; Ateeq, B. Dynamics of cellular plasticity in prostate cancer progression. Front. Mol. Biosci. 2020, 7, 130. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Montironi, R.; Jiang, Z.; Cheng, M.; Santoni, M.; Huang, K.; Massari, F.; Lu, X.; Cimadamore, A. Epigenetic modulations and lineage plasticity in advanced prostate cancer. Ann. Oncol. 2020, 31, 470–479. [Google Scholar] [CrossRef]
- Al Salhi, Y.; Sequi, M.B.; Valenzi, F.M.; Fuschi, A.; Martoccia, A.; Suraci, P.P.; Carbone, A.; Tema, G.; Lombardo, R.; Cicione, A. Cancer stem cells and prostate cancer: A narrative review. Int. J. Mol. Sci. 2023, 24, 7746. [Google Scholar] [CrossRef]
- Verma, S.; Shankar, E.; Kalayci, F.N.C.; Mukunda, A.; Alassfar, M.; Singh, V.; Chan, E.R.; MacLennan, G.T.; Gupta, S. Androgen deprivation induces transcriptional reprogramming in prostate cancer cells to develop stem cell-like characteristics. Int. J. Mol. Sci. 2020, 21, 9568. [Google Scholar] [CrossRef]
- Banerjee, P.; Kapse, P.; Siddique, S.; Kundu, M.; Choudhari, J.; Mohanty, V.; Malhotra, D.; Gosavi, S.W.; Gacche, R.N.; Kundu, G.C. Therapeutic implications of cancer stem cells in prostate cancer. Cancer Biol. Med. 2023, 20, 401–420. [Google Scholar] [CrossRef]
- Fatma, H.; Siddique, H.R. Cancer cell plasticity, stem cell factors, and therapy resistance: How are they linked? Cancer Metastasis Rev. 2024, 43, 423–440. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.-C.; Wongvipat, J.; Ku, S.-Y.; Gao, D.; Cao, Z. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53-and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef]
- Kainulainen, K.; Niskanen, E.A.; Kinnunen, J.; Mäki-Mantila, K.; Hartikainen, K.; Paakinaho, V.; Malinen, M.; Ketola, K.; Pasonen-Seppänen, S. Secreted factors from M1 macrophages drive prostate cancer stem cell plasticity by upregulating NANOG, SOX2, and CD44 through NFκB-signaling. Oncoimmunology 2024, 13, 2393442. [Google Scholar] [CrossRef]
- Linn, D.E.; Yang, X.; Sun, F.; Xie, Y.; Chen, H.; Jiang, R.; Chen, H.; Chumsri, S.; Burger, A.M.; Qiu, Y. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer 2010, 1, 908–916. [Google Scholar] [CrossRef]
- Chen, X. Super-enhancer in prostate cancer: Transcriptional disorders and therapeutic targets. npj Precis. Oncol. 2020, 4, 31. [Google Scholar] [CrossRef]
- AlAbdi, L.; Saha, D.; He, M.; Dar, M.S.; Utturkar, S.M.; Sudyanti, P.A.; McCune, S.; Spears, B.H.; Breedlove, J.A.; Lanman, N.A. Oct4-mediated inhibition of Lsd1 activity promotes the active and primed state of pluripotency enhancers. Cell Rep. 2020, 30, 1478–1490.e6. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, X.; Pauklin, S. 3D chromatin architecture and epigenetic regulation in cancer stem cells. Protein Cell 2021, 12, 440–454. [Google Scholar] [CrossRef]
- Kar, S.; Niharika, N.; Roy, A.; Patra, S.K. Overexpression of SOX2 gene by histone modifications: SOX2 enhances human prostate and breast cancer progression by prevention of apoptosis and enhancing cell proliferation. Oncology 2023, 101, 591–608. [Google Scholar] [CrossRef]
- Davies, A.; Nouruzi, S.; Ganguli, D.; Namekawa, T.; Thaper, D.; Linder, S.; Karaoğlanoğlu, F.; Omur, M.E.; Kim, S.; Kobelev, M. An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat. Cell Biol. 2021, 23, 1023–1034. [Google Scholar] [CrossRef]
- Logotheti, S.; Papadaki, E.; Zolota, V.; Logothetis, C.; Vrahatis, A.G.; Soundararajan, R.; Tzelepi, V. Lineage Plasticity and Stemness Phenotypes in Prostate Cancer: Harnessing the Power of Integrated “Omics” Approaches to Explore Measurable Metrics. Cancers 2023, 15, 4357. [Google Scholar] [CrossRef]
- Yasumizu, Y.; Rajabi, H.; Jin, C.; Hata, T.; Pitroda, S.; Long, M.D.; Hagiwara, M.; Li, W.; Hu, Q.; Liu, S. MUC1-C regulates lineage plasticity driving progression to neuroendocrine prostate cancer. Nat. Commun. 2020, 11, 338. [Google Scholar] [CrossRef]
- Shokraii, F.; Moharrami, M.; Motamed, N.; Shahhoseini, M.; Totonchi, M.; Ezzatizadeh, V.; Firouzi, J.; Khosravani, P.; Ebrahimi, M. Histone modification marks strongly regulate Cdh1 promoter in prostospheres as a model of prostate cancer stem like cells. Cell J. 2019, 21, 124. [Google Scholar]
- Saha, S.K.; Jeong, Y.; Cho, S.; Cho, S.-G. Systematic expression alteration analysis of master reprogramming factor OCT4 and its three pseudogenes in human cancer and their prognostic outcomes. Sci. Rep. 2018, 8, 14806. [Google Scholar] [CrossRef]
- Pacheco, M.B.; Camilo, V.; Henrique, R.; Jerónimo, C. Epigenetic editing in prostate cancer: Challenges and opportunities. Epigenetics 2022, 17, 564–588. [Google Scholar] [CrossRef]
- Grillo, G.; Keshavarzian, T.; Linder, S.; Arlidge, C.; Mout, L.; Nand, A.; Teng, M.; Qamra, A.; Zhou, S.; Kron, K.J. Transposable elements are co-opted as oncogenic regulatory elements by lineage-specific transcription factors in prostate cancer. Cancer Discov. 2023, 13, 2470–2487. [Google Scholar] [CrossRef]
- Kushwaha, P.P.; Verma, S.; Kumar, S.; Gupta, S. Role of prostate cancer stem-like cells in the development of antiandrogen resistance. Cancer Drug Resist. 2022, 5, 459. [Google Scholar] [CrossRef]
- Escudero-Lourdes, C.; Alvarado-Morales, I.; Tokar, E.J. Stem cells as target for prostate cancer therapy: Opportunities and challenges. Stem Cell Rev. Rep. 2022, 18, 2833–2851. [Google Scholar] [CrossRef]
- Verma, P.; Shukla, N.; Kumari, S.; Ansari, M.; Gautam, N.K.; Patel, G.K. Cancer stem cell in prostate cancer progression, metastasis and therapy resistance. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188887. [Google Scholar]
- Wolf, I.; Gratzke, C.; Wolf, P. Prostate cancer stem cells: Clinical aspects and targeted therapies. Front. Oncol. 2022, 12, 935715. [Google Scholar] [CrossRef]
- Murakami, S.; Ninomiya, W.; Sakamoto, E.; Shibata, T.; Akiyama, H.; Tashiro, F. SRY and OCT4 are required for the acquisition of cancer stem cell-like properties and are potential differentiation therapy targets. Stem Cells 2015, 33, 2652–2663. [Google Scholar] [CrossRef]
- Zhu, X.; Ding, C.-K.C.; Aggarwal, R.R. Emerging Therapeutic Targets of Neuroendocrine Prostate Cancer. Curr. Oncol. Rep. 2025, 27, 362–374. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Fay, A.P.; Matsubara, N.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.; Voog, E. Final overall survival (OS) with talazoparib (TALA)+ enzalutamide (ENZA) as first-line treatment in unselected patients with metastatic castration-resistant prostate cancer (mCRPC) in the phase 3 TALAPRO-2 trial. Am. Soc. Clin. Oncol. 2025, 43, 5. [Google Scholar] [CrossRef]
- Ramesh, S.; Selvakumar, P.; Ameer, M.Y.; Lian, S.; Abdullah Alzarooni, A.I.M.; Ojha, S.; Mishra, A.; Tiwari, A.; Kaushik, A.; Jung, Y.D. State-of-the-art therapeutic strategies for targeting cancer stem cells in prostate cancer. Front. Oncol. 2023, 13, 1059441. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, X.; Zheng, Z.; Huang, J.; Yang, X.; Shi, H. Capsaicin suppressed activity of prostate cancer stem cells by inhibition of Wnt/β-catenin pathway. Phytother. Res. 2020, 34, 817–824. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, P.; Cao, C.; Dai, G.; Xu, L.; Yang, D. Use of miR-145 and testicular nuclear receptor 4 inhibition to reduce chemoresistance to docetaxel in prostate cancer. Oncol. Rep. 2021, 45, 963–974. [Google Scholar] [CrossRef]
- Zhang, S. The mechanism of TR4 participate in prostate cancer metastasis and therapy. Theor. Nat. Sci. 2024, 61, 21–26. [Google Scholar]
- Mehravar, M.; Ghaemimanesh, F.; Poursani, E.M. An overview on the complexity of OCT4: At the level of DNA, RNA and protein. Stem Cell Rev. Rep. 2021, 17, 1121–1136. [Google Scholar] [CrossRef]
- Panayiotou, T.; Eftychiou, M.; Patera, E.; Promponas, V.J.; Strati, K. A paradigm for post-embryonic Oct4 re-expression: E7-induced hydroxymethylation regulates Oct4 expression in cervical cancer. J. Med. Virol. 2023, 95, e29264. [Google Scholar] [CrossRef]
- Lei, M.M.L.; Lee, T.K.W. Cancer stem cells: Emerging key players in immune evasion of cancers. Front. Cell Dev. Biol. 2021, 9, 692940. [Google Scholar] [CrossRef]
- Skvortsov, S.; Skvortsova, I.-I.; Tang, D.G.; Dubrovska, A. Concise review: Prostate cancer stem cells: Current understanding. Stem Cells 2018, 36, 1457–1474. [Google Scholar] [CrossRef]
- Su, H.; Huang, L.; Zhou, J.; Yang, G. Prostate cancer stem cells and their targeted therapies. Front. Cell Dev. Biol. 2024, 12, 1410102. [Google Scholar] [CrossRef]
- Deng, X.; Jiao, Y.; Hao, H.; Guo, Z.; An, G.; Zhang, W.; Xue, D.; Han, S. Dandelion extract suppresses the stem-like properties of triple-negative breast cancer cells by regulating CUEDC2/β-catenin/OCT4 signaling axis. J. Ethnopharmacol. 2025, 342, 119408. [Google Scholar] [CrossRef]
- Lin, T.-C.; Wang, K.-H.; Chuang, K.-H.; Kao, A.-P.; Kuo, T.-C. Oct-4 induces cisplatin resistance and tumor stem cell-like properties in endometrial carcinoma cells. Taiwan. J. Obstet. Gynecol. 2023, 62, 16–21. [Google Scholar] [CrossRef]
- Kasaju, M.; Mihailescu, M.-R. BPS2025-Investigation of miR-145-5p and FMRP binding to the 3′-UTR Oct4 mRNA G-quadruplex forming sequence. Biophys. J. 2025, 124, 87a. [Google Scholar] [CrossRef]
- Zou, J.; Chen, J.; Deng, L.; Xu, B.; Yu, T.; Wang, J.; He, C. Mechanistic Insights into SENP1 and OCT4 Interaction in Promoting Drug Resistance and Stem Cell Features in Colon Cancer. Am. J. Physiol.-Cell Physiol. 2025, 328, C1260–C1278. [Google Scholar] [CrossRef]

| Signaling Pathways | Key Role in Lineage Plasticity | Interaction with OCT4 | Downstream Effector | Cancer Type | Therapeutic Implication | Clinical Evidence Level | References |
|---|---|---|---|---|---|---|---|
| Wnt/β-catenin | Elevation in CSC-like properties, promotes dedifferentiation, induces transcription, and enhances epithelial–mesenchymal transition, Angiogenesis, and Antiapoptotic effect, drug resistance |
|
|
|
|
| [72,73,74,75,76,77,78,79,80] |
| PI3K/AKT/mTOR | Enhance proliferation, EMT, and plasticity |
|
|
|
|
| [23,81,82,83,84,85] |
| TGF-β | Induces EMT and plasticity |
|
|
|
|
| [74,76,86] |
| Notch1 | Drives CSC maintenance, tumor aggressiveness promotes therapy resistance, and integrates EMT with CSC self-renewal |
|
|
|
|
| [87,88,89,90,91,92,93,94,95] |
| JAK1-STAT3 | Promotes CSC plasticity and enhances tumor progression, viability, migration, and invasion. Contributes to chemoresistance and poor prognosis. |
|
|
|
|
| [96,97,98,99,100,101] |
| ERK/MAPK | Regulates tumor progression, differentiation, apoptosis, and EMT. Promotes CSC-like properties |
|
|
|
|
| [102,103,104,105,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esfini Farahani, M.; Zhang, Y.; Akinyemi, A.O.; Seilani, F.; Alam, M.R.; Liu, X. Unlocking the Role of OCT4 in Cancer Lineage Plasticity: A Cross-Cancer Perspective with an Emphasis on Prostate Cancer. Biomedicines 2025, 13, 1642. https://doi.org/10.3390/biomedicines13071642
Esfini Farahani M, Zhang Y, Akinyemi AO, Seilani F, Alam MR, Liu X. Unlocking the Role of OCT4 in Cancer Lineage Plasticity: A Cross-Cancer Perspective with an Emphasis on Prostate Cancer. Biomedicines. 2025; 13(7):1642. https://doi.org/10.3390/biomedicines13071642
Chicago/Turabian StyleEsfini Farahani, Mohammad, Yanquan Zhang, Amos Olalekan Akinyemi, Fatemeh Seilani, Md Rakibul Alam, and Xiaoqi Liu. 2025. "Unlocking the Role of OCT4 in Cancer Lineage Plasticity: A Cross-Cancer Perspective with an Emphasis on Prostate Cancer" Biomedicines 13, no. 7: 1642. https://doi.org/10.3390/biomedicines13071642
APA StyleEsfini Farahani, M., Zhang, Y., Akinyemi, A. O., Seilani, F., Alam, M. R., & Liu, X. (2025). Unlocking the Role of OCT4 in Cancer Lineage Plasticity: A Cross-Cancer Perspective with an Emphasis on Prostate Cancer. Biomedicines, 13(7), 1642. https://doi.org/10.3390/biomedicines13071642





