Vitamin D and Sarcopenia: Implications for Muscle Health
Abstract
1. Introduction
2. Vit D: Physiology and Metabolism
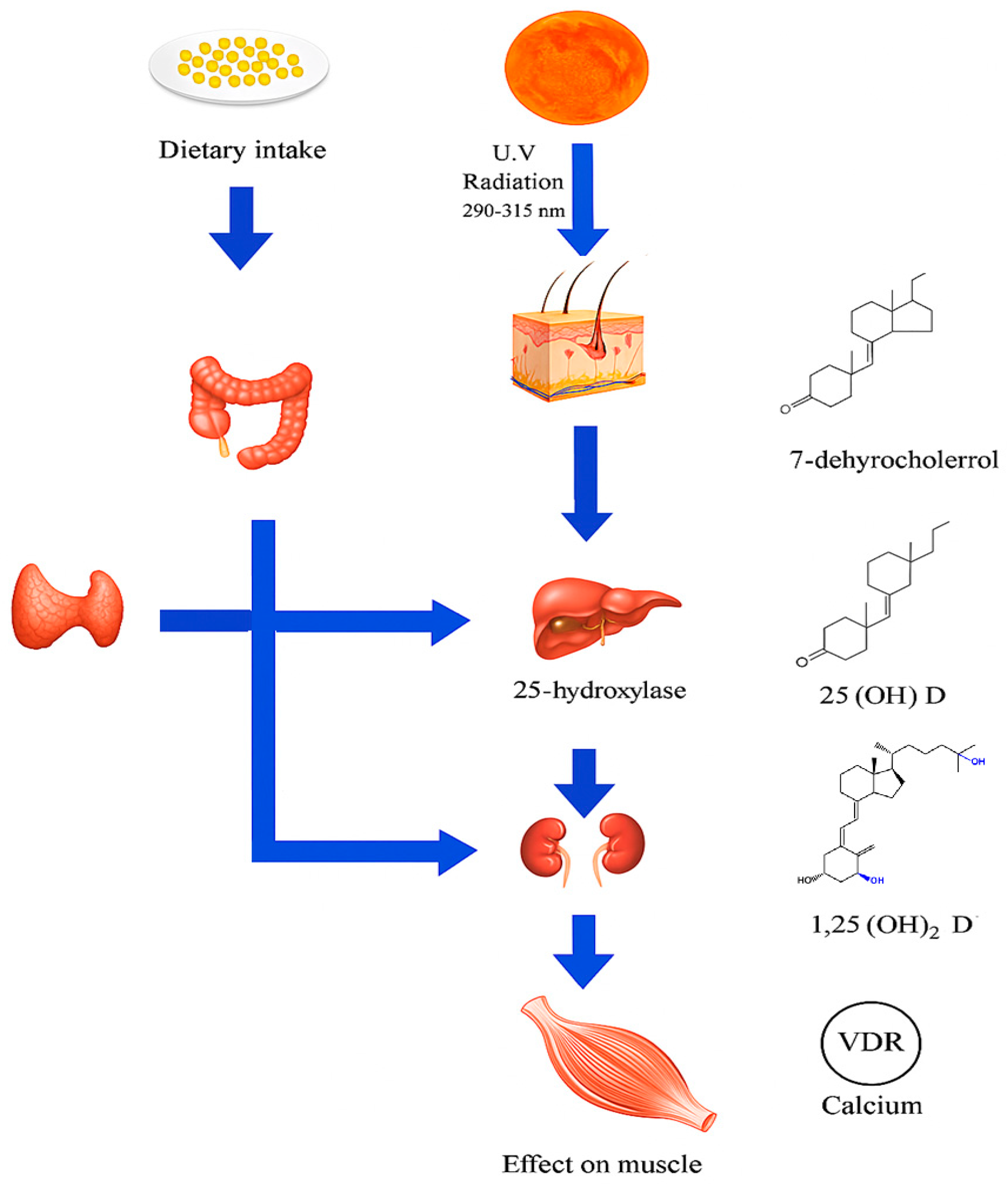
2.1. Cutaneous Synthesis and Dietary Sources
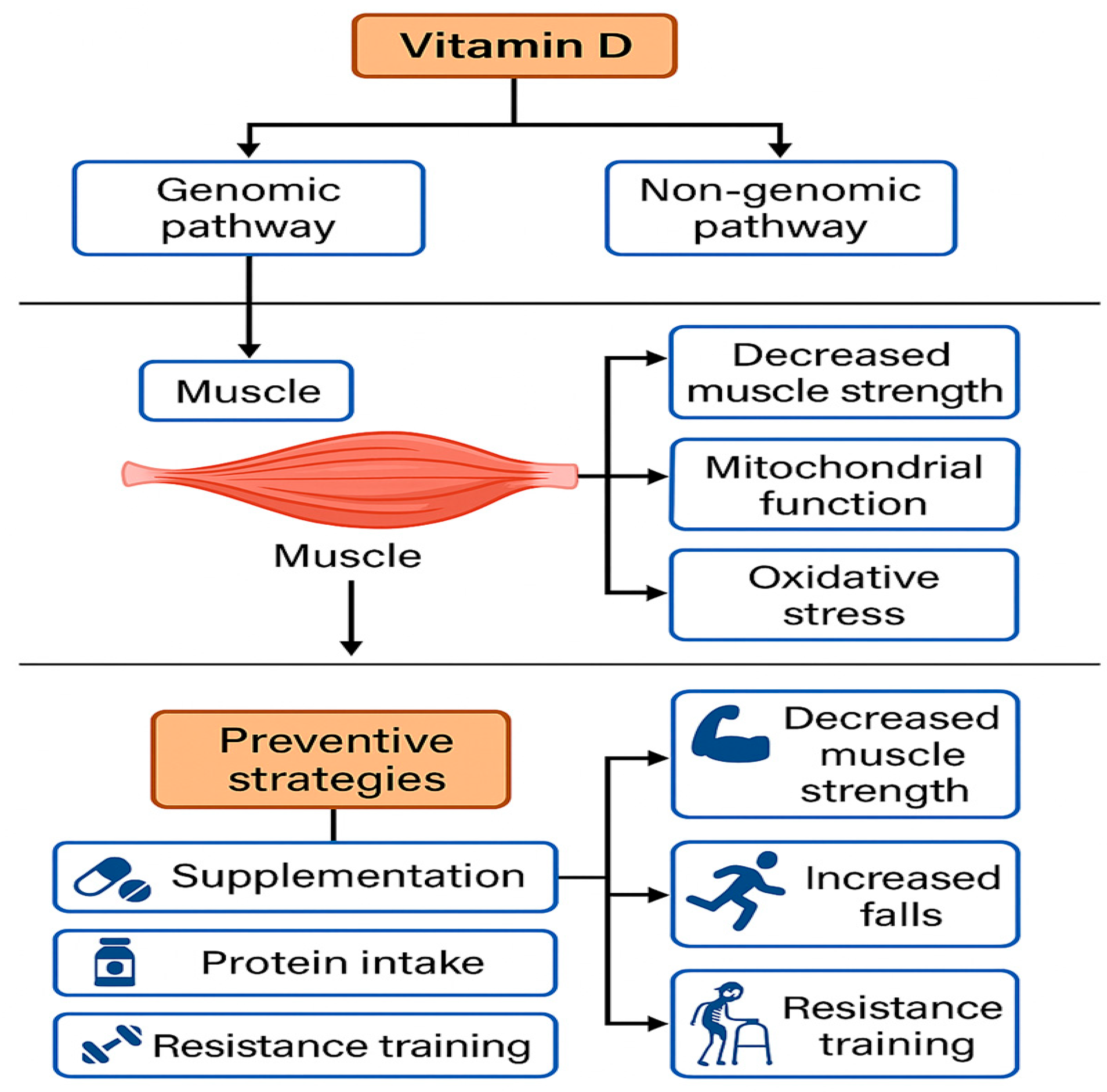
2.2. Vitamin D Receptors (VDRs) in Skeletal Muscle
3. Sarcopenia
3.1. Definition, Diagnostic Criteria, and Pathophysiology
3.2. Diagnostic Components: Muscle Mass, Strength, and Physical Performance
3.3. Pathophysiology and Etiological Factors: An Integrative Approach
4. Relationship Between Vit D and Sarcopenia
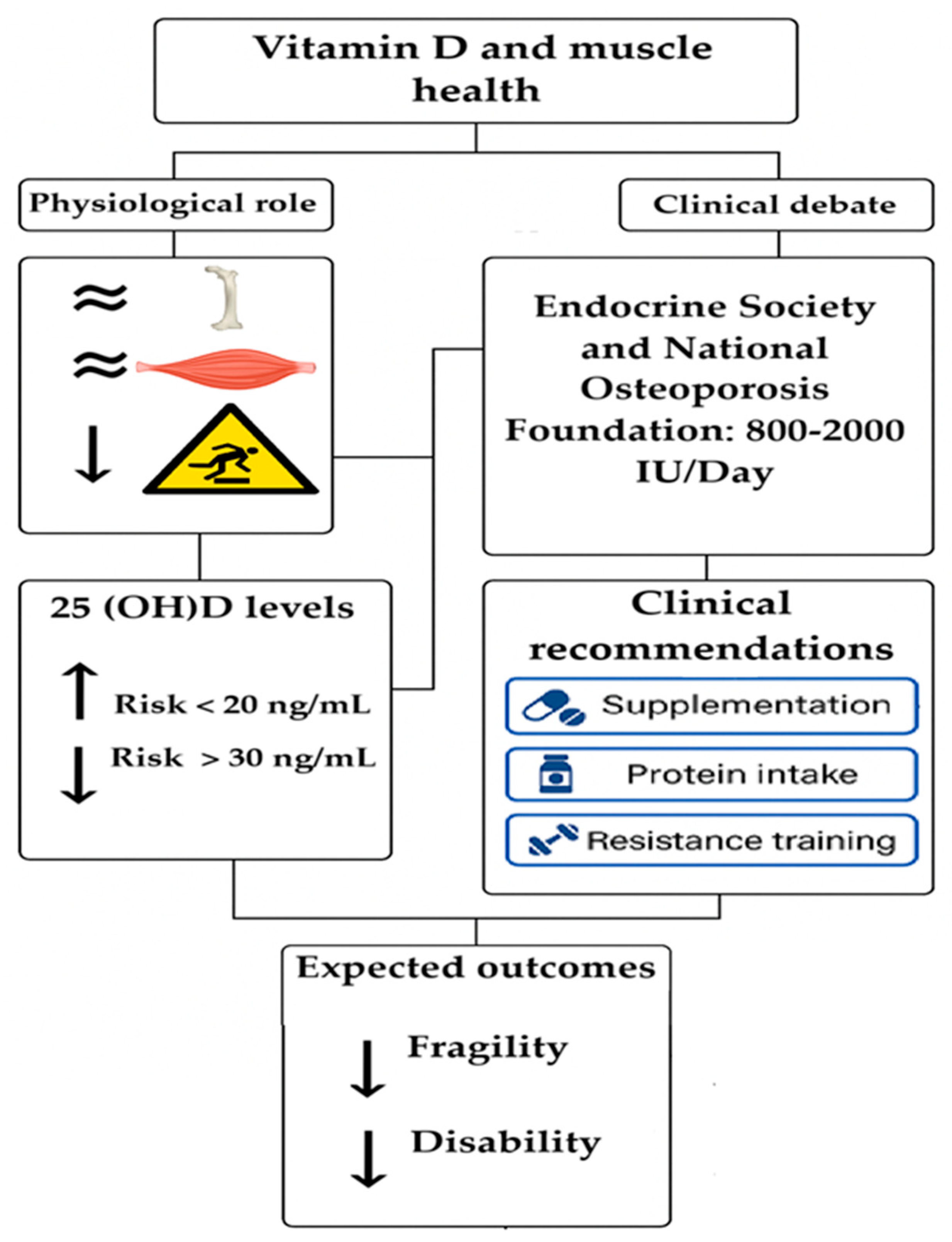
4.1. Vit D Levels
4.2. Recommendations for Vit D Intake
5. Controversies and Limitations of Current Evidence
6. Clinical Implications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D Metabolites: Analytical Challenges and Clinical Relevance. Calcif. Tissue Int. 2023, 112, 158–177. [Google Scholar] [CrossRef]
- Giustina, A.; Lazaretti-Castro, M.; Martineau, A.R.; Mason, R.S.; Rosen, C.J.; Schoenmakers, I. A view on vitamin D: A pleiotropic factor? Nat. Rev. Endocrinol. 2024, 20, 202–208. [Google Scholar] [CrossRef]
- Marazziti, D.; Parra, E.; Palermo, S.; Barberi, F.M.; Buccianelli, B.; Ricciardulli, S.; Cappelli, A.; Mucci, F.; Dell’Osso, L. Vitamin D: A Pleiotropic Hormone with Possible Psychotropic Activities. Curr. Med. Chem. 2021, 28, 3843–3864. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gorgojo, A.; Martin-Moreno, J.M. Insights into the Role of Vitamin D in the Prevention and Control of Cancer and Other Chronic Noncommunicable Diseases: Shedding Further Light on a Captivating Subject. Nutrients 2024, 16, 2166. [Google Scholar] [CrossRef] [PubMed]
- Argentieri, M.A.; Xiao, S.; Bennett, D.; Winchester, L.; Nevado-Holgado, A.J.; Ghose, U.; Albukhari, A.; Yao, P.; Mazidi, M.; Lv, J.; et al. Proteomic aging clock predicts mortality and risk of common age-related diseases in diverse populations. Nat. Med. 2024, 30, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Katsikas Triantafyllidis, K.; Kechagias, K.S.; Mesinovic, J.; Witard, O.C.; Scott, D. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 1642–1652. [Google Scholar] [CrossRef]
- Kuwabara, A.; Matsumoto, M.; Hatamoto, Y.; Fujita, S. Vitamin D and muscle health: Insights from recent studies. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bollen, S.E.; Bass, J.J.; Fujita, S.; Wilkinson, D.; Hewison, M.; Atherton, P.J. The Vitamin D/Vitamin D receptor (VDR) axis in muscle atrophy and sarcopenia. Cell. Signal. 2022, 96, 110355. [Google Scholar] [CrossRef]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, R.; Wan, Q.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med. 2020, 9, 7964–7978. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.V.; Paiva, A.E.G.; Silva, A.C.B.; de Castro, I.C.; Santiago, A.F.; de Oliveira, E.P.; Porto, L.C.J. Prevalence of sarcopenia according to EWGSOP1 and EWGSOP2 in older adults and their associations with unfavorable health outcomes: A systematic review. Aging Clin. Exp. Res. 2022, 34, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Coelho-Junior, H.J.; Calvani, R.; Marzetti, E.; Vetrano, D.L. Biomarkers shared by frailty and sarcopenia in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101530. [Google Scholar] [CrossRef]
- Kupisz-Urbańska, M.; Płudowski, P.; Marcinowska-Suchowierska, E. Vitamin D Deficiency in Older Patients-Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients 2021, 13, 1247. [Google Scholar] [CrossRef]
- Sha, T.; Wang, Y.; Zhang, Y.; Lane, N.E.; Li, C.; Wei, J.; Zeng, C.; Lei, G. Genetic Variants, Serum 25-Hydroxyvitamin D Levels, and Sarcopenia: A Mendelian Randomization Analysis. JAMA Netw. Open 2023, 6, e2331558. [Google Scholar] [CrossRef]
- Kressel, H.; Matsakas, A. Current Research on Vitamin D Supplementation against Sarcopenia: A Review of Clinical Trials. Int. J. Sports Med. 2023, 44, 843–856. [Google Scholar] [CrossRef]
- Gnoli, M.; Brizola, E.; Tremosini, M.; Di Cecco, A.; Sangiorgi, L. Vitamin D and Bone fragility in Individuals with Osteogenesis Imperfecta: A Scoping Review. Int. J. Mol. Sci. 2023, 24, 9416. [Google Scholar] [CrossRef]
- Herrera-Molina, E.; González, N.Y.; Low-Padilla, E.; Oliveros-Velásquez, J.D.; Mendivelso-Duarte, F.; Gómez-Gómez, O.V.; Castillo, A.N.; Barrero-Garzón, L.I.; Álvarez-Moreno, C.A.; Moscoso-Martínez, E.A. Recommendations for the rational use of he 25-hydroxyvitamin D test Policy Brief. Rev. Colomb. Nefrol. 2019, 6, 179–192. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2).; Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Qin, X.H.; Poon, C.C.; Wong, M.S.; Feng, R.; Wang, J.; Lin, F.H.; Sun, Y.L.; Liu, S.F.; Wang, Y.J.; et al. Vitamin D/Vitamin D Receptor Signaling Attenuates Skeletal Muscle Atrophy by Suppressing Renin-Angiotensin System. J. Bone Miner. Res. 2022, 37, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.C.; Emmert, S.; Boeckmann, L. Sunlight, Vitamin D, and Xeroderma Pigmentosum. Adv. Exp. Med. Biol. 2020, 1268, 319–331. [Google Scholar] [PubMed]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Albarri, E.M.A.; Alnuaimi, A.S.; Abdelghani, D. Effectiveness of Vitamin D2 Compared with Vitamin D3 Replacement Therapy in a Primary Healthcare Setting: A Retrospective Cohort Study. Qatar Med. J. 2022, 2022, 29. [Google Scholar]
- Fernández-Vicente, M.; Miján-de-la-Torre, A.; Vella-Ramírez, J.C.; Martí-Bonmatí, E.; Benito-Ibáñez, V.V.; Martínez-de-Arriba, R. Influencing variables on total and free 25(OH)D levels in healthy population. Rev. Clin. Esp. 2022, 222, 313–320. [Google Scholar] [CrossRef]
- Pineda-Lancheros, L.E.; Gálvez-Navas, J.M.; Rojo-Tolosa, S.; Membrive-Jiménez, C.; Valverde-Merino, M.I.; Martínez-Martínez, F.; Sánchez-Martín, A.; Ramírez-Tortosa, M.; Pérez-Ramírez, C.; Jiménez-Morales, A. Polymorphisms in VDR, CYP27B1, CYP2R1, GC and CYP24A1 Genes as Biomarkers of Survival in Non-Small Cell Lung Cancer: A Systematic Review. Nutrients 2023, 15, 1525. [Google Scholar] [CrossRef]
- Krasniqi, E.; Boshnjaku, A.; Wagner, K.H.; Wessner, B. Association between Polymorphisms in Vitamin D Pathway-Related Genes, Vitamin D Status, Muscle Mass and Function: A Systematic Review. Nutrients 2021, 13, 3109. [Google Scholar] [CrossRef]
- Bollen, S.E.; Bass, J.J.; Wilkinson, D.J.; Hewison, M.; Atherton, P.J. The impact of genetic variation within the vitamin D pathway upon skeletal muscle function: A systematic review. J. Steroid Biochem. Mol. Biol. 2023, 229, 106266. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Bolognesi, E.; Zanzottera, M.; Clerici, M. VDR Gene Single Nucleotide Polymorphisms and Autoimmunity: A Narrative Review. Biology 2023, 12, 916. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, G.; Wang, Q. Vitamin D attenuates lipopolysaccharide-induced inflammatory response in endothelial cells through inhibition of PI3K/Akt/NF-κB signaling pathway. Pharmazie 2019, 74, 412–417. [Google Scholar] [PubMed]
- Marampon, F.; Gravina, G.L.; Festuccia, C.; Popov, V.M.; Colapietro, A.; Sanità, P.; Musio, D.; De Felice, F.; Lenzi, A.; Jannini, E.A.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Investig. 2016, 39, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, L.; Yang, L.; Dong, Y.; Liu, T.; Wei, X.; Liu, D.; Gu, H.; Kong, J.; Yuan, Z.; et al. The vitamin D receptor regulates miR-140-5p and targets the MAPK pathway in bone development. Metabolism 2018, 85, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.G.; Assis, A.P.; Lautherbach, N.; Gonçalves, D.A.; Silveira, W.A.; Morgan, H.J.N.; Valentim, R.R.; Almeida, L.F.; Heck, L.C.; Zanon, N.M.; et al. Maternal vitamin D deficiency affects the morphology and function of glycolytic muscle in adult offspring rats. J. Cachexia Sarcopenia Muscle 2022, 13, 2175–2187. [Google Scholar] [CrossRef]
- Stratos, I.; Schleese, S.; Rinas, I.; Vollmar, B.; Mittlmeier, T. Effect of Calcitriol and Vitamin D Receptor Modulator 2 on Recovery of Injured Skeletal Muscle in Wistar Rats. Biomedicines 2023, 11, 2477. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Mondal, S.; Jana, S. Vitamin D3 supplementation improves spatial memory, muscle function, pain score, and modulates different functional physiological biomarkers in vitamin D3 deficiency diet (VDD)-induced rats model. BMC Nutr. 2023, 9, 108. [Google Scholar] [CrossRef]
- Fuentes-Barría, H.; Aguilera-Eguía, R.; Urbano-Cerda, S.; Vera-Aguirre, V.; González-Wong, C. El rol de la vitamina D en la prevención de caídas en sujetos con sarcopenia parte II: Requerimiento de vitamina D del adulto mayor. Rev. Chil. Nutr. 2020, 47, 830–835. [Google Scholar] [CrossRef]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.K.; et al. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef]
- Pratt, J.; Pessanha, L.; Narici, M.; Boreham, C.; De Vito, G. Handgrip strength asymmetry as a new biomarker for sarcopenia and individual sarcopenia signatures. Aging Clin. Exp. Res. 2023, 35, 2563–2571. [Google Scholar] [CrossRef]
- Moroni, A.; Gasparri, C.; Perna, S.; Rondanelli, M.; Micheletti Cremasco, M. Appendicular Skeletal Muscle Mass (ASMM) and Fat-Free Mass (FFM) DXA-BIA Estimations for the Early Identification of Sarcopenia/Low Muscle Mass in Middle-Aged Women. Nutrients 2024, 16, 3897. [Google Scholar] [CrossRef]
- Verstraeten, L.M.G.; de Haan, N.J.; Verbeet, E.; van Wijngaarden, J.P.; Meskers, C.G.M.; Maier, A.B. Handgrip strength rather than chair stand test should be used to diagnose sarcopenia in geriatric rehabilitation inpatients: REStORing health of acutely unwell adulTs (RESORT). Age Ageing 2022, 51, afac242. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Maietti, E.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Martone, A.M.; et al. Comparing EWGSOP2 and FNIH Sarcopenia Definitions: Agreement and 3-Year Survival Prognostic Value in Older Hospitalized Adults: The GLISTEN Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mao, L.; Feng, Y.; Ainsworth, B.E.; Liu, Y.; Chen, N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Angadi, S.S.; Bhargava, A.; Harper, J.; Hirschberg, A.L.; Levine, B.D.; Moreau, K.L.; Nokoff, N.J.; Stachenfeld, N.S.; Bermon, S. The Biological Basis of Sex Differences in Athletic Performance: Consensus Statement for the American College of Sports Medicine. Med. Sci. Sports Exerc. 2023, 55, 2328–2360. [Google Scholar] [CrossRef]
- Altinkaynak, M.; Gurel, E.; Oren, M.M.; Kilic, C.; Karan, M.A.; Bahat, G. Associations of EWGSOP1 and EWGSOP2 probable sarcopenia definitions with mortality: A comparative study. Clin. Nutr. 2023, 42, 2151–2158. [Google Scholar] [CrossRef]
- Tomkinson, G.R.; Lang, J.J.; Rubín, L.; McGrath, R.; Gower, B.; Boyle, T.; Klug, M.G.; Mayhew, A.J.; Blake, H.T.; Ortega, F.B.; et al. International norms for adult handgrip strength: A systematic review of data on 2.4 million adults aged 20 to 100+ years from 69 countries and regions. J. Sport. Health Sci. 2024, 14, 101014. [Google Scholar] [CrossRef]
- Pham, T.; McNeil, J.J.; Barker, A.L.; Orchard, S.G.; Newman, A.B.; Robb, C.; Ernst, M.E.; Espinoza, S.; Woods, R.L.; Nelson, M.R.; et al. Longitudinal association between handgrip strength, gait speed and risk of serious falls in a community-dwelling older population. PLoS ONE 2023, 18, e0285530. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Liu, N.; Hu, F.; Yu, J. Handgrip strength is positively associated with successful aging in older adults: A national cross-sectional study in China. J. Affect. Disord. 2023, 333, 30–37. [Google Scholar] [CrossRef]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L. Applied Aspects of Contemporary Motor Control Theories: A Natural Science Perspective. Mot. Control 2025, 29, 236–273. [Google Scholar] [CrossRef] [PubMed]
- Arosio, B.; Calvani, R.; Ferri, E.; Coelho-Junior, H.J.; Carandina, A.; Campanelli, F.; Ghiglieri, V.; Marzetti, E.; Picca, A. Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle-Brain Axis. Nutrients 2023, 15, 1853. [Google Scholar] [CrossRef]
- Ghiotto, L.; Muollo, V.; Tatangelo, T.; Schena, F.; Rossi, A.P. Exercise and physical performance in older adults with sarcopenic obesity: A systematic review. Front. Endocrinol. 2022, 13, 913953. [Google Scholar] [CrossRef]
- Park, C.M.; Jung, H.W.; Jang, I.Y.; Baek, J.Y.; Yoon, S.; Roh, H.; Lee, E. Comparison of Two Electronic Physical Performance Batteries by Measurement Time and Sarcopenia Classification. Sensors 2021, 21, 5147. [Google Scholar] [CrossRef]
- Mehmet, H.; Robinson, S.R.; Yang, A.W.H. Assessment of Gait Speed in Older Adults. J. Geriatr. Phys. Ther. 2020, 43, 42–52. [Google Scholar] [CrossRef]
- Zanker, J.; Scott, D.; Szoeke, C.; Vogrin, S.; Patel, S.; Blackwell, T.; Bird, S.; Kirk, B.; Center, J.; Alajlouni, D.A.; et al. Predicting Slow Walking Speed From a Pooled Cohort Analysis: Sarcopenia Definitions, Agreement, and Prevalence in Australia and New Zealand. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choo, P.L.; Pang, B.W.J.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Chen, K.K.; Ng, T.P.; Wee, S.L. SPPB reference values and performance in assessing sarcopenia in community-dwelling Singaporeans-Yishun study. BMC Geriatr. 2021, 21, 213. [Google Scholar] [CrossRef]
- Ortega-Bastidas, P.; Gómez, B.; Aqueveque, P.; Luarte-Martínez, S.; Cano-de-la-Cuerda, R. Instrumented Timed Up and Go Test (iTUG)—More Than Assessing Time to Predict Falls: A Systematic Review. Sensors 2023, 23, 3426. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, G.E.; Martínez-Gómez, L.E.; Martínez-Armenta, C.; Pineda, C.; Martínez-Nava, G.A.; Lopez-Reyes, A. Molecular Mechanisms of Inflammation in Sarcopenia: Diagnosis and Therapeutic Update. Cells 2022, 11, 2359. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mu, D.; Wang, Y.; Wang, Y.; Wang, C.; Zhang, X. Association between oxidative balance score and sarcopenia in US adults: NHANES 2011–2018. Front. Nutr. 2024, 11, 1342113. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, N.; Nanda, P.; Devi, S.; Mohapatra, S. Sarcopenia: An Age-Related Multifactorial Disorder. Curr. Aging Sci. 2022, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Mesinovic, J.; Fyfe, J.J.; Talevski, J.; Wheeler, M.J.; Leung, G.K.W.; George, E.S.; Hunegnaw, M.T.; Glavas, C.; Jansons, P.; Daly, R.M.; et al. Type 2 Diabetes Mellitus and Sarcopenia as Comorbid Chronic Diseases in Older Adults: Established and Emerging Treatments and Therapies. Diabetes Metab. J. 2023, 47, 719–742. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Li, S. Advances in nutritional supplementation for sarcopenia management. Front. Nutr. 2023, 10, 1189522. [Google Scholar] [CrossRef]
- Han, M.; Woo, K.; Kim, K. Association of Protein Intake with Sarcopenia and Related Indicators Among Korean Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 4350. [Google Scholar] [CrossRef]
- Cereda, E.; Pisati, R.; Rondanelli, M.; Caccialanza, R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef]
- Jia, S.; Zhao, W.; Hu, F.; Zhao, Y.; Ge, M.; Xia, X.; Yue, J.; Dong, B. Sex differences in the association of physical activity levels and vitamin D with obesity, sarcopenia, and sarcopenic obesity: A cross-sectional study. BMC Geriatr. 2022, 22, 898. [Google Scholar] [CrossRef]
- Francoz, C.; Sola, E. Assessment of renal function in cirrhosis: Sarcopenia, gender and ethnicity matter. J. Hepatol. 2019, 70, 828–830. [Google Scholar] [CrossRef]
- Wiegmann, S.; Felsenberg, D.; Armbrecht, G.; Dietzel, R. Longitudinal changes in muscle power compared to muscle strength and mass. J. Musculoskelet. Neuronal Interact. 2021, 21, 13–25. [Google Scholar]
- Cho, E.J.; Choi, Y.; Jung, S.J.; Kwak, H.B. Role of exercise in estrogen deficiency-induced sarcopenia. J. Exerc. Rehabil. 2022, 18, 2–9. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Morley, J.E.; Anker, S.D. From muscle wasting to sarcopenia and myopenia: Update 2012. J. Cachexia Sarcopenia Muscle 2012, 3, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef]
- Veronese, N.; Smith, L.; Koyanagi, A.; Hoffman, J.; Snoussi, M.; Prokopidis, K.; Dominguez, L.J.; Barbagallo, M. Prevalence of sarcopenia in Africa: A systematic review and meta-analysis of observational studies. Aging Clin. Exp. Res. 2024, 36, 12. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J. Physiol. 2021, 599, 963–979. [Google Scholar] [CrossRef]
- Matta Reddy, A.; Iqbal, M.; Chopra, H.; Urmi, S.; Junapudi, S.; Bibi, S.; Kumar Gupta, S.; Nirmala Pangi, V.; Singh, I.; Abdel-Daim, M.M. Pivotal role of vitamin D in mitochondrial health, cardiac function, and human reproduction. EXCLI J. 2022, 21, 967–990. [Google Scholar]
- Xu, Q.; Bu, F.; Song, Z.T.; Li, K.; Fang, C.; Luo, Y.; Zhang, L.; Pei, Y.F. Association of serum 25-hydroxyvitamin D with sarcopenic obesity risk: A longitudinal observational study from the UK Biobank. Obesity 2025, 33, 1136–1144. [Google Scholar] [CrossRef]
- Krasniqi, E.; Boshnjaku, A.; Ukëhaxhaj, A.; Wagner, K.H.; Wessner, B. Association between vitamin D status, physical performance, sex, and lifestyle factors: A cross-sectional study of community-dwelling Kosovar adults aged 40 years and older. Eur. J. Nutr. 2024, 63, 821–834. [Google Scholar] [CrossRef]
- Bachry, N.C.; Riviati, N.; Yulianto, K.; Kusnadi, E.; Bahar, E. Correlation of Vitamin D Serum Levels with Muscle Mass, Muscle Strength, and Physical Performance in the Elderly Community in Mohammad Hoesin General Hospital Palembang. Bioscmed. 2021, 5, 307–319. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Mølmen, K.S.; Hammarström, D.; Pedersen, K.; Lian Lie, A.C.; Steile, R.B.; Nygaard, H.; Khan, Y.; Hamarsland, H.; Koll, L.; Hanestadhaugen, M.; et al. Vitamin D3 supplementation does not enhance the effects of resistance training in older adults. J. Cachexia Sarcopenia Muscle 2021, 12, 599–628. [Google Scholar] [CrossRef]
- Fuentes-Barría, H.; Aguilera-Eguia, R.; González-Wong, C. El rol de la vitamina D en la prevención de caídas en sujetos con sarcopenia. Rev. Chil. Nutr. 2018, 45, 279–284. [Google Scholar] [CrossRef]
- Cheng, S.H.; Chen, K.H.; Chen, C.; Chu, W.C.; Kang, Y.N. The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3589. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Di Prazza, A.; Pollicino, F.; Carruba, L.; La Carrubba, A.; Dominguez, L.J. Effect of Calcifediol on Physical Performance and Muscle Strength Parameters: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1860. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoon, J.W.; Kim, Y.A.; Choi, H.J.; Yoon, B.W.; Seo, J.H. A Genome-Wide Association Study of Genetic Variants of Apolipoprotein A1 Levels and Their Association with Vitamin D in Korean Cohorts. Genes 2022, 13, 1553. [Google Scholar] [CrossRef]
- Fleet, J.C. Vitamin D-Mediated Regulation of Intestinal Calcium Absorption. Nutrients 2022, 14, 3351. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Nasimi, N.; Sohrabi, Z.; Nunes, E.A.; Sadeghi, E.; Jamshidi, S.; Gholami, Z.; Akbarzadeh, M.; Faghih, S.; Akhlaghi, M.; Phillips, S.M. Whey Protein Supplementation with or without Vitamin D on Sarcopenia-Related Measures: A Systematic Review and Meta-Analysis. Adv. Nutr. 2023, 14, 762–773. [Google Scholar] [CrossRef]
- Chang, M.C.; Choo, Y.J. Effects of Whey Protein, Leucine, and Vitamin D Supplementation in Patients with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 521. [Google Scholar] [CrossRef] [PubMed]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.W.; Tan, H.L.; Hsu, M.F.; Huang, H.L.; Chung, Y.C. Effect of non-pharmacological interventions on the prevention of sarcopenia in menopausal women: A systematic review and meta-analysis of randomized controlled trials. BMC Women’s Health 2023, 23, 606. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Yang, C.; Dai, Y.; Li, Z.; Peng, Y.; Zhang, L.; Jia, H. Relationship of Serum 25-Hydroxyvitamin D Levels with Sarcopenia and Body Composition in Community-Dwelling Older Adults: A Paired Case-Control Study. J. Am. Med. Dir. Assoc. 2023, 24, 1213–1219. [Google Scholar] [CrossRef]
- Fairfield, W.D.; Minton, D.M.; Elliehausen, C.J.; Nichol, A.D.; Cook, T.L.; Rathmacher, J.A.; Pitchford, L.M.; Paluska, S.A.; Kuchnia, A.J.; Allen, J.M.; et al. Small-Scale Randomized Controlled Trial to Explore the Impact of β-Hydroxy-β-Methylbutyrate Plus Vitamin D3 on Skeletal Muscle Health in Middle Aged Women. Nutrients 2022, 14, 4674. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int. J. Paleopathol. 2018, 23, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Leary, P.F.; Zamfirova, I.; Au, J.; McCracken, W.H. Effect of Latitude on Vitamin D Levels. J. Am. Osteopath. Assoc. 2017, 117, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kift, R.C.; Webb, A.R. Globally Estimated UVB Exposure Times Required to Maintain Sufficiency in Vitamin D Levels. Nutrients 2024, 16, 1489. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment, and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Australian Institute of Sport. Supplements: Group A. Available online: https://www.ausport.gov.au/ais/nutrition/supplements/group_a (accessed on 2 July 2025).
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef]
- Mangoo-Karim, R.; Da Silva Abreu, J.; Yanev, G.P.; Perez, N.N.; Stubbs, J.R.; Wetmore, J.B. Ergocalciferol versus Cholecalciferol for Nutritional Vitamin D Replacement in CKD. Nephron 2015, 130, 99–104. [Google Scholar] [CrossRef]
- Shirwaikar Thomas, A.; Criss, Z.K.; Shroyer, N.F.; Abraham, B.P. Vitamin D Receptor Gene Single Nucleotide Polymorphisms and Association With Vitamin D Levels and Endoscopic Disease Activity in Inflammatory Bowel Disease Patients: A Pilot Study. Inflamm. Bowel Dis. 2021, 27, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; di Filippo, L.; Allora, A.; Bikle, D.D.; Cavestro, G.M.; Feldman, D.; Latella, G.; Minisola, S.; Napoli, N.; Trasciatti, S.; et al. Vitamin D and malabsorptive gastrointestinal conditions: A bidirectional relationship? Rev. Endocr. Metab. Disord. 2023, 24, 121–138. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A. Vitamin D in geriatric patients. Internist 2020, 61, 535–540. [Google Scholar] [CrossRef]
- Chauhan, K.; Shahrokhi, M.; Huecker, M.R. Vitamin D. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441912/ (accessed on 23 July 2023).
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients 2022, 14, 2652. [Google Scholar] [CrossRef] [PubMed]
| Criteria | EWGSOP2 | AWGS | FNIH | IWGS |
|---|---|---|---|---|
| Operational Definition | I suspected with low muscle strength. Confirmed if low muscle mass is also present. Severe sarcopenia if physical performance is also impaired. | Sarcopenia is diagnosed with low muscle mass plus either low muscle strength or poor physical performance. | Sarcopenia is defined as low muscle strength combined with low muscle mass adjusted for BMI. | Sarcopenia is defined as the presence of low muscle mass and poor physical performance. |
| Diagnostic Stages | Sarcopenia probably: Low strength. Sarcopenia confirmed: + Low muscle mass. Serve Sarcopenia: + Low physical performance. | Not specified | Not specified | Not specified |
| Muscle Strength | Handgrip strength: Men: <27 kg Women: <16 kg | Handgrip strength: Men: <28 kg Women: <18 kg | Handgrip strength: Men: <26 kg Women: <16 kg | Not required |
| Muscle Mass | ASMI (DXA or BIA): Men: <7.0 kg/m2 Women: <5.5 kg/m2 | ASMI (DXA or BIA): Men: <7.0 kg/m2 Women: <5.7 kg/m2 | ASM / BMI: Men: <0.78 kg/m2 Women: <0.51 kg/m2 | ASMI (DXA): Men: <7.23 kg/m2 Women: <5.67 kg/m2 |
| Physical Performance | Gait speed: <0.8 m/s SPPB ≤8 Chair stand test: >15 s | Gait speed: <1.0 m/s SPPB ≤ 9 TUG ≥ 12 s | Not included | Gait speed: <1.0 m/s |
| Target Population | European adults ≥65 years | Asian adults ≥60 years | U.S. population ≥65 years | Older adults in general |
| Author (Ref) | Population and Country | Supplementation | Outcomes SMD (95% CI) | Conclusion |
|---|---|---|---|---|
| Prokopidis et al. [6]. | Adults ≥50 years. Europe, North America (United States), Australia and Brazil. | Vit D. | Short Physical Performance −0.23 (−0.40 to −0.06). Handgrip strength −0.07 (−0.70 to 0.55). Timed Up and Go 0.07 (−0.08 to 0.22). Appendicular lean mass 0.06 kg/m2 (−0.32 to 0.44). General muscle strength −0.01 (−0.17 to 0.15). General physical performance −0.02 (−0.23 to 0.18). | Vit D did not improve sarcopenia. |
| Cheng et al. [87]. | Older adults ≥ 60 years. Europe (Germany, Italy and Finland), Asia (Taiwan, Japan and China) and America. | Vit D. Protein. Exercise | Handgrip strength 3.86 (0.52 to 7.21). Chair stand time −1.32 (−1.98 to −0.65). | Vit D, protein, and exercise increase strength. |
| Barbagallo et al. [88]. | Older adults ≥ 60 years. United States, Italy and the Netherlands. | Calcifediol. | Gait speed improved 2.500 (1.768 to 3.223). Handgrip strength 0.532 (0.305 to 0.758). Leg extension 0.641 (0.346 to 0.935). | Calcifediol may improve muscle strength. |
| Nasimi et al. [93]. | Older adults ≥ 60 years. Europe, Brazil, United States, United Kingdom, Canada, Australia, China, Korea, Japan and Taiwan. | Whey protein. Vit D. | Physical function including whey protein 0.561 (0.256 to 0.865). Lean mass including whey protein 0.982 (0.228 to 1.736). Physical function including whey protein 1.211 (0.588 to 1.834). Lean mass including whey protein and Vit D 0.993 (0.112 to 1.874). Muscle Strength including whey protein and Vit D 2.005 (0.975 to 3.035). Physical function including whey protein and Vit D 3.038 (2.196 to 3.879). | Whey protein helps frail adults; Vit D enhances. |
| Chang et al. [94]. | Older adults ≥ 60 years. Europea (Belgium, Germany, Ireland, Italy and Sweden) and United Kingdom. | Whey protein. Leucine. Vit D. | Appendicular muscle mass global 0.27 (0.09 to 0.44). Appendicular muscle mass including exercise 0.45 (0.10 to 0.80). Appendicular muscle mass non-exercise 0.21 (0.01 to 0.41). Handgrip strength global 1.03 (−0.10 to 2.16). Handgrip strength including exercise 1.52 (0.62 to 2.41). Handgrip strength non-exercise 0.07 (−0.13 to 0.27). Short Physical Performance Battery global 1.01 (−0.86 to 2.88). Short Physical Performance Battery including exercise 1.97 (1.54 to 2.40). Short Physical Performance Battery non-exercise 0.06 (−0.14 to 0.26). | Whey protein, leucine, Vit D supplementation, and exercise improve muscle. |
| Gkekas et al. [95]. | Older adults ≥ 60 years. Europe (Italy) and Asia (Japan and China). | Vit D (100–1600 IU/day). Plus protein (10−44 g/day). | Handgrip strength 0.38 (0.01 to 0.75). Sit-to-stand time 0.25 (0.06 to 0.43). Skeletal muscle index 0.25 (−0.006 to 0.51). Appendicular muscle mass 0.25 (−0.406 to 0.91) | Vit D plus protein improves strength only. |
| Tan et al. [96]. | Women aged 40 to 60 at menopausal stage. Europe (Spain, Italy, Denmark and Finland), Asia (Thailand, China, Iran, Korea and Japan), America (Canada, United States and Brazil), Oceania (Australia) and Africa (Egypt). | Vit D. Exercise. ≥12 weeks. 3×/week. 60–90 min. | Body mass including exercise 0.232 (0.097 to 0.366). handgrip strength including exercise 0.901 (0.362 to 1.441). Knee extension strength including exercise 0.698 (0.384 to 1.013). Handgrip strength including Vit D 0.303 (0.130 to 0.476). | Exercise and Vit D improve strength. |
| Levels | Netherlands | Institute of Medicine | International Osteoporosis Foundation and American Geriatrics Society | Expert Opinion |
|---|---|---|---|---|
| Severe Deficiency | 10–12 ng/mL 25–30 nmol/L | 10–12 ng/mL 25–30 nmol/L | 10–12 ng/mL 25–30 nmol/L | 10–12 ng/mL 25–30 nmol/L |
| Slight Deficiency | N/A | <20 ng/mL <50 nmol/L | <30 ng/mL <75 nmol/L | <40 ng/mL <100 nmol/L |
| Adequate | >10–12 ng/mL >25–30 nmol/L | >20 ng/mL >50 nmol/L | >30 ng/mL >75 nmol/L | >40 ng/mL >100 nmol/L |
| City (Approximate Latitude) | Summer 10 a.m. or 2 p.m. | Winter 10 a.m. or 2 p.m. | Winter 12 p.m. |
|---|---|---|---|
| Cairns (~16.9° S). | 6 to 7 min. | 9 to 12 min. | 7 min. |
| Brisbane (~27.5° S). | 6 to 7 min. | 15 to 19 min. | 11 min. |
| Sydney (~33.9° S). | 6 to 8 min. | 26 to 28 min. | 16 min. |
| Melbourne (~37.8° S). | 6 to 8 min. | 32 to 52 min. | 25 min. |
| Hobart (~42.9° S). | 7 to 9 min. | 40 to 47 min. | 29 min. |
| Age (years) | Institute of Medicine | Deficiency Risk for the Endocrine Society | ||||
|---|---|---|---|---|---|---|
| AI (µg/UI) | EAR (µg/UI) | RDA (µg/IU) | UL (µg/IU) | IU | UL (IU) | |
| 19 to 30 | N/A | 10/400 | 15/600 | 100/4000 | 1500 to 2000 | 10,000 |
| 31 to 50 | N/A | 10/400 | 15/600 | 100/4000 | 1500 to 2000 | 10,000 |
| 51 to 70 | N/A | 10/400 | 15/600 | 100/4000 | 1500 to 2000 | 10,000 |
| >70 | N/A | 10/400 | 20/800 | 100/4000 | 1500 to 2000 | 10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Barría, H.; Aguilera-Eguía, R.; Angarita-Davila, L.; Rojas-Gómez, D.; Alarcón-Rivera, M.; López-Soto, O.; Maureira-Sánchez, J.; Bermúdez, V.; Rivera-Porras, D.; Contreras-Velázquez, J.C. Vitamin D and Sarcopenia: Implications for Muscle Health. Biomedicines 2025, 13, 1863. https://doi.org/10.3390/biomedicines13081863
Fuentes-Barría H, Aguilera-Eguía R, Angarita-Davila L, Rojas-Gómez D, Alarcón-Rivera M, López-Soto O, Maureira-Sánchez J, Bermúdez V, Rivera-Porras D, Contreras-Velázquez JC. Vitamin D and Sarcopenia: Implications for Muscle Health. Biomedicines. 2025; 13(8):1863. https://doi.org/10.3390/biomedicines13081863
Chicago/Turabian StyleFuentes-Barría, Héctor, Raúl Aguilera-Eguía, Lissé Angarita-Davila, Diana Rojas-Gómez, Miguel Alarcón-Rivera, Olga López-Soto, Juan Maureira-Sánchez, Valmore Bermúdez, Diego Rivera-Porras, and Julio Cesar Contreras-Velázquez. 2025. "Vitamin D and Sarcopenia: Implications for Muscle Health" Biomedicines 13, no. 8: 1863. https://doi.org/10.3390/biomedicines13081863
APA StyleFuentes-Barría, H., Aguilera-Eguía, R., Angarita-Davila, L., Rojas-Gómez, D., Alarcón-Rivera, M., López-Soto, O., Maureira-Sánchez, J., Bermúdez, V., Rivera-Porras, D., & Contreras-Velázquez, J. C. (2025). Vitamin D and Sarcopenia: Implications for Muscle Health. Biomedicines, 13(8), 1863. https://doi.org/10.3390/biomedicines13081863






