Pathophysiological Links Between Inflammatory Bowel Disease and Cardiovascular Disease: The Role of Dysbiosis and Emerging Biomarkers
Abstract
1. Introduction
2. Pathophysiological Links Between IBD and CVD
2.1. Hypercoagulability and Thrombosis Risk
2.2. Chronic Inflammation and Atherosclerosis
3. Impact of IBD Medications on Cardiovascular Health
3.1. Short-Chain Fatty Acids (SCFAs): Gut Metabolites with Systemic Impact
3.2. Calprotectin: Marker and Mediator of Inflammatory Risk
3.3. Zonulin: Intestinal Barrier Dysfunction and Cardiovascular Implications
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Alayo, Q.A.; Loftus EVJr Yarur, A.; Alvarado, D.; Ciorba, M.A.; de las Fuentes, L.; Deepak, P. Inflammatory Bowel Disease Is Associated with an Increased Risk of Incident Acute Arterial Events: Analysis of the United Kingdom Biobank. Clin. Gastroenterol. Hepatol. 2023, 21, 761–770.e13. [Google Scholar] [CrossRef]
- Singh, S.; Singh, H.; Loftus, E.V., Jr.; Pardi, D.S. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 382–393.e1. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hu, T.; Hao, H.; Hill, M.A.; Xu, C.; Liu, Z. Inflammatory Bowel Disease and Cardiovascular Diseases: A Concise Review. Eur. Heart J. Open 2022, 2, oeab029. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and Cardiovascular Diseases: A Clinical Review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef]
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut Microbiota and Inflammation in Chronic Kidney Disease and Their Roles in the Development of Cardiovascular Disease. Hypertens. Res. 2019, 42, 123–140. [Google Scholar] [CrossRef]
- Mafra, D.; Lobo, J.C.; Barros, A.F.; Koppe, L.; Vaziri, N.D.; Fouque, D. Role of Altered Intestinal Microbiota in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Future Microbiol. 2014, 9, 399–410. [Google Scholar] [CrossRef]
- Kumric, M.; Zivkovic, P.M.; Kurir, T.T.; Vrdoljak, J.; Vilovic, M.; Martinovic, D.; Bratanic, A.; Lizatovic, I.K.; Bozic, J. Role of B-Cell Activating Factor (Baff) in Inflammatory Bowel Disease. Diagnostics 2022, 12, 45. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Kumric, M.; Kurir, T.T.; Males, I.; Martinovic, D.; Vilovic, M.; Bozic, J. Effects of Wine Components in Inflammatory Bowel Diseases. Molecules 2021, 26, 5891. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Lana, R.; Taxonera, C.; Alba, C.; Izquierdo, S.; Díaz-Rubio, M. Extraintestinal Manifestations in Inflammatory Bowel Disease: Differences between Crohn’s Disease and Ulcerative Colitis. Med. Clin. 2005, 125, 297–300. [Google Scholar] [CrossRef]
- Villanacci, V.; Reggiani-Bonetti, L.; Salviato, T.; Leoncini, G.; Cadei, M.; Albarello, L.; Caputo, A.; Aquilano, M.C.; Battista, S.; Parente, P. Histopathology of IBD Colitis. A Practical Approach from the Pathologists of the Italian Group for the Study of the Gastrointestinal Tract (GIPAD). Pathologica 2021, 113, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, W.; Chen, H.; Zuo, T.; Wu, X. Immune Cell Landscaping Reveals Distinct Immune Signatures of Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 861790. [Google Scholar] [CrossRef] [PubMed]
- Brnic, D.; Martinovic, D.; Zivkovic, P.M.; Tokic, D.; Vilovic, M.; Rusic, D.; Tadin Hadjina, I.; Libers, C.; Glumac, S.; Supe-Domic, D.; et al. Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 4866. [Google Scholar] [CrossRef]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef]
- Caron, B.; Honap, S.; Peyrin-Biroulet, L. Epidemiology of Inflammatory Bowel Disease across the Ages in the Era of Advanced Therapies. J. Crohns Colitis 2024, 18, ii3–ii15. [Google Scholar] [CrossRef]
- The Lancet Gastroenterology & Hepatology. The Economic Burden of Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2023, 8, 391. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Sheth, T.; Pitchumoni, C.S.; Das, K.M. Management of Musculoskeletal Manifestations in Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2015, 2015, 387891. [Google Scholar] [CrossRef]
- He, R.; Zhao, S.; Cui, M.; Chen, Y.; Ma, J.; Li, J.; Wang, X. Cutaneous Manifestations of Inflammatory Bowel Disease: Basic Characteristics, Therapy, and Potential Pathophysiological Associations. Front. Immunol. 2023, 14, 1234535. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schlomann, B.H.; Parthasarathy, R. Timescales of Gut Microbiome Dynamics. Curr. Opin. Microbiol. 2019, 50, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dmytriv, T.R.; Storey, K.B.; Lushchak, V.I. Intestinal Barrier Permeability: The Influence of Gut Microbiota, Nutrition, and Exercise. Front. Physiol. 2024, 15, 1380713. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Semo, D.; Reinecke, H.; Godfrey, R. Gut Microbiome Regulates Inflammation and Insulin Resistance: A Novel Therapeutic Target to Improve Insulin Sensitivity. Signal Transduct. Target Ther. 2024, 9, 35. [Google Scholar] [CrossRef]
- Amini Khiabani, S.; Asgharzadeh, M.; Samadi Kafil, H. Chronic Kidney Disease and Gut Microbiota. Heliyon 2023, 9, e18991. [Google Scholar] [CrossRef] [PubMed]
- Manos, J. The Human Microbiome in Disease and Pathology. APMIS 2022, 130, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome–A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Behzadi, P.; Dodero, V.I.; Golubnitschaja, O. Systemic inflammation as the health-related communication tool between the human host and gut microbiota in the framework of predictive, preventive, and personalized medicine. In All Around Suboptimal Health: Advanced Approaches by Predictive, Preventive and Personalised Medicine for Healthy Populations; Springer Nature: Cham, Switzerland, 2024; pp. 203–241. [Google Scholar]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.P.; Roberts, H.R. Hypercoagulability in Venous and Arterial Thrombosis. Ann. Intern. Med. 1997, 126, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P. Acquired Risk Factors for Venous Thromboembolism in Medical Patients. Hematol. Am. Soc. Hematol. Educ. Program 2005, 2005, 458–461. [Google Scholar] [CrossRef]
- Zöller, B.; Svensson, P.J.; Dahlbäck, B.; Lind-Hallden, C.; Hallden, C.; Elf, J. Genetic Risk Factors for Venous Thromboembolism. Expert Rev. Hematol. 2020, 13, 971–981. [Google Scholar] [CrossRef]
- Gala, D.; Newsome, T.; Roberson, N.; Lee, S.M.; Thekkanal, M.; Shah, M.; Kumar, V.; Bandaru, P.; Gayam, V. Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview. Diseases 2022, 10, 73. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Nugent, Z.; Singh, H. Persistently High Rate of Venous Thromboembolic Disease in Inflammatory Bowel Disease: A Population-Based Study. Am. J. Gastroenterol. 2021, 116, 1476–1484. [Google Scholar] [CrossRef]
- Yazici, A. Thrombophilic Risk Factors in Patients with Inflammatory Bowel Disease. Gastroenterol. Res. 2010, 3, 112. [Google Scholar] [CrossRef][Green Version]
- Giannotta, M.; Tapete, G.; Emmi, G.; Silvestri, E.; Milla, M. Thrombosis in Inflammatory Bowel Diseases: What’s the Link? Thromb. J. 2015, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Alkim, H.; Ayaz, S.; Alkim, C.; Ulker, A.; Sahin, B. Continuous Active State of Coagulation System in Patients with Nonthrombotic Inflammatory Bowel Disease. Clin. Appl. Thromb./Hemost. 2011, 17, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Smalberg, J.H.; Kruip, M.J.H.A.; Janssen, H.L.A.; Rijken, D.C.; Leebeek, F.W.G.; De Maat, M.P.M. Hypercoagulability and Hypofibrinolysis and Risk of Deep Vein Thrombosis and Splanchnic Vein Thrombosis: Similarities and Differences. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Flemmig, M.; Melzig, M.F. Serine-Proteases as Plasminogen Activators in Terms of Fibrinolysis. J. Pharm. Pharmacol. 2012, 64, 1025–1039. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-Phase Proteins: As Diagnostic Tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen Activator Inhibitor-1 (PAI-1): A Key Factor Linking Fibrinolysis and Age-Related Subclinical and Clinical Conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef]
- Saibeni, S.; Ciscato, C.; Vecchi, M.; Boscolo Anzoletti, M.; Kaczmarek, E.; Caccia, S.; De Franchis, R.; Cugno, M. Antibodies to Tissue-Type Plasminogen Activator (t-PA) in Patients with Inflammatory Bowel Disease: High Prevalence, Interactions with Functional Domains of t-PA and Possible Implications in Thrombosis. J. Thromb. Haemost. 2006, 4, 1510–1516. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Huang, J.; Jiang, S.; Li, N.; Mu, H.H.; Xu, C. Plasminogen Activator Inhibitor-1 Potentiates Neutrophil Infiltration and Tissue Injury in Colitis. Int. J. Biol. Sci. 2023, 19, 2132–2149. [Google Scholar] [CrossRef]
- Jójárt, B.; Resál, T.; Kata, D.; Molnár, T.; Bacsur, P.; Szabó, V.; Varga, Á.; Szántó, K.J.; Pallagi, P.; Földesi, I.; et al. Plasminogen Activator Inhibitor 1 Is a Novel Faecal Biomarker for Monitoring Disease Activity and Therapeutic Response in Inflammatory Bowel Diseases. J. Crohns Colitis 2024, 18, 392–405. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Yang, W.; Zhu, Z.; Kong, W.; Zheng, S.; Jiang, L.; Fei, X.; Chen, Y.; Liu, J. D-Dimer Levels Are Correlated with Disease Activity in Crohn’s Patients. Oncotarget 2017, 8, 63971–63977. [Google Scholar] [CrossRef] [PubMed]
- Simes, J.; Robledo, K.P.; White, H.D.; Espinoza, D.; Stewart, R.A.; Sullivan, D.R.; Zeller, T.; Hague, W.; Nestel, P.J.; Glasziou, P.P.; et al. D-Dimer Predicts Long-Term Cause-Specific Mortality, Cardiovascular Events, and Cancer in Patients with Stable Coronary Heart Disease LIPID Study. Circulation 2018, 138, 712–723. [Google Scholar] [CrossRef] [PubMed]
- SriRajaskanthan, R.; Winter, M.; Muller, A.F. Venous Thrombosis in Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2005, 17, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Grainge, M.J.; West, J.; Card, T.R. Venous Thromboembolism during Active Disease and Remission in Inflammatory Bowel Disease: A Cohort Study. Lancet 2010, 375, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Papa, A.; Saibeni, S.; Repici, A.; Malesci, A.; Vecchi, M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am. J. Gastroenterol. 2007, 102, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 14, 258–264. [Google Scholar] [CrossRef]
- Brown, R.A.; Shantsila, E.; Varma, C.; Lip, G.Y.H. Current Understanding of Atherogenesis. Am. J. Med. 2017, 130, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Tobin, R.; Patel, N.; Tobb, K.; Weber, B.; Mehta, P.K.; Isiadinso, I. Atherosclerosis in Systemic Lupus Erythematosus. Curr. Atheroscler. Rep. 2023, 25, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Maki-Petaja, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular Consequences of Inflammation: A Position Statement Fromthe Eshworking Group Onvascular Structure and Function and Thearterysociety. J. Hypertens 2020, 38, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Phrommintikul, A.; Krittayaphong, R.; Wongcharoen, W.; Yamwong, S.; Boonyaratavej, S.; Kunjara-Na-Ayudhya, R.; Tatsanavivat, P.; Sritara, P. Management of Atherosclerosis Risk Factors for Patients at High Cardiovascular Risk in Real-World Practice: A Multicentre Study. Singapore Med. J. 2017, 58, 535–542. [Google Scholar] [CrossRef]
- Masson, W.; Fernández-Villar, G.; Martinez-Elhelou, S. Management of Atherosclerotic Cardiovascular Risk in Inflammatory Bowel Disease: Current Perspectives. Adv. Ther. 2025, 42, 2118–2134. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; Van Der Woude, C.J.; Verploegh, P.J.P.; Aribas, E.; Kavousi, M.; Roeters Van Lennep, J.E.; De Vries, A.C. Cardiovascular Risk Profiles in Patients with Inflammatory Bowel Disease Differ from Matched Controls from the General Population. Eur. J. Prev. Cardiol. 2023, 30, 1615–1622. [Google Scholar] [CrossRef]
- Jess, T.; Jensen, B.W.; Andersson, M.; Villumsen, M.; Allin, K.H. Inflammatory Bowel Diseases Increase Risk of Type 2 Diabetes in a Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2020, 18, 881–888.e1. [Google Scholar] [CrossRef]
- Ae Kang, E.; Han, K.; Chun, J.; Soh, H.; Park, S.; Im, J.P.; Kim, J.S. Increased Risk of Diabetes in Inflammatory Bowel Disease Patients: A Nationwide Population-Based Study in Korea. J. Clin. Med. 2019, 8, 343. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Cicero, A.F.G.; Janez, A.; Stoian, A.P.; Sonmez, A.; Rizzo, M. Atherosclerosis Development and Progression: The Role of Atherogenic Small, Dense LDL. Medicina 2022, 58, 299. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Hu, J.; Xu, F.; Lu, Y.; Zhu, L.; Shen, H. Association of Serum Lipids with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Front. Med. 2023, 10, 1198988. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, S.; Han, L.; Ba, X.; Shen, P.; Lin, W.; Li, T.; Zhang, R.; Huang, Y.; Huang, Y.; et al. Dyslipidemia in Rheumatoid Arthritis: The Possible Mechanisms. Front. Immunol. 2023, 14, 1254753. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid Paradox in Rheumatoid Arthritis: The Impact of Serum Lipid Measures and Systemic Inflammation on the Risk of Cardiovascular Disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Yuan, J. Abstract 14242: The Potential Impact of Inflammation on the Lipid Paradox in Patients with Acute Myocardial Infarction: A Multicenter Study. Circulation 2023, 148, 14242. [Google Scholar] [CrossRef]
- Venetsanopoulou, A.I.; Pelechas, E.; Voulgari, P.V.; Drosos, A.A. The Lipid Paradox in Rheumatoid Arthritis: The Dark Horse of the Augmented Cardiovascular Risk. Rheumatol. Int. 2020, 40, 1181–1191. [Google Scholar] [CrossRef]
- Arida, A.; Protogerou, A.D.; Kitas, G.D.; Sfikakis, P.P. Systemic Inflammatory Response and Atherosclerosis: The Paradigm of Chronic Inflammatory Rheumatic Diseases. Int. J. Mol. Sci. 2018, 19, 1890. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Oude Nijhuis, M.; van Keulen, J.; Pasterkamp, G.; Quax, P.; de Kleijn, D.V. Activation of the Innate Immune System in Atherosclerotic Disease. Curr. Pharm. Des. 2007, 13, 983–994. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The Emerging Role of Oxidative Stress in Inflammatory Bowel Disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Ong, G.; Logue, S.E. Unfolding the Interactions between Endoplasmic Reticulum Stress and Oxidative Stress. Antioxidants 2023, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Sha, X.; Song, X.; Zhang, X.; Xing, M.; Liu, S.; Xu, K.; Li, J. Targeted Therapy of Atherosclerosis Vulnerable Plaque by ROS-Scavenging Nanoparticles and MR/Fluorescence Dual-Modality Imaging Tracing. Int. J. Nanomedicine 2022, 17, 5413–5429. [Google Scholar] [CrossRef]
- Tsioufis, P.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. The Impact of Cytokines in Coronary Atherosclerotic Plaque: Current Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 15937. [Google Scholar] [CrossRef]
- Sterpetti, A.V. Inflammatory Cytokines and Atherosclerotic Plaque Progression. Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Muzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the Cytokine Profile in Inflammatory Bowel Diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Maharana, K.C.; Meenakshi, S.; Singh, S. Endothelial Dysfunction and Its Relation in Different Disorders: Recent Update. Health Sci. Rev. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, M.; Hao, H.; Hill, M.A.; Xu, C.; Liu, Z. Endothelial Dysfunction and Arterial Stiffness in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3179. [Google Scholar] [CrossRef]
- Ozturk, K.; Guler, A.K.; Cakir, M.; Ozen, A.; Demirci, H.; Turker, T.; Demirbas, S.; Uygun, A.; Gulsen, M.; Bagci, S. Pulse Wave Velocity, Intima Media Thickness, and Flow-Mediated Dilatation in Patients with Normotensive Normoglycemic Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Principi, M.; Mastrolonardo, M.; Scicchitano, P.; Gesualdo, M.; Sassara, M.; Guida, P.; Bucci, A.; Zito, A.; Caputo, P.; Albano, F.; et al. Endothelial Function and Cardiovascular Risk in Active Inflammatory Bowel Diseases. J. Crohns Colitis 2013, 7, e427–e433. [Google Scholar] [CrossRef] [PubMed]
- Bisoendial, R.J.; Kastelein, J.J.P.; Peters, S.L.M.; Levels, J.H.M.; Birjmohun, R.; Rotmans, J.I.; Hartman, D.; Meijers, J.C.M.; Levi, M.; Stroes, E.S.G. Effects of CRP Infusion on Endothelial Function and Coagulation in Normocholesterolemic and Hypercholesterolemic Subjects. J. Lipid Res. 2007, 48, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.W.; Singh, U.; Vasquez-Vivar, J.; Devaraj, S.; Kuo, L.; Jialal, I. Human C-Reactive Protein Induces Endothelial Dysfunction and Uncoupling of ENOS in Vivo. Atherosclerosis 2009, 206, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Coburn, L.A.; Horst, S.N.; Allaman, M.M.; Brown, C.T.; Williams, C.S.; Hodges, M.E.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T. L -Arginine Availability and Metabolism Is Altered in Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Baier, J.; Gänsbauer, M.; Giessler, C.; Arnold, H.; Muske, M.; Schleicher, U.; Lukassen, S.; Ekici, A.; Rauh, M.; Daniel, C.; et al. Arginase Impedes the Resolution of Colitis by Altering the Microbiome and Metabolome. J. Clin. Investig. 2020, 130, 5703–5720. [Google Scholar] [CrossRef]
- Horowitz, S.; Binion, D.G.; Nelson, V.M.; Kanaa, Y.; Javadi, P.; Lazarova, Z.; Andrekopoulos, C.; Kalyanaraman, B.; Otterson, M.F.; Rafiee, P. Increased Arginase Activity and Endothelial Dysfunction in Human Inflammatory Bowel Disease. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 292, G1323–G1336. [Google Scholar] [CrossRef]
- Gao, X.; Xu, X.; Belmadani, S.; Park, Y.; Tang, Z.; Feldman, A.M.; Chilian, W.M.; Zhang, C. TNF-α Contributes to Endothelial Dysfunction by Upregulating Arginase in Ischemia/Reperfusion Injury. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1269–1275. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding Light on the Mechanisms and Opportunities in Cardiovascular Diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef]
- Russo, I.; Barale, C.; Melchionda, E.; Penna, C.; Pagliaro, P. Platelets and Cardioprotection: The Role of Nitric Oxide and Carbon Oxide. Int. J. Mol. Sci. 2023, 24, 6107. [Google Scholar] [CrossRef]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Zivkovic, P.M.; Matetic, A.; Tadin Hadjina, I.; Rusic, D.; Vilovic, M.; Supe-Domic, D.; Borovac, J.A.; Mudnic, I.; Tonkic, A.; Bozic, J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial Dysfunction: The Early Predictor of Atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Tsiolakidou, G.; Koutroubakis, I.E. Thrombosis and Inflammatory Bowel Disease—The Role of Genetic Risk Factors. World J. Gastroenterol. 2008, 14, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Senchenkova, E.; Seifert, H.; Granger, D.N. Hypercoagulability and Platelet Abnormalities in Inflammatory Bowel Disease. Semin. Thromb. Hemost. 2015, 41, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Dallio, M.; Masarone, M.; Rosato, V.; Aglitti, A.; Persico, M.; Loguercio, C.; Federico, A. Vascular Endothelial Dysfunction in Inflammatory Bowel Diseases: Pharmacological and Nonpharmacological Targets. Oxid. Med. Cell Longev. 2018, 2018, 2568569. [Google Scholar] [CrossRef]
- Cibor, D.; Domagala-Rodacka, R.; Rodacki, T.; Jurczyszyn, A.; Mach, T.; Owczarek, D. Endothelial Dysfunction in Inflammatory Bowel Diseases: Pathogenesis, Assessment and Implications. World J. Gastroenterol. 2016, 22, 1067–1077. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Md Noor, S. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin, Regulation of Tight Junctions, and Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Mardanparvar, H. Aggravation of Hypertension by Gut Microbiota Dysbiosis; a Short-Review on New Concepts. J. Ren. Endocrinol. 2025, 11, e25185. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Wang, Z.; Yu, B. Gut Microbiota Dysbiosis in Human Hypertension: A Systematic Review of Observational Studies. Front. Cardiovasc. Med. 2021, 8, 650227. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.A.; Izar, M.C. Role of Inflammation in Cardiac Remodeling After Acute Myocardial Infarction. Front. Physiol. 2022, 13, 927163. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Lee, D.H. Inflammation and Resolution Signaling in Cardiac Repair and Heart Failure. EBioMedicine 2022, 79, 103992. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.R.; Yerly, A.; van der Vorst, E.P.C.; Baumgartner, I.; Bernhard, S.M.; Schindewolf, M.; Döring, Y. Inflammatory Mediators in Atherosclerotic Vascular Remodeling. Front. Cardiovasc. Med. 2022, 9, 868934. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, L.B.; Papamichael, K.; Cheifetz, A.S. Review Article: Emerging Drug Therapies in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2022, 55, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Murphy, M.; Malter, L. A Review of Available Medical Therapies to Treat Moderate-to-Severe Inflammatory Bowel Disease. Am. J. Gastroenterol. 2024, 119, 55–80. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Actis, G.C.; Pellicano, R. Inflammatory Bowel Disease: Efficient Remission Maintenance Is Crucial for Cost Containment. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 114–119. [Google Scholar] [CrossRef]
- Larussa, T.; Basile, A.; Palleria, C.; Iannelli, C.; Vero, A.; Giubilei, L.; De Sarro, C.; Suraci, E.; Marasco, R.; Imeneo, M.; et al. Real-Life Burden of Adverse Reactions to Biological Therapy in Inflammatory Bowel Disease: A Singlecentre Prospective Case Series. Med. Pharm. Rep. 2021, 94, 289–297. [Google Scholar] [CrossRef]
- Godat, S.; Fournier, N.; Safroneeva, E.; Juillerat, P.; Nydegger, A.; Straumann, A.; Vavricka, S.; Biedermann, L.; Greuter, T.; Fraga, M.; et al. Frequency and Type of Drug-Related Side Effects Necessitating Treatment Discontinuation in the Swiss Inflammatory Bowel Disease Cohort. Eur. J. Gastroenterol. Hepatol. 2018, 30, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Sholter, D.E.; Armstrong, P.W. Adverse Effects of Corticosteroids on the Cardiovascular System. Can. J. Cardiol. 2000, 16, 505–511. [Google Scholar] [PubMed]
- Tamez-Pérez, H.E. Steroid Hyperglycemia: Prevalence, Early Detection and Therapeutic Recommendations: A Narrative Review. World J. Diabetes 2015, 6, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Cummins, C.L. Fresh Insights into Glucocorticoid-Induced Diabetes Mellitus and New Therapeutic Directions. Nat. Rev. Endocrinol. 2022, 18, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Mebrahtu, T.F.; Morgan, A.W.; West, R.M.; Stewart, P.M.; Pujades-Rodriguez, M. Oral Glucocorticoids and Incidence of Hypertension in People with Chronic Inflammatory Diseases: A Population-Based Cohort Study. Can. Med. Assoc. J. 2020, 192, E295–E301. [Google Scholar] [CrossRef]
- Selinger, C.P.; Parkes, G.C.; Bassi, A.; Limdi, J.K.; Ludlow, H.; Patel, P.; Smith, M.; Saluke, S.; Ndlovu, Z.; George, B.; et al. Assessment of Steroid Use as a Key Performance Indicator in Inflammatory Bowel Disease—Analysis of Data from 2385 UK Patients. Aliment. Pharmacol. Ther. 2019, 50, 1009–1018. [Google Scholar] [CrossRef]
- Schroeder, K.W. Role of Mesalazine in Acute and Long-Term Treatment of Ulcerative Colitis and Its Complications. Scand. J. Gastroenterol. 2002, 37, 42–47. [Google Scholar] [CrossRef]
- Le Berre, C.; Roda, G.; Nedeljkovic Protic, M.; Danese, S.; Peyrin-Biroulet, L. Modern Use of 5-Aminosalicylic Acid Compounds for Ulcerative Colitis. Expert Opin. Biol. Ther. 2020, 20, 363–378. [Google Scholar] [CrossRef]
- Song, H.Y.; Seo, G.S. 5-Aminosalicylic Acid-Induced Myocarditis in a Patient with Atypical Ulcerative Colitis. Korean J. Gastroenterol. 2022, 79, 31–34. [Google Scholar] [CrossRef]
- Brown, G. 5-Aminosalicylic Acid-Associated Myocarditis and Pericarditis: A Narrative Review. Can. J. Hosp. Pharm. 2016, 69, 466–472. [Google Scholar] [CrossRef]
- Czubkowski, P.; Osiecki, M.; Szymańska, E.; Kierkuś, J. The Risk of Cardiovascular Complications in Inflammatory Bowel Disease. Clin. Exp. Med. 2020, 20, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Jensen, G.V.; Torp-Pedersen, C.; Nielsen, O.H.; Gislason, G.H.; Hansen, P.R. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death—A Danish nationwide cohort study. PLoS ONE 2013, 8, e56944. [Google Scholar] [CrossRef]
- Yewale, R.V.; Ramakrishna, B.S.; Doraisamy, B.V.; Basumani, P.; Venkataraman, J.; Jayaraman, K.; Murali, A.; Premkumar, K.; Kumar, A.S. Long-Term Safety and Effectiveness of Azathioprine in the Management of Inflammatory Bowel Disease: A Real-World Experience. JGH Open 2023, 7, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, M.; Herrmann, J.; Henkel, C.; Babic, M.; van der Giet, M.; Tölle, M. Long-Term Treatment of Azathioprine in Rats Induces Vessel Mineralization. Biomedicines 2021, 9, 327. [Google Scholar] [CrossRef]
- Nielsen, O.H. New Strategies for Treatment of Inflammatory Bowel Disease. Front. Med. 2014, 1, 3. [Google Scholar] [CrossRef]
- Xue, J.C.; Hou, X.T.; Zhao, Y.W.; Yuan, S. Biological Agents as Attractive Targets for Inflammatory Bowel Disease Therapeutics. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2025, 1871, 167648. [Google Scholar] [CrossRef]
- Banerjee, R.; Ali, R.A.R.; Wei, S.C.; Adsul, S. Biologics for the Management of Inflammatory Bowel Disease: A Review in Tuberculosis-Endemic Countries. Gut Liver 2020, 14, 685–698. [Google Scholar] [CrossRef]
- Rolski, F.; Tkacz, K.; Węglarczyk, K.; Kwiatkowski, G.; Pelczar, P.; Jaźwa-Kusior, A.; Bar, A.; Kuster, G.M.; Chłopicki, S.; Siedlar, M.; et al. TNF-α Protects from Exacerbated Myocarditis and Cardiac Death by Suppressing Expansion of Activated Heart-Reactive CD4+ T Cells. Cardiovasc. Res. 2024, 120, 82–94. [Google Scholar] [CrossRef]
- Hussain, A.; Tarahomi, T.; Singh, L.; Bollampally, M.; Heydari-Kamjani, M.; Kesselman, M.M. Cardiovascular Risk Associated With TNF Alpha Inhibitor Use in Patients With Rheumatoid Arthritis. Cureus 2021, 13, 17938. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-α, in Patients with Moderate-to-Severe Heart Failure: Results of the Anti-TNF Therapy against Congestive Heart Failure (ATTACH) Trial. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B. Infliximab in the Treatment of Crohn’s Disease: A User’s Guide for Clinicians. Am. J. Gastroenterol. 2002, 97, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; Alrashed, F.; Alkazemi, A.; Lakatos, P.L.; Bessissow, T. Impact of Biologic Therapies and Small Molecules on the Risk of Major Adverse Cardiovascular Events in Patients with Inflammatory Bowel Diseases: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277, Erratum in: Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Khachatryan, L.; Kondylis, A.; Battey, J.N.D.; Sierro, N.; Danilova, N.A.; Grigoryeva, T.V.; Markelova, M.I.; Khusnutdinova, D.R.; Laikov, A.V.; et al. Inflammatory Bowel Disease-Associated Changes in the Gut: Focus on Kazan Patients. Inflamm. Bowel Dis. 2021, 27, 418–433. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, S.; Yao, J.; Lin, X.; Li, Y.; Wang, W.; Weng, J.; Zou, Y.; Zhu, L.; Zhi, M. Correlation between Altered Gut Microbiota and Elevated Inflammation Markers in Patients with Crohn’s Disease. Front. Immunol. 2022, 13, 947313. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhao, H.L.; Guo, G.J.; Xu, J.; Zhou, Y.L.; Huang, H.L.; Nie, Y.Q. Characterization of Short-Chain Fatty Acids in Patients with Ulcerative Colitis: A Meta-Analysis. BMC Gastroenterol. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Firrman, J.; Liu, L.S.; Mahalak, K.; Tanes, C.; Bittinger, K.; Tu, V.; Bobokalonov, J.; Mattei, L.; Zhang, H.; Van Den Abbeele, P. The Impact of Environmental PH on the Gut Microbiota Community Structure and Short Chain Fatty Acid Production. FEMS Microbiol. Ecol. 2022, 98, fiac038. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhao, X.; Shang, C.; Xiang, M.; Li, L.; Cui, X. Microbiota-Derived Short-Chain Fatty Acids: Implications for Cardiovascular and Metabolic Disease. Front. Cardiovasc. Med. 2022, 9, 900381. [Google Scholar] [CrossRef]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-Chain Fatty Acids in Gut–Heart Axis: Their Role in the Pathology of Heart Failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef]
- Xu, J.; Moore, B.N.; Pluznick, J.L. Short-Chain Fatty Acid Receptors and Blood Pressure Regulation: Council on Hypertension Mid-Career Award for Research Excellence 2021. Hypertension 2022, 79, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Collard, D.; Prodan, A.; Levels, J.H.M.; Zwinderman, A.H.; Backhed, F.; Vogt, L.; Peters, M.J.L.; Muller, M.; Nieuwdorp, M.; et al. Associations between Gutmicrobiota, Faecal Short-Chain Fatty Acids, and Blood Pressure across Ethnic Groups: The HELIUS Study. Eur. Heart J. 2020, 41, 4259–4267. [Google Scholar] [CrossRef] [PubMed]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.; Capettini, L.S.; Lemos, V.S.; Santos, R.A.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Muradi, A.; Jasirwan, C.O.M.; Simanjuntak, C.D.; Pratama, D.; Suhartono, R.; Darwis, P.; Kekalih, A. The Correlation of Short-Chain Fatty Acids with Peripheral Arterial Disease in Diabetes Mellitus Patients. Life 2022, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Du, D.; Fu, T.; Han, Y.; Li, P.; Ju, H. Alterations of the Gut Microbiota in Patients With Severe Chronic Heart Failure. Front. Microbiol. 2022, 12, 813289. [Google Scholar] [CrossRef] [PubMed]
- Walsham, N.E.; Sherwood, R.A. Fecal Calprotectin in Inflammatory Bowel Disease. Clin. Exp. Gastroenterol. 2016, 9, 21–29. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From Biomarker to Biological Function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.S.; Mansfield, J.C. Can Faecal Calprotectin Predict Relapse in Inflammatory Bowel Disease: A Mini Review. Frontline Gastroenterol. 2018, 9, 23–28. [Google Scholar] [CrossRef]
- Heida, A.; Park, K.T.; Van Rheenen, P.F. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm. Bowel Dis. 2017, 23, 894–902. [Google Scholar] [CrossRef]
- Rasmussen, M.H.; Brodersen, J.B.; Brasen, C.L.; Madsen, J.S.; Knudsen, T.; Kjeldsen, J.; Jensen, M.D. The Diagnostic Accuracy of Plasma and Serum Calprotectin Is Inferior to C-Reactive Protein in Patients with Suspected Crohn’s Disease. Scand. J. Gastroenterol. 2025, 60, 235–242. [Google Scholar] [CrossRef]
- Ondriš, J.; Husťak, R.; Ďurina, J.; Malicherová Jurková, E.; Bošák, V. Serum Biomarkers in Diagnosis and Clinical Management of Inflammatory Bowel Disease: Anything New on the Horizon? Folia Biol. 2024, 70, 248–261. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Flores-Guerrero, J.L.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Sundrehagen, E.; Seidu, S.; Dullaart, R.P.F.; Bakker, S.J.L. Plasma Calprotectin and Risk of Cardiovascular Disease: Findings from the PREVEND Prospective Cohort Study. Atherosclerosis 2018, 275, 205–213. [Google Scholar] [CrossRef]
- Reshadmanesh, T.; Behnoush, A.H.; Farajollahi, M.; Khalaji, A.; Ghondaghsaz, E.; Ahangar, H. Circulating Levels of Calprotectin as a Biomarker in Patients With Coronary Artery Disease: A Systematic Review and Meta-Analysis. Clin. Cardiol. 2024, 47, e24315. [Google Scholar] [CrossRef] [PubMed]
- Hoy, C.K.; NaveenKumar, S.K.; Navaz, S.A.; Sugur, K.; Yalavarthi, S.; Sarosh, C.; Smith, T.; Kmetova, K.; Chong, E.; Peters, N.F.; et al. Calprotectin Impairs Platelet Survival in Patients With Primary Antiphospholipid Syndrome. Arthritis Rheumatol. 2024, 76, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Zlamal, J.; Singh, A.; Weich, K.; Jaffal, H.; Uzun, G.; Pelzl, L.; Althaus, K.; Bakchoul, T. Platelet Phosphatidylserine Is the Critical Mediator of Thrombosis in Heparin-Induced Thrombocytopenia. Haematologica 2023, 108, 2690–2702. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Perera, Y.R.; Chazin, W.J. A Structural Perspective on Calprotectin as a Ligand of Receptors Mediating Inflammation and Potential Drug Target. Biomolecules 2022, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.H.; Kan, B.; Roberts, H.; Wang, Y.; Walley, K.R. S100A8 and S100A9 Mediate Endotoxin-Induced Cardiomyocyte Dysfunction via the Receptor for Advanced Glycation End Products. Circ. Res. 2008, 102, 1239–1246. [Google Scholar] [CrossRef]
- Heinzel, S.; Jureczek, J.; Kainulainen, V.; Nieminen, A.I.; Suenkel, U.; von Thaler, A.K.; Kaleta, C.; Eschweiler, G.W.; Brockmann, K.; Aho, V.T.E.; et al. Elevated Fecal Calprotectin Is Associated with Gut Microbial Dysbiosis, Altered Serum Markers and Clinical Outcomes in Older Individuals. Sci. Rep. 2024, 14, 13513. [Google Scholar] [CrossRef]
- Shaw, K.A.; Bertha, M.; Hofmekler, T.; Chopra, P.; Vatanen, T.; Srivatsa, A.; Prince, J.; Kumar, A.; Sauer, C.; Zwick, M.E.; et al. Dysbiosis, Inflammation, and Response to Treatment: A Longitudinal Study of Pediatric Subjects with Newly Diagnosed Inflammatory Bowel Disease. Genome Med. 2016, 8, 75. [Google Scholar] [CrossRef]
- Ponziani, F.R.; De Luca, A.; Picca, A.; Marzetti, E.; Petito, V.; Del Chierico, F.; Reddel, S.; Paroni Sterbini, F.; Sanguinetti, M.; Putignani, L.; et al. Gut Dysbiosis and Fecal Calprotectin Predict Response to Immune Checkpoint Inhibitors in Patients with Hepatocellular Carcinoma. Hepatol. Commun. 2022, 6, 1492–1501. [Google Scholar] [CrossRef]
- Alili, R.; Belda, E.; Fabre, O.; Pelloux, V.; Giordano, N.; Legrand, R.; Lassen, P.B.; Swartz, T.D.; Zucker, J.D.; Clément, K. Characterization of the Gut Microbiota in Individuals with Overweight or Obesity during a Real-World Weight Loss Dietary Program: A Focus on the Bacteroides 2 Enterotype. Biomedicines 2022, 10, 16. [Google Scholar] [CrossRef]
- Vieira-Silva, S.; Sabino, J.; Valles-Colomer, M.; Falony, G.; Kathagen, G.; Caenepeel, C.; Cleynen, I.; van der Merwe, S.; Vermeire, S.; Raes, J. Quantitative Microbiome Profiling Disentangles Inflammation- and Bile Duct Obstruction-Associated Microbiota Alterations across PSC/IBD Diagnoses. Nat. Microbiol. 2019, 4, 1826–1831. [Google Scholar] [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of Human Zonulin, a Physiological Modulator of Tight Junctions, as Prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 2008, 135, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Østvik, A.E.; Granlund, A.V.B.; Bugge, M.; Nilsen, N.J.; Torp, S.H.; Waldum, H.L.; Damås, J.K.; Espevik, T.; Sandvik, A.K. Enhanced Expression of CXCL10 in Inflammatory Bowel Disease: Potential Role of Mucosal Toll-like Receptor 3 Stimulation. Inflamm. Bowel Dis. 2013, 19, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Schroepf, S.; Kappler, R.; Brand, S.; Prell, C.; Lohse, P.; Glas, J.; Hoster, E.; Helmbrecht, J.; Ballauff, A.; Berger, M.; et al. Strong Overexpression of CXCR3 Axis Components in Childhood Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2010, 16, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.J.; Arjomandi, A.; Delanoy, M.L.; Du Paty, E.; Galea, P.; Laune, D.; Rieunier, F.; Walker, R.P.; Binder, S.R. Development of Monoclonal Antibodies to Pre-Haptoglobin 2 and Their Use in an Enzyme-Linked Immunosorbent Assay (ELISA). J. Immunol. Methods 2014, 406, 7548. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef]
- Li, C.; Gao, M.; Zhang, W.; Chen, C.; Zhou, F.; Hu, Z.; Zeng, C. Zonulin Regulates Intestinal Permeability and Facilitates Enteric Bacteria Permeation in Coronary Artery Disease. Sci. Rep. 2016, 6, 802–819. [Google Scholar] [CrossRef]
- Ahmad, F.; Karim, A.; Khan, J.; Qaisar, R. Plasma Zonulin Correlates with Cardiac Dysfunction and Poor Physical Performance in Patients with Chronic Heart Failure. Life Sci. 2022, 311, 3. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of Gut Microbiome and Intestinal Epithelial Barrier Dysfunction in Patients with High Blood Pressure. Clin. Sci. 2018, 132, 95–100. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Dughera, F.; Ribaldone, D.G.; Rosso, C.; Abate, M.L.; Pellicano, R.; Bresso, F.; Smedile, A.; Saracco, G.M.; Astegiano, M. Serum Zonulin in Patients with Inflammatory Bowel Disease: A Pilot Study. Minerva Med. 2018, 110, 2472754. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; De Roos, N.M.; Hovenier, R.; Meijerink, J.; Besseling-Van Der Vaart, I.; Van Hemert, S.; Witteman, B.J.M. Intestinal Permeability Measured by Urinary Sucrose Excretion Correlates with Serum Zonulin and Faecal Calprotectin Concentrations in UC Patients in Remission. J. Nutr. Metab. 2019, 2019, w13557. [Google Scholar] [CrossRef]
- Burri, E.; Beglinger, C. Faecal calprotectin—A useful tool in the management of inflammatory bowel disease. Swiss. Med. Wkly 2012, 142, w13557. [Google Scholar] [CrossRef]
- Yan, D.; Ye, S.; He, Y.; Wang, S.; Xiao, Y.; Xiang, X.; Deng, M.; Luo, W.; Chen, X.; Wang, X. Fatty acids and lipid mediators in inflammatory bowel disease: From mechanism to treatment. Front. Immunol. 2023, 14, 1286667. [Google Scholar] [CrossRef]
- von Roon, A.C.; Karamountzos, L.; Purkayastha, S.; Reese, G.E.; Darzi, A.W.; Teare, J.P.; Paraskeva, P.; Tekkis, P.P. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am. J. Gastroenterol. 2007, 102, 803–813. [Google Scholar] [CrossRef]
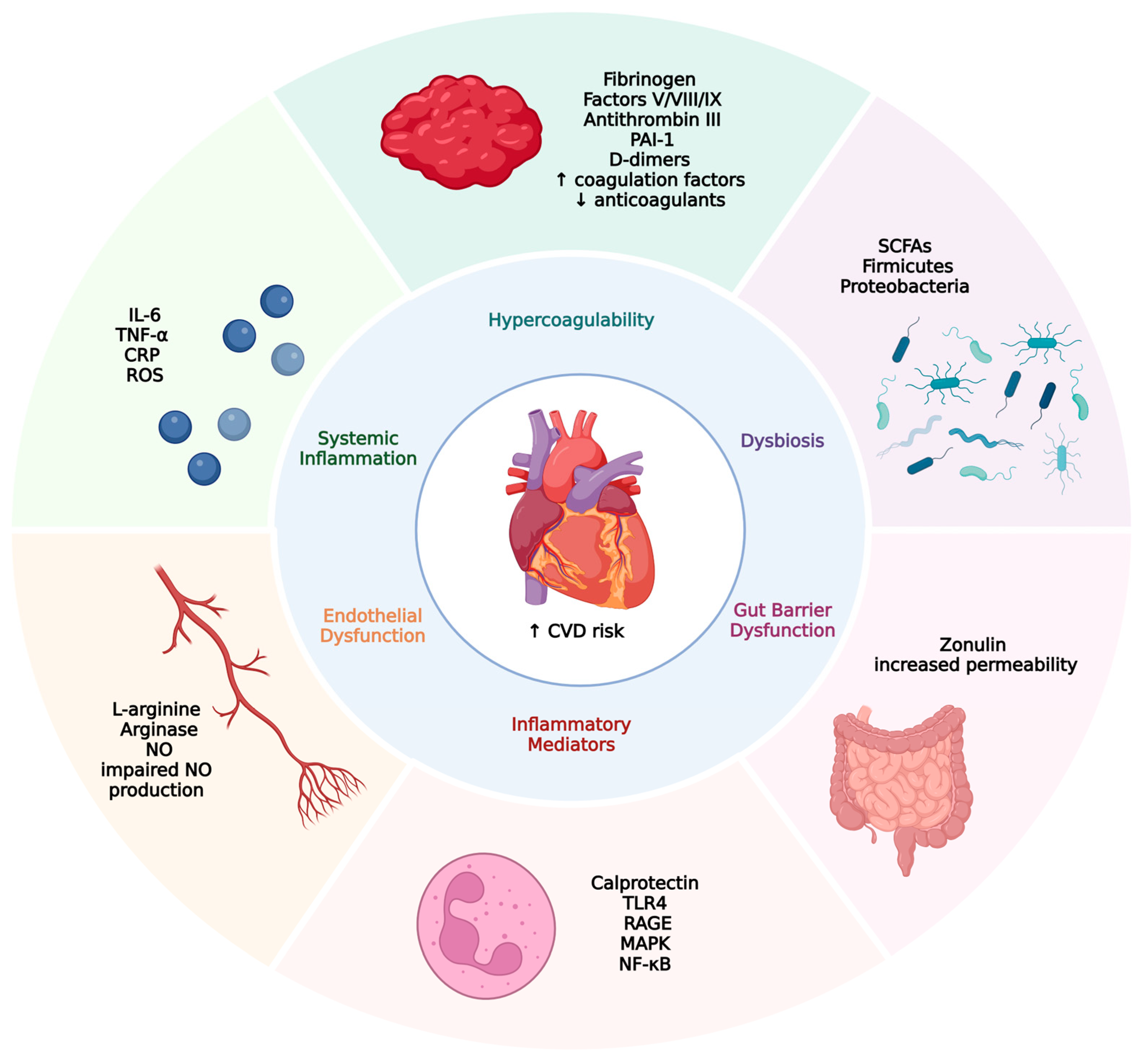
| Pathway | Mechanism | Key Biomarkers/Elements | Cardiovascular Consequence | Refs. |
|---|---|---|---|---|
| Hypercoagulability | ↑ Coagulation factors ↓ natural anticoagulants, hypofibrinolysis | Fibrinogen, Factors V/VIII/IX, Antithrombin III, PAI-1, D-dimers | Venous/arterial thrombosis ↑ VTE risk | [104,105] |
| Systemic Inflammation | Chronic inflammation ↑ proinflammatory cytokines | IL-6, TNF-α, CRP, ROS | Atherosclerosis, plaque instability ↑ level of tubules in stages VII/VIII | [105,106] |
| Endothelial Dysfunction | Impaired NO production ↑ Arginase ↓ eNOS activity | L-arginine, Arginase, NO | Early atherosclerosis, vascular tone dysregulation | [107,108,109] |
| Gut Barrier Dysfunction | Increased intestinal permeability due to dysbiosis | Zonulin | Endotoxemia, systemic inflammation | [110,111] |
| Dysbiosis | ↓ SCFA-producing bacteria ↑ proinflammatory taxa | SCFAs, Firmicutes, Proteobacteria | Hypertension, arterial stiffness, metabolic dysregulation | [112,113] |
| Inflammatory Mediators | Neutrophil activation, activation of NF-κB, and MAPK | Calprotectin, RAGE, Toll-like receptor 4 (TLR4) | Cardiac remodeling, ↑ CVD risk | [114,115,116] |
| Biomarker | Source | Main Producers/Origin | Role in Pathophysiology | Ref. |
|---|---|---|---|---|
| Short-chain fatty acids (SCFAs) | Gut microbiota (mainly Firmicutes: Ruminococcus, Akkermansia, Bifidobacterium, Lactobacillus, Succinivibrio, Roseburia, Clostridium, Eubacterium) | Produced by bacterial fermentation of dietary fibers and resistant carbohydrates in the colon (especially cecum) | Maintain the barrier, modulate inflammation, energy source for colonocytes, modulate immune homeostasis, reduce inflammation, regulate motility, promote mucus production, reduce colon cancer risk | [144] |
| Calprotectin | Neutrophils | Released during inflammation | Marker of intestinal inflammation, modulates leukocyte migration, cytokine expression, cell proliferation; acts intra- and extracellularly | [145] |
| Zonulin | Intestinal epithelium, liver | Pre-haptoglobin 2; upregulated by gliadin and bacteria via the CXCR3 receptor | Regulates gut permeability by modulating tight junctions; increased levels indicate barrier dysfunction | [146] |
| Biomarker | Sensitivity (%) | Specificity (%) | Predictive Value for CVD in IBD | Ref. |
|---|---|---|---|---|
| Short-chain fatty acids (SCFAs) | 80–95 | 70–85 | High for intestinal inflammation; indirect for CVD | [193] |
| Fecal Calprotectin | Not Available (N/A) | N/A | High for intestinal inflammation; indirect for CVD | [194] |
| Zonulin | 65–80 | 60–75 | Under investigation; linked to gut permeability and CVD | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šantić, R.; Pavlović, N.; Kumrić, M.; Vilović, M.; Božić, J. Pathophysiological Links Between Inflammatory Bowel Disease and Cardiovascular Disease: The Role of Dysbiosis and Emerging Biomarkers. Biomedicines 2025, 13, 1864. https://doi.org/10.3390/biomedicines13081864
Šantić R, Pavlović N, Kumrić M, Vilović M, Božić J. Pathophysiological Links Between Inflammatory Bowel Disease and Cardiovascular Disease: The Role of Dysbiosis and Emerging Biomarkers. Biomedicines. 2025; 13(8):1864. https://doi.org/10.3390/biomedicines13081864
Chicago/Turabian StyleŠantić, Roko, Nikola Pavlović, Marko Kumrić, Marino Vilović, and Joško Božić. 2025. "Pathophysiological Links Between Inflammatory Bowel Disease and Cardiovascular Disease: The Role of Dysbiosis and Emerging Biomarkers" Biomedicines 13, no. 8: 1864. https://doi.org/10.3390/biomedicines13081864
APA StyleŠantić, R., Pavlović, N., Kumrić, M., Vilović, M., & Božić, J. (2025). Pathophysiological Links Between Inflammatory Bowel Disease and Cardiovascular Disease: The Role of Dysbiosis and Emerging Biomarkers. Biomedicines, 13(8), 1864. https://doi.org/10.3390/biomedicines13081864








