Etiology of Delayed Lactogenesis in Obesity
Abstract
1. Introduction
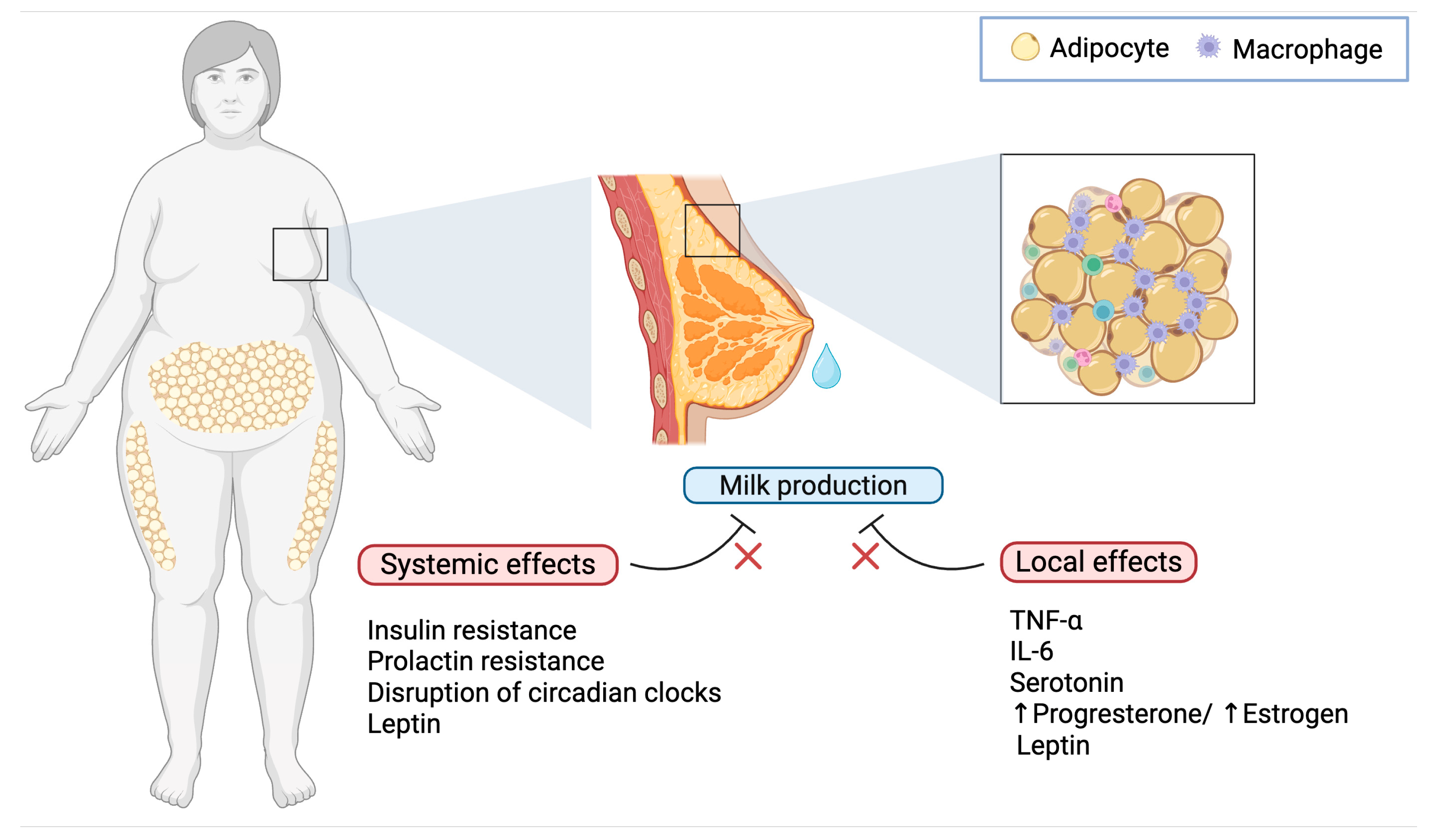
2. Inflammatory Mechanisms in Obesity-Related Delayed Lactogenesis
3. Hormonal and Endocrine Dysregulation
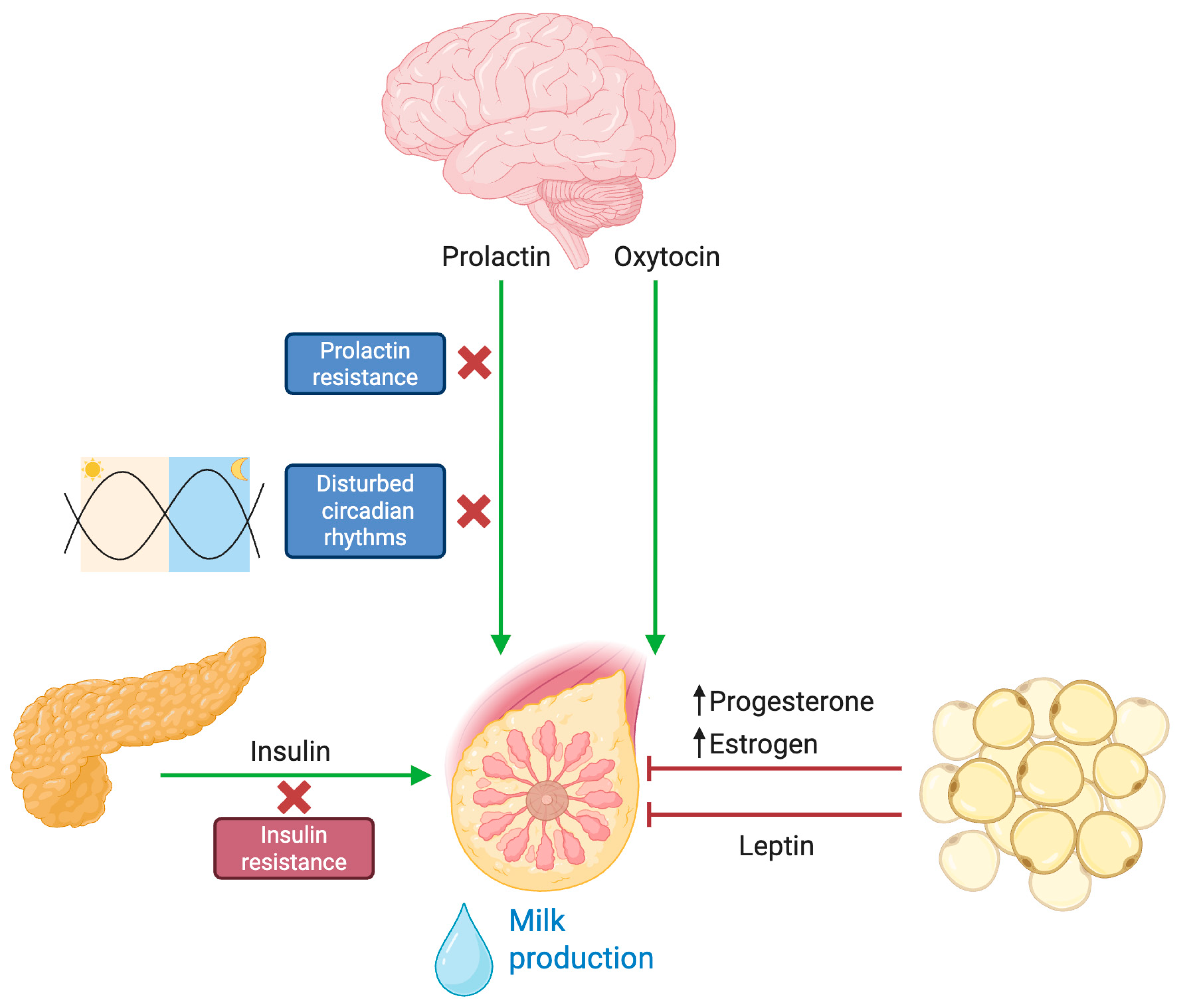
3.1. Blunted Prolactin Response
3.2. Progesterone Dynamics and Local Estrogen Production
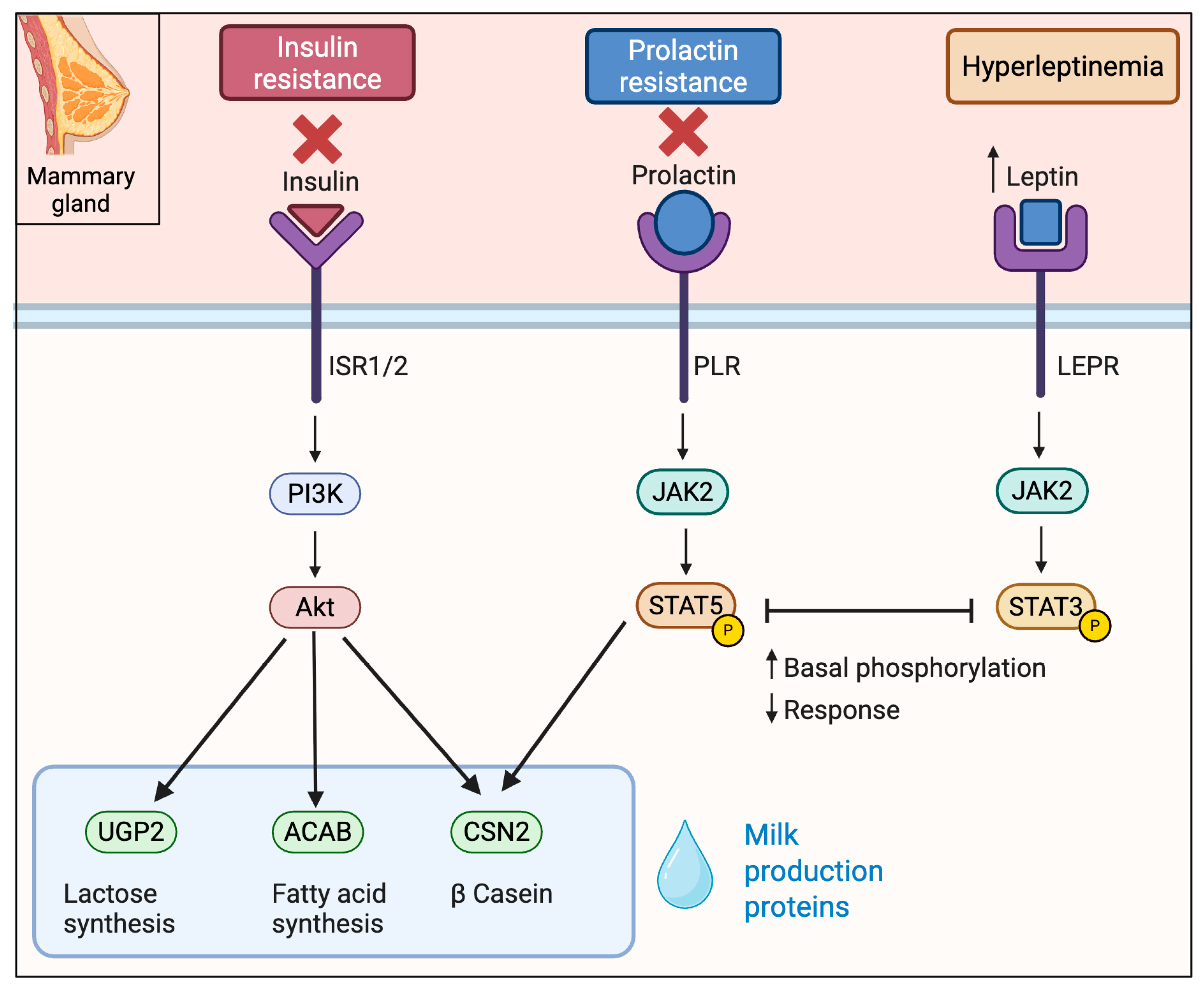
3.3. Insulin Resistance
3.4. Lepin and the Control of Lactogensis
4. Circadian Disruption and Its Impact on Lactogenesis
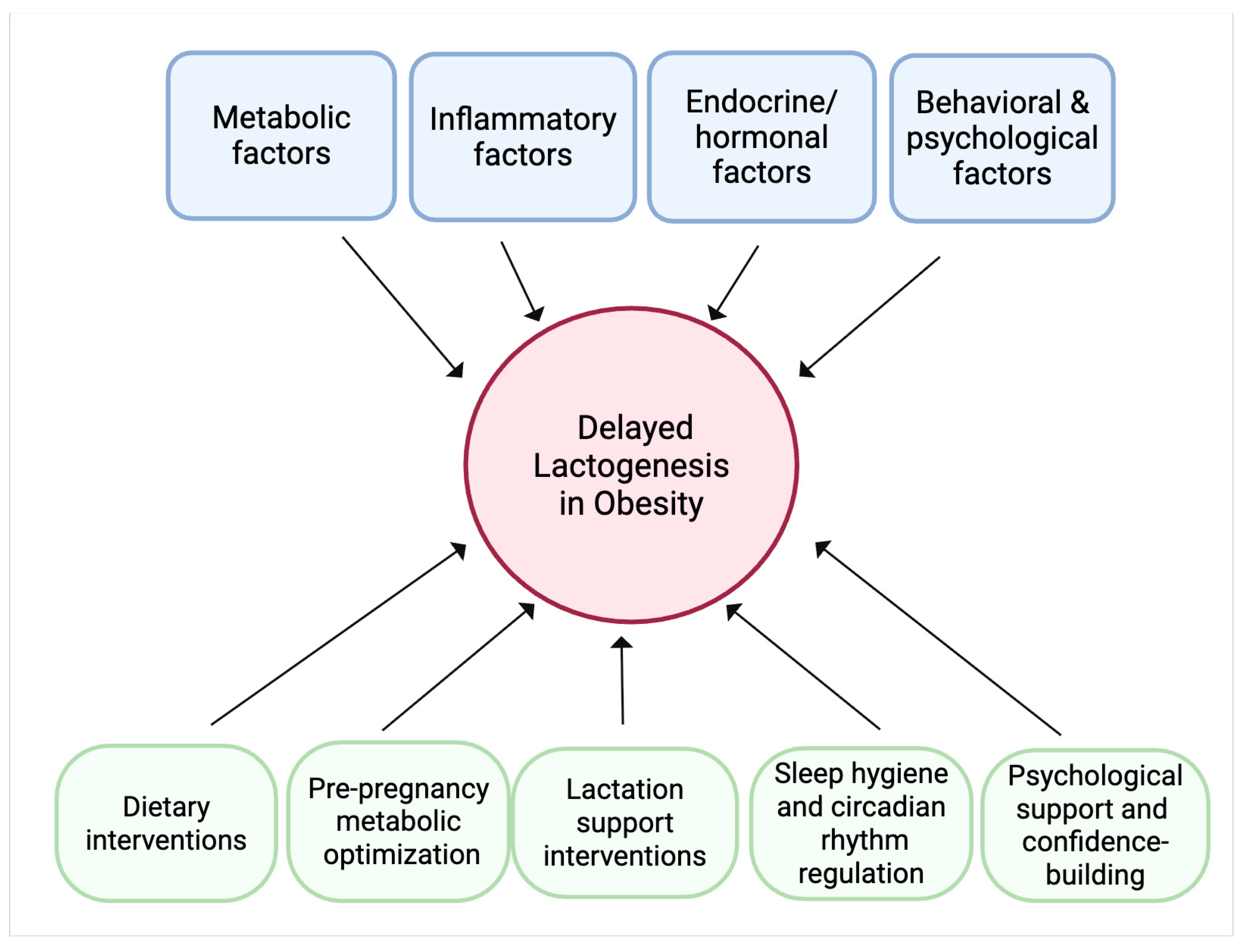
5. Mechanisms Underlying Impaired Lactogenesis in Obesity
6. Behavioral Influences and Clinical Implications
6.1. Mechanical and Ergonomic Challenges
6.2. Phycological and Sociocultural Barriers
6.3. Clinical Practice and Intervention Strategies
7. Discussion
8. Future Directions
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DLII | Delayed Lactogenesis II |
| OL | Onset of Lactogenesis |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| CLS | Crown-Like Structures |
| TPH1 | Tryptophan Hydroxylase 1 |
| STAT5 | Signal Transducer and Activator of Transcription 5 |
| CLOCK | Circadian Locomotor Output Cycles Kaput |
| INSR | Insulin Receptor |
| IRS1/2 | Insulin Receptor Substrate |
| PI3K | Phosphoinositide 3-Kinase |
| JAK2 | Janus Kinase 2 |
| AKT | Protein Kinase B |
| LEPR | Leptin Receptor |
| GLUT4 | Glucose Transporter Protein Type 4 |
| IGF | Insulin Growth Factor |
| IGFBP | Insulin Growth Factor Binding Protein |
References
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- European Society for Paediatric Gastroenterology, Hepatology & Nutrition(ESPGHAN); European Academy of Paediatrics (EAP); European Society for Paediatric Research (ESPR); European Academy for Allergy and Clinical Immunology(EAACI); Federation of International Societies for Paediatric Gastroenterology, Hepatology & Nutrition (FISPGHAN); Latin American Society for Pediatric Gastroenterology, Hepatology & Nutrition (LASPGHAN); Pan Arab Society for Pediatric Gastroenterology and Nutrition (PASPGHAN); Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology and Nutrition (AAPSGHAN); North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN); World Allergy Organization (WAO); et al. World Health Organization (WHO) guideline on the complementary feeding of infants and young children aged 6−23 months 2023: A multisociety response. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 181–188. [Google Scholar] [CrossRef]
- Sørensen, T.I.A.; Martinez, A.R.; Jørgensen, T.S.H. Epidemiology of Obesity. In From Obesity to Diabetes; Springer: Cham, Switzerland, 2022; Volume 274, pp. 3–27. [Google Scholar] [CrossRef]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult obesity complications: Challenges and clinical impact. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820934955. [Google Scholar] [CrossRef]
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023 Key Findings Data from the National Health and Nutrition Examination Survey. 2021. Available online: https://dx.doi.org/10.15620/cdc/159281 (accessed on 13 July 2025).
- Marques, A.; Peralta, M.; Naia, A.; Loureiro, N.; de Matos, M.G. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur. J. Public Health 2017, 28, 295–300. [Google Scholar] [CrossRef]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired Lactation: Review of Delayed Lactogenesis and Insufficient Lactation. J. Midwifery Women’s Health 2021, 66, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Ballesta-Castillejos, A.; Gomez-Salgado, J.; Rodriguez-Almagro, J.; Ortiz-Esquinas, I.; Hernandez-Martinez, A. Relationship between maternal body mass index with the onset of breastfeeding and its associated problems: An online survey. Int. Breastfeed. J. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A.; Chantry, C.J.; Peerson, J.M.; Cohen, R.J.; Dewey, K.G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr. 2010, 92, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Dewey, K.G.; Quesenberry, C.P.; Gunderson, E.P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am. J. Clin. Nutr. 2014, 99, 115–121. [Google Scholar] [CrossRef]
- Neville, M.C.; Morton, J. Physiology and endocrine changes underlying human lactogenesis II. J. Nutr. 2001, 131, 3005S–3008S. [Google Scholar] [CrossRef] [PubMed]
- Bigman, G.; Wilkinson, A.V.; Homedes, N.; Pérez, A. Body Image Dissatisfaction, Obesity and Their Associations with Breastfeeding in Mexican Women, a Cross-Sectional Study. Matern. Child Health J. 2018, 22, 1815–1825. [Google Scholar] [CrossRef]
- Dalrymple, K.V.; Briley, A.L.; Tydeman, F.A.S.; Seed, P.T.; Singh, C.M.; Flynn, A.C.; White, S.L.; Poston, L.; on behalf of the UPBEAT Consortium. Breastfeeding behaviours in women with obesity; associations with weight retention and the serum metabolome: A secondary analysis of UPBEAT. Int. J. Obes. 2024, 48, 1472–1480. [Google Scholar] [CrossRef]
- Darling, A.J.; Gatta, L.A.; Tucker, A.; Adkins, L.D.; Mitchell, C.; Reiff, E.; Dotters-Katz, S. Gestational weight gain and patterns of breastfeeding among patients with class III obesity. J. Matern. Neonatal Med. 2022, 35, 9851–9856. [Google Scholar] [CrossRef]
- Donath, S.M.; Amir, L.H. Does maternal obesity adversely affect breastfeeding initiation and duration? J. Paediatr. Child Health 2000, 36, 482–486. [Google Scholar] [CrossRef]
- Donath, S.M.; Amir, L.H. Maternal obesity and initiation and duration of breastfeeding: Data from the longitudinal study of Australian children. Matern. Child Nutr. 2008, 4, 163–170. [Google Scholar] [CrossRef]
- Fan, W.Q.; Molinaro, A. Maternal obesity adversely affects early breastfeeding in a multicultural, multi-socioeconomic Melbourne community. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Keitel-Korndörfer, A.; Bergmann, S.; Wendt, V.; von Klitzing, K.; Petroff, D. Breastfeeding in Obese versus Normal-Weight German Mothers of Various Socioeconomic Status. J. Hum. Lact. 2016, 32, 546–550. [Google Scholar] [CrossRef]
- Hawkins, M.A.W.; Colaizzi, J.; Rhoades-Kerswill, S.; Fry, E.D.; Keirns, N.G.; Smith, C.E. Earlier Onset of Maternal Excess Adiposity Associated with Shorter Exclusive Breastfeeding Duration. J. Hum. Lact. 2018, 35, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, X.; Zhang, Y.; Sun, G.; Zhong, C.; Wang, W.; Li, Q.; Li, X.; Yin, H.; Yang, X.; et al. Gestational weight gain is associated with delayed onset of lactogenesis in the TMCHC study: A prospective cohort study. Clin. Nutr. 2018, 38, 2436–2441. [Google Scholar] [CrossRef]
- Kair, L.R.; Nickel, N.C.; Jones, K.; Kornfeind, K.; Sipsma, H.L. Hospital breastfeeding support and exclusive breastfeeding by maternal prepregnancy body mass index. Matern. Child Nutr. 2019, 15, e12783. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, H.; Vaeth, M.; Rasmussen, K.M. Obesity and early cessation of breastfeeding in Denmark. Eur. J. Public Health 2012, 23, 316–322. [Google Scholar] [CrossRef]
- Lewkowitz, A.K.; López, J.D.; Stein, R.I.; Rhoades, J.S.; Schulz, R.C.; Woolfolk, C.L.; Macones, G.A.; Haire-Joshu, D.; Cahill, A.G. Effect of a Home-Based Lifestyle Intervention on Breastfeeding Initiation Among Socioeconomically Disadvantaged African American Women with Overweight or Obesity. Breastfeed. Med. 2018, 13, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Masho, S.W.; Cha, S.; Morris, M.R. Prepregnancy Obesity and Breastfeeding Noninitiation in the United States: An Examination of Racial and Ethnic Differences. Breastfeed. Med. 2015, 10, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Kido, M.; Tanabe, A.; Ando, K. Prepregnancy obesity as a risk factor for exclusive breastfeeding initiation in Japanese women. Nutrition 2019, 62, 93–99. [Google Scholar] [CrossRef]
- Oddy, W.H.; Li, J.; Landsborough, L.; Kendall, G.E.; Henderson, S.; Downie, J. The association of maternal overweight and obesity with breastfeeding duration. J. Pediatr. 2006, 149, 185–191. [Google Scholar] [CrossRef]
- Ramji, N.; Quinlan, J.; Murphy, P.; Crane, J.M. The Impact of Maternal Obesity on Breastfeeding. J. Obstet. Gynaecol. Can. 2016, 38, 703–711. [Google Scholar] [CrossRef]
- Tao, X.-Y.; Huang, K.; Yan, S.-Q.; Zuo, A.-Z.; Tao, R.-W.; Cao, H.; Gu, C.-L.; Tao, F.-B. Pre-pregnancy BMI, gestational weight gain and breast-feeding: A cohort study in China. Public Health Nutr. 2016, 20, 1001–1008. [Google Scholar] [CrossRef]
- Verret-Chalifour, J.; Giguère, Y.; Forest, J.-C.; Croteau, J.; Zhang, P.; Marc, I.; van Wouwe, J. Breastfeeding Initiation: Impact of Obesity in a Large Canadian Perinatal Cohort Study. PLoS ONE 2015, 10, e0117512. [Google Scholar] [CrossRef]
- Gomez-Casado, G.; Jimenez-Gonzalez, A.; Rodriguez-Muñoz, A.; Tinahones, F.J.; González-Mesa, E.; Murri, M.; Ortega-Gomez, A. Neutrophils as indicators of obesity-associated inflammation: A systematic review and meta-analysis. Obes. Rev. 2024, 26, e13868. [Google Scholar] [CrossRef]
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.L.; Grayson, B.E.; Yadav, E.; Seeley, R.J.; Horseman, N.D. High Fat Diet Alters Lactation Outcomes: Possible Involvement of Inflammatory and Serotonergic Pathways. PLoS ONE 2012, 7, e32598. [Google Scholar] [CrossRef]
- Buonfiglio, D.C.; Ramos-Lobo, A.M.; Freitas, V.M.; Zampieri, T.T.; Nagaishi, V.S.; Magalhães, M.; Cipolla-Neto, J.; Cella, N.; Donato, J. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci. Rep. 2016, 6, 22421. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Kjolhede, C.L. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 2004, 113, e465–e471. [Google Scholar] [CrossRef]
- Knight, C.H. An endocrine hypothesis to explain obesity-related lactation insufficiency in breastfeeding mothers. J. Dairy Res. 2020, 87, 78–81. [Google Scholar] [CrossRef]
- Lovelady, C.A. Is Maternal Obesity a Cause of Poor Lactation Performance? Nutr. Rev. 2005, 63, 352–355. [Google Scholar] [CrossRef]
- Garcia, A.H.; Voortman, T.; Baena, C.P.; Chowdhurry, R.; Muka, T.; Jaspers, L.; Warnakula, S.; Tielemans, M.J.; Troup, J.; Bramer, W.M.; et al. Maternal weight status, diet, and supplement use as determinants of breastfeeding and complementary feeding: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 490–516. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, A.; Zhang, J.; Wang, R.; Xia, H. Role of Perinatal Biological Factors in Delayed Lactogenesis II Among Women with Pre-pregnancy Overweight and Obesity. Biol. Res. Nurs. 2022, 24, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Nommsen-Rivers, L.A. Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Adv. Nutr. Int. Rev. J. 2016, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Suwaydi, M.A.; Wlodek, M.E.; Lai, C.T.; Prosser, S.A.; Geddes, D.T.; Perrella, S.L. Delayed secretory activation and low milk production in women with gestational diabetes: A case series. BMC Pregnancy Childbirth 2022, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jevitt, C.; Hernandez, I.; Groër, M. Lactation Complicated by Overweight and Obesity: Supporting the Mother and Newborn. J. Midwifery Women’s Health 2007, 52, 606–613. [Google Scholar] [CrossRef]
- Aoki, N.; Kawamura, M.; Matsuda, T. Lactation-dependent down regulation of leptin production in mouse mammary gland. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1999, 1427, 298–306. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, L.; Ahmed, A.; Plaut, K.; Haas, D.M.; Szucs, K.; Casey, T.M. Does Circadian Disruption Play a Role in the Metabolic–Hormonal Link to Delayed Lactogenesis II? Front. Nutr. 2015, 2, 131958. [Google Scholar] [CrossRef] [PubMed]
- Dolatshad, H.; Campbell, E.; O’hAra, L.; Maywood, E.; Hastings, M.; Johnson, M. Developmental and reproductive performance in circadian mutant mice. Hum. Reprod. 2005, 21, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Wakatsuki, Y.; Iigo, M.; Shibata, S. Circadian Clock Mutation in Dams Disrupts Nursing Behavior and Growth of Pups. Endocrinology 2006, 147, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.; Crodian, J.; Suárez-Trujillo, A.; Erickson, E.; Weldon, B.; Crow, K.; Cummings, S.; Chen, Y.; Shamay, A.; Mabjeesh, S.J.; et al. CLOCK regulates mammary epithelial cell growth and differentiation. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R1125–R1134. [Google Scholar] [CrossRef]
- Noh, J. The Effect of Circadian and Sleep Disruptions on Obesity Risk. J. Obes. Metab. Syndr. 2018, 27, 78–83. [Google Scholar] [CrossRef]
- Tian, M.; Qi, Y.; Zhang, X.; Wu, Z.; Chen, J.; Chen, F.; Guan, W.; Zhang, S. Regulation of the JAK2-STAT5 Pathway by Signaling Molecules in the Mammary Gland. Front. Cell Dev. Biol. 2020, 8, 604896. [Google Scholar] [CrossRef]
- Geng, Z.; Shan, X.; Lian, S.; Wang, J.; Wu, R. LPS-induced SOCS3 antagonizes the JAK2-STAT5 pathway and inhibits β-casein synthesis in bovine mammary epithelial cells. Life Sci. 2021, 278, 119547. [Google Scholar] [CrossRef]
- Bernardo, K.; Hovey, R.; Trott, J.; Wagner, E.; Karns, R.; Riddle, S.; Thompson, A.; Ward, L.; Nommsen-Rivers, L. Hormone-Sensitive Gene Signatures in the Mammary Epithelial Cells of Lactating Women with Persistent Low Milk Production. Curr. Dev. Nutr. 2021, 5, 720. [Google Scholar] [CrossRef]
- Flores-Quijano, M.E.; Pérez-Nieves, V.; Sámano, R.; Chico-Barba, G. Gestational Diabetes Mellitus, Breastfeeding, and Progression to Type 2 Diabetes: Why Is It So Hard to Achieve the Protective Benefits of Breastfeeding? A Narrative Review. Nutrients 2024, 16, 4346. [Google Scholar] [CrossRef]
- Rosa, S.C.d.S.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef]
- Wunderlich, C.M.; Hövelmeyer, N.; Wunderlich, F.T. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAK-STAT 2013, 2, e23878. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Cannata, D.; Lann, D.; Wu, Y.; Elis, S.; Sun, H.; Yakar, S.; Lazzarino, D.A.; Wood, T.L.; LeRoith, D. Elevated Circulating IGF-I Promotes Mammary Gland Development and Proliferation. Endocrinology 2010, 151, 5751–5761. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lee, E.J.; Kim, K.R.; Cha, B.S.; Song, Y.D.; Lim, S.K.; Lee, H.C.; Huh, K.B. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int. J. Obes. 1997, 21, 355–359. [Google Scholar] [CrossRef]
- Sun, Z.; Shushanov, S.; LeRoith, D.; Wood, T.L. Decreased IGF type 1 receptor signaling in mammary epithelium during pregnancy leads to reduced proliferation, alveolar differentiation, and expression of Insulin Receptor Substrate (IRS)-1 and IRS-2. Endocrinology 2011, 152, 3233–3245. [Google Scholar] [CrossRef]
- Dieterich, C.M.; McKenzie, S.A.; Devine, C.M.; Thornburg, L.L.; Rasmussen, K.M. Obese women experience multiple challenges with breastfeeding that are either unique or exacerbated by their obesity: Discoveries from a longitudinal, qualitative study. Matern. Child Nutr. 2017, 13, e12344. [Google Scholar] [CrossRef]
- Bever Babendure, J.; Reifsnider, E.; Mendias, E.; Moramarco, M.W.; Davila, Y.R. Reduced breastfeeding rates among obese mothers: A review of contributing factors, clinical considerations and future directions. Int. Breastfeed. J. 2015, 10, 21. [Google Scholar] [CrossRef]
- Mehta, U.J.; Siega-Riz, A.M.; Herring, A.H.; Adair, L.S.; Bentley, M.E. Maternal obesity, psychological factors, and breastfeeding initiation. Breastfeed. Med. 2011, 6, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ziomkiewicz, A.; Babiszewska, M.; Apanasewicz, A.; Piosek, M.; Wychowaniec, P.; Cierniak, A.; Barbarska, O.; Szołtysik, M.; Danel, D.; Wichary, S. Psychosocial stress and cortisol stress reactivity predict breast milk composition. Sci. Rep. 2021, 11, 11576. [Google Scholar] [CrossRef]
- Matyas, M.; Apanasewicz, A.; Krzystek-Korpacka, M.; Jamrozik, N.; Cierniak, A.; Babiszewska-Aksamit, M.; Ziomkiewicz, A. The association between maternal stress and human milk concentrations of cortisol and prolactin. Sci. Rep. 2024, 14, 28115. [Google Scholar] [CrossRef] [PubMed]
- Gussler, J.; Arensberg, M.B. Impact of maternal obesity on pregnancy and lactation: The health care challenge. Nutr. Today 2011, 46, 6–11. [Google Scholar] [CrossRef]
- Chapman, D.J.; Morel, K.; Bermúdez-Millán, A.; Young, S.; Damio, G.; Pérez-Escamilla, R. Breastfeeding education and support trial for overweight and obese women: A randomized trial. Pediatrics 2013, 131, e162–e170. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kelleher, S.L. Biological underpinnings of breastfeeding challenges: The role of genetics, diet, and environment on lactation physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Muñoz, Y.; Ortiz, M.; Maliqueo, M.; Chouinard-Watkins, R.; Valenzuela, R. Impact of Maternal Obesity on the Metabolism and Bioavailability of Polyunsaturated Fatty Acids during Pregnancy and Breastfeeding. Nutrients 2020, 13, 19. [Google Scholar] [CrossRef]
| Authors | Type of Study | Country | Groups of Study | N | Conclusions | Year | Data Collection | Comparison | Findings (OR, AOR, HR, Mean, %) | CI/SD | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [12] | Cross-sectional study | Mexico | Normal Weight | 863 | Higher BMI was associated with greater body image dissatisfaction, which in turn was associated with lower likelihood of currently breastfeeding or having breastfed. | 2018 | Survey (AOR of the associations between breastfeeding and maternal weight status) | ||||
| Overweight | 926 | Overweight (vs. normal weight) | 1.01 | 95%CI: 0.62–1.62 | n.s. | ||||||
| Obesity | 633 | Obese (vs. normal weight) | 0.60 | 0.38–0.96 | <0.05 | ||||||
| Total | 2422 | ||||||||||
| [13] | Randomized trial | UK | Obesity I (BMI 30.0–34.9 kg/m2) | 354 | The length of exclusive breastfeeding tended to be shorter with higher BMI. Breastfeeding-related changes in body size and shape were not noticeable among women of Black ethnicity | 2024 | Survey (difference in mean duration of breastfeeding in days) | Obesity II vs. I | −15.8 | 95%CI: −28.5, −3.1 | <0.05 |
| Obesity II (BMI 35.0–39.9 kg/m2) | 225 | ||||||||||
| Obesity III (BMI ≥ 40.0 kg/m2) | 136 | Obesity III vs. I | −16.7 | −32.0, −1.35 | <0.05 | ||||||
| Total | 715 | ||||||||||
| [14] | Retrospective cohort study | USA | Gestational weight gain over recommended | 117 | In women with class III obesity, excessive gestational weight gain did not affect the rates of exclusive breastfeeding at discharge or during the postpartum visit | 2022 | Survey (% exclusive breastfeeding at discharge in obesity class III) | At discharge (weight gain as vs. over recommended) | 66.7 vs. 70.9 | n.s. | |
| Gestational weight gain as recommended | 177 | At postpartum visit (weight gain as vs. over recommended) | 40.1 vs. 34.2 | n.s. | |||||||
| Total | 294 | ||||||||||
| [15] | Cross-sectional study | Australia | Underweight | 372 | Mothers with obesity breastfed for a significantly shorter duration compared to mothers without obesity. This difference persisted even after accounting for factors like maternal smoking, age, and other sociodemographic variables | 2000 | Survey (AOR of stopping breastfeeding according to BMI) | <25 kg/m2 | 1 | ||
| Normal weight | 1184 | ||||||||||
| Overweight | 490 | 25–30 kg/m2 | 1.15 | 95%CI: 1.01–1.31 | <0.05 | ||||||
| Obesity | 254 | >30 kg/m2 | 1.36 | 1.15–1.61 | <0.05 | ||||||
| Not known/stated | 312 | ||||||||||
| Total | 2612 | ||||||||||
| [16] | Cross-sectional study | Australia | Normal weight | 1567 | Overweight women had similar breastfeeding cessation rates in the first week and up to 6 months, whereas obese women were more likely to stop breastfeeding in the first week than later | 2008 | Survey (AOR of ceased lactation at first week vs. 6 months) | Normal weight | 1 | ||
| Overweight | 890 | Overweight | 1.15 | 95%CI: 1.01–1.31 | <0.05 | ||||||
| Obesity | 618 | Obesity | 1.36 | 1.15–1.61 | <0.05 | ||||||
| Total | 3075 | ||||||||||
| [17] | Cross-sectional study | Australia | Normal Weight | 108 | High maternal BMI has a significant negative effect on breastfeeding success during the early postnatal period | 2021 | Survey (OR of breastfeeding at 8 weeks) | Overweight vs. normal BMI | 1.48 | 95%CI: 0.79–2.78 | n.s. |
| Overweight | 103 | ||||||||||
| Obesity | 76 | Obesity vs. normal BMI | 3.97 | 2.08–7.55 | <0.05 | ||||||
| Morbid Obesity | 10 | ||||||||||
| Total | 297 | ||||||||||
| [18] | Case–control study | Germany | Normal Weight | 70 | A smaller proportion of mothers with obesity breastfed compared to normal-weight mothers, and their breastfeeding duration was significantly shorter-even after accounting for education and family income | 2016 | Survey (mean breastfeeding obesity vs. normal weight) | Breastfeeding practice (months) | 5.8 vs. 8.8 | SD: 4.5 vs. 5 | <0.05 |
| Obesity | 80 | Exclusive breastfeeding duration (months) | 3,9 vs. 5,1 | 2.5 vs. 2.1 | <0.05 | ||||||
| Total | 150 | ||||||||||
| [19] | Cross-sectional study | USA | Never obesity | 110 | Individuals who developed excess adiposity before or during puberty were 1.6 times more likely to have exclusive breastfeeding failure (less than 6 months) compared to those whose excess adiposity began after puberty | 2019 | Survey (exclusive breastfeeding < 6 moths) | Excess adiposity onset before or during puberty vs. after puberty | 1.58 | <0.05 | |
| Obesity after puberty | 991 | ||||||||||
| Obesity before/during puberty | 469 | ||||||||||
| Total | 1570 | ||||||||||
| [20] | Prospective cohort study | China | Underweight | 619 | Women with higher gestational weight gain throughout pregnancy are more likely to suffer from delayed onset of lactation in Chinese population | 2019 | Survey (AOR of maternal perception of noticeable breast fullness after 72 h postpartum among gestational weight gain quartiles) | Quartile 1 | 1 | ||
| Normal Weight | 2296 | Quartile 2 | 1.20 | 95%CI: 0.91–1.57 | n.s. | ||||||
| Overweight | 367 | Quartile 3 | 1.47 | 1.13–1.92 | <0.05 | ||||||
| Total | 3282 | Quartile 4 | 1.42 | 1.08–1.86 | <0.05 | ||||||
| [21] | Cross-sectional study | USA | Normal/underweight | 854 | Two practices: holding their babies skin-to-skin immediately after birth and being encouraged to breastfeed on demand were more strongly linked to exclusive breastfeeding in mothers with obesity than in other mothers. However, mothers with obesity reported practicing skin-to-skin contact significantly less frequently than others | 2019 | Survey (AOR of exclusive breastfeeding at 1 week and 3 months on skin-to-skin practice) | Normal/underweight | 1.07 and 0.87 | 95%CI: 0.76–1.51 and 0.62–1.21 | n.s. |
| Overweight | 378 | Overweight | 1.62 and 1.86 | 0.97–2.71 and 1.12–3.09 | <0.05 | ||||||
| Obesity | 274 | Obesity | 3.18 and 1.66 | 1.73–5.85 and 0.94–2.94 | ≤0.01 | ||||||
| Total | 1506 | Survey (AOR of exclusive breastfeeding at 1 week and 3 months on being encouraged to breasted on demand practice) | Normal/underweight | 1.16 and 1.08 | 95%CI: 0.80–1.69 and 0.75–1.56 | n.s. | |||||
| Overweight | 1.00 and 0.69 | 0.57–1.77 and 0.39–1.20 | n.s. | ||||||||
| Obesity | 2.32 and 2.29 | 1.09–4.56 and 1.10–4.78 | ≤0.01 | ||||||||
| [22] | Randomized trial | Denmark | Low BMI (<27 kg/m2) | 871 | Mothers with high postpartum BMI were more likely to stop exclusive breastfeeding early and often had socio-demographic, psychosocial, perinatal, and behavioral factors that increase the risk of early breastfeeding cessation compared to other mothers | 2013 | Survey (HR of breastfeeding cessation before 17 weeks postpartum) | Low BMI | 1 | ||
| Moderate (27 ≤ BMI < 32 kg/m2) | 344 | Moderate BMI | 1.16 | 95%CI: 0.84–1.24 | <0.05 | ||||||
| High (≥32 BMI kg/m2) | 160 | High BMI | 1.21 | 0.93–1.57 | <0.05 | ||||||
| Total | 1375 | ||||||||||
| [23] | Randomized trial | USA (African American Women) | Home-based parenting support | 59 | African American women with overweight or obesity who took part in a home-based educational program breastfed at rates higher than the national average for black women | 2018 | Survey (RR of breastfeeding after receiving home-based parenting support with or without) | With breastfeeding education vs. without | 1.05 | 95%CI: 0.86–1.28 | <0.05 |
| Home-based parenting support with additional content on breastfeeding | 59 | ||||||||||
| Total | 118 | ||||||||||
| [24] | Cross-sectional study | USA | Underweight | 213,108 | Overweight and obese non-Hispanic white women and obese non-Hispanic black women were more likely to not start breastfeeding. No link was found for Hispanic or other racial groups. | 2015 | Survey (AOR of breastfeeding cessation according to BMI depending in white, non-Hispanic women) | Underweight | 1.18 | 95%CI: 0.99–1.40 | n.s. |
| Normal Weight | 2,477,498 | Normal Weight | 1 | ||||||||
| Overweight ( | 1,186,841 | Overweight | 1.17 | 1.07–1.29 | <0.05 | ||||||
| Obesity Total | 1,041,886 | Obesity | 1.25 | 1.14–1.37 | <0.05 | ||||||
| 4,919,333 | Survey (COR of breastfeeding cessation according to pre-pregnancy BMI depending in Hispanic women= | Underweight | 1.36 | 95%CI: 0.77–2.39 | n.s | ||||||
| Normal Weight | |||||||||||
| Overweight | 1.00 | 0.77–1.30 | n.s | ||||||||
| Obesity | 1.32 | 1.02–1.71 | <0.05 | ||||||||
| [10] | Case–control study | USA | Timely OL | 588 | Maternal obesity, insulin therapy, and inadequate breastfeeding support in the hospital were major risk factors for delayed lactation onset. | 2014 | Survey (AOR of maternal perception of the onset of lactation) | Overweight | 1.26 | 95%CI: 0.85- 1.85 | n.s |
| Delayed OLs | 295 | Obesity | 1.56 | 1.07–2.29 | <0.05 | ||||||
| Total | 883 | ||||||||||
| [9] | Prospective cohort study | USA | Timing OL non-obesity | 102 | After accounting for prenatal feeding plans, having overweight or obesity were key factors linked to delayed onset of lactation | 2010 | Survey (AOR of reported delayed lactogenesis according to BMI) | Overweight vs. Normal Weight | 1.84 | 95%CI: 1.07–3.16 | <0.05 |
| Timing OL obesity | 34 | Obesity vs. Normal Weight | 2.21 | 1.24–3.94 | <0.05 | ||||||
| Delayed OL non-obesity | 49 | ||||||||||
| Delayed OL-obesity | 33 | ||||||||||
| Total | 218 | ||||||||||
| [25] | Retrospective cohort study | Japan | BMI at discharge: | Compared to women with normal weight, those with obesity were much less likely to successfully start exclusive breastfeeding. Gaining more weight during pregnancy also slightly reduced the chances of exclusive breastfeeding initiation | 2019 | Medical records (AOR of successful exclusive breastfeeding at discharge) | |||||
| Underweight | 404 | Underweight | 1.03 | 95%CI: 0.87–1.23 | n.s. | ||||||
| Normal Weight | 4.443 | Normal Weight | 1.00 | ||||||||
| Overweight | 224 | Overweight | 0.70 | 0.50–0.99 | <0.05 | ||||||
| Obesity | 54 | Obesity | 0.27 | 0.15–0.51 | <0.001 | ||||||
| BMI at 1 month after delivery: | (AOR of successful exclusive breastfeeding 1 month after delivery) | ||||||||||
| Underweight | 1.236 | Underweight | 0.99 | 95%CI: 0.85–1.16 | n.s. | ||||||
| Normal Weight | 3.900 | Normal Weight | 1.00 | ||||||||
| Overweight | 164 | Overweight | 0.81 | 0.56–1.16 | n.s. | ||||||
| Obesity | 48 | Obesity | 0.29 | 0.16–0.53 | <0.001 | ||||||
| Total | 10.473 | ||||||||||
| [26] | Prospective cohort study | Australia | Normal Weight | 1479 | Pre-pregnancy body mass index is linked to shorter breastfeeding duration, with overweight and obesity mothers tending to breastfeed for less time than normal-weight mothers, regardless of their socioeconomic or demographic background | 2006 | Survey (HR of breastfeeding duration with overweight or obesity) | Pre-pregnancy overweight or obesity vs. normal weight | 1.18 | 95%CI: 1.05–1.34 | <0.05 |
| Overweight | 211 | ||||||||||
| Obesity | 113 | ||||||||||
| Total | 1803 | ||||||||||
| [27] | Retrospective cohort study | Canada | Normal weight | 5685 | Women with obesity were less likely to breastfeed compared to those with normal weight, and obesity independently increased the risk of not breastfeeding at hospital discharge | 2016 | Database (AOR of breastfeeding at discharge) | Obesity vs. normal weight | 0.63 | 95%CI: 0.55–0.71 | <0.001 |
| Obesity | 3343 | ||||||||||
| Total | 9028 | ||||||||||
| [28] | Prospective cohort study | China | Underweight | 605 | In Chinese women, pre-pregnancy obesity raises the risk of delayed onset of milk production and early discontinuation of breastfeeding | 2017 | Survey (RR of delayed of maternal perception of breast fullness) | Underweight | 0.84 | 95%CI: 0.58–1.22 | n.s. |
| Normal Weight | 2209 | Overweight | 1.38 | 0.90–2.12 | n.s. | ||||||
| Overweight | 300 | Obesity | 1.89 | 1.04–3.4 | <0.05 | ||||||
| Obesity | 82 | ||||||||||
| Total | 3196 | ||||||||||
| [29] | Retrospective cohort study | Canada | Underweight | 337 | Maternal obesity was associated with a two-fold rate of non-initiation of breastfeeding. | 2015 | Survey (RR of non-initiation of breastfeeding) | Underweight | 0.97 | 95%CI: 0.75–1.25 | n.s. |
| Normal weight | 4105 | Normal weight | 1.00 | ||||||||
| Overweight | 1317 | Overweight | 1.07 | 0.92–1.24 | n.s. | ||||||
| Obesity | 833 | Obesity | 1.22 | 1.04–1.42 | <0.05 | ||||||
| Total | 6592 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Casado, G.; Saldaña-Garcia, N.; Gonzalez-Mesa, E.; Ortega-Gomez, A. Etiology of Delayed Lactogenesis in Obesity. Biomedicines 2025, 13, 1848. https://doi.org/10.3390/biomedicines13081848
Gomez-Casado G, Saldaña-Garcia N, Gonzalez-Mesa E, Ortega-Gomez A. Etiology of Delayed Lactogenesis in Obesity. Biomedicines. 2025; 13(8):1848. https://doi.org/10.3390/biomedicines13081848
Chicago/Turabian StyleGomez-Casado, Gema, Natalia Saldaña-Garcia, Ernesto Gonzalez-Mesa, and Almudena Ortega-Gomez. 2025. "Etiology of Delayed Lactogenesis in Obesity" Biomedicines 13, no. 8: 1848. https://doi.org/10.3390/biomedicines13081848
APA StyleGomez-Casado, G., Saldaña-Garcia, N., Gonzalez-Mesa, E., & Ortega-Gomez, A. (2025). Etiology of Delayed Lactogenesis in Obesity. Biomedicines, 13(8), 1848. https://doi.org/10.3390/biomedicines13081848







