Abstract
Obesity is a multifactorial condition that influences metabolic, endocrine, inflammatory, circadian, and behavioral systems. These disruptions can adversely affect the initiation of lactogenesis II—the critical process marking the onset of copious milk secretion following childbirth. In mothers with obesity, prolonged inflammation within the mammary gland, a blunted hormonal response (notably of prolactin), altered progesterone and estrogen dynamics, high leptin levels, and misaligned circadian rhythms contribute significantly to delayed lactogenesis. In addition, mechanical difficulties and psychological factors further hinder effective breastfeeding. This report synthesizes evidence from human epidemiological studies and animal models that elucidate the diverse mechanisms linking maternal obesity to delayed lactogenesis. We review the role of obesity-associated inflammatory mediators in impairing mammary tissue remodeling, the endocrine aberrations that impair lactogenic signaling, the consequences of circadian disruption on hormonal rhythmicity, and the behavioral influences that challenge effective breastfeeding. Finally, we discuss the clinical implications of these findings and propose future research directions targeting endocrine modulation, anti-inflammatory therapy, circadian interventions, and enhanced lactation support strategies for mothers with obesity.
1. Introduction
Breastmilk is widely recognized as the optimal source of nutrition for newborns, providing a perfectly balanced combination of nutrients essential for healthy growth and development during the early stages of life. Beyond its unparalleled nutritional value, breastmilk offers powerful immunological benefits that help protect infants against a wide range of infectious and chronic diseases. These include gastrointestinal and respiratory infections, ear infections, and a reduced risk of conditions such as asthma, allergies, and obesity later in life [1].
Due to its numerous health advantages, the World Health Organization (WHO) strongly recommends exclusive breastfeeding for the first six months of a child’s life. During this period, infants should receive only breastmilk, without any additional food or drink—not even water. This practice supports optimal physical and cognitive development while strengthening the bond between mother and baby. After the initial six months, the WHO advises the gradual introduction of complementary foods—nutritious solid or semi-solid foods appropriate for the infant’s age—while continuing to breastfeed. This combined feeding approach is encouraged up to two years of age or beyond, as it continues to contribute significantly to the child’s health, development, and immune resilience during the critical early years of life [2].
Currently, around 650 million adults and approximately 340 million children and adolescents aged 5 to 19 are affected by obesity. The condition tends to be more common among women and older individuals compared to men and younger populations [3]. Obesity in adults significantly exacerbates the four most prevalent non-communicable diseases: cardiovascular disease, type 2 diabetes, various forms of cancer, and chronic respiratory diseases. Excess body fat contributes to increased inflammation, insulin resistance, and hormonal imbalances, all of which are underlying mechanisms that worsen these chronic conditions [4]. Obesity affects a significant proportion of women of reproductive age worldwide, with prevalence estimates ranging from 8% to over 40%, depending on geographic region and population studied. In the United States, nearly 40% of women aged 20–39 years are classified as obese [5], while European rates range from 8% to 26% among women aged 18–44 years [6]. These figures underscore the public health importance of understanding obesity’s implications for reproductive outcomes, including lactation.
A well-documented consequence of maternal obesity is delayed lactogenesis II (DLII), defined as the initiation of copious milk secretion occurring later than 72 h post-delivery [7]. The initiation of lactogenesis is not only essential for immediate neonatal nutrition but also plays a critical role in the long-term health of both mother and child. Mothers with obesity are at an increased risk of encountering complications such as gestational diabetes, hypertensive disorders, and cesarean delivery—all of which further compromise lactation outcomes [8].
Age has been described as an independent risk factor in delayed lactogenesis. Older mothers with obesity are more likely to experience delayed onset of copious milk secretion compared to their younger or normal-weight counterparts, highlighting an age-related vulnerability in lactation physiology [9,10]. Age-related changes in endocrine function, such as altered prolactin secretion and increased insulin resistance, may exacerbate the metabolic and inflammatory disturbances associated with obesity, further impairing milk production [11].
Extensive clinical research has consistently reported that maternal obesity is associated with a delayed onset of lactogenesis II (OL) (Table 1). However, the underlying mechanisms remain relatively underexplored. The etiology of DLII in mothers with obesity is multifactorial. Recent research implicates systemic and localized inflammation, endocrine and metabolic dysregulation, circadian misalignment, and behavioral barriers as interrelated mechanisms that delay the transition from colostrum production to the establishment of a copious milk supply. This report examines the current state of knowledge regarding the impact of obesity on lactogenesis with a particular emphasis on the inflammatory pathways activated by excess adiposity, the hormonal disturbances that result from both altered adipose endocrine function and the persistence of progesterone, the disruption of circadian hormonal rhythms, molecular mechanisms, and the behavioral challenges inherent in breastfeeding among mothers with obesity.

Table 1.
Overview of studies investigating obesity in relation to breastfeeding.
2. Inflammatory Mechanisms in Obesity-Related Delayed Lactogenesis
Obesity is characterized by an expansion of adipose tissue that becomes infiltrated by immune cells, notably macrophages, which secrete proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) [30]. This systemic inflammatory state is further compounded by local inflammation within the mammary gland. The hypertrophy of mammary adipocytes leads to increased cell death and the formation of crown-like structures (CLS), where macrophages encircle necrotic adipocytes. Such localized inflammation interferes with the delicate remodeling of mammary tissue necessary for effective lactogenesis (Figure 1) [31]. Animal models have shown that inflammatory mediators not only impair the differentiation of mammary epithelial cells but also disrupt the expression of genes crucial for milk protein synthesis such as α-lactalbumin (milk protein gene important in regulation of milk volume) and β-casein (major milk protein, crucial for the development of the infant’s digestive and immune systems [32]. Furthermore, high-fat diet–induced obesity causes structural alterations in the mammary gland, including reduced alveolar development and parenchymal tissue, factors directly linked to delayed milk production [33].
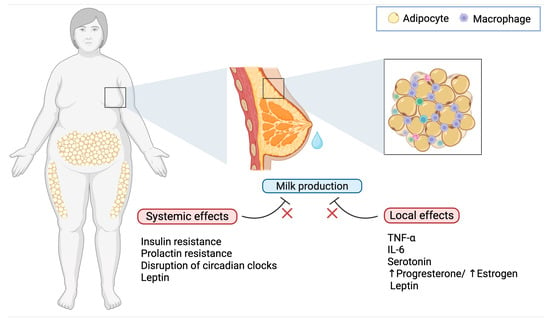
Figure 1.
Effects of obesity on delayed lactogenesis II. Obesity interferes with lactation through both systemic and local mechanisms: while hormonal dysregulation, chronic inflammation, and circadian disruption impair lactogenesis at a systemic level, the mammary microenvironment in mothers with obesity is characterized by elevated levels of TNF-α and IL-6 from adipose tissue-infiltrating macrophages, increased serotonin, an imbalance in progesterone and estrogen, and elevated levels of leptin, all of which contribute to impaired milk production.
Besides the overproduction of cytokines such as IL-6 and TNF-α within the inflamed mammary microenvironment, which has deleterious effects on mammary gland function, obesity is linked to increased synthesis of serotonin in the mammary gland by upregulating tryptophan hydroxylase 1 (TPH1). Elevated intramammary serotonin, via its receptor signaling, may mimic signals of involution and inhibit secretory function, thereby delaying the onset of lactogenesis II [32]. These findings from animal studies suggest that inflammatory processes triggered by obesity are a critical intermediary mechanism behind the perturbation of mammary gland structure and function, ultimately leading to delayed lactogenesis [33].
3. Hormonal and Endocrine Dysregulation
3.1. Blunted Prolactin Response
Prolactin is the key hormone that drives lactogenesis. In a normally functioning lactogenic system, the act of infant suckling elicits a rapid and robust release of prolactin, which in turn stimulates milk synthesis. However, multiple studies have indicated that mothers with obesity exhibit a provoked lower prolactin response to suckling in the early postpartum period [34]. Both clinical observations and animal models have provided evidence that obesity induces central and peripheral prolactin resistance. In obese murine models, high basal levels of phosphorylated STAT5 (a downstream mediator of prolactin receptor signaling) are noted, yet the acute stimulatory response to prolactin is markedly attenuated [33]. This hormonal insensitivity compromises the induction of lactogenesis II, resulting in a delay in the onset of copious milk production (Figure 2).
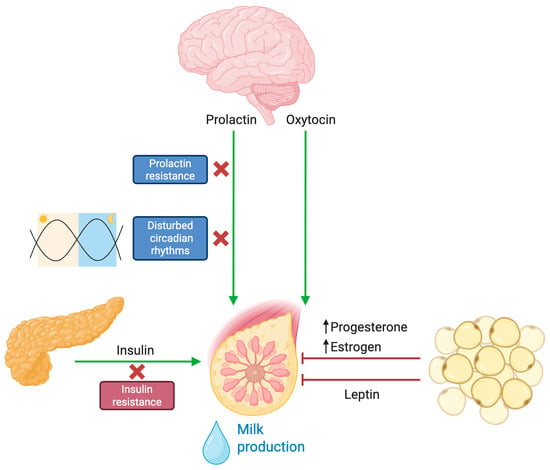
Figure 2.
Hormonal and endocrine dysregulation of lactogenesis in obesity. Under physiological conditions, infant suckling activates sensory pathways that stimulate the hypothalamus to release prolactin and oxytocin. These hormones are essential for initiating milk synthesis (prolactin) and milk ejection (oxytocin) from the mammary glands. In the context of obesity, multiple disruptions impair this finely tuned process. Prolactin signaling and insulin sensitivity are reduced, hindering the initiation and maintenance of adequate milk output. Additionally, altered circadian rhythms associated with obesity lead to hormonal imbalances that negatively affect lactation. Elevated leptin levels—commonly found in obesity—have been implicated in limiting the mammary gland’s ability to sustain robust lactation. Furthermore, excess adipose tissue acts as a hormonal reservoir for progesterone and estrogen, delaying the necessary postpartum decline in these hormones that is crucial for a successful onset of milk secretion.
3.2. Progesterone Dynamics and Local Estrogen Production
A critical trigger for lactogenesis II is the gradual withdrawal of progesterone following parturition. In normal physiology, the removal of the placental source of progesterone relieves its inhibitory effect on lactogenesis. In women with obesity, however, excess adipose tissue serves as a reservoir for progesterone, resulting in prolonged exposure to the hormone after delivery [34,35,36]. This persistence of progesterone delays the activation of the secretory apparatus within the mammary gland. In addition to progesterone issues, obesity increases the aromatization of androgens into estrogen within adipose tissue. The consequent rise in local estrogen is believed to antagonize prolactin signaling and downregulate genes necessary for milk synthesis [34,37]. These endocrine alterations—marked by both sustained progesterone levels and enhanced estrogenic activity—create an unfavorable hormonal milieu that postpones lactogenic transition, thereby delaying the onset of copious milk secretion [38].
3.3. Insulin Resistance
Insulin is recognized as an important regulator of mammary epithelial cell function and lactation. In women with obesity, the state of insulin resistance and hyperinsulinemia disrupts the normal metabolic reprogramming necessary for milk synthesis. This metabolic impairment hinders the delivery of glucose and other substrates that are vital for the synthetic machinery of milk production [39]. Moreover, the increased prevalence of gestational diabetes among mothers with obesity further exacerbates these impairments by altering insulin signaling cascades within the mammary tissue [40]. Consequently, the metabolic dysregulation intrinsic to obesity results in reduced availability of essential nutrients for lactogenesis, further compounding the delay in milk secretion.
3.4. Lepin and the Control of Lactogensis
Leptin, a hormone primarily known for its role in energy homeostasis, has emerged as a key factor in mammary gland physiology and lactogenesis. Adipose tissue is the main source of leptin production, and this includes adipose deposits within the mammary gland. During pregnancy, leptin acts similarly to a growth hormone, with maternal levels declining after birth [41]. Experimental evidence in mouse models indicates that while leptin mRNA is clearly present in the mammary epithelium, its production is downregulated during lactogenesis, suggesting that high local leptin levels might be counterproductive for maximal milk protein synthesis [42]. Leptin hinders milk ejection in part by directly targeting myoepithelial cells, which contract in response to oxytocin to help expel milk [33]. Women with obesity tend to have elevated leptin levels and may exhibit leptin resistance. Studies have shown that leptin concentrations remain significantly higher in obese women during lactation, specifically at 48 h and 7 days postpartum [34]. Consequently, high maternal leptin levels have been associated with delayed onset of lactogenesis II and attenuated milk ejection, likely because of leptin’s inhibitory effects on oxytocin-induced myometrial contractions [38].
4. Circadian Disruption and Its Impact on Lactogenesis
Circadian rhythms, regulated by the central clock in the suprachiasmatic nucleus of the hypothalamus, govern the periodic secretion of hormones and maintain metabolic homeostasis. In the context of lactation, these biological rhythms ensure that hormones such as prolactin, cortisol, and insulin are released in a coordinated manner to optimize milk production [43]. Peripheral clocks within the mammary gland further modulate local gene expression, aligning the gland’s functional activity with the body’s overall circadian schedule.
Studies in mice show that disrupting circadian clock genes, particularly through the Clock-Δ19 mutation, impairs lactation competence without significantly affecting fetal development [44]. Mutant dams exhibited abnormal nursing behavior, disrupted prolactin rhythms, reduced mammary gland development, and lower offspring survival. These effects were linked to peripheral rather than central clock dysfunction. Further experiments revealed that reducing CLOCK protein in mammary cells decreased the expression of genes involved in milk synthesis, indicating that proper circadian function is crucial for successful lactation, mammary differentiation, and milk production [45,46].
Obesity has been shown to disrupt normal circadian rhythms, primarily through behavioral factors such as irregular sleep patterns, exposure to artificial light at night, and altered meal timings. Such circadian misalignment blunts the nocturnal rise in melatonin and disturbs cortisol rhythms, which in turn have downstream effects on metabolic and reproductive hormone secretion [47]. These disrupted hormonal patterns hinder the normal pulsatile release of prolactin in response to suckling, thereby delaying lactogenesis [43]. The difficulty in synchronizing the timing of lactogenic signals due to circadian disturbance may add a layer of complexity to the endocrine challenges faced by mothers with obesity. While further studies in humans are needed to characterize the potential circadian rhythm disruption caused by obesity in lactating women, and current statements remain conjectural, this aspect could represent another piece of the puzzle in the etiology of delayed lactogenesis.
5. Mechanisms Underlying Impaired Lactogenesis in Obesity
Obesity-induced insulin resistance blunts anabolic signaling of the mammary gland. Under normal lactation, insulin acting via Insulin Receptor (INSR)—Insulin Receptor Substrate IRS1/2- Phosphoinositide 3-Kinase (PI3K)—Protein Kinase B (AKT) drives alveolar differentiation and milk synthesis, while prolactin signals via Prolactin Receptor (PRLR)- Janus Kinase 2 (JAK2)—(STAT5) to induce milk-protein genes (e.g., CSN2 for β-casein) [48,49]. In obese, insulin-resistant women, mammary insulin signaling is suppressed, e.g., IRS2 mRNA was ~2.3-fold lower in lactating obese vs. lean women [50]. Correspondingly, downstream anabolic genes are downregulated as follows: lactogenic genes ACACB (fatty acid synthesis), UGP2 (lactose synthesis), and β-casein (CSN2) are 3.1, 2.7, and 2.1 fold decreased. Notably, genes in the PRLR/JAK2/STAT5 pathway were unchanged, suggesting the defect is functional. Indeed, animal studies show obese dams have high basal mammary pSTAT5 but fail to respond to prolactin (true prolactin resistance) [33]. Thus, obesity creates an “insulin-resistant” gene signature in mammary without gross loss of PRL receptor, implicating downstream or cross-talk inhibition of STAT5 (Figure 3).
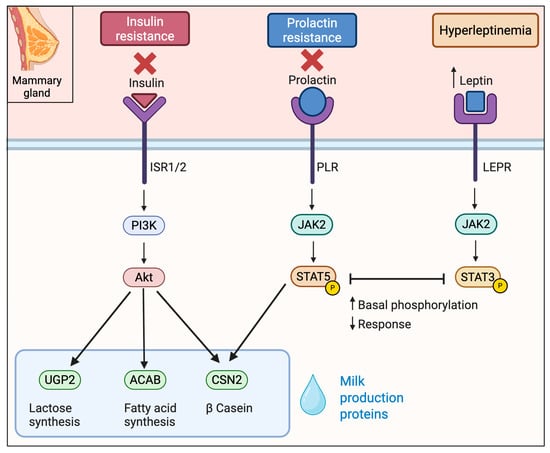
Figure 3.
Key signaling cascades in mammary gland affected by obesity in lactogenesis. Obesity promotes insulin and prolactin resistance and is characterized by elevated leptin levels. Insulin signaling through the PI3K–AKT pathway, essential for milk protein synthesis, is impaired. Simultaneously, high basal phosphorylation of STAT5 downstream of the prolactin receptor leads to a blunted prolactin response. Elevated leptin engages the JAK2–STAT3 pathway preventing further STAT5 activation and contributing to delayed lactogenesis.
Dysregulated adipose-derived signals in obesity impair lactogenic signaling via several pathways. In obesity, leptin is chronically elevated. Mammary epithelial cells express the long leptin receptor (LEPR), especially on myoepithelial cells. High leptin signaling activates JAK2-STAT3 pathways that antagonize prolactin/STAT5. For example, in obese mice leptin produced high basal mammary pSTAT5 and prevented further STAT5 activation by prolactin [33]. In humans, when maternal leptin levels are elevated, there is a diminished PRL output—obese mothers have a blunted prolactin response to suckling and lower milk volume with higher leptin [51] (Figure 3).
In obesity, skeletal muscle becomes insulin resistant with decreased IRS1 and Glucose Transporter protein type 4 (GLUT4), contributing to systemic hyperinsulinemia [52]. Muscle also secretes myokines, such as IL-6, which have been implicated in contributing to chronic JAK-STAT3-mediated insulin resistance under obese conditions [53].
Hepatic insulin resistance in obesity leads to elevated gluconeogenesis and dysregulated insulin growth factor/growth hormone (IGF/GH) signaling [54]. The liver is the main source of IGF-1 and binding proteins (IGFBPs) that support mammary development [55].
In obesity, altered IGF-1/IGFBP levels may impair IGF-1R-IRS/PI3K signaling in mammary tissue, compromising the production of milk [56,57].
6. Behavioral Influences and Clinical Implications
6.1. Mechanical and Ergonomic Challenges
In addition to the biochemical impairments, mothers with obesity frequently encounter behavioral and mechanical challenges during breastfeeding. Physical attributes associated with obesity, such as increased breast size and postpartum edema, can interfere with proper infant latch and positioning [58]. These mechanical difficulties can lead to ineffective milk removal, which prevents the necessary feedback loop that stimulates prolactin release. Research indicates that when mothers experience poor latch or insufficient suction, the reduced stimulation further blunts the hormonal signals required for lactogenesis, thereby exacerbating delayed milk production [59].
6.2. Phycological and Sociocultural Barriers
Obesity is also frequently accompanied by psychological stresses, including lower self-efficacy and body image concerns. These factors can have a profound impact on a mother’s confidence and willingness to initiate and continue breastfeeding [60]. The stigma associated with obesity may deter some mothers from seeking the specialized lactation support they require, further deepening the challenges associated with delayed lactogenesis. In addition, the psychological stress imposed by a negative body image may induce further endocrine disturbances, including elevated cortisol levels, which are known to interfere with lactogenic hormonal cascades and breastmilk composition [61,62].
6.3. Clinical Practice and Intervention Strategies
The clinical implications of obesity-induced delayed lactogenesis are significant. Mothers with obesity are more prone to obstetric complications such as cesarean delivery, gestational diabetes, and hypertensive disorders, all of which independently contribute to delays in lactogenesis [8,63]. In a clinical setting, early identification of at-risk mothers is essential. Interventions such as proactive lactation consultation, specialized breastfeeding education, and the utilization of mechanical aids to improve infant latching can mitigate some of the challenges posed by obesity [64]. The incorporation of multidisciplinary care, including endocrinologists, nutritionists, and mental health professionals, is also critical in addressing both the physiological and behavioral dimensions of delayed lactogenesis.
7. Discussion
The evidence presented in this report underscores that delayed lactogenesis in mothers with obesity is the product of a complex interplay among inflammatory, hormonal, circadian, and behavioral factors. Inflammation provoked by excess adipose tissue compromises mammary gland architecture and function by interfering with epithelial cell differentiation and reducing the expression of key milk synthesis genes [32,33]. At the same time, obesity dampens the prolactin response to suckling—a pivotal hormonal signal for lactogenesis—which is compounded by prolonged progesterone exposure and increased local estrogen production due to enhanced aromatization in adipose tissue [35,65]. Metabolic disturbances, including insulin resistance commonly seen in mothers with obesity, contribute further by limiting the availability of energy substrates necessary for milk production. In this hormonal balance, elevated leptin levels contribute to reduced milk secretion by counteracting the effects of oxytocin. Circadian misalignment appears as another significant factor; the disruption of the normal hormonal oscillations—particularly that of prolactin—impairs the synchronized activation of lactogenic processes [66]. Furthermore, behavioral influences—ranging from mechanical challenges in latch and infant positioning to psychosocial stress and lower self-esteem—create a self-reinforcing cycle that exacerbates lactational insufficiency. Ineffective breastfeeding practices reduce the suckling-induced prolactin surge, thereby further delaying milk production [59]. Importantly, these diverse factors do not operate in isolation; rather, they interact synergistically. For instance, inflammation may influence circadian clock gene expression, while endocrine imbalances may heighten psychological stress.
Despite the evidence identified between obesity and delayed lactogenesis, it is important to consider the concept of metabolically healthy obesity. A substantial proportion of mothers with obesity are able to initiate and sustain successful breastfeeding without requiring additional support. This highlights the heterogeneity within the population of women with obesity and suggests that not all individuals are equally affected by the metabolic and hormonal disruptions often linked to delayed lactogenesis.
While the primary factors contributing to the association between obesity and delayed lactogenesis—such as metabolic disturbances, hormonal imbalances, inflammation, and behavioral challenges—have been increasingly elucidated, further research is essential to fully complete the picture. Notably, there remains significant variability in how delayed lactogenesis is defined and measured across studies, as highlighted in Table 1, underscoring the urgent need for standardized diagnostic criteria and assessment practices. Moreover, current evidence often fails to distinguish between metabolically healthy obesity and obesity accompanied by metabolic dysfunction. The physiological differences between these phenotypes, and how they might differentially impact lactogenesis, remain largely unknown and represent a critical gap in our understanding that warrants future investigation.
8. Future Directions
Given the multifaceted nature of obesity-induced delayed lactogenesis, future research should adopt a multidisciplinary approach that encompasses molecular, physiological, and behavioral dimensions. Key areas for future investigation should include longitudinal studies that follow mothers with obesity from the prenatal period through postpartum lactation to fully characterize the interplay between metabolic, inflammatory, endocrine, and behavioral factors. By addressing these research directions, it will be possible to develop targeted therapeutic strategies that mitigate the adverse impacts of obesity on lactogenesis. Such approaches are essential not only for improving immediate breastfeeding outcomes but also for interrupting the intergenerational cycle of obesity and metabolic dysfunction.
Addressing delayed lactogenesis in mothers with obesity requires a comprehensive, multidisciplinary approach that targets the multifactorial pathways implicated in this condition. Therapeutic strategies should focus on mitigating systemic and local inflammation through dietary modifications rich in anti-inflammatory nutrients. Optimizing metabolic health prior to and during pregnancy—through interventions aimed at improving insulin sensitivity and controlling gestational diabetes—can help restore the anabolic signaling pathways essential for mammary gland function. Hormonal imbalances, including prolonged progesterone exposure and elevated leptin levels, might be managed through carefully timed hormonal therapies or lifestyle changes that support endocrine regulation. Behavioral and mechanical challenges can be alleviated by providing specialized lactation support, including expert breastfeeding education, ergonomic guidance to improve infant latch, and the use of assistive devices. Additionally, circadian rhythm stabilization through sleep hygiene interventions and controlled light exposure may enhance the coordinated release of lactogenic hormones such as prolactin and cortisol. Psychological support is equally critical to address body image concerns and reduce stress-related endocrine disruption, thereby improving maternal confidence and breastfeeding self-efficacy. Together, these strategies, tailored to individual needs, hold promise for overcoming the barriers posed by obesity and promoting timely onset of copious milk production, ultimately improving breastfeeding success and maternal-infant health outcomes (Figure 4).
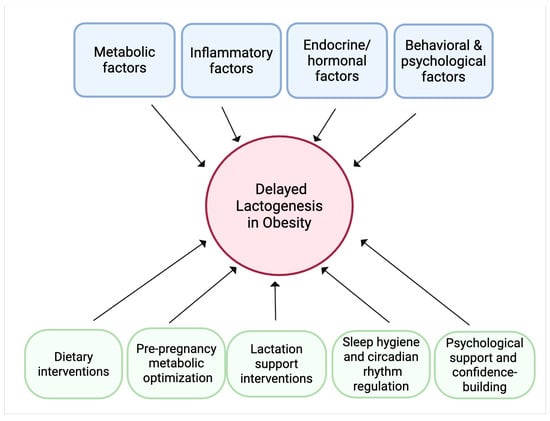
Figure 4.
Conceptual framework of the conclusions and future directions. Obesity can lead to delayed lactogenesis through the interplay of metabolic, inflammatory, endocrine/hormonal, and behavioral and psychological factors. Potential therapeutic strategies to mitigate these effects encompass dietary modifications rich in anti-inflammatory nutrients, metabolic optimization before and during pregnancy, hormonal therapies or lifestyle changes to support endocrine balance, sleep hygiene and circadian rhythm regulation, specialized lactation support, and psychological interventions to improve maternal confidence and reduce stress.
9. Conclusions
Obesity is associated with potential challenges in initiating lactogenesis due to multiple interconnected pathways. The chronic, low-grade inflammatory state associated with obesity disrupts the normal remodeling of the mammary gland, while hormonal dysregulation—manifested as a blunted prolactin response, prolonged progesterone presence, increased local estrogen production, and metabolic disturbances due to insulin resistance—further impairs the onset of copious milk production. In addition, obesity-related circadian misalignment contributes to the disruption of the timing of lactogenic hormonal release, and mechanical as well as psychosocial barriers reduce effective breastfeeding practices. Together, these factors culminate in a significant delay in lactogenesis II, undermining successful breastfeeding outcomes and potentially influencing long-term metabolic and developmental trajectories in the offspring and the mother.
Funding
This study was supported by research project PI22/01813 funded by the Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union, by Research Project ProyExcel_00962, from Consejería de Universidad, Investigación e Innovación de Andalucía to A.O-G. G.G-C. is supported by PFIS contract (FI23/00104), and A.O-G holds a Miguel Servet position (CP20/0060), both from ISCIII and co-funded by the European Union.
Acknowledgments
Figure 1, Figure 2, Figure 3 and Figure 4 Created in Biorender. Ortega, A. (2026). https://BioRender.com/jnw6at3, https://BioRender.com/2jm4682, https://BioRender.com/u8rxwea, https://BioRender.com/gbug8o5.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DLII | Delayed Lactogenesis II |
| OL | Onset of Lactogenesis |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| CLS | Crown-Like Structures |
| TPH1 | Tryptophan Hydroxylase 1 |
| STAT5 | Signal Transducer and Activator of Transcription 5 |
| CLOCK | Circadian Locomotor Output Cycles Kaput |
| INSR | Insulin Receptor |
| IRS1/2 | Insulin Receptor Substrate |
| PI3K | Phosphoinositide 3-Kinase |
| JAK2 | Janus Kinase 2 |
| AKT | Protein Kinase B |
| LEPR | Leptin Receptor |
| GLUT4 | Glucose Transporter Protein Type 4 |
| IGF | Insulin Growth Factor |
| IGFBP | Insulin Growth Factor Binding Protein |
References
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- European Society for Paediatric Gastroenterology, Hepatology & Nutrition(ESPGHAN); European Academy of Paediatrics (EAP); European Society for Paediatric Research (ESPR); European Academy for Allergy and Clinical Immunology(EAACI); Federation of International Societies for Paediatric Gastroenterology, Hepatology & Nutrition (FISPGHAN); Latin American Society for Pediatric Gastroenterology, Hepatology & Nutrition (LASPGHAN); Pan Arab Society for Pediatric Gastroenterology and Nutrition (PASPGHAN); Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology and Nutrition (AAPSGHAN); North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN); World Allergy Organization (WAO); et al. World Health Organization (WHO) guideline on the complementary feeding of infants and young children aged 6−23 months 2023: A multisociety response. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 181–188. [Google Scholar] [CrossRef]
- Sørensen, T.I.A.; Martinez, A.R.; Jørgensen, T.S.H. Epidemiology of Obesity. In From Obesity to Diabetes; Springer: Cham, Switzerland, 2022; Volume 274, pp. 3–27. [Google Scholar] [CrossRef]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult obesity complications: Challenges and clinical impact. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820934955. [Google Scholar] [CrossRef]
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023 Key Findings Data from the National Health and Nutrition Examination Survey. 2021. Available online: https://dx.doi.org/10.15620/cdc/159281 (accessed on 13 July 2025).
- Marques, A.; Peralta, M.; Naia, A.; Loureiro, N.; de Matos, M.G. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur. J. Public Health 2017, 28, 295–300. [Google Scholar] [CrossRef]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired Lactation: Review of Delayed Lactogenesis and Insufficient Lactation. J. Midwifery Women’s Health 2021, 66, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Ballesta-Castillejos, A.; Gomez-Salgado, J.; Rodriguez-Almagro, J.; Ortiz-Esquinas, I.; Hernandez-Martinez, A. Relationship between maternal body mass index with the onset of breastfeeding and its associated problems: An online survey. Int. Breastfeed. J. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A.; Chantry, C.J.; Peerson, J.M.; Cohen, R.J.; Dewey, K.G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr. 2010, 92, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Dewey, K.G.; Quesenberry, C.P.; Gunderson, E.P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am. J. Clin. Nutr. 2014, 99, 115–121. [Google Scholar] [CrossRef]
- Neville, M.C.; Morton, J. Physiology and endocrine changes underlying human lactogenesis II. J. Nutr. 2001, 131, 3005S–3008S. [Google Scholar] [CrossRef] [PubMed]
- Bigman, G.; Wilkinson, A.V.; Homedes, N.; Pérez, A. Body Image Dissatisfaction, Obesity and Their Associations with Breastfeeding in Mexican Women, a Cross-Sectional Study. Matern. Child Health J. 2018, 22, 1815–1825. [Google Scholar] [CrossRef]
- Dalrymple, K.V.; Briley, A.L.; Tydeman, F.A.S.; Seed, P.T.; Singh, C.M.; Flynn, A.C.; White, S.L.; Poston, L.; on behalf of the UPBEAT Consortium. Breastfeeding behaviours in women with obesity; associations with weight retention and the serum metabolome: A secondary analysis of UPBEAT. Int. J. Obes. 2024, 48, 1472–1480. [Google Scholar] [CrossRef]
- Darling, A.J.; Gatta, L.A.; Tucker, A.; Adkins, L.D.; Mitchell, C.; Reiff, E.; Dotters-Katz, S. Gestational weight gain and patterns of breastfeeding among patients with class III obesity. J. Matern. Neonatal Med. 2022, 35, 9851–9856. [Google Scholar] [CrossRef]
- Donath, S.M.; Amir, L.H. Does maternal obesity adversely affect breastfeeding initiation and duration? J. Paediatr. Child Health 2000, 36, 482–486. [Google Scholar] [CrossRef]
- Donath, S.M.; Amir, L.H. Maternal obesity and initiation and duration of breastfeeding: Data from the longitudinal study of Australian children. Matern. Child Nutr. 2008, 4, 163–170. [Google Scholar] [CrossRef]
- Fan, W.Q.; Molinaro, A. Maternal obesity adversely affects early breastfeeding in a multicultural, multi-socioeconomic Melbourne community. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Keitel-Korndörfer, A.; Bergmann, S.; Wendt, V.; von Klitzing, K.; Petroff, D. Breastfeeding in Obese versus Normal-Weight German Mothers of Various Socioeconomic Status. J. Hum. Lact. 2016, 32, 546–550. [Google Scholar] [CrossRef]
- Hawkins, M.A.W.; Colaizzi, J.; Rhoades-Kerswill, S.; Fry, E.D.; Keirns, N.G.; Smith, C.E. Earlier Onset of Maternal Excess Adiposity Associated with Shorter Exclusive Breastfeeding Duration. J. Hum. Lact. 2018, 35, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, X.; Zhang, Y.; Sun, G.; Zhong, C.; Wang, W.; Li, Q.; Li, X.; Yin, H.; Yang, X.; et al. Gestational weight gain is associated with delayed onset of lactogenesis in the TMCHC study: A prospective cohort study. Clin. Nutr. 2018, 38, 2436–2441. [Google Scholar] [CrossRef]
- Kair, L.R.; Nickel, N.C.; Jones, K.; Kornfeind, K.; Sipsma, H.L. Hospital breastfeeding support and exclusive breastfeeding by maternal prepregnancy body mass index. Matern. Child Nutr. 2019, 15, e12783. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, H.; Vaeth, M.; Rasmussen, K.M. Obesity and early cessation of breastfeeding in Denmark. Eur. J. Public Health 2012, 23, 316–322. [Google Scholar] [CrossRef]
- Lewkowitz, A.K.; López, J.D.; Stein, R.I.; Rhoades, J.S.; Schulz, R.C.; Woolfolk, C.L.; Macones, G.A.; Haire-Joshu, D.; Cahill, A.G. Effect of a Home-Based Lifestyle Intervention on Breastfeeding Initiation Among Socioeconomically Disadvantaged African American Women with Overweight or Obesity. Breastfeed. Med. 2018, 13, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Masho, S.W.; Cha, S.; Morris, M.R. Prepregnancy Obesity and Breastfeeding Noninitiation in the United States: An Examination of Racial and Ethnic Differences. Breastfeed. Med. 2015, 10, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Kido, M.; Tanabe, A.; Ando, K. Prepregnancy obesity as a risk factor for exclusive breastfeeding initiation in Japanese women. Nutrition 2019, 62, 93–99. [Google Scholar] [CrossRef]
- Oddy, W.H.; Li, J.; Landsborough, L.; Kendall, G.E.; Henderson, S.; Downie, J. The association of maternal overweight and obesity with breastfeeding duration. J. Pediatr. 2006, 149, 185–191. [Google Scholar] [CrossRef]
- Ramji, N.; Quinlan, J.; Murphy, P.; Crane, J.M. The Impact of Maternal Obesity on Breastfeeding. J. Obstet. Gynaecol. Can. 2016, 38, 703–711. [Google Scholar] [CrossRef]
- Tao, X.-Y.; Huang, K.; Yan, S.-Q.; Zuo, A.-Z.; Tao, R.-W.; Cao, H.; Gu, C.-L.; Tao, F.-B. Pre-pregnancy BMI, gestational weight gain and breast-feeding: A cohort study in China. Public Health Nutr. 2016, 20, 1001–1008. [Google Scholar] [CrossRef]
- Verret-Chalifour, J.; Giguère, Y.; Forest, J.-C.; Croteau, J.; Zhang, P.; Marc, I.; van Wouwe, J. Breastfeeding Initiation: Impact of Obesity in a Large Canadian Perinatal Cohort Study. PLoS ONE 2015, 10, e0117512. [Google Scholar] [CrossRef]
- Gomez-Casado, G.; Jimenez-Gonzalez, A.; Rodriguez-Muñoz, A.; Tinahones, F.J.; González-Mesa, E.; Murri, M.; Ortega-Gomez, A. Neutrophils as indicators of obesity-associated inflammation: A systematic review and meta-analysis. Obes. Rev. 2024, 26, e13868. [Google Scholar] [CrossRef]
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.L.; Grayson, B.E.; Yadav, E.; Seeley, R.J.; Horseman, N.D. High Fat Diet Alters Lactation Outcomes: Possible Involvement of Inflammatory and Serotonergic Pathways. PLoS ONE 2012, 7, e32598. [Google Scholar] [CrossRef]
- Buonfiglio, D.C.; Ramos-Lobo, A.M.; Freitas, V.M.; Zampieri, T.T.; Nagaishi, V.S.; Magalhães, M.; Cipolla-Neto, J.; Cella, N.; Donato, J. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci. Rep. 2016, 6, 22421. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Kjolhede, C.L. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 2004, 113, e465–e471. [Google Scholar] [CrossRef]
- Knight, C.H. An endocrine hypothesis to explain obesity-related lactation insufficiency in breastfeeding mothers. J. Dairy Res. 2020, 87, 78–81. [Google Scholar] [CrossRef]
- Lovelady, C.A. Is Maternal Obesity a Cause of Poor Lactation Performance? Nutr. Rev. 2005, 63, 352–355. [Google Scholar] [CrossRef]
- Garcia, A.H.; Voortman, T.; Baena, C.P.; Chowdhurry, R.; Muka, T.; Jaspers, L.; Warnakula, S.; Tielemans, M.J.; Troup, J.; Bramer, W.M.; et al. Maternal weight status, diet, and supplement use as determinants of breastfeeding and complementary feeding: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 490–516. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, A.; Zhang, J.; Wang, R.; Xia, H. Role of Perinatal Biological Factors in Delayed Lactogenesis II Among Women with Pre-pregnancy Overweight and Obesity. Biol. Res. Nurs. 2022, 24, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Nommsen-Rivers, L.A. Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Adv. Nutr. Int. Rev. J. 2016, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Suwaydi, M.A.; Wlodek, M.E.; Lai, C.T.; Prosser, S.A.; Geddes, D.T.; Perrella, S.L. Delayed secretory activation and low milk production in women with gestational diabetes: A case series. BMC Pregnancy Childbirth 2022, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jevitt, C.; Hernandez, I.; Groër, M. Lactation Complicated by Overweight and Obesity: Supporting the Mother and Newborn. J. Midwifery Women’s Health 2007, 52, 606–613. [Google Scholar] [CrossRef]
- Aoki, N.; Kawamura, M.; Matsuda, T. Lactation-dependent down regulation of leptin production in mouse mammary gland. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1999, 1427, 298–306. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, L.; Ahmed, A.; Plaut, K.; Haas, D.M.; Szucs, K.; Casey, T.M. Does Circadian Disruption Play a Role in the Metabolic–Hormonal Link to Delayed Lactogenesis II? Front. Nutr. 2015, 2, 131958. [Google Scholar] [CrossRef] [PubMed]
- Dolatshad, H.; Campbell, E.; O’hAra, L.; Maywood, E.; Hastings, M.; Johnson, M. Developmental and reproductive performance in circadian mutant mice. Hum. Reprod. 2005, 21, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Wakatsuki, Y.; Iigo, M.; Shibata, S. Circadian Clock Mutation in Dams Disrupts Nursing Behavior and Growth of Pups. Endocrinology 2006, 147, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.; Crodian, J.; Suárez-Trujillo, A.; Erickson, E.; Weldon, B.; Crow, K.; Cummings, S.; Chen, Y.; Shamay, A.; Mabjeesh, S.J.; et al. CLOCK regulates mammary epithelial cell growth and differentiation. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R1125–R1134. [Google Scholar] [CrossRef]
- Noh, J. The Effect of Circadian and Sleep Disruptions on Obesity Risk. J. Obes. Metab. Syndr. 2018, 27, 78–83. [Google Scholar] [CrossRef]
- Tian, M.; Qi, Y.; Zhang, X.; Wu, Z.; Chen, J.; Chen, F.; Guan, W.; Zhang, S. Regulation of the JAK2-STAT5 Pathway by Signaling Molecules in the Mammary Gland. Front. Cell Dev. Biol. 2020, 8, 604896. [Google Scholar] [CrossRef]
- Geng, Z.; Shan, X.; Lian, S.; Wang, J.; Wu, R. LPS-induced SOCS3 antagonizes the JAK2-STAT5 pathway and inhibits β-casein synthesis in bovine mammary epithelial cells. Life Sci. 2021, 278, 119547. [Google Scholar] [CrossRef]
- Bernardo, K.; Hovey, R.; Trott, J.; Wagner, E.; Karns, R.; Riddle, S.; Thompson, A.; Ward, L.; Nommsen-Rivers, L. Hormone-Sensitive Gene Signatures in the Mammary Epithelial Cells of Lactating Women with Persistent Low Milk Production. Curr. Dev. Nutr. 2021, 5, 720. [Google Scholar] [CrossRef]
- Flores-Quijano, M.E.; Pérez-Nieves, V.; Sámano, R.; Chico-Barba, G. Gestational Diabetes Mellitus, Breastfeeding, and Progression to Type 2 Diabetes: Why Is It So Hard to Achieve the Protective Benefits of Breastfeeding? A Narrative Review. Nutrients 2024, 16, 4346. [Google Scholar] [CrossRef]
- Rosa, S.C.d.S.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef]
- Wunderlich, C.M.; Hövelmeyer, N.; Wunderlich, F.T. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAK-STAT 2013, 2, e23878. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Cannata, D.; Lann, D.; Wu, Y.; Elis, S.; Sun, H.; Yakar, S.; Lazzarino, D.A.; Wood, T.L.; LeRoith, D. Elevated Circulating IGF-I Promotes Mammary Gland Development and Proliferation. Endocrinology 2010, 151, 5751–5761. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lee, E.J.; Kim, K.R.; Cha, B.S.; Song, Y.D.; Lim, S.K.; Lee, H.C.; Huh, K.B. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int. J. Obes. 1997, 21, 355–359. [Google Scholar] [CrossRef]
- Sun, Z.; Shushanov, S.; LeRoith, D.; Wood, T.L. Decreased IGF type 1 receptor signaling in mammary epithelium during pregnancy leads to reduced proliferation, alveolar differentiation, and expression of Insulin Receptor Substrate (IRS)-1 and IRS-2. Endocrinology 2011, 152, 3233–3245. [Google Scholar] [CrossRef]
- Dieterich, C.M.; McKenzie, S.A.; Devine, C.M.; Thornburg, L.L.; Rasmussen, K.M. Obese women experience multiple challenges with breastfeeding that are either unique or exacerbated by their obesity: Discoveries from a longitudinal, qualitative study. Matern. Child Nutr. 2017, 13, e12344. [Google Scholar] [CrossRef]
- Bever Babendure, J.; Reifsnider, E.; Mendias, E.; Moramarco, M.W.; Davila, Y.R. Reduced breastfeeding rates among obese mothers: A review of contributing factors, clinical considerations and future directions. Int. Breastfeed. J. 2015, 10, 21. [Google Scholar] [CrossRef]
- Mehta, U.J.; Siega-Riz, A.M.; Herring, A.H.; Adair, L.S.; Bentley, M.E. Maternal obesity, psychological factors, and breastfeeding initiation. Breastfeed. Med. 2011, 6, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ziomkiewicz, A.; Babiszewska, M.; Apanasewicz, A.; Piosek, M.; Wychowaniec, P.; Cierniak, A.; Barbarska, O.; Szołtysik, M.; Danel, D.; Wichary, S. Psychosocial stress and cortisol stress reactivity predict breast milk composition. Sci. Rep. 2021, 11, 11576. [Google Scholar] [CrossRef]
- Matyas, M.; Apanasewicz, A.; Krzystek-Korpacka, M.; Jamrozik, N.; Cierniak, A.; Babiszewska-Aksamit, M.; Ziomkiewicz, A. The association between maternal stress and human milk concentrations of cortisol and prolactin. Sci. Rep. 2024, 14, 28115. [Google Scholar] [CrossRef] [PubMed]
- Gussler, J.; Arensberg, M.B. Impact of maternal obesity on pregnancy and lactation: The health care challenge. Nutr. Today 2011, 46, 6–11. [Google Scholar] [CrossRef]
- Chapman, D.J.; Morel, K.; Bermúdez-Millán, A.; Young, S.; Damio, G.; Pérez-Escamilla, R. Breastfeeding education and support trial for overweight and obese women: A randomized trial. Pediatrics 2013, 131, e162–e170. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kelleher, S.L. Biological underpinnings of breastfeeding challenges: The role of genetics, diet, and environment on lactation physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Muñoz, Y.; Ortiz, M.; Maliqueo, M.; Chouinard-Watkins, R.; Valenzuela, R. Impact of Maternal Obesity on the Metabolism and Bioavailability of Polyunsaturated Fatty Acids during Pregnancy and Breastfeeding. Nutrients 2020, 13, 19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).