Implementation of Medical Therapy in Different Stages of Heart Failure with Reduced Ejection Fraction: An Analysis of the VIENNA-HF Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Patient and Public Involvement
2.3. Assessing GDMT in HF
2.4. Definition of Clinical Factors Limiting GDMT Up-Titration—Adverse Events
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Up-Titration of HF Medication
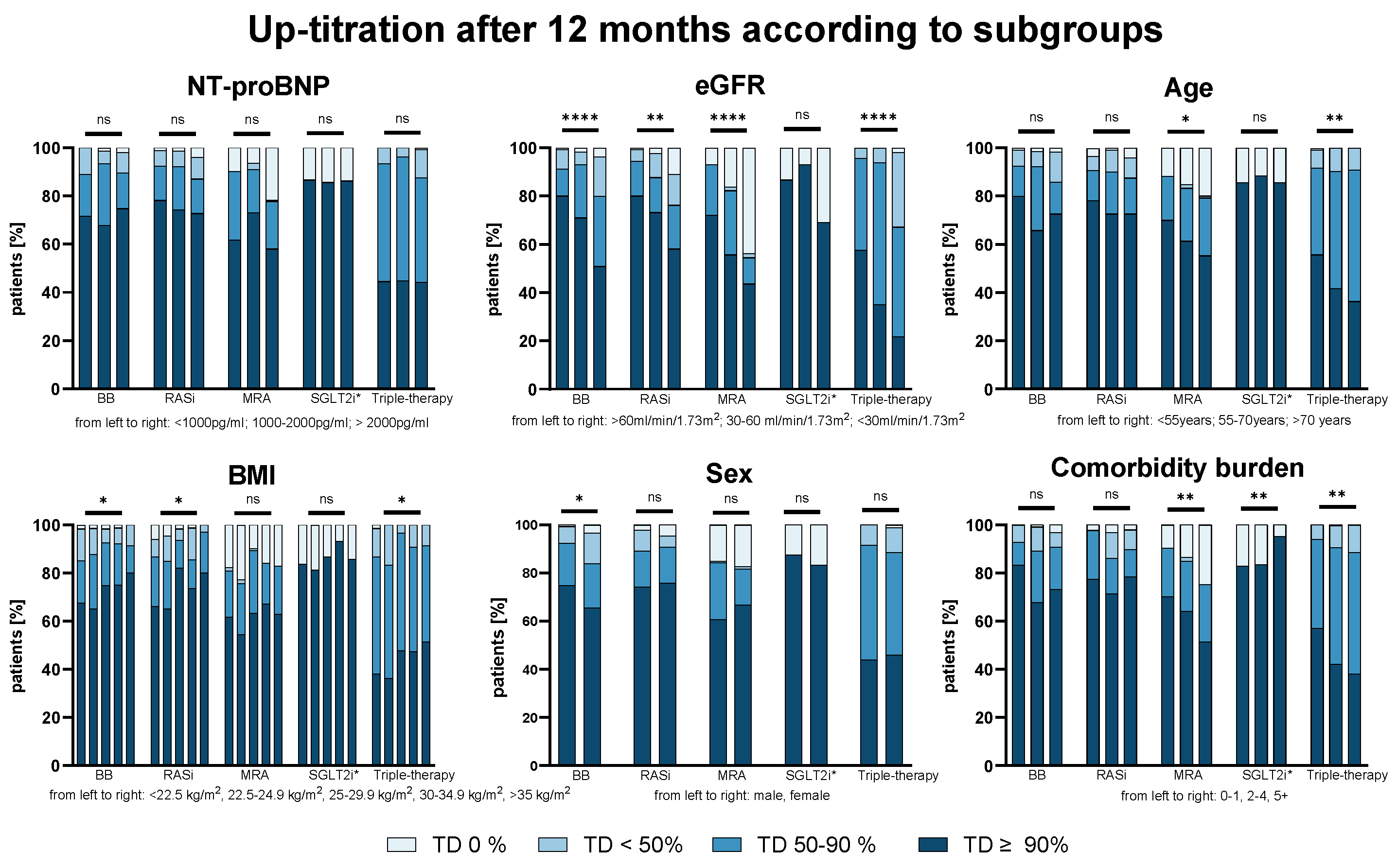
3.3. Predictors for Successful Up-Titration—Impact of Disease Severity and Patient Characteristics
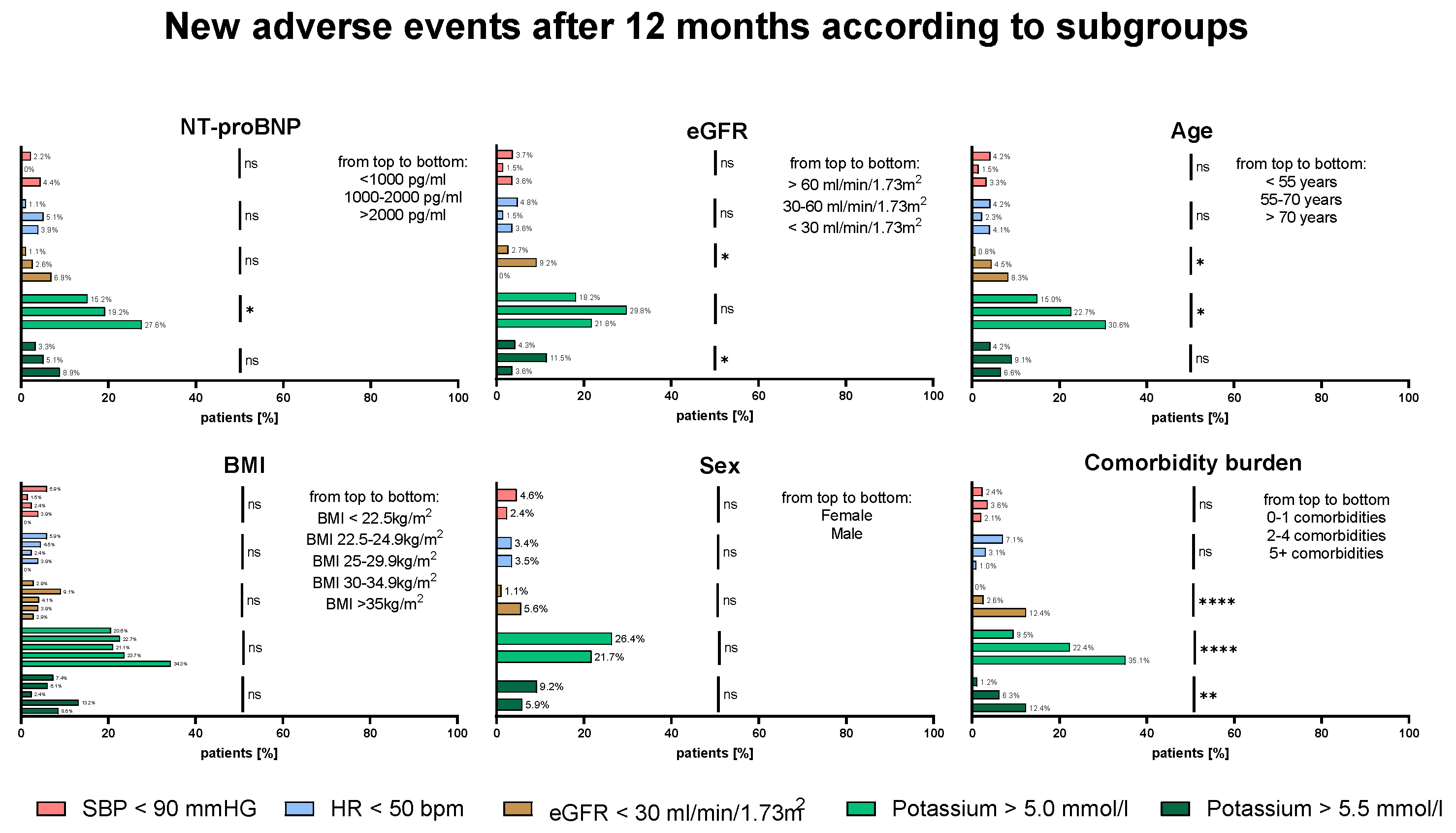
3.4. Frequency of AEs—Impact of Disease Severity and Patient Characteristics
3.5. Reasons for No Up-Titration (TD ≤ 50%) at 12 M
4. Discussion
4.1. GDMT Evidence in AHF
4.2. GDMT Implementation and Clinical Patient Profiles in AHF
4.3. Characteristics Predisposing Patients to Suboptimal GDMT
4.4. Development of Adverse Events During Up-Titration
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEi | Angiotensin-Converting Enzyme Inhibitor |
| AHF | Advanced Heart Failure |
| ARB | Angiotensin Receptor Blocker |
| ARNI | Angiotensin Receptor-Neprilysin Inhibitor |
| BB | Beta-blocker |
| eGFR | Estimated Glomerular Filtration Rate |
| GDMT | Guideline Directed Medical Therapy |
| HF | Heart Failure |
| HFA | Heart Failure Association |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| HK | Hyperkalemia |
| IQR | Interquartile Range |
| LVEF | Left Ventricular Ejection Fraction |
| MRA | Mineralocorticoid Receptor Antagonist |
| NYHA | New York Heart Association |
| RASi | Renin Angiotensin System Inhibitor |
| RHR | Resting Heart Rate |
| SGLT2i | Sodium–Glucose Transporter 2 Inhibitor |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Mamas, M.A.; Sperrin, M.; Watson, M.C.; Coutts, A.; Wilde, K.; Burton, C.; Kadam, U.T.; Kwok, C.S.; Clark, A.B.; Murchie, P.; et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017, 19, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Fiuzat, M.; Ezekowitz, J.; Alemayehu, W.; Westerhout, C.M.; Sbolli, M.; Cani, D.; Whellan, D.J.; Ahmad, T.; Adams, K.; Piña, I.L.; et al. Assessment of Limitations to Optimization of Guideline-Directed Medical Therapy in Heart Failure from the GUIDE-IT Trial: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2020, 5, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lindberg, F.; Christodorescu, R.M.; Ferrini, M.; Kumler, T.; Toutoutzas, K.; Dattilo, G.; Bayes-Genis, A.; Moura, B.; Amir, O.; et al. Physician perceptions, attitudes, and strategies towards implementing guideline-directed medical therapy in heart failure with reduced ejection fraction. A survey of the Heart Failure Association of the ESC and the ESC Council for Cardiology Practice. Eur. J. Heart Fail. 2024, 26, 1408–1418. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Voors, A.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; Lang, C.C.; ter Maaten, J.M.; et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: A prospective European study. Eur. Heart J. 2017, 38, 1883–1890. [Google Scholar] [CrossRef]
- Sumarsono, A.; Xie, L.; Keshvani, N.; Zhang, C.; Patel, L.; Alonso, W.W.; Thibodeau, J.T.; Fonarow, G.C.; Van Spall, H.G.C.; Messiah, S.E.; et al. Sex Disparities in Longitudinal Use and Intensification of Guideline-Directed Medical Therapy among Patients with Newly Diagnosed Heart Failure with Reduced Ejection Fraction. Circulation 2024, 149, 510–520. [Google Scholar] [CrossRef]
- Walsh, M.N.; Jessup, M.; Lindenfeld, J.A. Women With Heart Failure: Unheard, Untreated, and Unstudied. J. Am. Coll. Cardiol. 2019, 73, 41–43. [Google Scholar] [CrossRef]
- Peterson, P.N.; Rumsfeld, J.S.; Liang, L.; Hernandez, A.F.; Peterson, E.D.; Fonarow, G.C.; Masoudi, F.A. Treatment and risk in heart failure: Gaps in evidence or quality? Circ. Cardiovasc. Qual. Outcomes 2010, 3, 309–315. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Cotter, G.; Deniau, B.; Davison, B.; Edwards, C.; Adamo, M.; Arrigo, M.; Barros, M.; Biegus, J.; Celutkiene, J.; Čerlinskaitė-Bajorė, K.; et al. Optimization of Evidence-Based Heart Failure Medications After an Acute Heart Failure Admission: A Secondary Analysis of the STRONG-HF Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- The Consensus Trial Study Group. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N. Engl. J. Med. 1987, 316, 1429–1435. [Google Scholar] [CrossRef]
- Packer, M.; Fowler, M.B.; Roecker, E.B.; Coats, A.J.S.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Staiger, C.; et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: Results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002, 106, 2194–2199. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Brunner-La Rocca, H.P.; Linssen, G.C.; Smeele, F.J.; van Drimmelen, A.A.; Schaafsma, H.J.; Westendorp, P.H.; Rademaker, P.C.; van de Kamp, H.J.; Hoes, A.W.; Brugts, J.J. Contemporary Drug Treatment of Chronic Heart Failure with Reduced Ejection Fraction: The CHECK-HF Registry. JACC Heart Fail. 2019, 7, 13–21. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Hülsmann, M.; Wurm, R.; Eskandary, F.; Neuhold, S.; Zuckermann, A.; Bojic, A.; Strunk, G.; Pacher, R. Outcome of conservative management vs. assist device implantation in patients with advanced refractory heart failure. Eur. J. Clin. Investig. 2016, 46, 34–41. [Google Scholar] [CrossRef]
- Cowie, M.R.; Schöpe, J.; Wagenpfeil, S.; Tavazzi, L.; Böhm, M.; Ponikowski, P.; Anker, S.D.; Filippatos, G.S.; Komajda, M. Patient factors associated with titration of medical therapy in patients with heart failure with reduced ejection fraction: Data from the QUALIFY international registry. ESC Heart Fail. 2021, 8, 861. [Google Scholar] [CrossRef]
- Greene, S.J.; Fonarow, G.C.; DeVore, A.D.; Sharma, P.P.; Vaduganathan, M.; Albert, N.M.; Duffy, C.I.; Hill, C.L.; McCague, K.; Patterson, J.H.; et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 73, 2365–2383. [Google Scholar] [CrossRef]
- Lavalle, C.; Mariani, M.V.; Severino, P.; Palombi, M.; Trivigno, S.; D’Amato, A.; Silvetti, G.; Pierucci, N.; Di Lullo, L.; Chimenti, C.; et al. Efficacy of Modern Therapies for Heart Failure with Reduced Ejection Fraction in Specific Population Subgroups: A Systematic Review and Network Meta-Analysis. Cardiorenal Med. 2024, 14, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L.; Mark, D.B.; Piña, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide–Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef]
- Harrington, J.; Fonarow, G.C.; Khan, M.S.; Hernandez, A.; Anker, S.; Böhm, M.; Greene, S.J.; Felker, G.M.; Vaduganathan, M.; Butler, J. Medication-Attributable Adverse Events in Heart Failure Trials. JACC Heart Fail. 2023, 11, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Grobbee, D.E.; Filippatos, G.; Desai, N.R.; Coats, A.J.S.; Pinto, F.; Rosano, G.M.C.; Cleland, J.G.F.; Kammerer, J.; de Arellano, A.R. Epidemiology and risk factors for hyperkalaemia in heart failure. ESC Heart Fail. 2024, 11, 1821. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Anker, S.D.; Lund, L.H.; Coats, A.J.S.; Filippatos, G.; Siddiqi, T.J.; Friede, T.; Fabien, V.; Kosiborod, M.; Metra, M.; et al. Patiromer for the management of hyperkalemia in heart failure with reducedejection fraction: The DIAMOND trial. Eur. Heart J. 2022, 43, 4362. [Google Scholar] [CrossRef]
- Weir, M.R.; Rossignol, P.; Pitt, B.; Lund, L.H.; Coats, A.J.S.; Filippatos, G.; Perrin, A.; Waechter, S.; Budden, J.; Kosiborod, M.; et al. Patiromer-Facilitated Renin-Angiotensin-Aldosterone System Inhibitor Utilization in Patients with Heart Failure with or without Comorbid Chronic Kidney Disease: Subgroup Analysis of DIAMOND Randomized Trial. Am. J. Nephrol. 2024, 55, 672–689. [Google Scholar] [CrossRef]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.F.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P. Renin–Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef]
- Guidetti, F.; Lund, L.H.; Benson, L.; Hage, C.; Musella, F.; Stolfo, D.; Mol, P.G.M.; Flammer, A.J.; Ruschitzka, F.; Dahlstrom, U.; et al. Safety of continuing mineralocorticoid receptor antagonist treatment in patients with heart failure with reduced ejection fraction and severe kidney disease: Data from Swedish Heart Failure Registry. Eur. J. Heart Fail. 2023, 25, 2164–2173. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Damman, K.; Gori, M.; Claggett, B.; Jhund, P.S.; Senni, M.; Lefkowitz, M.P.; Prescott, M.F.; Shi, V.C.; Rouleau, J.L.; Swedberg, K.; et al. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC Heart Fail. 2018, 6, 489–498. [Google Scholar] [CrossRef]
- Le, D.; Grams, M.E.; Coresh, J.; Shin, J.I. Sacubitril-Valsartan in Patients Requiring Hemodialysis. JAMA Netw. Open 2024, 7, e2429237. [Google Scholar] [CrossRef]


| Baseline Characteristics | Total Cohort | NT-proBNP < 1000 pg/mL | NT-proBNP 1000–2000 pg/mL | NT-proBNP > 2000 pg/mL | p-Value |
|---|---|---|---|---|---|
| Basic demographics | |||||
| Age, years (IQR) | 62 (50–72) | 57 (48–64) | 62 (49–70) | 66 (54–73) | <0.001 |
| Male sex, n (%) | 286 (76.7%) | 70 (76.1%) | 63 (80.8%) | 153 (75.4%) | 0.637 |
| BMI, kg/m2 (IQR) | 26.7 (23.4–30.8) | 28.4 (24.9–31.1) | 26.6 (23.2–30.4) | 26.2 (23.2–30.8) | 0.103 |
| Systolic BP, mmHg (IQR) | 124 (110–140) | 125 (113–135) | 120 (114–141) | 124 (108–140) | 0.531 |
| Diastolic BP, mmHg (IQR) | 80 (70–89) | 79 (70–85) | 80 (72–90) | 78 (70–90) | 0.502 |
| Heart rate, bpm (IQR) | 74 (65–85) | 68 (60–77) | 74 (64–85) | 76 (67–88) | <0.001 |
| NYHA class, n (%) † | |||||
| I | 29 (7.8%), n = 370 | 16 (17.4%) | 5 (6.4%) | 8 (4.0%) | <0.001 |
| II | 191 (51.6%), n = 370 | 58 (63.0%) | 43 (55.1%) | 90 (45.0%) | |
| III | 145 (39.2%), n = 370 | 18 (19.6%) | 30 (38.5%) | 97 (48.5%) | |
| IV | 5 (1.4%), n = 370 | - | - | 5 (2.5%) | |
| Comorbidities | |||||
| Arterial hypertension, n (%) | 176 (47.2%) | 38 (41.3%) | 37 (47.4%) | 101 (49.8%) | 0.397 |
| CAD, n (%) | 175 (46.9%) | 35 (38%) | 41 (52.6%) | 99 (48.8%) | 0.120 |
| Atrial fibrillation, n (%) | 168 (45.0%) | 30 (32.6%) | 36 (46.2%) | 102 (50.2%) | 0.018 |
| Diabetes mellitus type II, n (%) | 146 (39.1%) | 26 (28.3%) | 30 (38.5%) | 90 (44.3%) | 0.031 |
| Chronic kidney disease, n (%) | 186 (49.9%) | 20 (21.7%) | 35 (44.9%) | 131 (64.5%) | <0.001 |
| COPD, n (%) | 44 (11.8%) | 7 (7.6%) | 9 (11.5%) | 28 (13.8%) | 0.319 |
| PAD, n (%) | 48 (12.9%) | 4 (4.3%) | 11 (14.1%) | 33 (16.3%) | 0.010 |
| Carotid artery disease, n (%) | 24 (6.4%) | 3 (3.3%) | 5 (6.4%) | 16 (7.9%) | 0.366 |
| Stroke, n (%) | 24 (6.4%) | 6 (6.5%) | 3 (3.8%) | 15 (7.4%) | 0.636 |
| Iron deficiency, n (%) † | 147 (50.2%), n = 293 | 26 (32.1%) | 32 (54.2%) | 89 (58.2%) | 0.001 |
| Any malignant disease, n (%) | 57 (15.3%) | 10 (10.9%) | 7 (9.0%) | 40 (19.7%) | 0.035 |
| Medication | |||||
| Beta-blocker, n (%) | 347 (93%) | 85 (92.4%) | 72 (92.3%) | 190 (93.6%) | 0.890 |
| Target dose ≥ 50%, n (%) | 243 (65.1%) | 65 (70.7%) | 45 (57.7%) | 113 (65.5%) | 0.214 |
| RASi, n (%) | 331 (88.7%) | 86 (93.5%) | 73 (93.6%) | 172 (84.7%) | 0.031 |
| Target dose ≥ 50%, n (%) | 202 (54.2%) | 63 (68.5%) | 51 (65.4%) | 88 (43.3%) | <0.001 |
| MRA, n (%) | 271 (72.7%) | 72 (78.3%) | 63 (80.8%) | 136 (67.0%) | 0.028 |
| Target dose ≥ 50%, n (%) | 264 (70.8%) | 71 (77.2%) | 61 (78.2%) | 132 (65.0%) | 0.030 |
| SGLT2i, n (%) * | 85 (52.8%) | 22/37 (59.5%) | 20/42 (47.6%) | 43/82 (52.4%) | 0.558 |
| Diuretics, n (%) | 212 (56.8%) | 49 (53.3%) | 51 (65.4%) | 112 (55.2%) | 0.221 |
| Devices | |||||
| ICD, n (%) | 144 (38.6%) | 29 (31.5%) | 35 (44.9%) | 80 (39.4%) | 0.203 |
| CRT, n (%) | 89 (23.9%) | 19 (20.7%) | 21 (26.9%) | 49 (24.1%) | 0.622 |
| Laboratory parameters | |||||
| NT-proBNP pg/mL (IQR) | 2363 (1014–5009) | 508 (378–707) | 1430 (1216–1719) | 4730 (3199–8307) | <0.001 |
| Creatinine, mg/dL (IQR) | 1.17 (0.96–1.57) | 0.98 (0.81–1.12) | 1.16 (0.99–1.35) | 1.39 (1.06–2.04) | <0.001 |
| eGFR, ml/min/1.73 m2 (IQR) | 60.23 (42.55–77.26) | 74.99 (63.29–89.29) | 62.74 (48.75–77.06) | 47.57 (31.47–68.01) | <0.001 |
| Potassium, mmol/L (IQR) | 4.73 (4.40–5.03) | 4.69 (4.36–5.03) | 4.77 (4.44–5.11) | 4.71 (4.39–4.99) | 0.257 |
| BUN, mg/dL (IQR) † | 22.1 (16.3–33.1), n = 339 | 17.4 (13.9–23.7) | 19.2 (15.8–28.1) | 27.1 (18.9–38.7) | <0.001 |
| Variable | ß-Coefficient | Standard Error | T-Value | p-Value | Odds Ratio | 95% CI | Cox and Snell Pseudo R2 |
|---|---|---|---|---|---|---|---|
| Intercept | −0.298 | 0.150 | |||||
| eGFR (baseline) * | 0.679 | 0.143 | 4.733 | <0.001 | 1.972 | 1.488–2.612 | |
| Triple-therapy TD (baseline) * | 0.523 | 0.118 | 4.432 | <0.001 | 1.686 | 1.338–2.125 | |
| Potassium (baseline) * | −0.240 | 0.121 | 1.975 | 0.048 | 0.787 | 0.620–0.998 |
| Reasons | BB TD ≤ 50% (n = 86/373) | RASi TD ≤ 50% (n = 84/373) | MRA TD ≤ 50% (n = 141/373) |
|---|---|---|---|
| Asymptomatic hypotension | 16/86 (18.6%) | 17/84 (20.2%) | - |
| Bradycardia | 32/86 (37.2%) | - | - |
| Hyperkalemia | - | 26/84 (30.9%) | 61/141 (43.2%) |
| Impaired renal function | - | 8/84 (9.5%) | 12/141 (8.5%) |
| Hyperkalemia and impaired renal function | - | 6/84 (7.1%) | 21/141 (14.9%) |
| Dizziness | 3/86 (3.5%) | 1/84 (11.9%) | - |
| Symptomatic hypotension | 9/86 (10.5%) | 8/84 (9.5%) | - |
| Symptomatic bradycardia | 3/86 (3.5%) | - | - |
| Syncope/presyncope | 2/86 (2.3%) | - | - |
| Intolerance/patient request/allergy | 6/86 (6.9%) | 3/84 (3.5%) | 6/141 (4.3%) |
| Medication discontinued elsewhere | 8/86 (9.3%) | 7/84 (8.3%) | 5/141 (3.5%) |
| Total number of reasons | 79/86 (91.9%) | 76/84 (90.5%) | 105/141 (74.5%) |
| No reason | 7/86 (8.1%) | 8 (9.5%) | 36 (25.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotides, N.G.; Weidenhammer, A.; Prausmüller, S.; Stadler, M.; Spinka, G.; Heitzinger, G.; Arfsten, H.; Strunk, G.; Bartko, P.E.; Goliasch, G.; et al. Implementation of Medical Therapy in Different Stages of Heart Failure with Reduced Ejection Fraction: An Analysis of the VIENNA-HF Registry. Biomedicines 2025, 13, 1846. https://doi.org/10.3390/biomedicines13081846
Panagiotides NG, Weidenhammer A, Prausmüller S, Stadler M, Spinka G, Heitzinger G, Arfsten H, Strunk G, Bartko PE, Goliasch G, et al. Implementation of Medical Therapy in Different Stages of Heart Failure with Reduced Ejection Fraction: An Analysis of the VIENNA-HF Registry. Biomedicines. 2025; 13(8):1846. https://doi.org/10.3390/biomedicines13081846
Chicago/Turabian StylePanagiotides, Noel G., Annika Weidenhammer, Suriya Prausmüller, Marc Stadler, Georg Spinka, Gregor Heitzinger, Henrike Arfsten, Guido Strunk, Philipp E. Bartko, Georg Goliasch, and et al. 2025. "Implementation of Medical Therapy in Different Stages of Heart Failure with Reduced Ejection Fraction: An Analysis of the VIENNA-HF Registry" Biomedicines 13, no. 8: 1846. https://doi.org/10.3390/biomedicines13081846
APA StylePanagiotides, N. G., Weidenhammer, A., Prausmüller, S., Stadler, M., Spinka, G., Heitzinger, G., Arfsten, H., Strunk, G., Bartko, P. E., Goliasch, G., Hengstenberg, C., Hülsmann, M., & Pavo, N. (2025). Implementation of Medical Therapy in Different Stages of Heart Failure with Reduced Ejection Fraction: An Analysis of the VIENNA-HF Registry. Biomedicines, 13(8), 1846. https://doi.org/10.3390/biomedicines13081846







