Is TGF-β Associated with Cytokines and Other Biochemical or Clinical Risk Parameters in Early-Onset CAD Patients?
Abstract
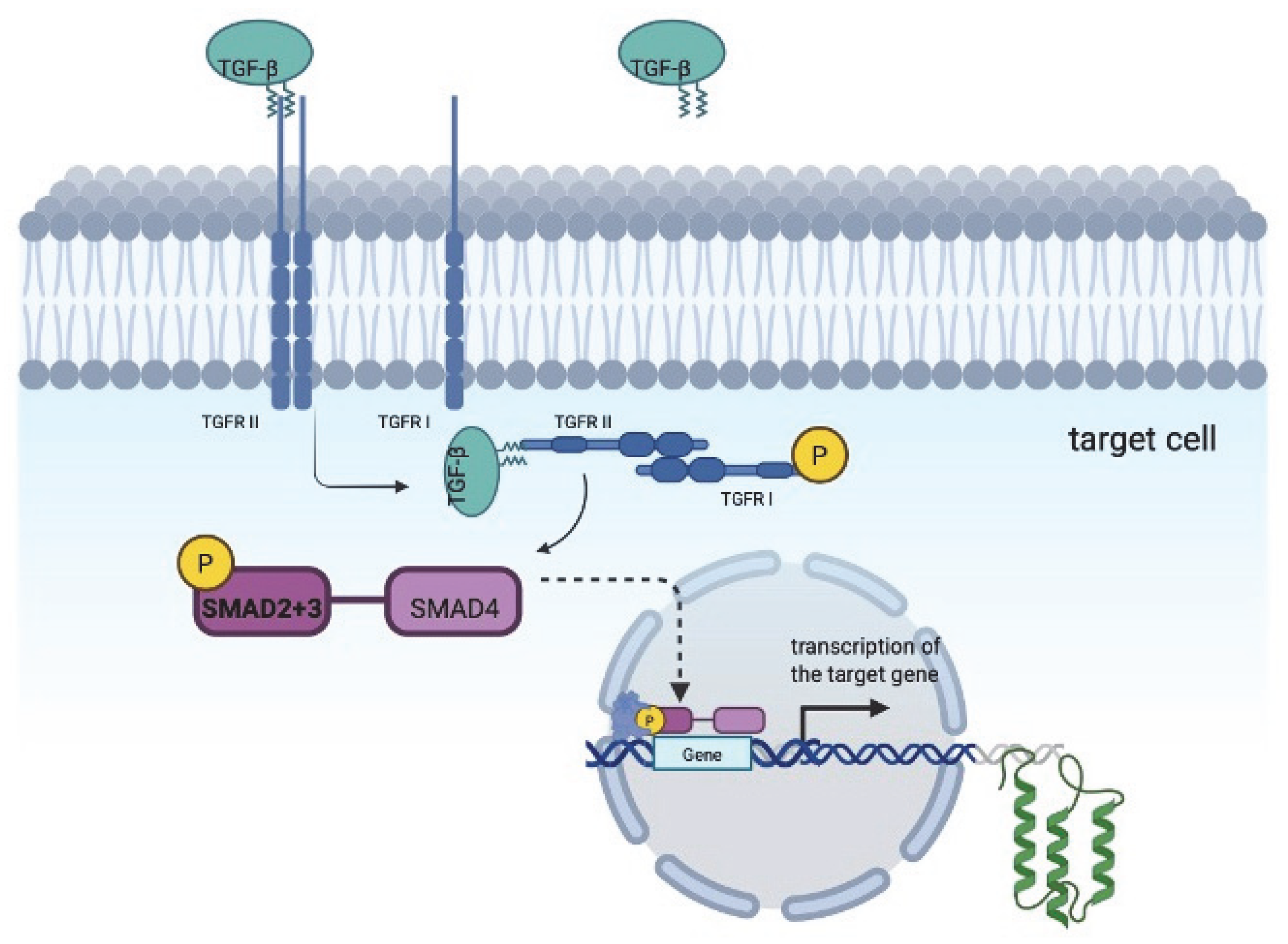
1. Introduction
2. Materials and Methods
2.1. Study Group Characteristic
2.2. Diagnostic Tests
2.2.1. Physical Examination
2.2.2. Biochemistry
2.2.3. Testing Plasma TGF-β and Other Protein Levels by the ELISA Method
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Heldin, C.H.; Moustakas, A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P.; Mueller, T.D.; Springer, T.A. Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2016, 8, a022103. [Google Scholar] [CrossRef] [PubMed]
- Groppe, J.; Hinck, C.S.; Samavarchi-Tehrani, P.; Zubieta, C.; Schuermann, J.P.; Taylor, A.B.; Schwarz, P.M.; Wrana, J.L.; Hinck, A.P. Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 2008, 29, 157–168. [Google Scholar] [CrossRef]
- Bai, P.; Lyu, L.; Yu, T.; Zuo, C.; Fu, J.; He, Y.; Wan, Q.; Wan, N.; Jia, D.; Lyu, A. Macrophage-Derived Legumain Promotes Pulmonary Hypertension by Activating the MMP (Matrix Metalloproteinase)-2/TGF (Transforming Growth Factor)-β1 Signaling. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e130–e145. [Google Scholar] [CrossRef]
- Nickel, J.; ten Dijke, P.; Mueller, T.D. TGF-β family co-receptor function and signaling. Acta Biochim. Biophys. Sin. 2018, 50, 12–36. [Google Scholar] [CrossRef]
- Mitchell, H.; Choudhury, A.; Pagano, R.E.; Leof, E.B. Ligand-dependent and -independent transforming growth factor-β receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol. Biol. Cell 2004, 15, 4166–4178. [Google Scholar] [CrossRef] [PubMed]
- Doré, J.J., Jr.; Yao, D.; Edens, M.; Garamszegi, N.; Sholl, E.L.; Leof, E.B. Mechanisms of transforming growth factor-β receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol. Biol. Cell 2001, 12, 675–684. [Google Scholar] [CrossRef]
- Yakymovych, I.; Yakymovych, M.; Zang, G.; Mu, Y.; Bergh, A.; Landström, M.; Heldin, C.H. CIN85 modulates TGFβ signaling by promoting the presentation of TGFβ receptors on the cell surface. J. Cell Biol. 2015, 210, 319–332. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Aashaq, S.; Batool, A.; Mir, S.A.; Beigh, M.A.; Andrabi, K.I.; Shah, Z.A. TGF-beta signaling: A recap of SMAD-independent and SMAD-dependent pathways. J. Cell Physiol. 2022, 237, 59–85. [Google Scholar] [CrossRef]
- Stępień-Wyrobiec, O.; Hrycek, A.; Wyrobiec, G. Transforming growth factor beta (TGF-beta): Its structure, function, and role in the pathogenesis of systemic lupus erythematosus. Postepy Hig. Med. Dosw. (Online) 2008, 62, 688–693. [Google Scholar]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- David, C.J.; Massagué, J. Publisher Correction: Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 479. [Google Scholar] [CrossRef]
- Buss, A.; Pech, K.; Kakulas, B.A.; Martin, D.; Schoenen, J.; Noth, J.; Brook, G.A. TGF-beta1 and TGF-beta2 expression after traumatic human spinal cord injury. Spinal Cord 2008, 46, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Bax, N.A.; van Oorschot, A.A.M.; Maas, S.; Braun, J.; van Tuyn, J.; de Vries, A.A.F.; Gittenberger-de Groot, A.C.; Goumans, M.J. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFβ-signaling and WT1. Basic Res. Cardiol. 2011, 106, 829–847. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, B.; Fan, J.; Guan, S.; Li, Y.; Chen, M.; Woodley, D.T.; Li, W. A“traf c control” role for TGFbeta3: Orchestrating dermal and epidermal cell motility during wound healing. J. Cell Biol. 2006, 172, 1093–1105. [Google Scholar] [CrossRef]
- Lagace, T.A. PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and incells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef]
- Rac, M. Human CD36: Gene Regulation, Protein Function, and Its Role in Atherosclerosis Pathogenesis. Genes 2025, 16, 705. [Google Scholar] [CrossRef] [PubMed]
- Jarząbek, K.; Sobczyk, A.; Sobczyk, W.; Łabuzek, K.; Belowski, D.; Gabryel, B. The concept of unstable atherosclerotic plaque and pharmacological therapeutic strategies. Chir. Pol. 2015, 17, 49–68. [Google Scholar]
- Rac, M.; Rac, M.; Krzystolik, A.; Safranow, K.; Chlubek, D.; Dziedziejko, V. Evaluation of Plasma E-Selectin Concentration as a Risk Marker for Atherosclerotic Vascular Damage in Patients with Early CAD. Biomolecules 2024, 15, 22. [Google Scholar] [CrossRef]
- Bialecka, M.; Rac, M.; Dziedziejko, V.; Safranow, K.; Chlubek, D.; Rać, M.E. An Evaluation of Plasma TNF, VEGF-A, and IL-6 Determination as a Risk Marker of Atherosclerotic Vascular Damage in Early-Onset CAD Patients. J. Clin. Med. 2024, 13, 1742. [Google Scholar] [CrossRef]
- Bryk, D.; Olejarz, W.; Zapolska-Downar, D. Mitogen-activated protein kinases in atherosclerosis. Postepy Hig. Med. Dosw. (Online) 2014, 68, 10–22. [Google Scholar] [CrossRef]
- Christiansen, M.K. Early-onset Coronary Artery Disease Clinical and Hereditary Aspects. Dan. Med. J. 2017, 64, B5406. [Google Scholar]
- Khoja, A.; Andraweera, P.H.; Lassi, Z.S.; Zheng, M.; Pathirana, M.M.; Ali, A.; Aldridge, E.; Wittwer, M.R.; Chaudhuri, D.D.; Tavella, R.; et al. Risk factors for premature coronary artery disease (PCAD) in adults: A systematic review protocol. F1000Research 2021, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Early-onset or Premature Coronary Artery Disease. Curr. Med. Chem. 2025, 32, 1040–1064. [Google Scholar] [CrossRef] [PubMed]
- Khoja, A.; Andraweera, P.H.; Lassi, Z.S.; Ali, A.; Zheng, M.; Pathirana, M.M.; Aldridge, E.; Wittwer, M.R.; Chaudhuri, D.D.; Tavella, R.; et al. Risk Factors for Early-Onset Versus Late-Onset Coronary Heart Disease (CHD): Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, 1277–1311. [Google Scholar] [CrossRef]
- Dziedziejko, V.; Pauli, N.; Kuligowska, A.; Safranow, K.; Goschorska, M.; Chlubek, D.; Rać, M. Significant limitations associated with the analysis of human plasma soluble CD36 performed by ELISA. Pomeranian J. Life Sci. 2018, 64, 5–7. [Google Scholar] [CrossRef]
- Totoń-Żurańska, J.; Mikolajczyk, T.P.; Saju, B.; Guzik, T.J. Vascular remodelling in cardiovascular diseases: Hypertension, oxidation, and inflammation. Clin. Sci. 2024, 138, 817–850. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Du, M.; Li, S.; Ding, J.; Wang, J.; Wang, Y.; Liu, P. Identification of diagnostic biomarkers and therapeutic targets in peripheral immune landscape from coronary artery disease. J. Transl. Med. 2022, 20, 399. [Google Scholar] [CrossRef]
- Malinowski, D.; Bochniak, O.; Luterek-Puszyńska, K.; Puszyński, M.; Pawlik, A. Genetic Risk Factors Related to Coronary Artery Disease and Role of Transforming Growth Factor Beta 1 Polymorphisms. Genes 2023, 14, 1425. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Wu, C.; Lu, X.; Huang, J. MicroRNAs involved in the TGF-β signaling pathway in atherosclerosis. Biomed. Pharmacother. 2022, 146, 112499. [Google Scholar] [CrossRef]
- Goumans, M.J.; Dijke, P.T. TGF-β Signaling in Control of Cardiovascular Function. Cold Spring Harb. Perspect. Biol. 2018, 10, a022210. [Google Scholar] [CrossRef]
- Chen, C.; Lei, W.; Chen, W.; Zhong, J.; Gao, X.; Li, B.; Wang, H.; Huang, C. Serum TGF-β1 and SMAD3 levels are closely associated with coronary artery disease. BMC Cardiovasc. Disord. 2014, 14, 18. [Google Scholar] [CrossRef]
- Pauli, N.; Kuligowska, A.; Krzystolik, A.; Dziedziejko, V.; Safranow, K.; Rać, M.; Chlubek, D.; Rac, M. The circulating vascular endothelial growth factor is only marginally associated with an increased risk for atherosclerosis. Minerva Cardioangiol. 2020, 68, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Krzystolik, A.; Dziedziejko, V.; Safranow, K.; Kurzawski, G.; Rać, M.; Sagasz-Tysiewicz, D.; Poncyljusz, W.; Jakubowska, K.; Chlubek, D.; Rac, M. Is plasma soluble CD36 associated with cardiovascular risk factors in early onset coronary artery disease patients? Scand. J. Clin. Lab. Investig. 2015, 75, 398–406. [Google Scholar] [CrossRef]
- Abdallah, H.Y.; Fareed, A.; Abdelmaogood, A.K.K.; Allam, S.; Abdelgawad, M.; El Deen, L.A.T. Introducing Circulating Vasculature-Related Transcripts as Biomarkers in Coronary Artery Disease. Mol. Diagn. Ther. 2023, 27, 243–259. [Google Scholar] [CrossRef]
- Indumathi, B.; Oruganti, S.S.; Naushad, S.M.; Kutala, V.K. Probing the epigenetic signatures in subjects with coronary artery disease. Mol. Biol. Rep. 2020, 47, 6693–6703. [Google Scholar] [CrossRef] [PubMed]
- Anlar, G.G.; Pedersen, S.; Al Ashmar, S.; Krzyslak, H.; Kamareddine, L.; Zeidan, A. Unraveling the proteomic signatures of coronary artery disease and hypercholesterolemia. Biomol. Biomed. 2025, 25, 1280–1292. [Google Scholar] [CrossRef]
- Ahmadi, J.; Hosseini, E.; Kargar, F.; Ghasemzadeh, M. Stable CAD patients show higher levels of platelet-borne TGF-β1 associated with a superior pro-inflammatory state than the pro-aggregatory status; Evidence highlighting the importance of platelet-derived TGF-β1 in atherosclerosis. J. Thromb. Thrombolysis 2023, 55, 102–115. [Google Scholar] [CrossRef]
- Sepehri, Z.; Masoumi, M.; Ebrahimi, N.; Kiani, Z.; Nasiri, A.A.; Kohan, F.; Fathollahi, M.S.; Arababadi, M.K.; Asadikaram, G. Atorvastatin, Losartan and Captopril Lead to Upregulation of TGF-β, and Downregulation of IL-6 in Coronary Artery Disease and Hypertension. PLoS ONE 2016, 11, e0168312. [Google Scholar] [CrossRef]
- Rath, D.; Chatterjee, M.; Müller, I.; Müller, K.; Böckmann, C.; Droppa, M.; Stimpfle, F.; Karathanos, A.; Borst, O.; Seizer, P.; et al. Platelet expression of transforming growth factor beta 1 is enhanced and associated with cardiovascular prognosis in patients with acute coronary syndrome. Atherosclerosis 2014, 237, 754–759. [Google Scholar] [CrossRef]
- Saita, E.; Kishimoto, Y.; Aoyama, M.; Ohmori, R.; Kondo, K.; Momiyama, Y. Low Plasma Levels of Soluble Endoglin and Cardiovascular Events in Patients Undergoing Coronary Angiography. Biomedicines 2023, 11, 2975. [Google Scholar] [CrossRef]
- Garganeeva, A.; Kuzheleva, E.; Tukish, O.; Kondratiev, M.; Vitt, K.; Andreev, S.; Bogdanov, Y.; Ogurkova, O. Predictors of Adverse Cardiovascular Events After CABG in Patients with Previous Heart Failure. Life 2025, 15, 387. [Google Scholar] [CrossRef]
- Dąbek, J.; Bochenek, A.; Kułach, A.; Szota, J.; Gąsior, Z. Transcriptome of TGF-b family, TGF-b receptors, and TGF-b receptor-activated signaling pathways in advanced coronary artery disease (CAD) and post-MI patients. Chir. Pol. 2008, 10, 158–163. [Google Scholar]
- Lee, C.H.; Lee, C.Y.; You, H.L.; Wu, Y.T.; Chen, D.P. The growth factor content as an indicator of platelet counts in platelet-rich plasma. Clin. Chim. Acta 2025, 564, 119901. [Google Scholar] [CrossRef] [PubMed]
- Bonowicz, K.; Jerka, D.; Ławkowska, K.; Łuniewska-Bury, J.; Wrońska, I.; Bai, Y.; Gagat, M. TGF-β: The Molecular Mechanisms of Atherosclerosis—Insights into SMAD Pathways and Gene Therapy Prospects. Curr. Med. Chem. 2025, in press. [Google Scholar] [CrossRef] [PubMed]



| Parameter in CAD (n = 100) | Quantity |
|---|---|
| Age of patient (years) | 49.9 ± 5.91 |
| Gender (% males) | 75% |
| Past MI | 70% |
| After first MI (years) | 44.0 ± 5.6 |
| Hypertension presence | 66% |
| After hypertension diagnosis (years) | 42.6 ± 8.6 |
| Diabetes type 2 | 13% |
| Cigarette smokers | 89% |
| Years smoking | 18.9 ± 9.8 |
| CABG | 37% |
| PTCA | 71% |
| Anti-platelet drugs (Aspirin) | 90% |
| Statins | 96% |
| Calcium channel blockers | 18% |
| Beta-blockers | 88% |
| Diuretics | 31% |
| ACEI | 80% |
| ARB | 17% |
| Heart rate (1/min) | 70.7 ± 12.1 |
| Systolic BP (mmHg) | 127 ± 14.2 |
| Diastolic BP (mmHg) | 77.0 ± 9.01 |
| MAP (mmHg) | 93.8 ± 9.35 |
| Weight (kg) | 83.4 ± 17.0 |
| BMI (kg/m2) | 28.1 ± 3.98 |
| WHR | 0.96 ± 0.09 |
| Waist (cm) | 98.3 ± 12.5 |
| Hip (cm) | 103 ± 9 |
| TGF-β (ng/mL) | 31.45 ± 0.74 |
| TNF-α (pg/mL) | 1.33 ± 0.36 |
| VEGF (pg/mL) | 236 ± 17.2 |
| IL-6 (pg/mL) | 1.69 ± 0.77 |
| sCD36 (µg/mL) | 15.8 ±12.9 |
| PCSK9 (ng/mL) | 358 ±10.7 |
| E-selectin (ng/mL) | 35.58 ± 1.21 |
| Platelets (G/L) | 218 ± 44.6 |
| Hemoglobin (g/dL) | 14.8 ± 1.14 |
| WBC (G/L) | 6.80 ± 0.22 |
| PTC (%) | 0.23 ± 0.01 |
| hsCRP (mg/L) | 1.82 ± 2.7 |
| Glucose (mg/dL) | 107 ± 24.8 |
| Triacylglycerols (mg/dL) | 136 ± 57.1 |
| LDL-cholesterol (mg/dL) | 102 ± 36.2 |
| HDL-cholesterol (mg/dL) | 48.4 ± 11.5 |
| Total cholesterol (mg/dL) | 173 ± 40.4 |
| ApoB/ApoA1 | 0.53 ± 0.15 |
| ApoB (mg/dL) | 74.0 ± 2.25 |
| ApoA1 (mg/dL) | 154 ± 38.4 |
| Lp(a) (mg/dL) | 40.3 ± 49.3 |
| Group | Mean ± Sem | p-Value |
|---|---|---|
| CAD | 31.45 ± 0.74 | 0.68 |
| control | 31.14 ± 0.74 | |
| CAD females | 30.81 ± 1.25 | 0.70 |
| CAD males | 31.92 ± 0.91 | |
| control females | 31.16 ± 1.70 | 0.83 |
| control males | 30.70 ± 1.07 |
| Parameters | Correlations for Early-Onset CAD Patients (n = 100) | |
|---|---|---|
| Rs | p-Value | |
| Age of patients (years) | −0.15 | 0.16 |
| Past first MI (years) | −0.06 | 0.62 |
| Age of hypertension diagnosis (years) | −0.23 | 0.06 |
| Systolic BP * (mmHg) | −0.03 | 0.77 |
| Diastolic BP (mmHg) | −0.08 | 0.48 |
| MAP (mmHg) | −0.06 | 0.59 |
| Heart rate (1/min) | −0.07 | 0.54 |
| BMI (kg/m2) | 0.22 | 0.035 |
| Weight (kg) | 0.17 | 0.11 |
| WHR | 0.12 | 0.29 |
| Waist (cm) | 0.19 | 0.07 |
| Hip (cm) | 0.15 | 0.17 |
| Parameter | p-Value |
|---|---|
| Gender (% males) | 0.70 |
| Past MI | 0.81 |
| History of hypertension | 0.25 |
| Presence diabetes type 2 | 0.52 |
| Past CABG | 0.83 |
| Past PTCA | 0.57 |
| Metabolic syndrome | 0.23 |
| Anti-platelet drugs (Aspirin) | 0.84 |
| Statins | 0.44 |
| Calcium channel blockers | 0.45 |
| Beta-blockers | 0.14 |
| Diuretics | 0.07 |
| ACEI | 0.19 |
| ARB | 0.73 |
| Anti-arrhythmic drugs | 0.30 |
| Digitalis | 0.78 |
| Nitrates | 0.00025 |
| Fibrates | 0.63 |
| Trimetazidine | 0.34 |
| NSAIDs * | 0.19 |
| Parameters | Correlations | |
|---|---|---|
| Rs | p-Value | |
| TNF-α (pg/mL) | 0.34 | 0.0006 |
| IL6 (pg/mL) | 0.08 | 0.44 |
| VEGF (pg/mL) | 0.22 | 0.03 |
| sCD36 (µg/mL) | −0.25 | 0.02 |
| PCSK9 (ng/mL) | 0.11 | 0.29 |
| E-selectin (ng/mL) | 0.15 | 0.15 |
| Glucose (mg/dL) | 0.28 | 0.002 |
| Platelets (G/L) | 0.38 | 0.0002 |
| Hemoglobin (g/dL) | 0.19 | 0.07 |
| WBC (G/L) | 0.32 | 0.002 |
| PTC | 0.37 | 0.0003 |
| hsCRP (mg/dL) | 0.17 | 0.11 |
| Triacylglycerol (mg/dL) | 0.35 | 0.0005 |
| LDL-cholesterol (mg/dL) | 0.28 | 0.005 |
| HDL-cholesterol (mg/dL) | −0.13 | 0.22 |
| Total cholesterol (mg/dL) | 0.23 | 0.02 |
| Lp(a) (mg/dL) | −0.08 | 0.46 |
| ApoB/ApoA1 | 0.26 | 0.01 |
| ApoB (mg/dL) | 0.32 | 0.002 |
| ApoA (mg/dL) | −0.01 | 0.94 |
| Variables | p-Value | β Coefficient (95%CI) |
|---|---|---|
| TNF-α | 0.00061 | +0.33 (+0.15–+0.52) |
| Platelets | 0.0048 | +0.27 (+0.08–+0.45) |
| Triacylglycerols | 0.07 | +0.17 (−0.02–+0.36) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakoczy, B.; Dziedziejko, V.; Safranow, K.; Rac, M. Is TGF-β Associated with Cytokines and Other Biochemical or Clinical Risk Parameters in Early-Onset CAD Patients? Biomedicines 2025, 13, 1840. https://doi.org/10.3390/biomedicines13081840
Rakoczy B, Dziedziejko V, Safranow K, Rac M. Is TGF-β Associated with Cytokines and Other Biochemical or Clinical Risk Parameters in Early-Onset CAD Patients? Biomedicines. 2025; 13(8):1840. https://doi.org/10.3390/biomedicines13081840
Chicago/Turabian StyleRakoczy, Bartosz, Violetta Dziedziejko, Krzysztof Safranow, and Monika Rac. 2025. "Is TGF-β Associated with Cytokines and Other Biochemical or Clinical Risk Parameters in Early-Onset CAD Patients?" Biomedicines 13, no. 8: 1840. https://doi.org/10.3390/biomedicines13081840
APA StyleRakoczy, B., Dziedziejko, V., Safranow, K., & Rac, M. (2025). Is TGF-β Associated with Cytokines and Other Biochemical or Clinical Risk Parameters in Early-Onset CAD Patients? Biomedicines, 13(8), 1840. https://doi.org/10.3390/biomedicines13081840






