Abstract
Chronic Obstructive Pulmonary Disease (COPD) is a multifactorial condition associated with significant systemic complications such as cardiovascular disease (CVD), metabolic disorders, muscle wasting, and sarcopenia. While Body Mass Index (BMI) is a well-established indicator of obesity and has prognostic value in COPD, its role in predicting disease outcomes is complex. Muscle wasting is prevalent in COPD patients and exacerbates disease severity, contributing to poor physical performance, reduced quality of life, and increased mortality. Additionally, COPD is linked to metabolic disorders, such as dyslipidemia and diabetes, which contribute to systemic inflammation and worse prognosis and, therefore, should be treated. The systemic inflammatory response plays a central role in the development of sarcopenia. In this review, we highlight the mixed efficacy of statins in managing dyslipidemia in COPD, considering side effects, including muscle toxicity in such a frail population. Alternative lipid-lowering therapies and nutraceuticals, in addition to standard treatment, have the potential to target hypercholesterolemia, which is a coexisting condition present in more than 50% of all COPD patients, without worsening muscle wasting. The interference between adipose tissue and lung, and particularly the potential protective role of adiponectin, an adipocytokine with anti-inflammatory properties, is also reviewed. Respiratory, metabolic and muscular health in COPD is comprehensively assessed. Identifying and managing dyslipidemia and paying attention to other relevant COPD comorbidities, such as sarcopenia and muscle wasting, is important to improve the quality of life and to reduce the clinical burden of COPD patients. Future research should focus on understanding the relationships between these intimate mechanisms to facilitate specific treatment for systemic involvement of COPD.
1. Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous lung condition characterized by persistent and progressive airflow obstruction [1,2]. COPD is associated with significant comorbidities, such as cardiovascular disease (CVD), metabolic disorders, cancer, osteoporosis, skeletal muscle dysfunction, anxiety/depression, cognitive impairment, gastrointestinal (GI) diseases, and muscle wasting [3,4,5]. The systemic effects of COPD not only compromise pulmonary function but also exacerbate the risk of CVD, leading to increased morbidity and mortality [6]. Indeed, in patients with COPD who also have multiple comorbidities, the risk of mortality is greater due to the comorbidities rather than the severity of the COPD itself [7]. Cardiovascular disease represents a significant concern for patients with COPD, playing a substantial role in the elevated mortality rates associated with this condition [8]. Recent research highlights dyslipidemia as a significant comorbidity among COPD patients, suggesting its potential role in disease progression is through mechanisms, such as endothelial injury [9]. In this context, adiponectin, an adipocytokine with anti-inflammatory properties, may play a protective role in the cardiovascular health of COPD patients [10]. Although the impact of dyslipidemia on the progression of COPD is not yet fully understood, it represents a risk factor for the onset of cardiovascular disease in these individuals [9]. A comprehensive nationwide cohort study conducted in Taiwan has revealed a significant association between hyperlipidemia and an increased incidence of COPD among individuals [11]. Despite solid evidence for the efficacy of statins in reducing risk factors for CVD, its role in managing dyslipidemia and outcome improvement in COPD remains controversial and requires further investigation [12]. Understanding the relationship between cholesterol lowering strategies, muscle condition, and COPD may lead to improving clinical outcomes and enhance patient quality of life [13]. Dyslipidemia, muscle wasting, and sarcopenia are all expressions of systemic consequences of COPD and treatments are required to balance protection and side effects [14].
This review aims to explore the interplay between dyslipidemia, muscle wasting, and sarcopenia in COPD. Furthermore, as dyslipidemia has to be considered a modifiable risk factor that also requires treatment in COPD patients, we explored the effect of therapies in patients with muscle wasting and sarcopenia. In this context, we have considered the potential efficacy of alternative treatments, such as nutraceuticals and non-statin lipid-lowering agents, in preserving muscle mass whilst reducing cardiovascular risk factors and improving disease outcomes. A schematic study summary is represented in Figure 1.
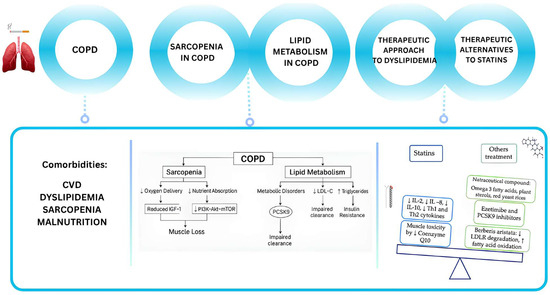
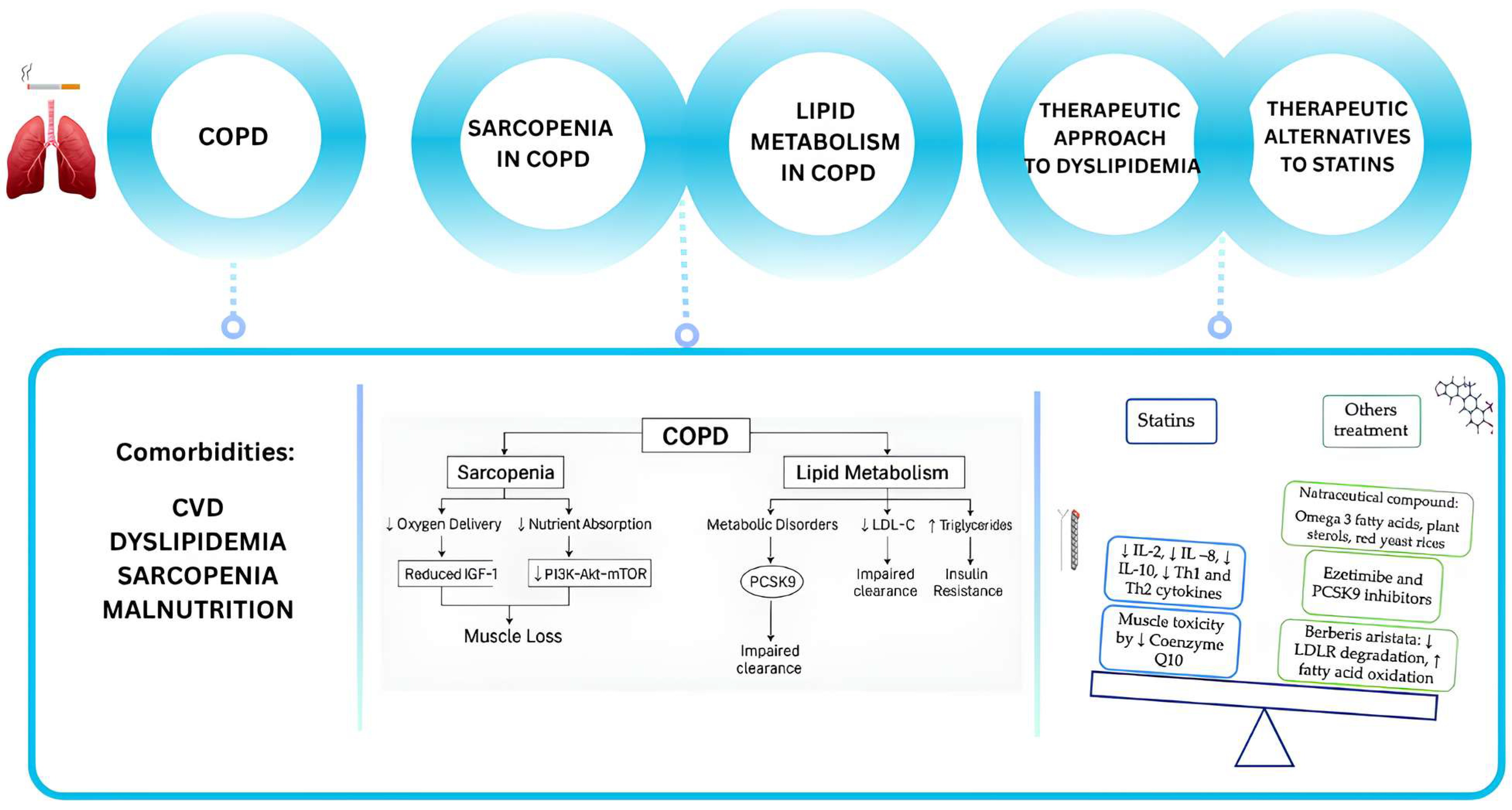
Figure 1.
Schematic overview of the study. COPD is associated with significant comorbidities, such as cardiovascular disease (CVD), metabolic disorders, muscle wasting and sarcopenia. In particular, sarcopenia, poor nutrition, and pulmonary cachexia are associated with COPD and can lead to a worse prognosis. Sarcopenia in COPD involves reduced anabolic signaling, such as impaired IGF-1/PI3K/Akt/mTOR pathways, leading to decreased protein synthesis. Moreover, mitochondrial dysfunction and increased oxidative stress further exacerbate muscle loss. According to the guidelines, statins are the main medication recommended for hypocholesterolemia. Statin therapy is associated with a reduction in IL-2, IL-8, IL-10, Th1, and Th2 cytokines in COPD patients but the most frequent side effect (the muscle toxicity) is due to a reduction in coenzyme Q10. Alternative medications can be the nutraceuticals (like the Barberis aristata, an alkaloid that reduce LDL receptor degradation).
Methods
In this context, the objective of this narrative review is threefold: (i.) to investigate the interference of dyslipidaemia in muscle wasting and sarcopenia in COPD; (ii.) to assess the impact of therapies on patients with muscle wasting and sarcopenia; (iii.) to examine the potential of alternative treatments to statins, including nutraceuticals and non-statin lipid-lowering agents, in preserving muscle mass and improving clinical outcomes in this population. A comprehensive literature search was conducted by MEDLINE, Embase, and the Cochrane Database, including articles published in the last 20 years (from 2005 to 2025). Only the articles written in English were included. The following MESH terms were used, tailored to each of the three research aims: COPD, chronic obstructive pulmonary disease, dyslipidaemia, sarcopenia, muscle wasting, Hydroxymethylglutaryl-CoA Reductase Inhibitors, statin, Nutraceuticals, Nutraceutical, Dietary Supplement, Functional Food, Natural Compound, Polyphenol, and Omega-3 Fatty Acid. This review provides a narrative synthesis of the evidence identified in the literature search.
2. Cardiopulmonary Risk Associated with COPD: Mechanisms Contributing and Underlying Pathogenesis
COPD and CVD frequently coexist [12]. Among patients with COPD, the prevalence of cardiac disease ranges between 30% and 70% and a high proportion of patients with mild or moderate COPD die of cardiovascular events, including myocardial infarction, stroke, heart failure, or arrhythmia [13,14,15]. In patients with severe COPD, the deaths are mainly due to respiratory causes. Other than the increased risk of mortality, patients with COPD and CVD also have increased morbidity, worse quality of life and a greater number of hospitalizations with a longer length of stay [16,17]. COPD and CVD share common risk factors, such as smoking history, exposure to air pollution, more advanced age, physical inactivity, poor diet, or low socioeconomic status [18,19]. Furthermore, complex pathophysiological links exist between these two conditions.
Hyperinflation, characterized by elevated end-expiratory lung volume, is a hallmark of COPD, contributing to worse exertional dyspnea and exercise capacity. Airflow limitation may lead to lung hyperinflation with consequent increased falls in intrathoracic pressures, right-ventricular dysfunction, compromised left-ventricular filling, and reduced cardiac output [20,21]. In patients with COPD, chronic hypoxemia, due to a ventilation/perfusion mismatch, provokes pulmonary vasoconstriction and vascular remodeling, with augmented pulmonary vascular resistance and right-ventricular diastolic dysfunction [22]. Hypoxia and lung hyperinflation can cause pulmonary hypertension [23]. Hypoxia also increases systemic inflammation and oxidative stress [24]. Persistent low-grade systemic inflammation in COPD contributes to the formation and progression of atherosclerotic plaque. Several studies have shown that patients with stable COPD and CVD have higher levels of systemic inflammatory biomarkers, such as fibrinogen, C-reactive protein (CRP), interleukin (IL)-6, and IL-8 [25,26]. Levels of inflammatory biomarkers further increase during COPD exacerbation (AECOPD). A recent meta-analysis identified an increased risk of acute CV events after AECOPD, mainly severe, and in particular, the risk of acute myocardial was increased immediately after COPD exacerbation and remained high for up to a year later [27]. Possible mechanisms related to the risk of acute CV events after COPD exacerbation include systemic inflammation, acute hypoxemia, oxidative stress, and increased platelet activity [28,29]. These conditions may increase the risk of plaque rupture with subsequent acute cardiovascular event [30]. Another interesting finding is that patients with COPD have increased arterial stiffness, a strong predictive value for CV events, in comparison with age and smoking matched controls [31]. A possible explanation is that degradation of elastin occurs not only in the lung, resulting in emphysema, but also in the arterial walls, resulting in more pronounced arterial stiffness.
The presence of comorbid CVD inevitably complicates the management of COPD and specific recommendations are necessary for the management of CVD in patients with COPD. For example, β-blockers are widely prescribed in the treatment of CVD, but they are often underused in COPD patients due to their potential antagonism with β2-agonists inducing bronchoconstriction [32]. Cardio selective β-blockers (e.g., atenolol, bisoprolol, and metoprolol) should be recommended in patients with COPD. On the other hand, long-acting β2-agonists (LABAs) represent the mainstay pharmacological treatment of COPD, but they have been associated with an increased CV risk, possibly due to the stimulation of the sympathetic nervous system [33]. There are also data that suggest favorable effects of LABAs on CV risk, due to reducing lung hyperinflation and the rate of COPD exacerbations [34,35]. Similarly, long-acting muscarinic antagonists (LAMAs) reduce lung hyperinflation but they could induce cardiac arrhythmias, suppressing parasympathetic control of heart rate [36,37]. Other drugs routinely prescribed in patients with CVD are angiotensin conversion enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs). A study suggests that the use of ACE-Is or ARBs slow the progression of emphysema, but a side effect of ACE-I is coughing [38]. In addition, an observational cohort study among patients with COPD found that ARBs were associated with lower risk of severe exacerbations and mortality, in comparison to ACEIs [39]. This finding may suggest that ARBs are a preferred choice for patients with COPD. Patients with COPD often receive statins for the prevention of cardiovascular risk. Many studies suggest positive effects of statin use in patients with COPD, reducing lung function decline and exacerbations and improving symptoms [40,41,42]. Antiplatelet therapy may be beneficial after an exacerbation when platelet activation occurs [43].
3. Additional Mechanisms Implicated in COPD: From Muscle Wasting to Sarcopenia
Body Mass Index (BMI) is an accepted indicator of obesity, and it is associated with COPD [15]. Patients with lower BMI had more severe COPD symptoms, including worse airflow obstruction, hyperinflation, and osteoporosis. Conversely, patients with an increase in BMI had a higher prevalence of cardiovascular comorbidities and systemic inflammation, indicating that adipose tissue might modulate disease expression differently in these two groups [16]. In a large cohort of the Korean population, the authors demonstrated that a decrease in BMI is associated with an increased risk of severe exacerbations and all-cause mortality in COPD patients. Notably, the relationship between BMI reduction and mortality is dose dependent. Conversely, an increase in BMI is linked to a higher risk of death only among obese COPD patients [17]. Abnormal lipid metabolism in COPD patients can impair immunity, airway repair, and tissue remodeling, while excessive fat accumulation worsens inflammation and metabolic dysfunction [18]. These findings highlight the importance of monitoring BMI as part of non-pharmacological management and as a predictor of COPD outcomes.
Although it is a lung disease, COPD often leads to systemic complications, such as muscle wasting and sarcopenia [19]. Muscle wasting can progress to sarcopenia, a multifactorial condition characterized by progressive loss of skeletal muscle mass, impaired neuromuscular junction stability, reduced anabolic signaling, increased proteolysis, mitochondrial dysfunction, and chronic inflammation [20]. Central to its pathogenesis is decreased anabolic signaling—particularly a reduction in IGF-1–induced activation of the PI3K–Akt–mTOR axis—which leads to diminished protein synthesis, while concurrently allows FOXO transcription factors to up-regulate E3 ubiquitin ligases (Atrogin-1, MuRF-1), thus enhancing proteasomal degradation [44,45]. Mitochondrial dysfunction and oxidative stress further exacerbate atrophy, as reactive oxygen species impair mitochondrial quality control and reduce PGC-1α-mediated biogenesis. Additionally, neuromuscular junction destabilization and satellite cell senescence compromise muscle regeneration [46]. In the context of COPD, these age-associated mechanisms are significantly amplified by systemic and muscle-specific inflammation (elevated TNF-α, IL-6), chronic hypoxia, oxidative imbalances, and corticosteroid exposure—all contributing to a hypercatabolic state [47]. Crucially, COPD muscle biopsies show an overactivation of the myostatin–Smad2/3 pathway, which inhibits Akt signaling and promotes atrophy signaling via FOXO [48] (Figure 2). Concurrently, markers of autophagy and protein turnover are elevated in patients with COPD, indicating excessive degradation.
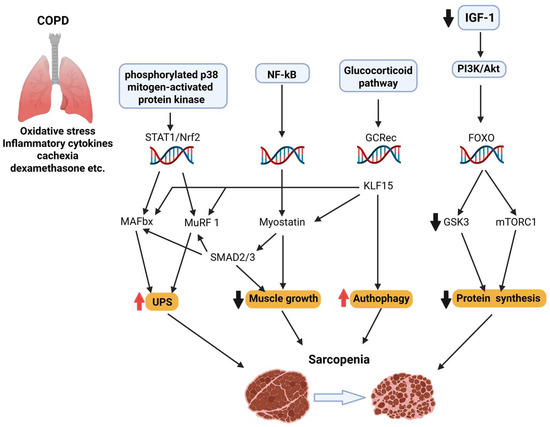
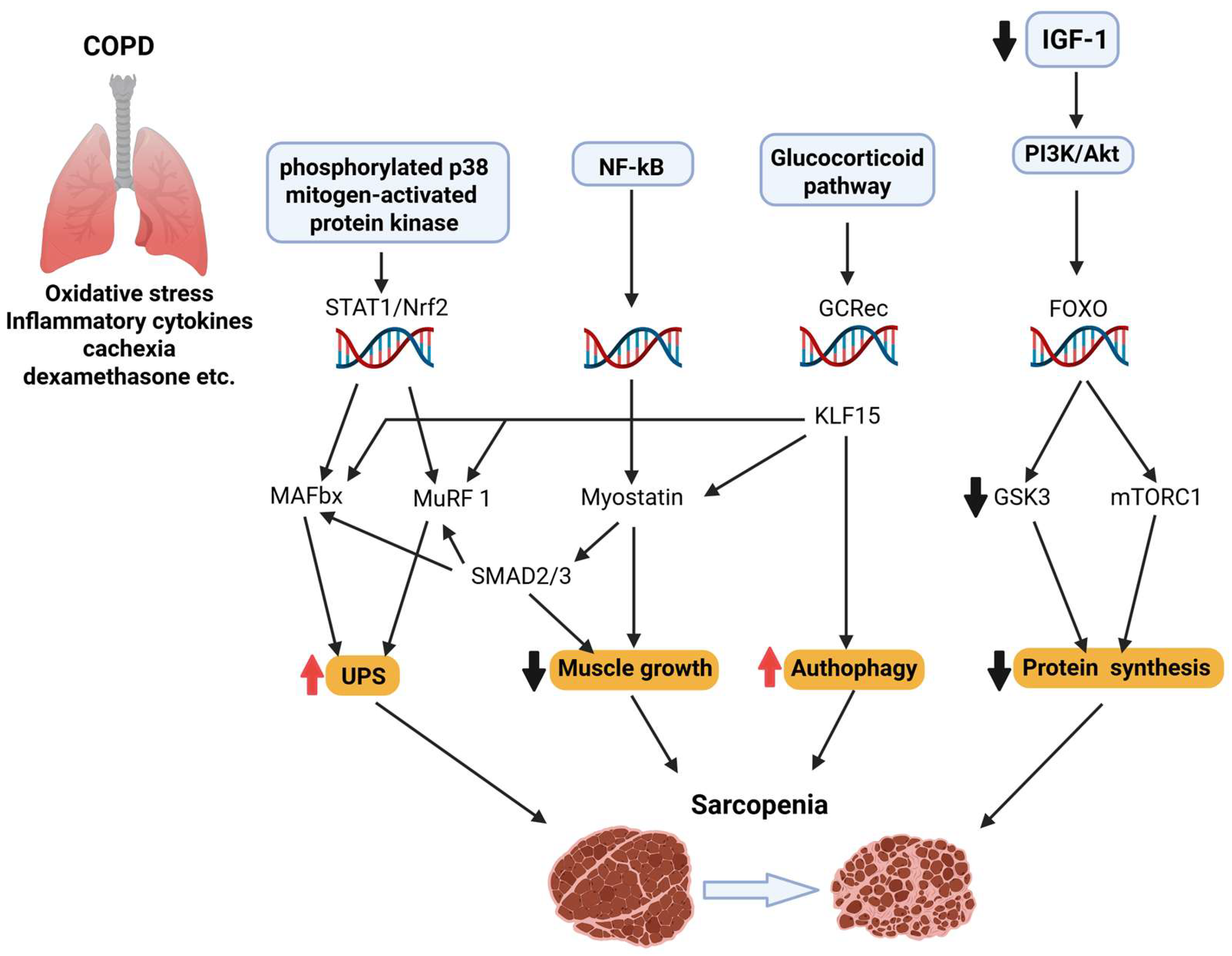
Figure 2.
Sarcopenia is a multifactorial condition marked by the progressive loss of skeletal muscle mass and function. It involves impaired neuromuscular junction stability, reduced IGF-1/PI3K/Akt/mTOR signaling, increased FOXO-driven proteolysis via Atrogin-1 and MuRF1, mitochondrial dysfunction, and chronic inflammation. In COPD, these mechanisms are exacerbated by systemic inflammation, hypoxia, corticosteroids, and myostatin–Smad2/3 signaling, promoting a hypercatabolic state and excessive autophagy.
According to the European Working Group on Sarcopenia in Older People (EWGSOP), for the diagnosis of sarcopenia, three parameters should be evaluated: low muscle strength, low muscle quantity or quality, and low physical performance; moreover, if these three parameters are present together, sarcopenia can be classified as severe [21].
In general, several factors contribute to reduced muscle strength and endurance, including chronic inflammation, oxidative stress, physical inactivity, hypoxemia, hormonal imbalances, deficiencies in essential nutrients, like protein and vitamin D, and the use of systemic corticosteroids [22,23]. However, in COPD patients, sarcopenia is reported to occur in about 25% of cases and is linked to reduced lung function and a decline in overall health status [25]. Sarcopenia can negatively affect COPD patients by reducing quality of life, increasing the risk of hospitalization, and raising mortality rates [26]. The main pathogenic feature is the systemic inflammatory response with the releasing of TNFα and IL-6, which correlate negatively with grip strength (HGS) skeletal muscle mass index (SMMI) in COPD patients compared to patients without muscle loss [27]. The presence of systemic inflammation is closely linked to complications that can impact prognosis, including weight loss, cachexia, and cardiovascular disease [28]. Byun et al. examined the correlations between sarcopenia and the systemic inflammatory biomarkers IL-6 and high sensitivity (hs)TNFα. Their findings revealed significant relationships between muscle strength, as measured by HGS and muscle mass, assessed through skeletal muscle mass index (SMMI), with levels of IL-6 and hsTNFα. Multivariate analysis indicated that higher hsTNFα was a significant predictor of sarcopenia [10].
Additionally, the reduced oxygen delivery and nutrient absorption seen in advanced stages of COPD exacerbate muscle loss. The resultant sarcopenia not only diminishes physical strength and endurance but also contributes to a vicious cycle of worsening respiratory function and decreased quality of life [30]. Clinical outcomes, such as the severity of COPD, the extent of dyspnea, and the presence of CVD, are strongly related to sarcopenia in these patients [31].
Sarcopenia and HGS are linked to the frequency of AECOPD [32,33]. Perrot et al. found that the prevalence of sarcopenia was notably high among COPD patients during an acute exacerbation (48%) and remained significant after recovery (30%) [34]. Notably, De Blasio et al. reported that 58.7% of COPD patients with sarcopenia were also malnourished. This condition is common in patients with progression of COPD and, in particular, in patients with systemic inflammation, like cachectic patients. According to them, malnourished patients with sarcopenia exhibited a notable decline in BMI, fat-free mass, and HGS compared to those without sarcopenia [35,36,37,38].
Furthermore, hormonal dysregulation, including reduced levels of testosterone and insulin-like growth factor 1 (IGF-1), can hinder muscle regeneration and maintenance. These pathways illustrate the link between respiratory and muscular health in COPD, emphasizing the need for strategies targeting both lung function and muscle strength [39].
While smoking is widely acknowledged as a significant and established risk factor for COPD, the direct relationship between smoking and sarcopenia remains a subject of debate and investigation [41]. Patients with sarcopenia demonstrated lower predicted forced expiratory volume in the first second (FEV1) and had reduced exercise tolerance and lower quality of life compared to those without sarcopenia. Sarcopenia is common among individuals with COPD and has a detrimental effect on key clinical outcomes. However, further research is needed to assess its impact on mortality in this population [42].
4. Adipokines in Metabolic Disorders of COPD
In the last few years, it has been recognized that adipose tissue plays a central role in the control of immune and inflammatory responses with cross-talk between adipose tissue and the lung. The functional role of adipose tissue is strictly related to its endocrine functions, capable of secreting hormones known as adipokines. It is plausible that the contribution of adipose tissue in the lung inflammatory state is the molecular basis of the cross-talk between the two organs and that the secretion of adipokines is part of a very complex inflammatory milieu that is established in the lungs of COPD patients sustained by neutrophils, macrophages, and CD8+ T cells, with increased production of chemokines and cytokines [49].
Adiponectin and leptin are among the principal adipokines. It is believed that adiponectin is the main adipokine, being secreted in serum at very high levels between 5 and 30 μg/mL. It is generally recognized that adiponectin levels are increased in COPD patients, with even higher levels in those without bronchiectasis and worse prognosis [43]. For this reason, adiponectin has been proposed as a biomarker to identify patients with advanced COPD patients and to monitor severity and progression of the disease [43]. Functionally, such up-regulation of adiponectin levels might be traceable to multiple aspects of COPD, i.e., the sustained inflammation as well as alteration in body composition. It is proposed that the increased production of adiponectin represents a biological response in an attempt to counteract inflammation. In addition to the inflammatory response, body composition is another important factor in COPD patients, especially in controlling disease progression and prognosis. Indeed, metabolic alterations are found to be more frequent in COPD. It has been shown that BMI and fat free mass index (FFM) are inversely related to mortality in patients with COPD [50,51]. Oliveira et al. reported that adiponectin levels were significantly and positively correlated with fat mass and the fat mass index while negatively correlated with fat-free mass and the fat-free mass index in patients with bronchiectasis [52]. It has also been shown that high BMI values are associated with high levels of C-reactive protein (CRP) and a recent study identified that this association is present even in obese patients with COPD [53]. Therefore, it is plausible that systemic inflammation is one of the potential mechanisms responsible for both COPD and metabolic syndrome, as suggested by the presence of various inflammatory markers in different biological samples such as plasma, sputum, and bronchoalveolar fluid. This suggests that inflammatory response and body composition are both regulated by adipokines—with a specific regard to adiponectin—in COPD diseases participating in the control of the progression and prognosis.
Leptin is a protein product of the ob gene, synthesized and secreted mainly by white adipose tissue but expressed also by the human lung, including bronchial epithelial cells and alveolar type II pneumocytes and macrophages [54]. Systemic leptin may be associated with greater COPD prevalence and severity; Breyer et al. demonstrated a positive correlation between serum concentrations of leptin and C-reactive protein in women with COPD but not in men [55]. Schols et al. reported a positive correlation between plasma concentrations of leptin (adjusted for fat mass) and of soluble TNF receptor, a marker of systemic inflammation, among stable male patients [56]. On the other hand, other case-control studies did not confirm such an association between leptin and activity of the TNF-alpha system [57]. Such data suggest a regulation of leptin in stable/unstable COPD with a clear correlation to the control of the inflammatory response. In accordance with that, leptin concentrations rise during acute COPD exacerbations and return to baseline in the stable state following the resolution of the exacerbation [58,59]. Serum leptin levels (ng/mL) were significantly higher in obese COPD cases compared to controls and non-obese cases and during exacerbations, which indicates that leptin plays a role in the systemic inflammatory process [60]. Regarding the relationship between leptin, body weight, and composition in COPD, several studies outlined that the cause of weight loss in some patients is not due to increased circulating leptin in COPD. Instead, leptin remains regulated in COPD and further decreases in patients with low BMI, probably as a compensatory mechanism in an attempt to preserve body fat content [61]. Serum leptin hormone level is positively correlated with BMI (kg/m2). In this context, it is fundamental to consider the leptin/adiponectin ratio since it is recognized as a functional biomarker of adipose tissue functioning [62]. Metabolically unhealthy COPD patients have higher levels of leptin, lower levels of adiponectin, and increased insulin resistance, compared with patients without metabolic syndrome. These individuals constitute a subgroup of patients with a specific COPD phenotype characterized by an increased leptin–adiponectin imbalance and insulin resistance. Patients with stable COPD have also been shown to have increased leptin levels [63], while an increased leptin/adiponectin ratio has been reported during COPD exacerbations [59]. Besides the metabolic regulation of adiponectin in this group of COPD, Watz et al. reported that in COPD patients, the presence of metabolic syndrome is associated with an increased inflammatory profile; this coexistence would suggest a role for adiponectin in the regulation of both inflammation and body composition [64].
Adiponectin and leptin are two central hormones in COPD and can be considered as potential biomarkers for both disease severity and prognosis, as well as for the presence of concomitant metabolic disorders. The functional role of adiponectin up-regulation in the above-mentioned conditions is still a matter of debate but it is likely that this adipokine plays a role in t counteracting chronic inflammation, a hallmark of both COPD and metabolic disorders [65,66]. On the other hand, the adiponectin–leptin ratio represents a promising diagnostic and prognostic index in COPD in relation to body composition.
5. Targets for Lipid Related Risk: Risk Assessment Tools
Airway inflammation is a consistent feature of COPD and is implicated in the pathogenesis and progression of disease [67]. COPD is most associated with the activation of innate immune pathways, including T1 and T17 responses, but in a subset of patients—especially those with severe disease-, eosinophil-mediated, and autoimmune responses have also been implicated [5,67]. Adiponectin, an insulin sensitizing hormone, also takes part in the regulation of inflammation [68]. The serum haptoglobin level is simultaneously and significantly increased in COPD rather than in controls [69]. In addition, its expression is significantly and negatively correlated with FEV1 both in COPD and in controls, and for haptoglobin, also strongly supports the role of a pro-inflammatory cytokine in the immune systems of COPD patients [70,71]. A high proportion of patients with COPD have CVD, but there is also evidence that COPD is a risk factor for adverse outcomes in CVD [72]. This connection between COPD and cardiovascular risk appears to be linked to the increase in lipoproteins [73]. There are six major lipoproteins in the blood: chylomicrons, very low-density lipoprotein (VLDL), intermediate density lipoprotein (IDL), low-density lipoprotein (LDL); lipoprotein(a) (Lp(a)), and high-density lipoprotein (HDL) [74]. Lipoproteins in the plasma transport lipids to tissues and consist of esterified and non-esterified cholesterol, TG, and phospholipids, and protein components called apolipoproteins that act as structural components, ligands for binding to cellular receptors, and enzyme activators or inhibitors [74,75]. All ApoB-containing lipoproteins with diameter <70 nm, including the smaller TG-rich lipoproteins and their residual particles, can cross the endothelial barrier, especially in the presence of endothelial dysfunction [76] where they can remain retained in the arterial wall causing a complex process leading to lipid deposition [76,77], the initiation of an atheroma and the subsequent growth and more rapid progression of atherosclerotic plaques. Patients with severe and very severe COPD have higher levels of cholesterol and LDL in the blood and lower levels of HDL in the blood [78]. Accumulation of cholesterol and its oxidized derivatives (oxysterols) in macrophages activates the NLRP3 inflammasome, promoting the release of IL-1β and IL-18 and fostering a chronic pro-inflammatory environment [79]. Additionally, elevated membrane cholesterol enhances the formation of lipid rafts that facilitate Toll-like receptor (TLR) signaling, particularly TLR4, thereby amplifying innate immune responses [80,81]. Among lipoproteins, oxidized LDL (oxLDL) has strong pro-inflammatory effects: it induces endothelial activation, increases cytokine production, and engages TLRs, contributing to atherogenesis and systemic inflammation [82,83]. Conversely, HDL is generally anti-inflammatory, inhibiting LDL oxidation, promoting cholesterol efflux via ABCA1 and ABCG1 transporters, and suppressing inflammatory signaling; however, during aging or chronic disease, HDL becomes dysfunctional and may lose these protective effects [84,85]. ApoA-I, the main apolipoprotein in HDL, directly inhibits NF-κB signaling in macrophages [86], while enzymes, like Lp-PLA2, can further modulate the inflammatory response depending on lipid substrate availability [87]. These inflammatory and lipid-related processes are closely linked to sarcopenia. Disrupted lipid metabolism, particularly ectopic deposition of toxic lipid species, such as ceramides and diacylglycerols in skeletal muscle, impairs mitochondrial function and insulin signaling, leading to anabolic resistance and muscle protein degradation [88,89]. Systemic low-grade inflammation—often exacerbated by dyslipidemia—promotes catabolic pathways in muscle through increased cytokines like TNF-α and IL-6 [90,91]. Another potential target is represented by polyunsaturated fatty acids (PUFAs) [92]. Their biosynthetic products, including eicosanoids and docosanoids, modulate processes involved in CVD, such as inflammatory processes, endothelial dysfunction, and immune modulation [93]. Additionally, mitochondrial dysfunction and reactive oxygen species (ROS) production impair muscle homeostasis, partly by activating redox-sensitive transcription factors like NF-κB [94,95]. Insulin resistance, driven in part by lipid accumulation, further impairs muscle protein synthesis via the suppression of PI3K-Akt-mTOR signaling [96]. In a large meta-analysis of randomized trials, triglyceride lowering was associated with lower ASCVD risk, which was somewhat lower than seen for LDL-C [78,97]. In a comprehensive systematic review and meta-analysis of risk factors for premature myocardial infarction, a mild elevation of triglycerides (>150 mg/dL) was associated with a two- to three-fold increased risk of premature myocardial infarction, like that magnitude of risk noted for total cholesterol > 200 mg/dL or HDL-C < 60 mg/dL [98,99]. Furthermore, triglycerides are an important risk marker for premature CHD events in women, with a stronger risk magnitude than LDL cholesterol or non-HDL cholesterol [99,100].
6. Prevalence of Dyslipidemia and Cardiovascular Risk Among COPD Patients
The occurrence of CVD among COPD patients varies widely, ranging from 20% to 70%, depending on the specific type of CVD assessed [101,102,103]. A systematic review by Chen et al. found that individuals with COPD have nearly double the risk of developing CVD compared to the general population (OR 2.46, 95% CI 2.02–3.00) [11,101,104]. This elevated risk persists even after adjusting for factors such as age and smoking [101,104]. The GOLD guideline recommends the use of the National Heart and Lung Institute’s global risk calculator for evaluating cardiovascular risk in COPD patients [105]. Dyslipidemia, a common risk factor for cardiovascular disease, is also frequently seen in COPD patients, although its influence on COPD progression is not yet fully understood [103]. A large-scale study in Taiwan, which included over 100,000 patients, found that individuals with hyperlipidemia had a higher likelihood of developing COPD later on compared to those without hyperlipidemia. This finding suggests a potential link between COPD progression and endothelial damage associated with dyslipidemia [11,106]. The efficacy of statins and anti-inflammatory treatments for dyslipidemia in enhancing COPD outcomes remains a topic of debate, with observational studies showing mixed results, likely influenced by various study biases [12]. Two randomized controlled trials have specifically investigated the impact of statins on COPD: one reported no effect of simvastatin on COPD exacerbations, while the other found that simvastatin delayed the onset of first exacerbations and reduced exacerbation frequency [107]. These findings highlight the need for further research to clarify the precise role of statins in COPD management.
7. The Use of Medications for Dyslipidemia: Clinical Significance and Implications
According to the guidelines, statins are the main medication recommended for hypocholesterolemia [74,75]. The mechanisms of action of these drugs are to reduce cholesterol synthesis, increase hepatic expression of the LDL receptor and uptake of Apo-B-containing lipoproteins by inhibiting 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA) determining a reduction in plasma LDL-C levels and triglycerides [108,109]. Sanja et al. studied the possible effect of statin therapy on the expression of inflammatory cytokines involved in COPD; for example, IL-1β, IL-2, IL-4, IL-8, IL-10, IL-12p70, and TNF-α and the possible associations between cytokines and BMI [67]. The authors observed a reduction in these cytokines in COPD patients in treatment with statin compared to COPD patients not receiving statin therapy. In particular, COPD patients with increased BMI (>25) had reduced concentrations of IL-2 (p = 0.038), IL-8 (p = 0.039), and IL-10 (p = 0.005) compared to patients with normal BMI (20–25). Moreover, statin therapy is associated with the reduced expression of selected Th1 and Th2 cytokines in COPD, and this effect may be relevant in COPD patients with increased CVD [73]. A systematic review and meta-analysis by M. Abbasifard showed that statins significantly reduce serum TNF-α levels in CVD patients (SMD = −0.99 pg/mL; 95% CI: −1.43 to −0.55; p < 0.001) [110]. Since TNF-α and proinflammatory interleukins contribute to COPD progression [111], lowering TNF-α and IL-1, IL-8, and IL-10 may improve both systemic inflammation in COPD and cardiovascular risk. Although they are drugs with a very good safety profile, the most important side effects include myopathy, myalgia, muscle weakness, and rhabdomyolysis [75,112,113], which are dose-dependent and usually resolve with dose reduction or discontinuation of treatment [114]. A recent meta-analysis of 176 studies with 4,143,517 patients showed that the worldwide prevalence of statin intolerance, defined according to the International Lipid Expert Panel (ILEP), National Lipid Association (NLA), and Luso-Latin American Consortium (LLAC) criteria, is 9.1% [115]. In a 3-year study monitoring 774 older adults, Scott et al. [116] associated a greater decrease in muscle strength and an increased risk of falls in patients treated with statins compared to those not treated [108,116]. Therefore, for the secondary prevention of cardiovascular disease, evidence suggests that statin treatments are highly cost-effective [112,117,118,119] while their use in primary prevention is controversial [72]. Indeed, in primary prevention, 25–50% of patients with a new statin prescription discontinue therapy during the first year, with a worsening trend over time showing an adherence rate of only 25% after two years [120]. Therapeutic adherence and persistence are key factors for the success of all drug therapies.
8. Therapeutic Alternatives to Statins
While statins have long been considered the standard treatment for managing cholesterol levels and lowering the associated risks, many patients either do not reach their cholesterol targets or cannot tolerate statins due to side effects [121]. The muscle toxicity potentially associated with statins has been linked to a reduction in Coenzyme Q10, a crucial component of the mitochondrial respiratory chain [122]. In a three-year study involving 774 older patients, Scott et al. found that patients treated with statins experienced a greater decline in muscle strength and a higher risk of falls compared to patients who were not treated [108,115]. Recent studies have shown that non-statin cholesterol-lowering medications, such as ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, offer cardiovascular benefits [123].
In particular, PCSK9 inhibitors are monoclonal antibodies that target PCSK9 [123], a protein involved in LDL cholesterol regulation. By binding to LDL receptors on hepatocytes, PCSK9 promotes their degradation via intracellular pathways [124]. Thus, PCSK9 inhibitors block this interaction, enhancing receptor recycling and increasing the clearance of circulating LDLcholesterol [123,124].
In this context, great interest in recent years has been around nutraceuticals and functional foods in terms of a reduction in cholesterol levels [125,126,127]. The International Lipid Expert Panel emphasized the potential use of specific nutraceuticals, such as Omega-3 Fatty Acids, plant sterols, red yeast rice, and soluble fiber, to complement standard pharmacological approaches in managing dyslipidemia. The panel highlights that while these natural compounds may help in reducing low-density lipoprotein cholesterol (LDL-C) and triglyceride levels, their efficacy can vary based on individual patient profiles, dietary habits, and genetic predispositions [128]. The potential for combining different nutraceuticals stems from the possibility of enhancing lipid-lowering effects through their complementary actions and the ability to lower the doses needed for effectiveness while ensuring tolerability [129,130]. From reviewing the literature, the combination of fiber and phytosterols in individuals with normal lipid levels and those with moderate–high cholesterol levels indicates that this combination can lead to an average reduction of about 8% in total cholesterol and 11% in LDL cholesterol. Furthermore, two studies have suggested that using both components together results in a slightly more pronounced reduction in cholesterol levels compared to using each component individually [109,131]. In this setting, Berberis aristata/Silybum marianum (BBR) is a benzylisoquinoline quaternary alkaloid which, inhibiting the proprotein convertase subtilisin/kexin type 9 (PCSK9), leads to increased levels of hepatic LDL receptors (LDLR) and reduced LDLR degradation [132,133]. Moreover, BBR activates AMP-activated protein kinase (AMPK), promoting fatty acid oxidation and inhibiting lipogenic gene expression [134,135]. A recent meta-analysis confirmed the efficacy of BBR on the reduction in the lipid level and appeared to be additive to those of statins with an improvement on glucose metabolism and blood pressure [136]. A Palimerica study evaluated the efficacy of a nutraceutical compound useful in regulating the metabolism of LDL-C and triglycerides [137]. Thanks to the combined effect of berberine, selective optichol, artichoke, fistosterols, and fenugreek, Derosa et al. demonstrated a significant control by the product not only on total cholesterol and LDL levels, but also on triglycerides and carbohydrate profile. The study included two treatment arms, one and two capsules per day, respectively. The primary objective was achieved in the treatment group with two capsules/day, in which after 3 months of treatment there was a 25% reduction in LDL-C equal to 40 mg/dl [75,137]. The guidelines state that a reduction in LDL cholesterol of 40 mg/dL reduces CV risk by about 20% [75]. Furthermore, the results of the secondary endpoints after 3 months of treatment showed a reduction of total cholesterol by 11% (27 mg/dl) in the group treated with one capsule/day and by 19% (45 mg/dl) in the group with two capsules/day, a reduction of triglycerides by 11% (19 mg/dl) vs. 16% (29 mg/dl), a reduction of glycemia by 8% (8 mg/dl) vs. 14% (13 mg/dl) and also a reduction of insulinemia by 15% (2 μUI/mL) vs. 21% (3 μUI/mL) [137].
An overview of a different therapeutic class for dyslipidemia and the role in the treatment in COPD patients is represented in Table 1 (below).

Table 1.
Different therapeutic class for the treatment of dyslipidemia and the additional role in COPD patients. We provide a summary of different class of drugs according to the mechanism of action, lipid effects, side effects, and the correlation with real life evidence.
Therefore, the decision to suggest nutraceutical products to control LDL-C levels in patients in primary prevention can be taken by the physician in specific conditions [126]. In secondary prevention, the use of nutraceuticals or functional foods can be a valuable tool to reduce LDL-C levels (and, therefore, the cumulative LDL-C load) when target LDL-C levels are not reached with statins as they have an additive action. Therapeutic alternatives to statins for managing cholesterol levels and cardiovascular risk include several classes of medications and lifestyle interventions. Engaging in physical activity, especially aerobic exercise, has been shown to enhance cardiovascular outcomes by positively affecting various risk factors for CV, including dyslipidemias [109,138].
9. Conclusions
The complex relationship between COPD and its comorbidities underscores the importance of comprehensive management strategies that focus not only on respiratory function but also systemic complications such as muscle wasting and dyslipidemia. Statins have shown promise in reducing inflammation and improving cardiovascular outcomes in COPD patients, yet concerns regarding their side effects, especially muscle toxicity, highlight the need for alternative approaches. Therapies aimed to normalize cholesterol levels without muscle toxicity may be beneficial to avoid muscle waste progression towards sarcopenia in COPD. A comprehensive assessment and targeted optimization of nutritional status and lipid profiles are crucial to achieving effective management and improving outcomes in COPD patients. Emphasizing lifestyle modifications, integrating nutraceuticals, and exploring innovative lipid-lowering therapies could provide favorable adjuncts to traditional pharmacological treatments. However, potential confounding factors in the reviewed studies, such as rehabilitation interventions and corticosteroid use, should be considered. Furthermore, recognizing and treating dyslipidemia and sarcopenia in these patients should be a priority for improving their overall quality of life and reducing morbidity and mortality. Future studies are required to clarify the interactions between these factors to enable the development of integrated therapeutic strategies tailored to the patients with COPD.
Author Contributions
Conceptualization, A.B. and F.P.; methodology, A.B.; software, F.S.; validation, R.P., V.D., and D.F.M.; formal analysis, R.P.; investigation, A.S.; resources, R.C.; data curation, R.P.; writing—original draft preparation, A.B., R.P., and F.P.; writing—review and editing, E.N. and A.D.; visualization, F.S.; supervision, F.P.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Nucera, F.; Bianco, A.; David, T.; Salvato, I.; Adcock, I.M.; Caramori, G. Treatable traits in COPDpatients. Minerva Medica 2022, 113, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Negewo, N.A.; Gibson, P.G.; McDonald, V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015, 20, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; D’aGnano, V.; Scialò, F.; Komici, K.; Allocca, V.; Nucera, F.; Salvi, R.; Stella, G.M.; Bianco, A. Evolving concepts in COPD and lung cancer: A narrative review. Minerva Medica 2022, 113, 436–448. [Google Scholar] [CrossRef]
- Mariniello, D.F.; D’Agnano, V.; Cennamo, D.; Conte, S.; Quarcio, G.; Notizia, L.; Pagliaro, R.; Schiattarella, A.; Salvi, R.; Bianco, A.; et al. Comorbidities in COPD: Current and Future Treatment Challenges. J. Clin. Med. 2024, 13, 743. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef]
- Iglesias, J.R.; Díez-Manglano, J.; García, F.L.; Peromingo, J.A.D.; Almagro, P.; Aguilar, J.M.V. Management of the COPD Patient with Comorbidities: An Experts Recommendation Document. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1015–1037. [Google Scholar] [CrossRef]
- Berry, C.E.; Wise, R.A. Mortality in COPD: Causes, risk factors, and prevention. COPD: J. Chronic Obstr. Pulm. Dis. 2010, 7, 375–382. [Google Scholar] [CrossRef]
- Isago, H. The Association between Dyslipidemia and Pulmonary Diseases. J. Atheroscler. Thromb. 2024, 31, 1249–1259. [Google Scholar] [CrossRef]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Hu, L.-Y.; Chen, H.-J.; Chen, R.-Y.; Hu, C.-K.; Shen, C.-C. Increased Risk of Chronic Obstructive Pulmonary Disease in Patients with Hyperlipidemia: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 12331. [Google Scholar] [CrossRef]
- Sule, N.O.; Suissa, S. Statins and Mortality in COPD: A Methodological Review of Observational Studies. COPD J. Chronic Obstr. Pulm. Dis. 2023, 20, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Dobler, C.C.; Wong, K.K.; Marks, G.B. Associations between statins and COPD: A systematic review. BMC Pulm. Med. 2009, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Lewandowicz, M.; Kaczmarek, B.; Wieczorowska-Tobis, K. Malnutrition, sarcopenia, and malnutrition-sarcopenia syndrome in older adults with COPD. Nutrients 2022, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; von Haehling, S.; Doehner, W.; Sarc, I.; Jeric, T.; Ziherl, K.; Kosnik, M.; Anker, S.D.; Suskovic, S. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J. Cachex-Sarcopenia Muscle 2011, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cabrera, C.; Casanova, C.; Marin, J.; Pinto-Plata, V.; de-Torres, J.; Zulueta, J.; Zagaceta, J.; Sanchez-Salcedo, P.; Berto, J.; et al. Comorbidity Distribution, Clinical Expression and Survival in COPD Patients with Different Body Mass Index. Chronic Obstr. Pulm. Dis. J. COPD Found. 2014, 1, 229–238. [Google Scholar] [CrossRef][Green Version]
- Kim, T.; Shin, S.H.; Kim, H.; Im, Y.; Cho, J.; Kang, D.; Park, H.Y. Longitudinal BMI change and outcomes in Chronic Obstructive Pulmonary Disease: A nationwide population-based cohort study. Respir. Res. 2024, 25, 150. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Q.; Li, Z.; Li, F. Body Composition and COPD: A New Perspective. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 79–97. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, M.J.; Shin, Y.B.; Kim, K.U. Sarcopenia associated with chronic obstructive pulmonary disease. J. Bone Metab. 2019, 26, 65–74. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Marquis, K.; Debigaré, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaré, R.; Dekhuijzen, P.N.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American thoracic society/European respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Tsepis, E.; Billis, E.; Gliatis, J. Sarcopenia in patients with chronic obstructive pulmonary disease: A study of prevalence and associated factors in Western Greek population. Lung India 2020, 37, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Martínez, M.; Rodríguez-García, W.; González-Islas, D.; Orea-Tejeda, A.; Keirns-Davis, C.; Salgado-Fernández, F.; Hernández-López, S.; Jiménez-Valentín, A.; Ríos-Pereda, A.V.; Márquez-Cordero, J.C.; et al. Impact of Body Composition and Sarcopenia on Mortality in Chronic Obstructive Pulmonary Disease Patients. J. Clin. Med. 2023, 12, 1321. [Google Scholar] [CrossRef]
- Ma, K.; Huang, F.; Qiao, R.; Miao, L. Pathogenesis of sarcopenia in chronic obstructive pulmonary disease. Front. Physiol. 2022, 13, 850964. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Byun, M.K.; Na Cho, E.; Chang, J.; Ahn, C.M.; Kim, H.J. Sarcopenia correlates with systemic inflammation in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 669–675. [Google Scholar] [CrossRef]
- Widjanantie, S.C.; Lestari, F.; Nusdwinuringtyas, N.; Susanto, A.D. Rehabilitation Management for Sarcopenia in Chronic Obstructive Pulmonary Disease: A Literature Review. Respir. Sci. 2024, 4, 232–250. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Yao, J.; Wang, Y. Prevalence of sarcopenia in patients with COPD through different musculature measurements: An updated meta-analysis and meta-regression. Front. Nutr. 2023, 10, 1137371. [Google Scholar] [CrossRef]
- Lee, C.-T.; Wang, P.-H. Handgrip strength during admission for COPD exacerbation: Impact on further exacerbation risk. BMC Pulm. Med. 2021, 21, 245. [Google Scholar] [CrossRef]
- Abdulai, R.M.; Jensen, T.J.; Patel, N.R.; Polkey, M.I.; Jansson, P.; Celli, B.R.; Rennard, S.I. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 197, 433–449. [Google Scholar] [CrossRef]
- Perrot, L.; Greil, A.; Boirie, Y.; Farigon, N.; Mulliez, A.; Costes, F.; Caillaud, D. Prevalence of sarcopenia and malnutrition during acute exacerbation of COPD and after 6 months recovery. Eur. J. Clin. Nutr. 2020, 74, 1556–1564. [Google Scholar] [CrossRef]
- de Blasio, F.; Di Gregorio, A.; de Blasio, F.; Bianco, A.; Bellofiore, B.; Scalfi, L. Malnutrition and sarcopenia assessment in patients with chronic obstructive pulmonary disease according to international diagnostic criteria, and evaluation of raw BIA variables. Respir. Med. 2018, 134, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scalfi, L.; Di Gregorio, A.; Alicante, P.; Bianco, A.; Tantucci, C.; Bellofiore, B.; de Blasio, F. Raw Bioelectrical Impedance Analysis Variables Are Independent Predictors of Early All-Cause Mortality in Patients with COPD. Chest 2019, 155, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- de Blasio, F.; Santaniello, M.G.; Mazzarella, G.; Bianco, A.; Lionetti, L.; Franssen, F.M.E.; Scalfi, L. Raw BIA variables are predictors of muscle strength in patients with chronic obstructive pulmonary disease. Eur. J. Clin. Nutr. 2017, 71, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- De Blasio, F.; de Blasio, F.; Berlingieri, G.M.; Bianco, A.; La Greca, M.; Franssen, F.M.E.; Scalfi, L. Evaluation of body composition in COPD patients using multifrequency bioelectrical impedance analysis. Int. J. COPD 2016, 11, 2419–2426. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Mou, K.; Chan, S.M.; Vlahos, R. Musculoskeletal crosstalk in chronic obstructive pulmonary disease and comorbidities: Emerging roles and therapeutic potentials. Pharmacol. Ther. 2024, 257, 108635. [Google Scholar] [CrossRef]
- Lee, L.-W.; Lin, C.-M.; Li, H.-C.; Hsiao, P.-L.; Chung, A.-C.; Hsieh, C.-J.; Wu, P.-C.; Hsu, S.-F.; Loukides, S. Body composition changes in male patients with chronic obstructive pulmonary disease: Aging or disease process? PLoS ONE 2017, 12, e0180928. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Loyola, W.; Osadnik, C.; Phu, S.; Morita, A.A.; Duque, G.; Probst, V.S. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Saini, V.; Kaur, J.; Gupta, S.; Kaur, H.; Garg, K. Association of adiponectin with lung function impairment and disease severity in chronic obstructive pulmonary disease. Int. J. Appl. Basic Med. Res. 2018, 8, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt Pathway Prevents Expression of Muscle Atrophy-Induced Ubiquitin Ligases by Inhibiting FOXO Transcription Factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Gellhaus, B.; Böker, K.O.; Schilling, A.F.; Saul, D. Therapeutic Consequences of Targeting the IGF-1/PI3K/AKT/FOXO3 Axis in Sarcopenia: A Narrative Review. Cells 2023, 12, 2787. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y. Muscle-Bone Crosstalk in Chronic Obstructive Pulmonary Disease. Front. Endocrinol. 2021, 12, 724911. [Google Scholar] [CrossRef]
- Núñez-Robainas, A.; Guitart, M.; López-Postigo, A.; Sancho-Muñoz, A.; Barreiro, E. Myostatin/Smad2/Smad3 pathway define a differential clinical phenotype in COPD-associated sarcopenia. ERJ Open Res. 2025, 11, 00772–2024. [Google Scholar] [CrossRef]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef]
- Slinde, F.; Grönberg, A.; Engström, C.-P.; Rossander-Hulthén, L.; Larsson, S. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir. Med. 2005, 99, 1004–1009. [Google Scholar] [CrossRef]
- Vestbo, J.; Prescott, E.; Almdal, T.; Dahl, M.; Nordestgaard, B.G.; Andersen, T.; Sørensen, T.I.A.; Lange, P. Body Mass, Fat-Free Body Mass, and Prognosis in Patients with Chronic Obstructive Pulmonary Disease from a Random Population Sample. Am. J. Respir. Crit. Care Med. 2006, 173, 79–83. [Google Scholar] [CrossRef]
- Rodrigues, S.d.O.; da Cunha, C.M.C.; Soares, G.M.V.; Silva, P.L.; Silva, A.R.; Gonçalves-De-Albuquerque, C.F. Mechanisms, pathophysiology and currently proposed treatments of chronic obstructive pulmonary disease. Pharmaceuticals 2021, 14, 979. [Google Scholar] [CrossRef]
- Meeuwsen, S.; Horgan, G.; Elia, M. The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin. Nutr. 2010, 29, 560–566. [Google Scholar] [CrossRef]
- Ali Assad, N.; Sood, A. Leptin, adiponectin and pulmonary diseases. Biochimie 2012, 94, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.-K.; Rutten, E.P.; Vernooy, J.H.; Spruit, M.A.; Dentener, M.A.; van der Kallen, C.; Vangreevenbroek, M.M.; Wouters, E.F. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): A pivotal role of leptin. Respir. Med. 2011, 105, 1046–1053. [Google Scholar] [CrossRef]
- Schols, A.M.W.J.; Creutzberg, E.C.; Buurman, W.A.; Campfield, L.A.; Saris, W.H.M.; Wouters, E.F.M. Plasma Leptin Is Related to Proinflammatory Status and Dietary Intake in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.C.; Barton, R.L.; Singh, J.; Morgan, D.L. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: A randomised controlled trial. Thorax 2003, 58, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kythreotis, P.; Kokkini, A.; Avgeropoulou, S.; Hadjioannou, A.; Anastasakou, E.; Rasidakis, A.; Bakakos, P. Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm. Med. 2009, 9, 11. [Google Scholar] [CrossRef]
- Krommidas, G.; Kostikas, K.; Papatheodorou, G.; Koutsokera, A.; Gourgoulianis, K.I.; Roussos, C.; Koulouris, N.G.; Loukides, S. Plasma leptin and adiponectin in COPD exacerbations: Associations with inflammatory biomarkers. Respir. Med. 2010, 104, 40–46. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Omar, M.M.; Hibah, N.A.A.; Issa, H.A. Leptin hormone in obese and non-obese stable and exacerbated cases of chronic obstructive pulmonary disease. Egypt. J. Chest Dis. Tuberc. 2015, 64, 557–565. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Chanez, P.; Chiappara, G.; Siena, L.; Giammanco, S.; Gjomarkaj, M.; Bonsignore, G.; Bousquet, J.; Vignola, A.M. Does leptin play a cytokine-like role within the airways of COPD patients? Eur. Respir. J. 2005, 26, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Waschki, B.; Kirsten, A.; Müller, K.-C.; Kretschmar, G.; Meyer, T.; Holz, O.; Magnussen, H. The Metabolic Syndrome in Patients With Chronic Bronchitis and COPD: Frequency and Associated Consequences for Systemic Inflammation and Physical Inactivity. Chest 2009, 136, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; De Rosa, A.; Nigro, E.; Scudiero, O.; Capasso, M.; Masullo, M.; de Laurentiis, G.; Oriani, G.; Sofia, M.; Bianco, A. Adiponectin oligomerization state and adiponectin receptors airway expression in chronic obstructive pulmonary disease. Int. J. Biochem. Cell Biol. 2012, 44, 563–569. [Google Scholar] [CrossRef]
- Nigro, E.; Mosella, M.; Daniele, A.; Mallardo, M.; Accardo, M.; Bianco, A.; Perrotta, F.; Scialò, F. Adiponectin Increase in Patients Affected by Chronic Obstructive Pulmonary Disease with Overlap of Bronchiectasis. Life 2023, 13, 444. [Google Scholar] [CrossRef]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Templeton, S.P. Regulation of lung inflammation by adiponectin. Front. Immunol. 2023, 14, 1244586. [Google Scholar] [CrossRef]
- Cuttitta, G.; Ferraro, M.; Cibella, F.; Alfano, P.; Bucchieri, S.; Patti, A.M.; Muratori, R.; Pace, E.; Bruno, A. Relationship among Body Composition, Adipocytokines, and Irisin on Exercise Capacity and Quality of Life in COPD: A Pilot Study. Biomolecules 2023, 13, 48. [Google Scholar] [CrossRef]
- Higham, A.; Baker, J.M.; Jackson, N.; Shah, R.; Lea, S.; Singh, D. Dysregulation of the CD163-haptoglobin axis in the airways of COPD patients. Cells 2022, 11, 2. [Google Scholar] [CrossRef]
- Lee, P.-L.; Lee, K.-Y.; Cheng, T.-M.; Chuang, H.-C.; Wu, S.-M.; Feng, P.-H.; Liu, W.-T.; Chen, K.-Y.; Ho, S.-C. Relationships of Haptoglobin Phenotypes with Systemic Inflammation and the Severity of Chronic Obstructive Pulmonary Disease. Sci. Rep. 2019, 9, 189. [Google Scholar]
- Singh, D.; Han, M.K.; Hawkins, N.M.; Hurst, J.R.; Kocks, J.W.H.; Skolnik, N.; Stolz, D.; El Khoury, J.; Gale, C.P. Implications of Cardiopulmonary Risk for the Management of COPD: A Narrative Review. Adv. Ther. 2024, 41, 2151–2167. [Google Scholar] [CrossRef] [PubMed]
- Sanja, M.; Jozsef, P.; Sanja, P.G.; Ivana, C.; Ivana, G.; Lana, G.; Gordana, S.; Renata, L.; Lepej Snjezana, Z. Cytokines and statin therapy in chronic obstructive pulmonary disease patients. Scand. J. Clin. Lab. Investig. 2018, 78, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Infect. Dis. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Dugani, S.B.; Hydoub, Y.M.; Ayala, A.P.; Reka, R.; Nayfeh, T.; Ding, J.; McCafferty, S.N.; Alzuabi, M.; Farwati, M.; Murad, M.H.; et al. Risk Factors for Premature Myocardial Infarction: A Systematic Review and Meta-analysis of 77 Studies. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 783–794. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Kulkarni, R.; Wiemer, E.A.C.; Chang, W. Role of Lipid Rafts in Pathogen-Host Interaction—A Mini Review. Front. Immunol. 2022, 12, 815020. [Google Scholar] [CrossRef]
- Rosenberger, C.M.; Brumell, J.H.; Finlay, B. Microbial pathogenesis: Lipid rafts as pathogen portals. Curr. Biol. 2000, 10, R823–R825. [Google Scholar] [CrossRef]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.C.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- De Beer, M.C.; Ji, A.; Jahangiri, A.; Vaughan, A.M.; De Beer, F.C.; Van Der Westhuyzen, D.R.; Webb, N.R. ATP binding cassette G1-dependent cholesterol efflux during inflammation. J. Lipid Res. 2011, 52, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Ananthramaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fonarow, G.C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; et al. The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J. Lipid Res. 2004, 45, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Cochran, B.J.; Ong, K.-L.; Manandhar, B.; Rye, K.-A. APOA1: A Protein with Multiple Therapeutic Functions. Curr. Atheroscler. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Chen, S.; Zhang, Y.; He, R.; Wang, X.; Ding, F.; Hu, W.; Dai, Y.; Lu, L.; et al. Serum levels of lipoprotein-associated phospholipase A2 are associated with coronary atherosclerotic plaque progression in diabetic and non-diabetic patients. BMC Cardiovasc. Disord. 2024, 24, 251. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal Muscle Lipid Content and Insulin Resistance: Evidence for a Paradox in Endurance-Trained Athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, B.; Zhang, K.; Zhu, W.; Lian, X.; Xu, Y.; Chen, Z.; Liu, L.; Guo, Z. The association of lipid metabolism and sarcopenia among older patients: A cross-sectional study. Sci. Rep. 2023, 13, 17538. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory Markers and Onset of Cardiovascular Events. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Lee-Okada, H.-C.; Xue, C.; Yokomizo, T. Recent advances on the physiological and pathophysiological roles of polyunsaturated fatty acids and their biosynthetic pathway. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2025, 1870, 159564. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J. Free radicals in skin and muscle: Damaging agents or signals for adaptation? Proc. Nutr. Soc. 1999, 58, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.-M.; Adhihetty, P.J.; Wawrzyniak, N.R.; Wohlgemuth, S.E.; Picca, A.; Kujoth, G.C.; Prolla, T.A.; Leeuwenburgh, C.; Johannsen, D. Dysregulation of Mitochondrial Quality Control Processes Contribute to Sarcopenia in a Mouse Model of Premature Aging. PLoS ONE 2013, 8, e69327. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef]
- Zafirova-Ivanovska, B.; Stojkovikj, J.; Dokikj, D.; Anastasova, S.; Debresliovska, A.; Zejnel, S.; Stojkovikj, D. The level of cholesterol in COPD patients with severe and very severe stage of the disease. Open Access Maced. J. Med. Sci. 2016, 4, 277–282. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’dOnoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef]
- Dugani, S.B.; Moorthy, M.V.; Li, C.; Demler, O.V.; Alsheikh-Ali, A.A.; Ridker, P.M.; Glynn, R.J.; Mora, S. Association of Lipid, Inflammatory, and Metabolic Biomarkers With Age at Onset for Incident Coronary Heart Disease in Women. JAMA Cardiol. 2021, 6, 437–447. [Google Scholar] [CrossRef]
- Stone, N.J.; Smith, S.C.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef]
- Onishi, K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J. Cardiol. 2017, 70, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.; Pavasini, R.; Malagù, M.; Mascetti, S.; Biscaglia, S.; Ceconi, C.; Papi, A.; Contoli, M. Chronic Obstructive Pulmonary Disease and Ischemic Heart Disease Comorbidity: Overview of Mechanisms and Clinical Management. Cardiovasc. Drugs Ther. 2015, 29, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: What are the implications for clinical practice? Ther. Adv. Respir. Dis. 2018, 12, 1753465817750524. [Google Scholar] [CrossRef]
- Goedemans, L.; Bax, J.J.; Delgado, V. COPD and acute myocardial infarction. Eur. Respir. Rev. 2020, 29, 190139. [Google Scholar] [CrossRef]
- Polverino, F.; Celli, B.R.; Owen, C.A. COPD as an endothelial disorder: Endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045894018758528. [Google Scholar] [CrossRef]
- Criner, G.J.; Connett, J.E.; Aaron, S.D.; Albert, R.K.; Bailey, W.C.; Casaburi, R.; Cooper, J.A.D.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; et al. Simvastatin for the Prevention of Exacerbations in Moderate-to-Severe COPD. N. Engl. J. Med. 2014, 370, 2201–2210. [Google Scholar] [CrossRef]
- Campins, L.; Camps, M.; Riera, A.; Pleguezuelos, E.; Yebenes, J.C.; Serra-Prat, M. Oral drugs related with muscle wasting and sarcopenia. A review. Pharmacology 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Figorilli, F.; Mannarino, M.R.; Bianconi, V.; Pirro, M. Cholesterol-Lowering Therapy in Patients at Low-to-Moderate Cardiovascular Risk. High Blood Press. Cardiovasc. Prev. 2022, 29, 327–336. [Google Scholar] [CrossRef]
- Abbasifard, M.; Kandelouei, T.; Aslani, S.; Razi, B.; Imani, D.; Fasihi, M.; Cicero, F.G.; Sahebkar, A. Effect of statins on the plasma/serum levels of inflammatory markers in patients with cardiovascular disease; a systematic review and meta-analysis of randomized clinical trials. Inflammopharmacology 2022, 30, 369–383. [Google Scholar] [CrossRef]
- Celli, B.R.; Anzueto, A.; Singh, D.; Hanania, N.A.; Fabbri, L.; Martinez, F.J.; Soler, X.; Djandji, M.; Jacob-Nara, J.A.; Rowe, P.J.; et al. The Emerging Role of Alarmin-Targeting Biologics in the Treatment of Patients With COPD. Chest 2025, 167, 1346–1355. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, R.; Yao, J.; Xu, X.; Teng, Y.; Cheng, N. Effectiveness of long-term using statins in COPD—A network meta-analysis. Respir. Res. 2019, 20, 17. [Google Scholar] [CrossRef]
- Corrao, G.; Scotti, L.; Zambon, A.; Baio, G.; Nicotra, F.; Conti, V.; Capri, S.; Tragni, E.; Merlino, L.; Catapano, A.L.; et al. Cost-effectiveness of enhancing adherence to therapy with statins in the setting of primary cardiovascular prevention. Evidence from an empirical approach based on administrative databases. Atherosclerosis 2011, 217, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Šimić, I.; Reiner, Ž. Adverse effects of statins—Myths and reality. Curr. Pharm. Des. 2015, 21, 1220–1226. [Google Scholar] [CrossRef]
- Scott, D.; Blizzard, L.; Fell, J.; Jones, G. Statin therapy, muscle function and falls risk in community-dwelling older adults. Qjm Int. J. Med. 2009, 102, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Penson, P.E.; Mikhailidis, D.P.; Wong, N.D.; Hernandez, A.V.; Sahebkar, A.; Thompson, P.D.; Mazidi, M.; Rysz, J.; Pella, D.; et al. Prevalence of statin intolerance: A meta-analysis. Eur. Heart J. 2022, 43, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.J.; Coxson, P.G.; Penko, J.; Pletcher, M.J.; Goldman, L.; Odden, M.C.; Kazi, D.S.; Bibbins-Domingo, K. Evaluating the impact and cost-effectiveness of statin use guidelines for primary prevention of coronary heart disease and stroke. Circulation 2017, 136, 1087–1098. [Google Scholar] [CrossRef]
- Davies, G.M.; Vyas, A.; Baxter, C.A. Economic evaluation of ezetimibe treatment in combination with statin therapy in the United States. J. Med. Econ. 2017, 20, 723–731. [Google Scholar] [CrossRef]
- Peura, P.; Martikainen, J.; Soini, E.; Hallinen, T.; Niskanen, L. Cost-effectiveness of statins in the prevention of coronary heart disease events in middle-aged Finnish men. Curr. Med. Res. Opin. 2008, 24, 1823–1832. [Google Scholar] [CrossRef]
- May, H.T.; Knowlton, K.U.; Anderson, J.L.; Lappé, D.L.; Bair, T.L.; Muhlestein, J.B. High-statin adherence over 5 years of follow-up is associated with improved cardiovascular outcomes in patients with atherosclerotic cardiovascular disease: Results from the IMPRES study. Eur. Hear. J. Qual. Care Clin. Outcomes 2022, 8, 352–360. [Google Scholar] [CrossRef]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Marcoff, L.; Thompson, P.D. The Role of Coenzyme Q10 in Statin-Associated Myopathy: A Systematic Review. J. Am. Coll. Cardiol. 2007, 49, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Bardolia, C.; Amin, N.S.; Turgeon, J. Emerging Non-statin Treatment Options for Lowering Low-Density Lipoprotein Cholesterol. Front. Cardiovasc. Med. 2021, 8, 789931. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.N.; Low Wang, C.C.; Hiatt, W.R. PCSK9 Inhibitors: Mechanisms of Action, Metabolic Effects, and Clinical Outcomes. Annu. Rev. Med. 2018, 29, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Catapano, A.L.; Corsini, A.; Manzato, E.; Werba, J.P.; Catena, G.; Cetin, I.; Cicero, A.F.; Cignarella, A.; Colivicchi, F.; et al. LDL-cholesterol control in the primary prevention of cardiovascular diseases: An expert opinion for clinicians and health professionals. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 245–257. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the Management of Dyslipidemia: Which, When, and for Whom? Could Nutraceuticals Help Low-Risk Individuals with Non-optimal Lipid Levels? Curr. Atheroscler. Rep. 2021, 23, 57. [Google Scholar] [CrossRef]
- Penson, P.E.; Banach, M. Natural compounds as anti-atherogenic agents: Clinical evidence for improved cardiovascular outcomes. Atherosclerosis 2021, 316, 58–65. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2017, 5, 965–1005. [Google Scholar] [CrossRef]
- Pirro, M.; Vetrani, C.; Bianchi, C.; Mannarino, M.; Bernini, F.; Rivellese, A. Joint position statement on “Nutraceuticals for the treatment of hypercholesterolemia” of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr. Metab. Cardiovasc. Dis. 2017, 27, 2–17. [Google Scholar] [CrossRef]
- Mannarino, M.R.; Ministrini, S.; Pirro, M. Nutraceuticals for the treatment of hypercholesterolemia. Eur. J. Intern. Med. 2014, 25, 592–599. [Google Scholar] [CrossRef]
- Castellanos-Jankiewicz, A.; del Bosque-Plata, L.; Tejero, M.E. Combined Effect of Plant Sterols and Dietary Fiber for the Treatment of Hypercholesterolemia. Plant Foods Hum. Nutr. 2014, 69, 93–100. [Google Scholar] [CrossRef]
- Liu, C.-S.; Zheng, Y.-R.; Zhang, Y.-F.; Long, X.-Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Zhao, Z.-X.; Huang, M.; Feng, R.; He, C.-Y.; Ma, C.; Luo, S.-H.; Fu, J.; Wen, B.-Y.; Ren, L.; et al. Effect of Berberine on promoting the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters. J. Transl. Med. 2015, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.-R.; Oh, G.T.; Park, H.-S.; Lee, K.-U.; Lane, M.D.; et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef]
- Qiang, X.; Xu, L.; Zhang, M.; Zhang, P.; Wang, Y.; Wang, Y.; Zhao, Z.; Chen, H.; Liu, X.; Zhang, Y. Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochem. Biophys. Res. Commun. 2016, 472, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 2016, 35, 1091–1095. [Google Scholar] [CrossRef]
- Derosa, G. Evidence from a novel nutraceutical in patients with hypercholesterolemia [oral presentation]. In Proceedings of the 84th Congress of the Italian Society of Cardiology, Rome, Italy, 14–16 December 2023. [Google Scholar]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).