Circulating Beta-Defensin 2 Levels Correlate with Conventional Inflammatory Markers in Infection-Free Individuals with Overweight and Obesity: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical and Laboratory Evaluation
2.3. Statistical Analysis
2.4. Ethical Aspects
3. Results
3.1. Characteristics of the Study Subjects
3.2. Inflammatory Markers in the Study Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262, Erratum in Lancet Diabetes Endocrinol. 2025, 13, e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stephenson, J.; Smith, C.M.; Kearns, B.; Haywood, A.; Bissell, P. The association between obesity and quality of life: A retrospective analysis of a large-scale population-based cohort study. BMC Public Health 2021, 21, 1990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Lavikainen, P.; Wikström, K.; Laatikainen, T. Trajectories of Body Mass Index and Risk for Diabetes Complications and All-Cause Mortality in Finnish Type 2 Diabetes Patients. Clin. Epidemiol. 2024, 16, 203–212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Z.; Efremov, L.; Mikolajczyk, R. Differences in the levels of inflammatory markers between metabolically healthy obese and other obesity phenotypes in adults: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Gosti, F. Journey to the past: Molecular de-extinction enables the discovery of ancient β-defensins and highlights their evolutionary history. Trends Biochem. Sci. 2025, 50, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Human β-Defensin 2 and Its Postulated Role in Modulation of the Immune Response. Cells 2021, 10, 2991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rüb, A.M.; Tsakmaklis, A.; Gräfe, S.K.; Simon, M.C.; Vehreschild, M.J.; Wuethrich, I. Biomarkers of human gut microbiota diversity and dysbiosis. Biomark. Med. 2021, 15, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Kapel, N.; Benahmed, N.; Morali, A.; Svahn, J.; Canioni, D.; Goulet, O.; Ruemmele, F.M. Fecal beta-defensin-2 in children with inflammatory bowel diseases. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.H.; Lee, K.W.; Kim, W.B.; Choi, H.W.; Moon, J.E.; Moon, A.; Kim, Y.H. β-Defensin 2, an Antimicrobial Peptide, as a Novel Biomarker for Ulcerative Interstitial Cystitis; Can β-Defensin 2 Suspect the Dysbiosis of Urine Microbiota? Diagnostics 2021, 11, 2082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Busetto, L.; Dicker, D.; Frühbeck, G.; Halford, J.C.G.; Sbraccia, P.; Yumuk, V.; Goossens, G.H. A new framework for the diagnosis, staging and management of obesity in adults. Nat. Med. 2024, 30, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masson, S.; Caironi, P.; Fanizza, C.; Thomae, R.; Bernasconi, R.; Noto, A.; Oggioni, R.; Pasetti, G.S.; Romero, M.; Tognoni, G.; et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial. Intensive Care Med. 2015, 41, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zografou, I.; Kouroupis, D.; Dimakopoulos, G.; Doukelis, P.; Doumas, M.; Koufakis, T. Correlation Between Presepsin Levels and Continuous Glucose Monitoring Metrics in Infection-Free Individuals With Type 1 Diabetes. J. Diabetes Sci. Technol. 2025, 19, 277–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kouroupis, D.; Zografou, I.; Balaska, A.; Reklou, A.; Varouktsi, A.; Paschala, A.; Pyrpasopoulou, A.; Stavropoulos, K.; Vogiatzis, K.; Sarvani, A.; et al. Presepsin Levels in Infection-Free Subjects with Diabetes Mellitus: An Exploratory Study. Biomedicines 2024, 12, 1960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kouroupis, D.; Zografou, I.; Doukelis, P.; Patoulias, D.; Popovic, D.S.; Karakasis, P.; Pyrpasopoulou, A.; Stavropoulos, K.; Papadopoulos, C.; Giouleme, O.; et al. Presepsin: An Emerging Biomarker in the Management of Cardiometabolic Disorders. J. Pers. Med. 2025, 15, 125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koufakis, T.; Kouroupis, D.; Dimakopoulos, G.; Georgiadis, T.; Kourti, A.; Doukelis, P.; Zografou, I.; Patoulias, D.; Popovic, D.S.; Pyrpasopoulou, A.; et al. Obesity, but Not Overweight, Is Associated with Increased Presepsin Levels in Infection-Free Individuals: An Exploratory Study. Biomedicines 2025, 13, 701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filipe Rosa, L.; Rings, A.; Stolzer, I.; Koeninger, L.; Wehkamp, J.; Beisner, J.; Günther, C.; Nordkild, P.; Jensen, B.A.H.; Bischoff, S.C. Human α-Defensin 51-9 and Human β-Defensin 2 Improve Metabolic Parameters and Gut Barrier Function in Mice Fed a Western-Style Diet. Int. J. Mol. Sci. 2023, 24, 13878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Megawati, G.; Indraswari, N.; Johansyah, A.A.; Kezia, C.; Herawati, D.M.D.; Gurnida, D.A.; Musfiroh, I. Comparison of hs-CRP in Adult Obesity and Central Obesity in Indonesia Based on Omega-3 Fatty Acids Intake: Indonesian Family Life Survey 5 (IFLS 5) Study. Int. J. Environ. Res. Public Health 2023, 20, 6734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngwa, D.N.; Pathak, A.; Agrawal, A. IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms. Mol. Immunol. 2022, 146, 50–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat. Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Faraj, M.; Bastard, J.P.; St-Pierre, D.H.; Brochu, M.; Prud’homme, D.; Rabasa-Lhoret, R. The metabolically healthy but obese individual presents a favorable inflammation profile. J. Clin. Endocrinol. Metab. 2005, 90, 4145–4150. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Yaghootkar, H. Identifying obesity subtypes: A review of studies utilising clinical biomarkers and genetic data. Diabet. Med. 2023, 40, e15226. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian. J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phalitakul, S.; Okada, M.; Hara, Y.; Yamawaki, H. Vaspin prevents TNF-α-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-κB and PKCθ activation in cultured rat vascular smooth muscle cells. Pharmacol. Res. 2011, 64, 493–500. [Google Scholar] [CrossRef] [PubMed]
- de Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussain, S.; Yadav, S.S.; Sawlani, K.K.; Usman, K.; Khattri, S. Evaluating Pro- and Anti-inflammatory Biomarkers for Predicting Type 2 Diabetes Mellitus in the Geriatric Population and Correlation With Clinical and Biochemical Parameters. Cureus 2025, 17, e77896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikonomidis, I.; Michalakeas, C.A.; Lekakis, J.; Paraskevaidis, I.; Kremastinos, D.T. Multimarker approach in cardiovascular risk prediction. Dis. Markers 2009, 26, 273–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feinstein, A.R. P-values and confidence intervals: Two sides of the same unsatisfactory coin. J. Clin. Epidemiol. 1998, 51, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.; Morey, R.D.; Rouder, J.N.; Wagenmakers, E.J. Robust misinterpretation of confidence intervals. Psychon. Bull. Rev. 2014, 21, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Biomarker | Group | Mean | SD | Median | Minimum | Maximum | 95% CI for the Median | p-Value |
|---|---|---|---|---|---|---|---|---|
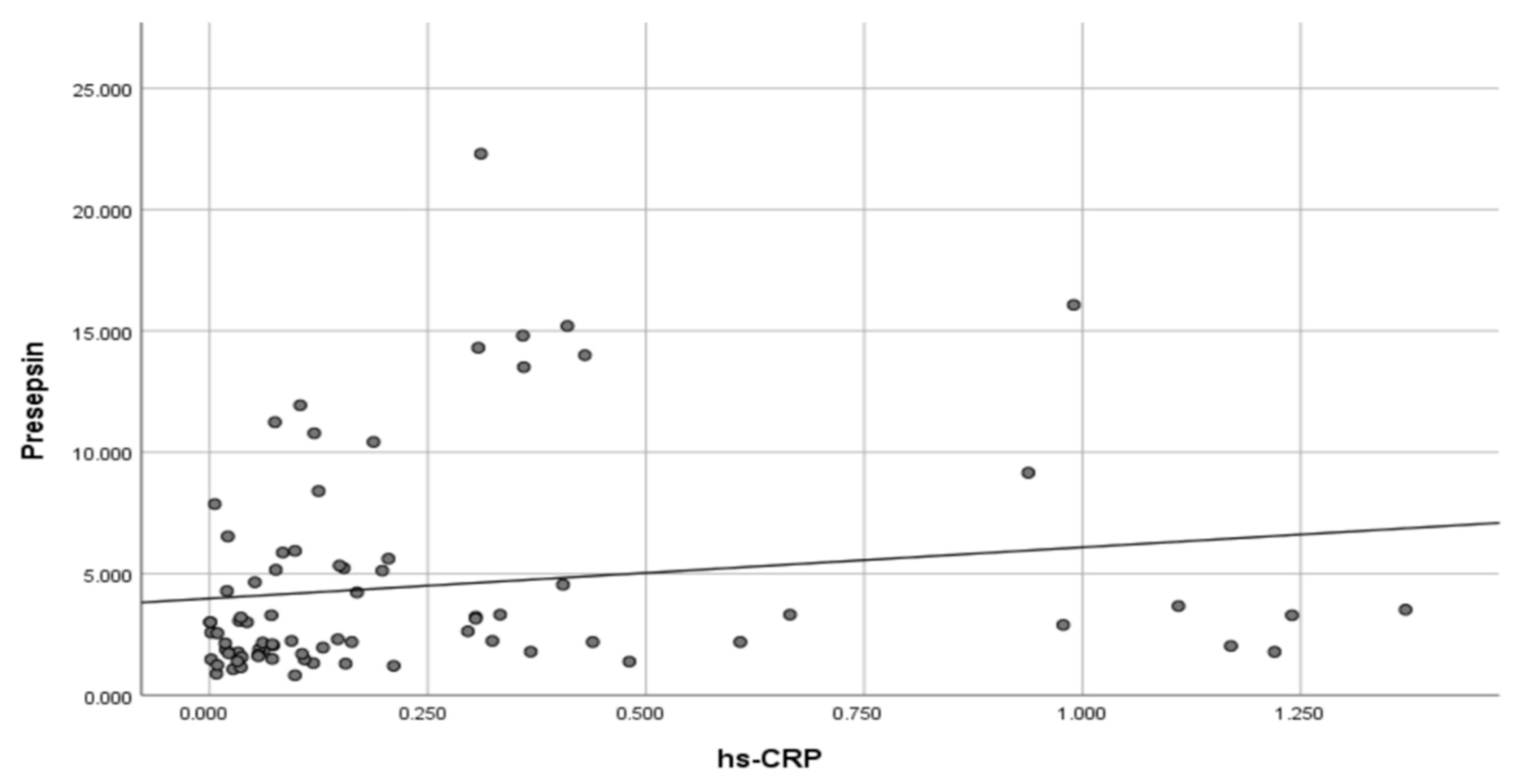
| Presepsin (ng/mL) | Control or Overweight | 3.97 | 3.98 | 2.56 | 0.82 | 22.30 | 1.79–3.00 | 0.175 |
| Obesity | 5.61 | 5.00 | 3.18 | 1.24 | 16.07 | 2.10–6.54 | ||
| Beta-defensin 2 (pg/mL) | Control or Overweight | 181.99 | 122.94 | 152.95 | 136.45 | 919.50 | 146.55–162.55 | 0.936 |
| Obesity | 165.00 | 30.52 | 157.40 | 136.70 | 267.70 | 146.17–165.93 | ||
| Ferritin (pg/mL) | Control or Overweight | 85.28 | 69.31 | 71.34 | 6.71 | 320.80 | 51.10–92.64 | 0.237 |
| Obesity | 113.38 | 85.74 | 90.39 | 8.87 | 329.10 | 49.70–153.55 | ||
| IL-6 (pg/mL) | Control or Overweight | 3.38 | 3.71 | 2.00 | 1.50 | 22.51 | 1.50–2.68 | 0.341 |
| Obesity | 3.76 | 2.98 | 2.21 | 1.50 | 11.11 | 1.60–4.28 | ||
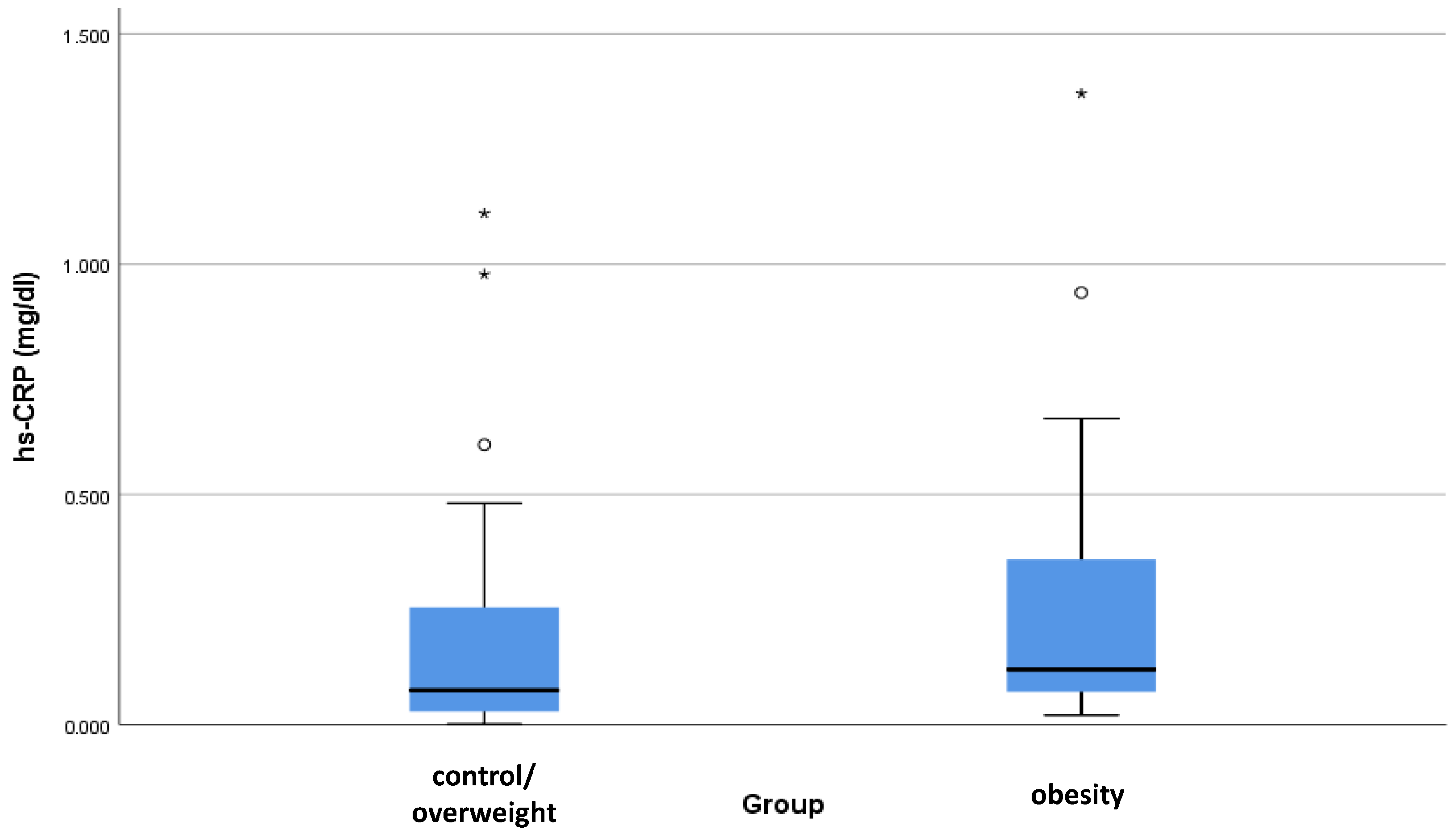
| hs-CRP (mg/dL) | Control or Overweight | 0.20 | 0.27 | 0.10 | 0.00 | 1.24 | 0.06–0.15 | 0.076 |
| Obesity | 0.36 | 0.42 | 0.13 | 0.01 | 1.37 | 0.09–0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koufakis, T.; Kouroupis, D.; Dimakopoulos, G.; Georgiadis, T.; Kourti, A.; Karalazou, P.; Thisiadou, K.; Doukelis, P.; Zografou, I.; Patoulias, D.; et al. Circulating Beta-Defensin 2 Levels Correlate with Conventional Inflammatory Markers in Infection-Free Individuals with Overweight and Obesity: An Exploratory Study. Biomedicines 2025, 13, 1800. https://doi.org/10.3390/biomedicines13081800
Koufakis T, Kouroupis D, Dimakopoulos G, Georgiadis T, Kourti A, Karalazou P, Thisiadou K, Doukelis P, Zografou I, Patoulias D, et al. Circulating Beta-Defensin 2 Levels Correlate with Conventional Inflammatory Markers in Infection-Free Individuals with Overweight and Obesity: An Exploratory Study. Biomedicines. 2025; 13(8):1800. https://doi.org/10.3390/biomedicines13081800
Chicago/Turabian StyleKoufakis, Theocharis, Dimitrios Kouroupis, Georgios Dimakopoulos, Theofylaktos Georgiadis, Areti Kourti, Paraskevi Karalazou, Katerina Thisiadou, Panagiotis Doukelis, Ioanna Zografou, Dimitrios Patoulias, and et al. 2025. "Circulating Beta-Defensin 2 Levels Correlate with Conventional Inflammatory Markers in Infection-Free Individuals with Overweight and Obesity: An Exploratory Study" Biomedicines 13, no. 8: 1800. https://doi.org/10.3390/biomedicines13081800
APA StyleKoufakis, T., Kouroupis, D., Dimakopoulos, G., Georgiadis, T., Kourti, A., Karalazou, P., Thisiadou, K., Doukelis, P., Zografou, I., Patoulias, D., Popovic, D. S., Pyrpasopoulou, A., Fousteris, E., Argyrakopoulou, G., Kokkinos, A., Giouleme, O., Kotsa, K., Doumas, M., & Makedou, K. (2025). Circulating Beta-Defensin 2 Levels Correlate with Conventional Inflammatory Markers in Infection-Free Individuals with Overweight and Obesity: An Exploratory Study. Biomedicines, 13(8), 1800. https://doi.org/10.3390/biomedicines13081800










