Obesity, but Not Overweight, Is Associated with Increased Presepsin Levels in Infection-Free Individuals: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Collection
2.3. Biochemical Analysis
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Characteristics of the Study Population
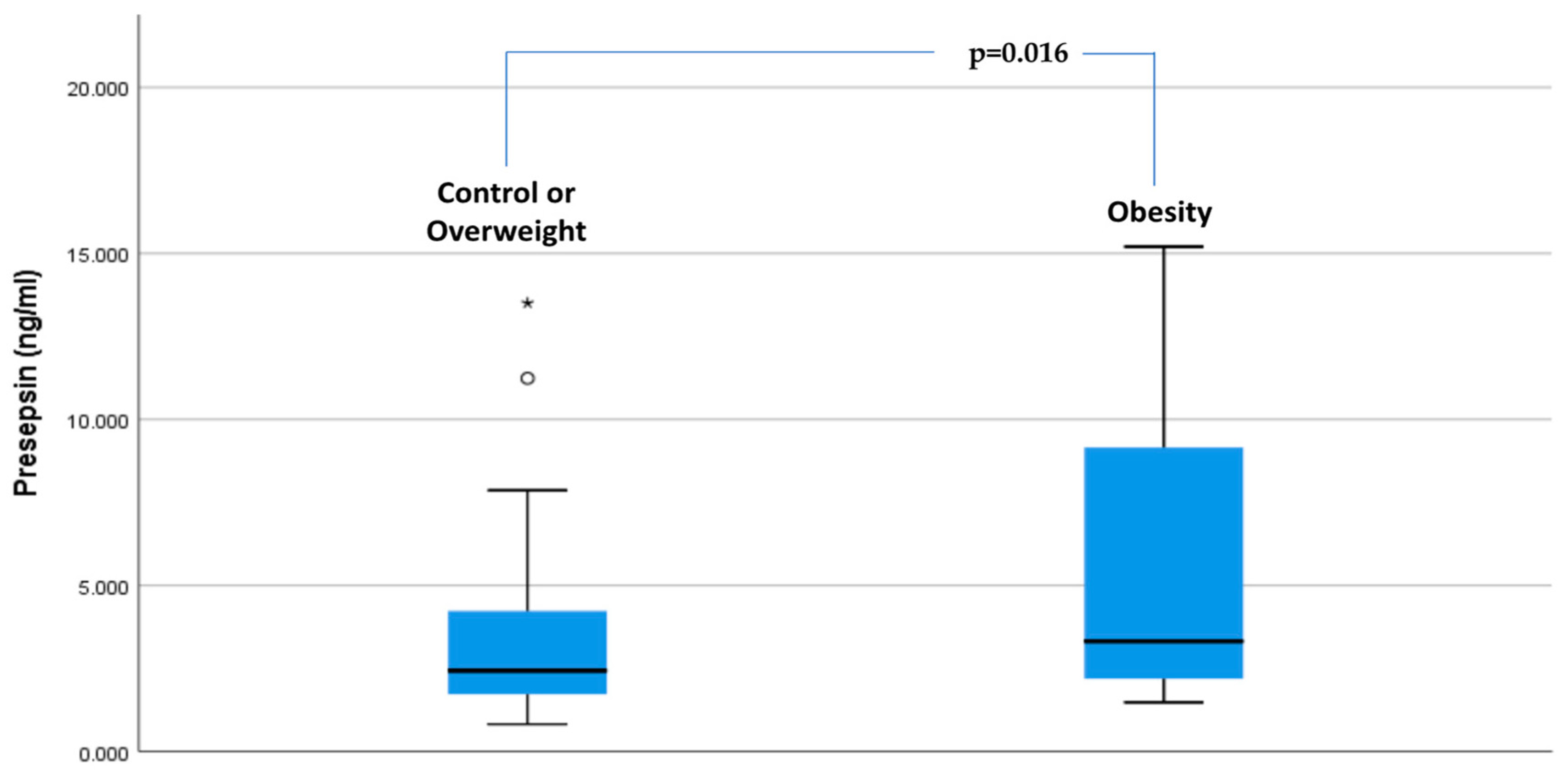
3.2. Presepsin Levels in Study Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. The Physiology of Hunger. N. Engl. J. Med. 2025, 392, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Hamjane, N.; Mechita, M.B.; Nourouti, N.G.; Barakat, A. Gut microbiota dysbiosis -associated obesity and its involvement in cardiovascular diseases and type 2 diabetes. A systematic review. Microvasc. Res. 2024, 151, 104601. [Google Scholar] [CrossRef] [PubMed]
- Awoyemi, A.; Trøseid, M.; Arnesen, H.; Solheim, S.; Seljeflot, I. Markers of metabolic endotoxemia as related to metabolic syndrome in an elderly male population at high cardiovascular risk: A cross-sectional study. Diabetol. Metab. Syndr. 2018, 10, 59. [Google Scholar] [CrossRef]
- Trøseid, M.; Nestvold, T.K.; Rudi, K.; Thoresen, H.; Nielsen, E.W.; Lappegård, K.T. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: Evidence from bariatric surgery. Diabetes Care 2013, 36, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.G.; Møller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Drăgoescu, A.N.; Pădureanu, V.; Stănculescu, A.D.; Chiuțu, L.C.; Florescu, D.N.; Gheonea, I.A.; Pădureanu, R.; Stepan, A.; Streba, C.T.; Drocaș, A.I.; et al. Presepsin as a Potential Prognostic Marker for Sepsis According to Actual Practice Guidelines. J. Pers. Med. 2020, 11, 2. [Google Scholar] [CrossRef]
- Yaegashi, Y.; Shirakawa, K.; Sato, N.; Suzuki, Y.; Kojika, M.; Imai, S.; Takahashi, G.; Miyata, M.; Furusako, S.; Endo, S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J. Infect. Chemother. 2005, 11, 234–238. [Google Scholar] [CrossRef]
- Alzohily, B.; AlMenhali, A.; Gariballa, S.; Munawar, N.; Yasin, J.; Shah, I. Unraveling the complex interplay between obesity and vitamin D metabolism. Sci. Rep. 2024, 14, 7583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr. Issues Mol. Biol. 2024, 46, 13514–13525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Busebee, B.; Ghusn, W.; Cifuentes, L.; Acosta, A. Obesity: A Review of Pathophysiology and Classification. Mayo Clin. Proc. 2023, 98, 1842–1857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagata, T.; Yasuda, Y.; Ando, M.; Abe, T.; Katsuno, T.; Kato, S.; Tsuboi, N.; Matsuo, S.; Maruyama, S. Clinical impact of kidney function on presepsin levels. PLoS ONE 2015, 10, e0129159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chenevier-Gobeaux, C.; Trabattoni, E.; Roelens, M.; Borderie, D.; Claessens, Y.E. Presepsin (sCD14-ST) in emergency department: The need for adapted threshold values? Clin. Chim. Acta 2014, 427, 34–36. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [CrossRef] [PubMed] [PubMed Central]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Postmus, D.; Gansevoort, R.T.; de Jong, P.E.; Gans, R.O.; Struck, J.; Hillege, H.L.; Stolk, R.P.; Navis, G.; et al. Plasma procalcitonin is associated with obesity, insulin resistance, and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2010, 95, E26–E31. [Google Scholar] [CrossRef] [PubMed]
- Koufakis, T.; Dimitriadis, G.; Metallidis, S.; Zebekakis, P.; Kotsa, K. The role of autoimmunity in the pathophysiology of type 2 diabetes: Looking at the other side of the moon. Obes. Rev. 2021, 22, e13231. [Google Scholar] [CrossRef] [PubMed]
- Kouroupis, D.; Zografou, I.; Balaska, A.; Reklou, A.; Varouktsi, A.; Paschala, A.; Pyrpasopoulou, A.; Stavropoulos, K.; Vogiatzis, K.; Sarvani, A.; et al. Presepsin Levels in Infection-Free Subjects with Diabetes Mellitus: An Exploratory Study. Biomedicines 2024, 12, 1960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vieira-Silva, S.; Falony, G.; Belda, E.; Nielsen, T.; Aron-Wisnewsky, J.; Chakaroun, R.; Forslund, S.K.; Assmann, K.; Valles-Colomer, M.; Nguyen, T.T.D.; et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 2020, 581, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Zografou, I.; Kouroupis, D.; Dimakopoulos, G.; Doukelis, P.; Doumas, M.; Koufakis, T. Correlation Between Presepsin Levels and Continuous Glucose Monitoring Metrics in Infection-Free Individuals with Type 1 Diabetes. J. Diabetes Sci. Technol. 2025, 19, 277–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Oliveira, C.; Biddulph, J.P.; Hirani, V.; Schneider, I.J.C. Vitamin D and inflammatory markers: Cross-sectional analyses using data from the English Longitudinal Study of Ageing (ELSA). J. Nutr. Sci. 2017, 6, e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Schurman, S.H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D.M., 3rd; Sen, R.; Ferrucci, L. Aging and Inflammation. Cold Spring Harb. Perspect. Med. 2024, 14, a041197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Si, H.; Ma, Y.; Li, S.; Gao, L.; Liu, K.; Liu, X. Vitamin D3 affects the gut microbiota in an LPS-stimulated systemic inflammation mouse model. Microbes Infect. 2023, 25, 105180. [Google Scholar] [CrossRef] [PubMed]
- Heinsberg, L.W.; Weeks, D.E. Post hoc power is not informative. Genet. Epidemiol. 2022, 46, 390–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikegame, A.; Kondo, A.; Kitaguchi, K.; Sasa, K.; Miyoshi, M. Presepsin production in monocyte/macrophage-mediated phagocytosis of neutrophil extracellular traps. Sci. Rep. 2022, 12, 5978. [Google Scholar] [CrossRef]
- Toprak, K.; Inanır, M.; Memioğlu, T.; Kaplangoray, M.; Palice, A.; Tascanov, M.B. Could Zonulin and Presepsin Be Biomarkers and Therapeutic Targets for Acute Myocarditis? Arq. Bras. Cardiol. 2023, 120, e20230017. [Google Scholar] [CrossRef] [PubMed]
- Aulin, L.B.S.; Kleijburg, A.; Moerland, M.; van Hasselt, J.G.C. Characterizing the kinetics of presepsin and associated inflammatory biomarkers in human endotoxemia. Inflamm. Res. 2022, 71, 999–1001. [Google Scholar] [CrossRef] [PubMed]

| Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 20) | Overweight (n = 34) | Obesity (n = 27) | ||||||||
| Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | p-Value | |
| Age (years) | 43.45 | 11.98 | 46 | 49.88 | 13.01 | 50 | 47.44 | 10.09 | 50 | 0.542 |
| Weight (kg) | 64.75 | 8.88 | 64 | 78.50 | 11.22 | 75 | 98.07 | 21.79 | 90 | <0.01 |
| Height (m) | 1.69 | 0.09 | 1.67 | 1.69 | 0.12 | 1.67 | 1.67 | 0.10 | 1.67 | 0.752 |
| BMI (kg/m2) | 22.53 | 1.19 | 22.9 | 27.45 | 1.31 | 27.5 | 35.18 | 5.86 | 33.4 | <0.01 |
| Duration of intake (months) | 6.07 | 10.80 | 0.5 | 1.90 | 5.74 | 0.0 | 1.44 | 2.98 | 0.0 | 0.324 |
| 25(OH)D (ng/mL) | 28.19 | 9.21 | 29.2 | 25.63 | 7.02 | 25.3 | 24.24 | 5.28 | 23.2 | 0.179 |
| Presepsin (ng/mL) | 4.45 | 3.24 | 3.30 | 4.01 | 6.94 | 2.16 | 8.09 | 13.79 | 3.80 | 0.067 |
| Parameter | BETA | 95% Lower Bound | 95%Upper Bound | p-Value |
|---|---|---|---|---|
| Constant | 2.150 | −4.621 | 8.922 | 0.527 |
| Group | 1.271 | 0.224 | 2.323 | 0.016 |
| Age | −0.003 | −0.091 | 0.086 | 0.954 |
| Sex | −0.068 | −2.281 | 2.146 | 0.951 |
| 25(OH)D | 0.008 | −0.129 | 0.144 | 0.908 |
| Vitamin D intake | −0.293 | −2.502 | 1.917 | 0.792 |
| Smoking | 0.321 | −2.131 | 1.453 | 0.832 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koufakis, T.; Kouroupis, D.; Dimakopoulos, G.; Georgiadis, T.; Kourti, A.; Doukelis, P.; Zografou, I.; Patoulias, D.; Popovic, D.S.; Pyrpasopoulou, A.; et al. Obesity, but Not Overweight, Is Associated with Increased Presepsin Levels in Infection-Free Individuals: An Exploratory Study. Biomedicines 2025, 13, 701. https://doi.org/10.3390/biomedicines13030701
Koufakis T, Kouroupis D, Dimakopoulos G, Georgiadis T, Kourti A, Doukelis P, Zografou I, Patoulias D, Popovic DS, Pyrpasopoulou A, et al. Obesity, but Not Overweight, Is Associated with Increased Presepsin Levels in Infection-Free Individuals: An Exploratory Study. Biomedicines. 2025; 13(3):701. https://doi.org/10.3390/biomedicines13030701
Chicago/Turabian StyleKoufakis, Theocharis, Dimitrios Kouroupis, Georgios Dimakopoulos, Theofylaktos Georgiadis, Areti Kourti, Panagiotis Doukelis, Ioanna Zografou, Dimitrios Patoulias, Djordje S. Popovic, Athina Pyrpasopoulou, and et al. 2025. "Obesity, but Not Overweight, Is Associated with Increased Presepsin Levels in Infection-Free Individuals: An Exploratory Study" Biomedicines 13, no. 3: 701. https://doi.org/10.3390/biomedicines13030701
APA StyleKoufakis, T., Kouroupis, D., Dimakopoulos, G., Georgiadis, T., Kourti, A., Doukelis, P., Zografou, I., Patoulias, D., Popovic, D. S., Pyrpasopoulou, A., Busetto, L., Kokkinos, A., Tsimihodimos, V., Kotsa, K., Doumas, M., & Makedou, K. (2025). Obesity, but Not Overweight, Is Associated with Increased Presepsin Levels in Infection-Free Individuals: An Exploratory Study. Biomedicines, 13(3), 701. https://doi.org/10.3390/biomedicines13030701










