Enhancement of Oral Mucosal Regeneration Using Human Exosomal Therapy in SD Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
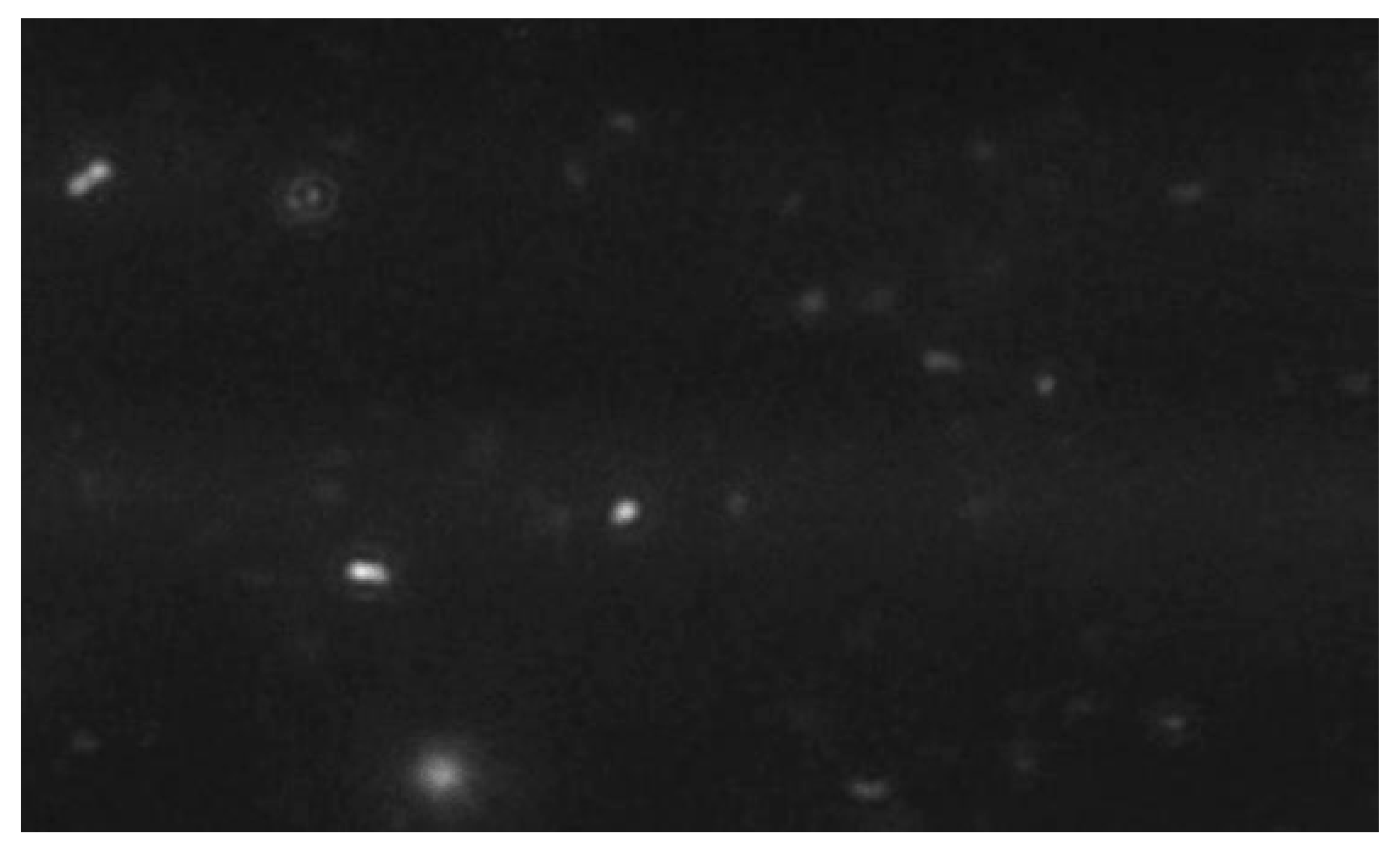
2.2. Exosome Characterization
- Size Distribution Analysis:
2.3. Surgical Methodology and Treatment Administration
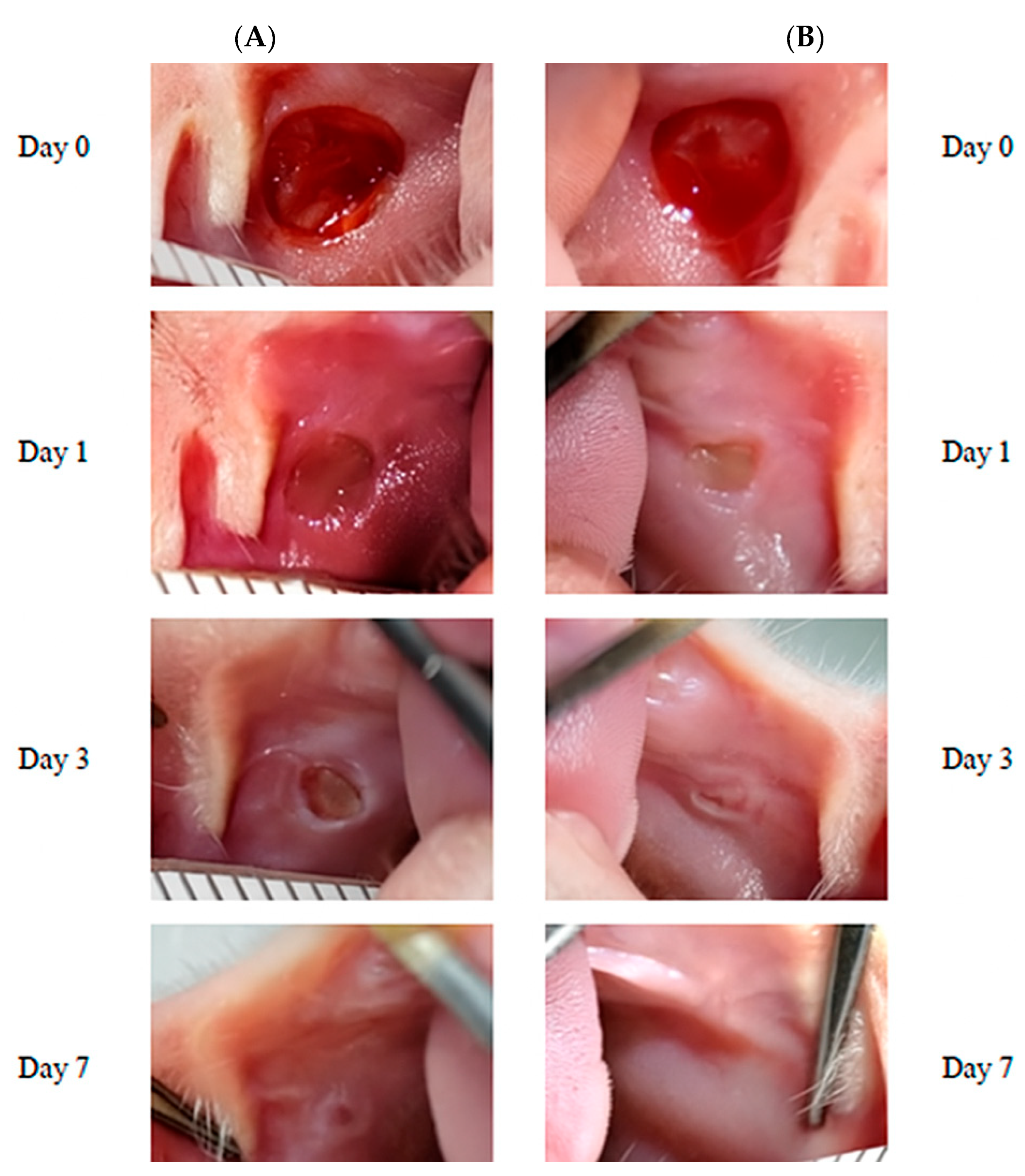
2.4. Macroscopic Assessment
2.5. Tissue Collection and Processing
2.6. Histological Evaluation
- Epithelial regeneration: measured as the epithelial thickness (μm) at the wound center and margins and evaluated for the completeness of coverage, cellular organization, and stratification;
- Inflammatory response: quantified through inflammatory cell counts (neutrophils, macrophages, and lymphocytes) in the wound bed and margins;
- Collagen deposition and organization: evaluated for density, fiber orientation, and architectural pattern using a semi-quantitative scoring system (1 = minimal/disorganized to 4 = abundant/well organized).
2.7. Data Analysis
3. Results
3.1. Macroscopic Healing Progression
3.2. Histological Observations
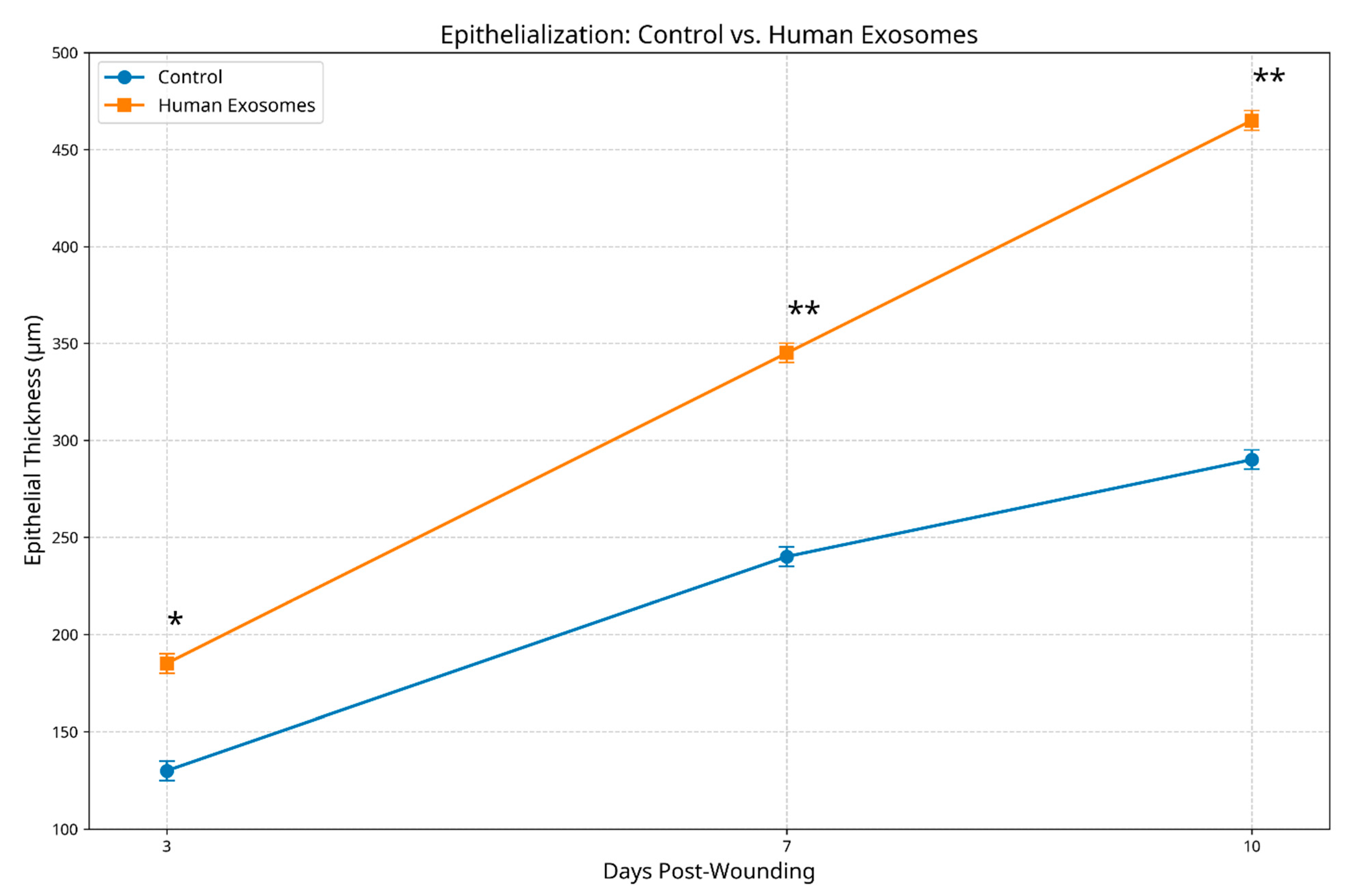
3.2.1. Epithelial Regenerative Patterns
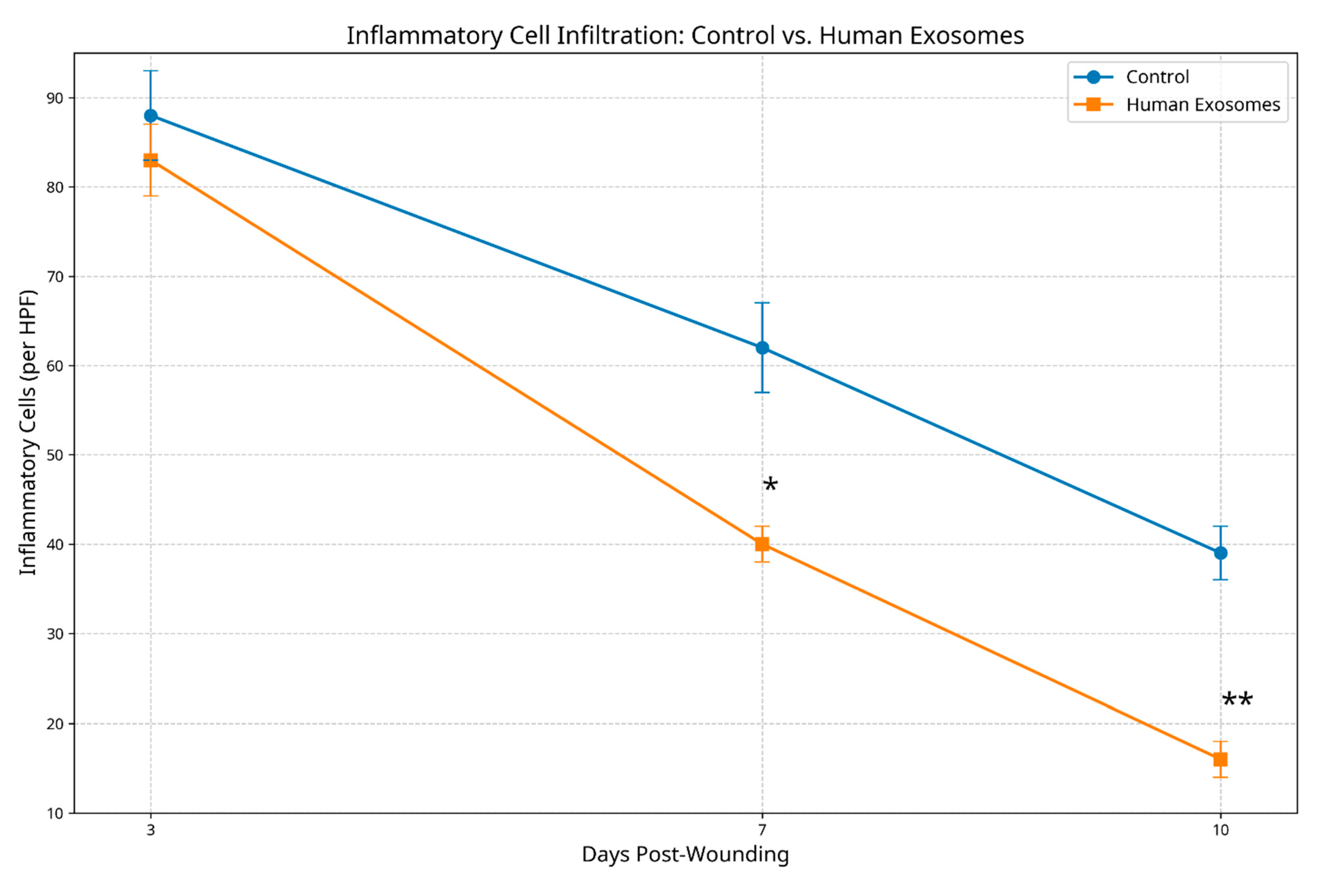
3.2.2. Inflammatory Response Patterns
3.2.3. Collagen Architectural Patterns
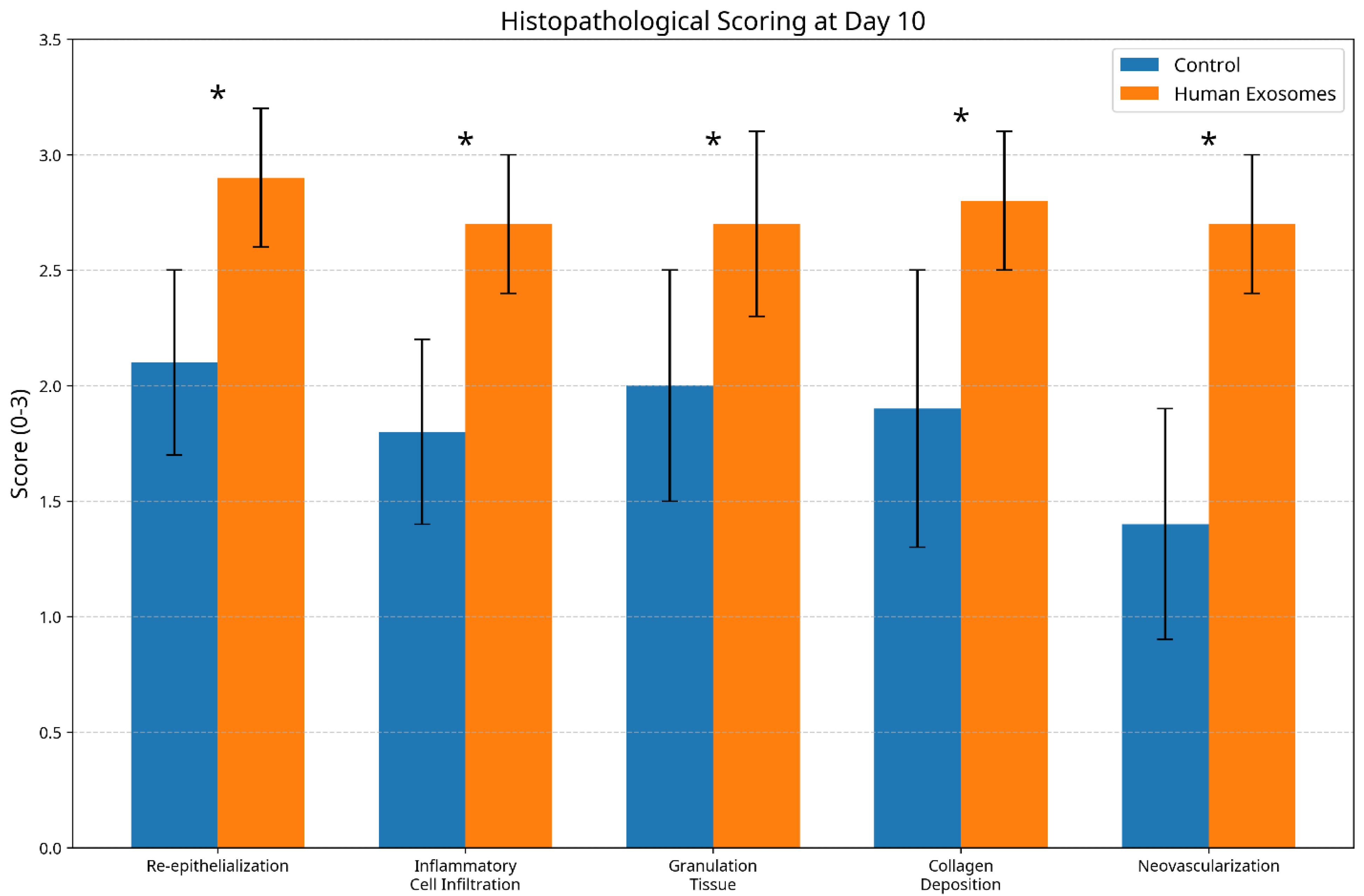
3.3. Histological Scoring Analysis
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, X.; Zhang, Y.; Liang, X.; Ding, Y.; Xu, Y.; Fang, Z.; Zhang, F. Enhanced wound healing with bioactive wound dressings: A comprehensive review of emerging technologies. Bioact. Mater. 2022, 18, 460–476. [Google Scholar]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J. Mol. Histol. 2017, 48, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 6977. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Sargent, I.L.; Harrison, P. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sharma, S. Oral biopsy: A dental gawk. J. Surg. Tech. Case Rep. 2010, 2, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chang, T.M.; Huang, H.C. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Attenuate Mast Cell Activation. Antioxidants 2022, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.L.; Liu, D.W. Mesenchymal stem cell-derived exosomes: An emerging therapeutic strategy for normal and chronic wound healing. World J. Clin. Cases 2021, 9, 6218–6233. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ritter, T. The Exosome—A Naturally Secreted Nanoparticle and its Application to Wound Healing. Adv. Mater. 2016, 28, 5542–5552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 234. [Google Scholar]
- Politis, C.; Schoenaers, J.; Jacobs, R.; Agbaje, J.O. Wound healing problems in the mouth. Front. Physiol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yichuan, L.; Keyu, Z.; Wenlin, J.; Kingjun, G.; Ting, W.; Song, G.; Zhanyong, Z. Exosomes from mesenchymal stem cells: Potential applications in wound healing. Life Sci. 2024, 357, 123066. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Liu, H.; Wang, Q.; Li, R.; Zhang, Q.; Hu, Q. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Wound Healing Through the WNT/β-catenin Signaling Pathway in Dermal Fibroblasts. Stem Cell Rev. Rep. 2022, 18, 1532–1546. [Google Scholar]
- Zhao, H.; Li, Z.; Wang, Y.; Zhou , K.; Li, H.; Bi, S.; Wang, Y.; Wu, W.; Huang, Y.; Peng, B.; et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front. Cell Dev. Biol. 2023, 11, 1029671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Wang, H.; Wu, Z.; Li, Z.; Wang, H.; Zhao, H.; Wang, H.; Liang, S. The functions and clinical application potential of exosomes derived from mesenchymal stem cells on wound repair: A review of recent research advances. Front. Immunol. 2023, 14, 1256687. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Li, S.; Xie, X.; Qin, L.; Zhang, Q.; Yang, Y.; Wang, T.; Zhang, Y. Exosomes: Compositions, biogenesis, and mechanisms in diabetic wound healing. J. Nanobiotechnology 2024, 22, 463. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Gong, S.; Liu, J.; Aghayants, S.; Liu, Y.; Wu, M.; Wu, Y.; Song , J.; Lin, Z. Adipose-derived stem cell exosomes: Mechanisms and therapeutic potentials in wound healing. Biomark. Res. 2025, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Fu, X.; Han, W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China Life Sci. 2016, 59, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Fakouri, A.; Razavi, Z.S.; Mohammed, A.T.; Hussein, A.H.A.; Afkhami, H.; Hooshiar, M.H. Applications of mesenchymal stem cell-exosome components in wound healing: New insights. Burns Trauma 2024, 12, 021. [Google Scholar]
- Geiger, A.; Walker, A.; Nissen, E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem. Biophys. Res. Commun. 2015, 467, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, H.; Mahdipour, M.; Ghaffari-Nasab, A.; Rahbarghazi, R. Xenogeneic Transplantation Promoted Human Exosome Sequestration in Rat Specific Organs. Adv. Pharm. Bull. 2023, 14, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Zheng, C.X.; Sui, B.D.; Liu, W.J.; Jin, Y. Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration. World J. Stem Cells 2022, 14, 318–329. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.M.; Hussain, Q.; Chuang, K.P.; Minh, H. Enhancement of Oral Mucosal Regeneration Using Human Exosomal Therapy in SD Rats. Biomedicines 2025, 13, 1785. https://doi.org/10.3390/biomedicines13071785
Lee CM, Hussain Q, Chuang KP, Minh H. Enhancement of Oral Mucosal Regeneration Using Human Exosomal Therapy in SD Rats. Biomedicines. 2025; 13(7):1785. https://doi.org/10.3390/biomedicines13071785
Chicago/Turabian StyleLee, Chien Ming, Qasim Hussain, Kuo Pin Chuang, and Hoang Minh. 2025. "Enhancement of Oral Mucosal Regeneration Using Human Exosomal Therapy in SD Rats" Biomedicines 13, no. 7: 1785. https://doi.org/10.3390/biomedicines13071785
APA StyleLee, C. M., Hussain, Q., Chuang, K. P., & Minh, H. (2025). Enhancement of Oral Mucosal Regeneration Using Human Exosomal Therapy in SD Rats. Biomedicines, 13(7), 1785. https://doi.org/10.3390/biomedicines13071785




